Abstract
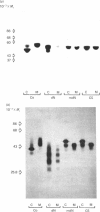
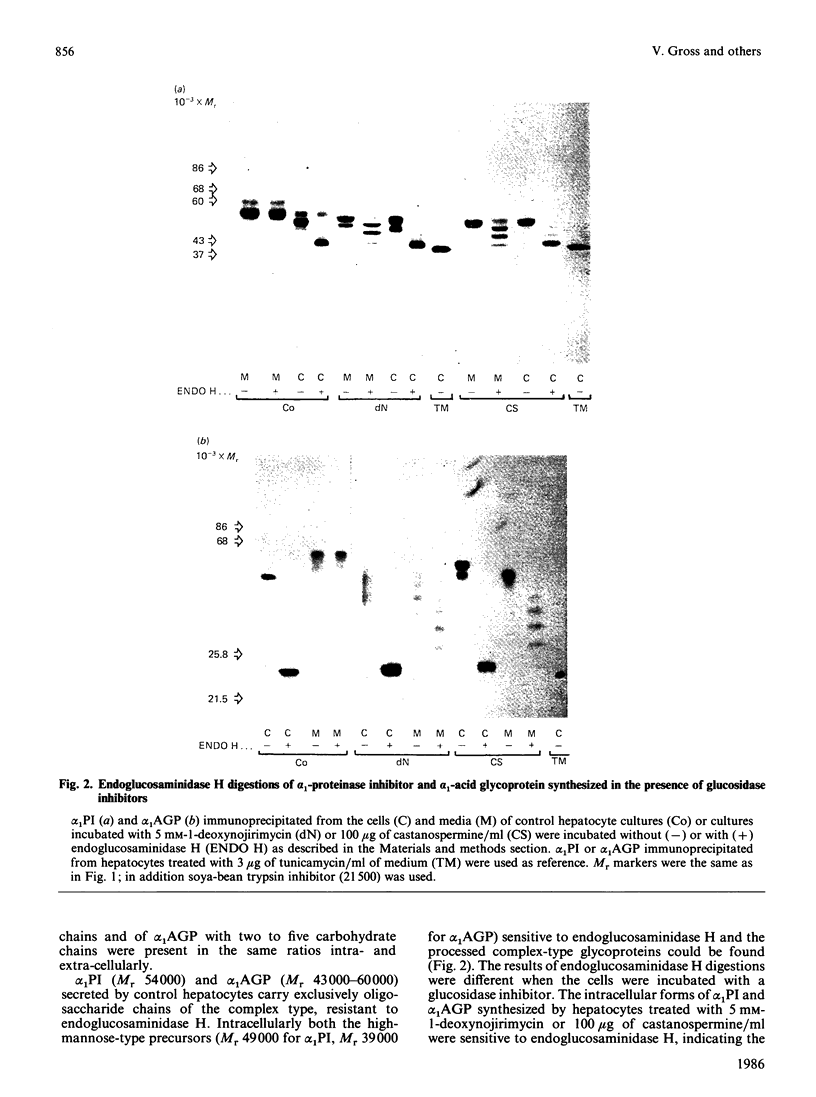
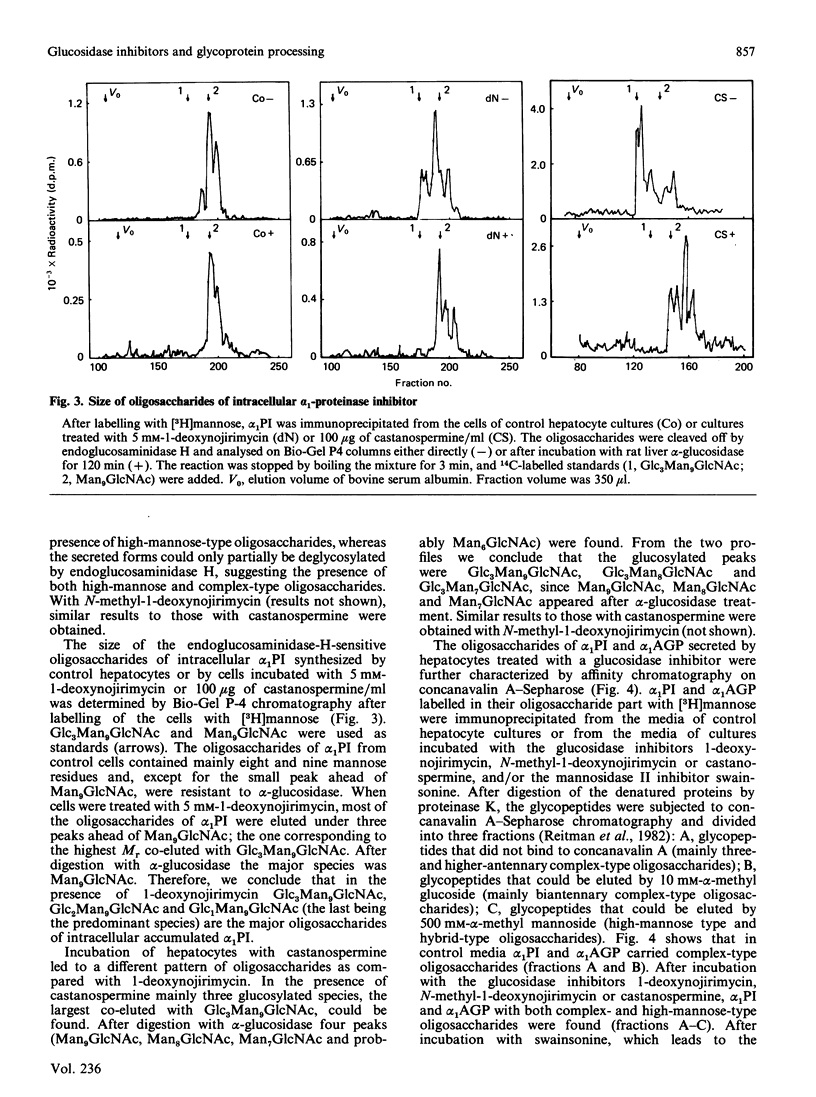
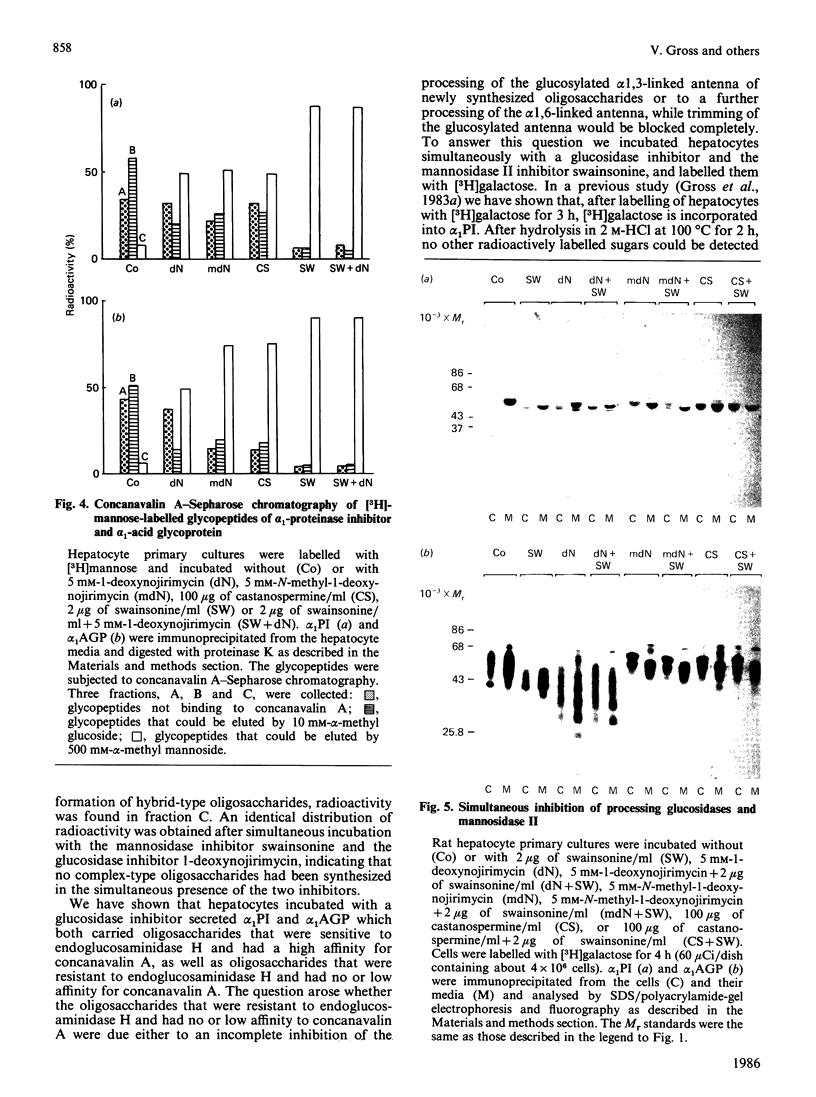
The glucosidase inhibitors 1-deoxynojirimycin, N-methyl-1-deoxynojirimycin and castanospermine were used to inhibit oligosaccharide processing in primary cultures of rat hepatocytes. Their effect on the glycosylation of alpha 1-proteinase inhibitor (alpha 1PI) and alpha 1-acid glycoprotein (alpha 1AGP) was studied. Of the three glucosidase inhibitors examined, 1-deoxynojirimycin inhibited not only oligosaccharide trimming but also glycosylation de novo of newly synthesized proteins, resulting in the formation of alpha 1PI with two and three (normally carrying three) and alpha 1AGP with two to five (normally carrying six) oligosaccharide side chains. In the presence of the glucosidase inhibitors, glucosylated high-mannose-type oligosaccharides accumulated. Whereas most of the endoglucosaminidase-H-sensitive oligosaccharides formed in the presence of 1-deoxynojirimycin contained only one glucose residue, N-methyl-1-deoxynojirimycin and castanospermine led mainly to the formation of oligosaccharides with three glucose residues. None of the three glucosidase inhibitors completely prevented the formation of complex-type oligosaccharides. Thus, in their presence, alpha 1PI and alpha 1AGP with a mixture of both high-mannose and complex-type oligosaccharides were secreted.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Jahreis G. P. Glucose starvation leads in rat hepatoma cells to partially N-glycosylated glycoproteins including alpha 1-acid glycoproteins. Identification by endoglycolytic digestions in polyacrylamide gels. J Biol Chem. 1983 Mar 25;258(6):3942–3949. [PubMed] [Google Scholar]
- Bischoff E., Wilkening J., Tran-Thi T. A., Decker K. Differentiation of the nucleotide pyrophosphatases of rat-liver plasma membranes and endoplasmic reticulum by enzymic iodination. Eur J Biochem. 1976 Feb 16;62(2):279–283. doi: 10.1111/j.1432-1033.1976.tb10158.x. [DOI] [PubMed] [Google Scholar]
- Bischoff J., Kornfeld R. Evidence for an alpha-mannosidase in endoplasmic reticulum of rat liver. J Biol Chem. 1983 Jul 10;258(13):7907–7910. [PubMed] [Google Scholar]
- Bischoff J., Kornfeld R. The effect of 1-deoxymannojirimycin on rat liver alpha-mannosidases. Biochem Biophys Res Commun. 1984 Nov 30;125(1):324–331. doi: 10.1016/s0006-291x(84)80371-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burke B., Matlin K., Bause E., Legler G., Peyrieras N., Ploegh H. Inhibition of N-linked oligosaccharide trimming does not interfere with surface expression of certain integral membrane proteins. EMBO J. 1984 Mar;3(3):551–556. doi: 10.1002/j.1460-2075.1984.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. M., Touster O. Purification and characterization of glucosidase II, an endoplasmic reticulum hydrolase involved in glycoprotein biosynthesis. J Biol Chem. 1982 Sep 10;257(17):9990–10000. [PubMed] [Google Scholar]
- Carlson J., Stenflo J. The biosynthesis of rat alpha 1-antitrypsin. J Biol Chem. 1982 Nov 10;257(21):12987–12994. [PubMed] [Google Scholar]
- Datema R., Romero P. A., Legler G., Schwarz R. T. Inhibition of formation of complex oligosaccharides by the glucosidase inhibitor bromoconduritol. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6787–6791. doi: 10.1073/pnas.79.22.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R., Romero P. A., Rott R., Schwarz R. T. On the role of oligosaccharide trimming in the maturation of Sindbis and influenza virus. Arch Virol. 1984;81(1-2):25–39. doi: 10.1007/BF01309294. [DOI] [PubMed] [Google Scholar]
- Datema R., Schwarz R. T. Effect of energy depletion on the glycosylation of a viral glycoprotein. J Biol Chem. 1981 Nov 10;256(21):11191–11198. [PubMed] [Google Scholar]
- Elbein A. D., Mitchell M., Sanford B. A., Fellows L. E., Evans S. V. The pyrrolidine alkaloid, 2,5-dihydroxymethyl-3,4-dihydroxypyrrolidine, inhibits glycoprotein processing. J Biol Chem. 1984 Oct 25;259(20):12409–12413. [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Legler G., Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984 Feb 23;307(5953):755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Mecke D. Perifused monolayer cultures of rat hepatocytes as an improved in vitro system for studies on ureogenesis. Exp Cell Res. 1979 Dec;124(2):349–359. doi: 10.1016/0014-4827(79)90210-6. [DOI] [PubMed] [Google Scholar]
- Grinna L. S., Robbins P. W. Glycoprotein biosynthesis. Rat liver microsomal glucosidases which process oligosaccharides. J Biol Chem. 1979 Sep 25;254(18):8814–8818. [PubMed] [Google Scholar]
- Gross V., Andus T., Tran-Thi T. A., Bauer J., Decker K., Heinrich P. C. Induction of acute phase proteins by dexamethasone in rat hepatocyte primary cultures. Exp Cell Res. 1984 Mar;151(1):46–54. doi: 10.1016/0014-4827(84)90354-9. [DOI] [PubMed] [Google Scholar]
- Gross V., Andus T., Tran-Thi T. A., Schwarz R. T., Decker K., Heinrich P. C. 1-deoxynojirimycin impairs oligosaccharide processing of alpha 1-proteinase inhibitor and inhibits its secretion in primary cultures of rat hepatocytes. J Biol Chem. 1983 Oct 25;258(20):12203–12209. [PubMed] [Google Scholar]
- Gross V., Geiger T., Tran-Thi T. A., Gauthier F., Heinrich P. C. Biosynthesis and secretion of alpha 1-antitrypsin in primary cultures of rat hepatocytes. Characterization of differently glycosylated intracellular and extracellular forms. Eur J Biochem. 1982 Dec 15;129(2):317–323. doi: 10.1111/j.1432-1033.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- Gross V., Kaiser C., Tran-Thi T. A., Schmelzer E., Witt I., Plummer T. H., Jr, Heinrich P. C. N-terminal amino acid sequences of precursor and mature forms of alpha-1-antitrypsin. FEBS Lett. 1983 Jan 24;151(2):201–205. doi: 10.1016/0014-5793(83)80069-6. [DOI] [PubMed] [Google Scholar]
- Gross V., Steube K., Tran-Thi T. A., McDowell W., Schwarz R. T., Decker K., Gerok W., Heinrich P. C. Secretion of high-mannose-type alpha 1-proteinase inhibitor and alpha 1-acid glycoprotein by primary cultures of rat hepatocytes in the presence of the mannosidase I inhibitor 1-deoxymannojirimycin. Eur J Biochem. 1985 Jul 1;150(1):41–46. doi: 10.1111/j.1432-1033.1985.tb08985.x. [DOI] [PubMed] [Google Scholar]
- Gross V., Tran-Thi T. A., Vosbeck K., Heinrich P. C. Effect of swainsonine on the processing of the asparagine-linked carbohydrate chains of alpha 1-antitrypsin in rat hepatocytes. Evidence for the formation of hybrid oligosaccharides. J Biol Chem. 1983 Mar 25;258(6):4032–4036. [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of membrane and secretory glycoproteins. Arch Biochem Biophys. 1981 Oct 1;211(1):1–19. doi: 10.1016/0003-9861(81)90423-9. [DOI] [PubMed] [Google Scholar]
- Hettkamp H., Legler G., Bause E. Purification by affinity chromatography of glucosidase I, an endoplasmic reticulum hydrolase involved in the processing of asparagine-linked oligosaccharides. Eur J Biochem. 1984 Jul 2;142(1):85–90. doi: 10.1111/j.1432-1033.1984.tb08253.x. [DOI] [PubMed] [Google Scholar]
- Hori H., Pan Y. T., Molyneux R. J., Elbein A. D. Inhibition of processing of plant N-linked oligosaccharides by castanospermine. Arch Biochem Biophys. 1984 Feb 1;228(2):525–533. doi: 10.1016/0003-9861(84)90019-5. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Ikehara Y., Miyasato M., Ogata S., Oda K. Multiple forms of rat-serum alpha 1-protease inhibitor. Involvement of sialic acid in the multiplicity of three original forms. Eur J Biochem. 1981 Apr;115(2):253–260. doi: 10.1111/j.1432-1033.1981.tb05231.x. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Cathepsin D and beta-hexosaminidase synthesized in the presence of 1-deoxynojirimycin accumulate in the endoplasmic reticulum. J Biol Chem. 1984 Aug 25;259(16):10129–10135. [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Glucose removal from N-linked oligosaccharides is required for efficient maturation of certain secretory glycoproteins from the rough endoplasmic reticulum to the Golgi complex. J Cell Biol. 1984 May;98(5):1720–1729. doi: 10.1083/jcb.98.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima M., Urban J., Schreiber G. Intrahepatic precursor form of rat alpha 1-acid glycoprotein. Isolation and properties. J Biol Chem. 1980 May 25;255(10):4951–4956. [PubMed] [Google Scholar]
- Pan Y. T., Hori H., Saul R., Sanford B. A., Molyneux R. J., Elbein A. D. Castanospermine inhibits the processing of the oligosaccharide portion of the influenza viral hemagglutinin. Biochemistry. 1983 Aug 2;22(16):3975–3984. doi: 10.1021/bi00285a038. [DOI] [PubMed] [Google Scholar]
- Peyrieras N., Bause E., Legler G., Vasilov R., Claesson L., Peterson P., Ploegh H. Effects of the glucosidase inhibitors nojirimycin and deoxynojirimycin on the biosynthesis of membrane and secretory glycoproteins. EMBO J. 1983;2(6):823–832. doi: 10.1002/j.1460-2075.1983.tb01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M. L., Trowbridge I. S., Kornfeld S. A lectin-resistant mouse lymphoma cell line is deficient in glucosidase II, a glycoprotein-processing enzyme. J Biol Chem. 1982 Sep 10;257(17):10357–10363. [PubMed] [Google Scholar]
- Romero P. A., Datema R., Schwarz R. T. N-methyl-1-deoxynojirimycin, a novel inhibitor of glycoprotein processing, and its effect on fowl plague virus maturation. Virology. 1983 Oct 15;130(1):238–242. doi: 10.1016/0042-6822(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Romero P. A., Friedlander P., Herscovics A. Deoxynojirimycin inhibits the formation of Glc3Man9GlcNAc2-PP-dolichol in intestinal epithelial cells in culture. FEBS Lett. 1985 Apr 8;183(1):29–32. doi: 10.1016/0014-5793(85)80947-9. [DOI] [PubMed] [Google Scholar]
- Romero P. A., Saunier B., Herscovics A. Comparison between 1-deoxynojirimycin and N-methyl-1-deoxynojirimycin as inhibitors of oligosaccharide processing in intestinal epithelial cells. Biochem J. 1985 Mar 15;226(3):733–740. doi: 10.1042/bj2260733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul R., Chambers J. P., Molyneux R. J., Elbein A. D. Castanospermine, a tetrahydroxylated alkaloid that inhibits beta-glucosidase and beta-glucocerebrosidase. Arch Biochem Biophys. 1983 Mar;221(2):593–597. doi: 10.1016/0003-9861(83)90181-9. [DOI] [PubMed] [Google Scholar]
- Saunier B., Kilker R. D., Jr, Tkacz J. S., Quaroni A., Herscovics A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J Biol Chem. 1982 Dec 10;257(23):14155–14161. [PubMed] [Google Scholar]
- Schlesinger S., Malfer C., Schlesinger M. J. The formation of vesicular stomatitis virus (San Juan strain) becomes temperature-sensitive when glucose residues are retained on the oligosaccharides of the glycoprotein. J Biol Chem. 1984 Jun 25;259(12):7597–7601. [PubMed] [Google Scholar]
- Snider M. D., Robbins P. W. Synthesis and processing of asparagine-linked oligosaccharides of glycoproteins. Methods Cell Biol. 1981;23:89–100. doi: 10.1016/s0091-679x(08)61493-4. [DOI] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. alpha-D-Mannosidases of rat liver Golgi membranes. Mannosidase II is the GlcNAcMAN5-cleaving enzyme in glycoprotein biosynthesis and mannosidases Ia and IB are the enzymes converting Man9 precursors to Man5 intermediates. J Biol Chem. 1982 Apr 10;257(7):3660–3668. [PubMed] [Google Scholar]
- Ugalde R. A., Staneloni R. J., Leloir L. F. Action of glycosidases on the saccharide moiety of the glucose--containing dolichyl diphosphate oligosaccharide. FEBS Lett. 1978 Jul 15;91(2):209–212. doi: 10.1016/0014-5793(78)81174-0. [DOI] [PubMed] [Google Scholar]
- Yoshima H., Matsumoto A., Mizuochi T., Kawasaki T., Kobata A. Comparative study of the carbohydrate moieties of rat and human plasma alpha 1-acid glycoproteins. J Biol Chem. 1981 Aug 25;256(16):8476–8484. [PubMed] [Google Scholar]