Abstract
Because the persistence of human immunodeficiency virus (HIV) in cellular reservoirs presents an obstacle to viral eradication, we evaluated whether tumor necrosis factor-related apoptosis-inducing ligand (TRAIL/Apo2L) induces apoptosis in such reservoirs. Lymphocytes and monocyte-derived macrophages (MDM) from uninfected donors do not die following treatment with either leucine zipper human TRAIL (LZhuTRAIL) or agonistic anti-TRAIL receptor antibodies. By contrast, such treatment induces apoptosis of in vitro HIV-infected MDM as well as peripheral blood lymphocytes from HIV-infected patients, including CD4+ CD45RO+ HLA-DR− lymphocytes. In addition, LZhuTRAIL-treated cells produce less viral RNA and p24 antigen than untreated controls. Whereas untreated cultures produce large amounts of HIV RNA and p24 antigen, of seven treated CD4+ CD45RO+ HLA-DR− cell cultures, viral RNA production was undetectable in all, p24 antigen was undetectable in six, and proviral DNA was undetectable in four. These data demonstrate that TRAIL induces death of cells from HIV-infected patients, including cell types which harbor latent HIV reservoirs.
Peripheral blood lymphocytes (PBL) isolated from patients infected with human immunodeficiency virus (HIV), as well as cells infected with HIV in vitro, exhibit alterations in the physiological mechanisms controlling T-cell apoptosis (3, 35). Although only a minority of CD4 T cells become infected by HIV, most that are infected undergo apoptotic cell death (29). Furthermore, a significant number of uninfected CD4 and CD8 T cells die by apoptosis induced either by immunological activation, by the effects of HIV proteins, or by elevated levels of death-inducing ligands produced by infected cells (reviewed in reference 3). In contrast to the usual fate of HIV-infected T cells, some cells do not die following direct infection. As well, in a small fraction of CD4+ T cells, infection with HIV does not result in apoptosis, but in a state of latent infection that appears to be critical in the persistence of HIV infection (16, 17).
The development of postintegration latency has been postulated to be a reversion of activated HIV-infected CD4+ T cells to resting memory cells in which viral transcription is absent (16) and HIV is retained as an integrated provirus. These infected resting memory (CD4+ CD45RO+ HLA-DR−) T cells have an estimated half-life of more than 6 months (16), and the unique resistance of such latently infected T cells and HIV-infected macrophages to HIV-induced apoptosis may be the critical step required for the development of viral reservoirs (17). It is the presence of latently infected CD4 T cells and HIV-infected macrophages that prevents complete virus eradication by standard antiretroviral therapies. Recent attempts to eradicate HIV reservoirs by using agents including interleukin-2, an anti-CD45RO immunotoxin, an anti-CD3 antibody, and a therapeutic HIV vaccine (7, 8, 36; M. Van Praag, J. Prins, I. Berge, P. Schellekens, and J. Lange, presented at the Fifth Conference on Retroviruses and Opportunistic Infections, 31 January to 4 February 1999, Chicago, Ill.) have so far been unsuccessful and highlight the need for novel therapeutic approaches.
TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) is a member of the tumor necrosis factor (TNF) superfamily and was identified by sequence homology with Fas ligand (FasL) and TNF (1). There are five cognate receptors for TRAIL/Apo2L, yet only two (TRAIL-R1 and TRAIL-R2) contain death domains that trigger the apoptotic caspase cascade (22). In contrast, TRAIL-R3, TRAIL-R4, and osteoprotegerin lack functional death-signaling domains (12, 14, 53). TRAIL/Apo2L can induce apoptosis in tumor cells and cytomegalovirus-infected cells (47) but is not cytotoxic to normal cells and does not induce tissue injury following injection in murine and nonhuman primate models (1, 21, 22, 48, 50). The ability of TRAIL to kill transformed cells, as well as the resistance of normal cells to TRAIL, has led to its preclinical evaluation as a potential therapy for selected human malignancies.
The regulation of TRAIL/Apo2L and TRAIL receptors in HIV infection is undefined. Reports that TRAIL/Apo2L may contribute to HIV-associated activation-induced cell death (32, 34) suggest that the regulation of TRAIL and its receptors may be altered in patients with HIV infection. Furthermore, it has recently been proposed that TRAIL may be involved in CD4 T-cell depletion in a Hu-PBL-SCID model (38). In the present study, we have demonstrated altered regulation of TRAIL/Apo2L and TRAIL receptor expression in T cells infected with HIV in vitro as well as in T cells from HIV-infected patients. Therefore, we have evaluated the potential therapeutic utility of TRAIL/Apo2L. Further, TRAIL/Apo2L treatment in vitro induces apoptosis, significantly reduces the amount of replication-competent HIV in treated compared to untreated cells, and significantly decreases the amount of integrated HIV provirus in resting memory cells, in some cases to undetectable levels. Thus, TRAIL/Apo2L may offer a new therapeutic approach toward eradication of HIV in infected patients.
MATERIALS AND METHODS
Study patients.
HIV-infected patients and HIV-negative healthy donors were recruited from the Ottawa Hospital, General Campus, following informed consent. The study protocol was reviewed and approved by the institutional review board. For experiments assessing TRAIL sensitivity, HIV patients were randomly selected; some were on therapy, and some had suppressed viral replication. For coculture experiments, only patients with suppressed viral replication (less than 50 copies/ml) for >12 months were chosen.
In vitro Jurkat HIV infection.
Jurkat T cells (American Type Culture Collection [ATCC]) were maintained in RPMI 1640 containing 10% heat-inactivated fetal bovine serum and 2 mM (each) l-glutamine, penicillin, and streptomycin. All cell culture products were purchased from Canadian Life Technology (Montreal, Quebec, Canada) unless otherwise stated. Cells were either infected with 100 ng of HIVIIIB (National Institutes of Health [NIH] AIDS Research and Reference Reagent Program)/ml (2) or mock infected in the presence of 10 ng of Polybrene/ml for 4 h, washed twice in complete medium, and incubated at 37°C in a humidified 5.0% CO2 environment.
Cell culture.
Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation in Ficoll (Pharmacia, Toronto, Ontario, Canada), washed once with phosphate-buffered saline (PBS), and resuspended in medium containing RPMI 1640 supplemented with 10% heat inactivated AB serum (Sigma, Grand Island, N.Y.) and 2 mM (each) l-glutamine, penicillin, and streptomycin. To obtain PBL, monocytes were depleted by adherence for 1 h. Cells were kept at 37°C in a humidified 5% CO2 environment. Where indicated, cells were treated with recombinant gp120 (1 μg/ml) (NIH AIDS Research and Reference Reagent Program).
Detection of TRAIL receptor mRNA expression by RT-PCR.
Total mRNA was isolated using the RNeasy Mini Prep (Qiagen, Toronto, Ontario, Canada) and quantified by UV spectrophotometry (Becton Dickinson, Toronto, Ontario, Canada). cDNA synthesis was performed by using Superscript reverse transcriptase PCR (RT-PCR) (Canadian Life Technology) with conditions and primers described and used previously for estimation of message intensity (24, 47). Prior to our experiments, we confirmed that these conditions allow detection of the PCR product within the linear range of the assay (24, 47). Samples were resolved on a 1.0% agarose gel and visualized by ethidium bromide. Surface expression of TRAIL receptors was determined by flow cytometry and analyzed using 1.0 μg of mouse monoclonal antibodies (MAbs) to TRAIL-R1 (clone M271, immunoglobulin G2a [IgG2a]), TRAIL-R2 (clone M412, IgG1), TRAIL-R3 (clone M430, IgG1), and TRAIL-R4 (clone 445, IgG1) (all from Immunex Corporation) (21). A total of 106 cells were incubated with primary MAbs in PBS–1% bovine serum albumin (BSA) for 1 h on ice, washed, and stained sequentially, first with 1:100 biotinylated goat-anti-mouse IgG1/IgG2a (Immunotech, Toronto, Ontario, Canada) and then with 1:500 streptavidin-phycoerythrin (PE) (Pharmingen, Toronto, Ontario, Canada). For each sample, isotype IgG1/IgG2a (Immunotech)-matched controls were used. For detection of TRAIL receptors on macrophages, culture medium was poured off, followed by the addition of 10 ml of ice-cold PBS. Macrophages were scraped off T75 flasks, and 106 cells were used for isolation of RNA or flow cytometry. RT-PCR assessment of macrophage expression of TRAIL receptors was performed as described above for T cells, and flow cytometry of TRAIL receptor expression was performed as described above with the following modification: prior to primary antibody staining, monocyte-derived macrophages (MDM) were incubated in PBS–10% AB serum for 30 min at 4°C.
Preparation of MDM.
PBMC were isolated from healthy donors, and monocytes were isolated by adherence in T125 flasks for 2 h in RPMI 1640 containing 10% heat-inactivated AB serum. Cells were scraped and counted, and 106 cells were then plated on microslides (Nalge, Naperville, Ill.). Fifty percent of the medium was changed every 3 days. On day 6, cells were either mock infected or infected with 100 pg of HIVBal (NIH AIDS Research and Reference Reagent Program)/ml, and on day 16, where appropriate, 100 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF)/ml was added to each culture. One microgram of leucine zipper human TRAIL (LZhuTRAIL) was added on day 17, and apoptosis was measured 12 h following treatment by using terminal deoxyuridine nucleotide end labeling (TUNEL) (Intergen, Purchase, N.Y.) according to the manufacturer's instructions. Three hundred cells from each condition were counted individually by three different laboratory personnel, all of whom were unaware of the treatment conditions, and the average scores were used for data analysis.
LZhuTRAIL treatment.
MDM or PBL were treated for 12 h with LZhuTRAIL at 1 μg/ml unless otherwise stated (Immunex Corporation). LZhuTRAIL is a recombinant preparation of human TRAIL that forms trimers due to a terminal leucine zipper motif (49, 50). For studies using agonistic MAbs to induce apoptosis, 1.0 μg of the MAb or isotype control (Immunotech) was plated in 24-well culture dishes in PBS for 1 h at 4°C and washed twice with PBS before addition of cells.
Detection of apoptosis.
Apoptotic cell death was determined using Hoechst 33342 (Molecular Probes, Eugene, Oreg.) or TUNEL (Intergen) staining. For Hoechst staining, cells were washed in ice-cold PBS, resuspended in PBS–1% BSA, and stained for 20 min at 4°C in 5 μl of various antibody combinations as follows (unless otherwise stated, all antibodies were purchased from Becton Dickinson, Oakville, Ontario, Canada): anti-CD4–PE/Texas Red (Coulter), anti-CD8–PE (Immunotech), anti-CD45RO allophycocyanin (APC), anti-CD62L–fluorescein isothiocyanate, anti-CD45RA–fluorescein isothiocyanate allophycocyanin (APC), and anti-HLA-DR–PE APC. Stained cells were washed with ice-cold PBS and incubated with 1 μg of Hoechst stain for exactly 7 min (2, 28). After two washes with ice-cold PBS, cells were analyzed by flow cytometry (Coulter Epics Altra). For apoptosis detection using TUNEL (ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit; Intergen), all assays were performed according to the manufacturer's instructions with the following modifications. Cells were fixed and permeabilized using Fix and Perm reagent (Cedarline Products) according to the manufacturer's specifications. Data are expressed as median TRAIL-specific apoptosis (TSA), calculated as percent apoptosis following TRAIL/Apo2L treatment minus percent apoptosis in the control sample.
Isolation of latently infected CD4+ T cells.
Resting memory CD4+ HLA-DR− CD45RO+ T cells were isolated from PBMC using magnetic bead separation (Miltenyi Biotec) as described elsewhere (16). All antibodies for magnetic bead separation were purchased from Becton Dickinson. A second purification step was performed by sorting flow cytometry using anti-CD4-ECD (Coulter) and anti-HLA-DR–PE (Becton Dickinson, Oakville, Ontario, Canada). The purity of final CD4+ HLA-DR− CD45RO+ cell suspensions was ≥98%.
Quantitative micrococulture assay.
To determine the frequency of latently infected cells, highly purified resting memory cells were subjected to a high-input quantitative micrococulture assay as previously described (7). We used the maximum available number of cells in each coculture for each patient. The starting cell concentrations ranged from 3.4 × 106 to 5.0 × 107 cells. Following overnight LZhuTRAIL (or mock) treatments, cells were washed three times and then coincubated with irradiated feeder cells for 14 days. For the macrophage coculture, we isolated MDM from healthy donors and mock- or HIVBal-infected samples on day 6 following isolation. The following day, cells were treated with 1 μg of LZhuTRAIL overnight, washed three times, and resuspended in culture medium. On day 14, p24 and viral RNA levels were measured. p24 antigen was measured in duplicate using a commercial enzyme-linked immunosorbent assay (NEN, Life Sciences Products, Boston, Mass.). In independent experiments we determined that LZhuTRAIL treatment does not impair the ability of resting T cells to become activated (data not shown).
HIV viral load testing.
Tissue culture supernatants were processed and stored at −80°C until the time of testing. Testing was performed either by the Amplicor assay for studies involving MDM or by the Quantiplex bDNA assay for studies using PBL or sorted cells from HIV-infected patients.
(i) Amplicor assay.
The Amplicor HIV monitor 1.5 assay (Roche Diagnostics, Laval, Quebec, Canada) was performed according to the manufacturer's instructions, and all samples were run singly following a three-step workflow: specimen preparation (including viral lysis, RNA precipitation, RNA washing, and suspension of purified RNA in buffer), amplification, and detection. This assay has a range of sensitivity from 400 to 750,000 HIV RNA copies/ml.
(ii) Quantiplex assay.
The Quantiplex bDNA 3.0 assay (Bayer Diagnostics, Markham, Ontario, Canada) was performed in conjunction with the semiautomated Quantiplex 340 system according to the manufacturer's instructions, and all samples were run singly. Samples with values between 50 and 500,000 RNA copies/ml were within the limit of quantitation for this assay.
Detection of proviral HIV DNA. (i) DNA extraction from cell pellets.
DNA was extracted from cell pellets using the extraction reagent from the Amplicor Whole Blood Specimen Preparation Kit (Roche Diagnostics). Two hundred fifty microliters of extraction reagent was added to each pellet, and the pellets were incubated in a dry-heat block for 30 min at 100°C. The samples were vortexed briefly and stored at −20°C until further use.
(ii) DQ-α or DQ-γ gene amplification.
The presence and quality of the DNA were determined by PCR amplification of the human DQ-γ gene by the method of Ehrlich et al. (13). Briefly, PCR mixtures contained, per 50 μl, 5 μl of 10× PCR buffer (Perkin-Elmer, Mississauga, Ontario, Canada), 200 μM each deoxynucleoside triphosphate (Perkin-Elmer), 1 μM (each) primer GH26 (5′-GTGCTGCAGGTGTAAACTTGTACCAG-3′) and primer GH27 (5′-CACGGATCCGGTAGCAGCGGTAGAGTTG-3′), 1 U of AmpliTaq DNA polymerase (Perkin-Elmer), and 12.5 μl of sample. Samples were amplified for 35 cycles (96°C for 30 s, 60°C for 30 s, and 72°C for 30 s). Amplified PCR products (243 bp) were separated by 1% agarose gel electrophoresis for 30 min at 100 V and visualized by ethidium bromide staining and UV transillumination of the gel. Amplification and detection of HIV-1-specific samples were analyzed using the Amplicor HIV-1 amplification and detection kits (Roche Diagnostics) according to the manufacturer's instructions. As a positive control, and to assess the sensitivity of the assay, 8E5 cells which contain 1 proviral copy/cell were used. Limiting-dilution analysis revealed a sensitivity of 2 copies in this PCR. To eliminate the possibility of false-negative results, all negative results from the Roche assay were subjected to nested PCR using primers specific to the pol region (18). PCR mixtures contained, per 50 μl, 5 μl of 10× PCR buffer (Perkin-Elmer), 200 μM each deoxynucleoside triphosphate (Perkin Elmer), 1 μM each primer, 1 U of AmpliTaq DNA polymerase (Perkin-Elmer), and 2.25 mM MgCl2 (Perkin-Elmer). The outer primer pair consisted of HPOL4235 (5′-CCCTACAATCCCCAAAGTCAAGG-3′) and HPOL4538 (5′-TACTGCCCCTTCACCTTTCCA-3′), and 12.5 μl of sample was used in the first-round reaction. Two microliters of the first-round product was added to the second-round PCR mixture using the inner primers HPOL4327 (5′-TAAGACAGCAGTACAAATGGCAG-3′) and HPOL4481 (5′-GCTGTCCCTGTAATAAACCCG-3′). In both the first and second rounds, samples were amplified for 35 cycles (96°C for 30 s, 65°C for 30 s, and 72°C for 30 s). Second-round PCR products (175 bp) were separated by 1% agarose gel electrophoresis for 30 min at 100 V, followed by ethidium bromide staining and UV transillumination of the gel. Limiting-dilution analysis reveals a sensitivity of 1 copy of the 8E5 gag DNA, as previously described (31).
Statistics.
Infectious units per million (IUPM) were estimated, using data from limiting-dilution cocultures, according to maximum-likelihood methods (39). Statistical comparisons between treatment groups and control groups were performed using a standard Student's t test. The 95% confidence intervals for individuals determinations spanned 1.1 log units.
RESULTS
TRAIL/Apo2L and TRAIL receptor expression are altered following HIV infection in vitro and in vivo.
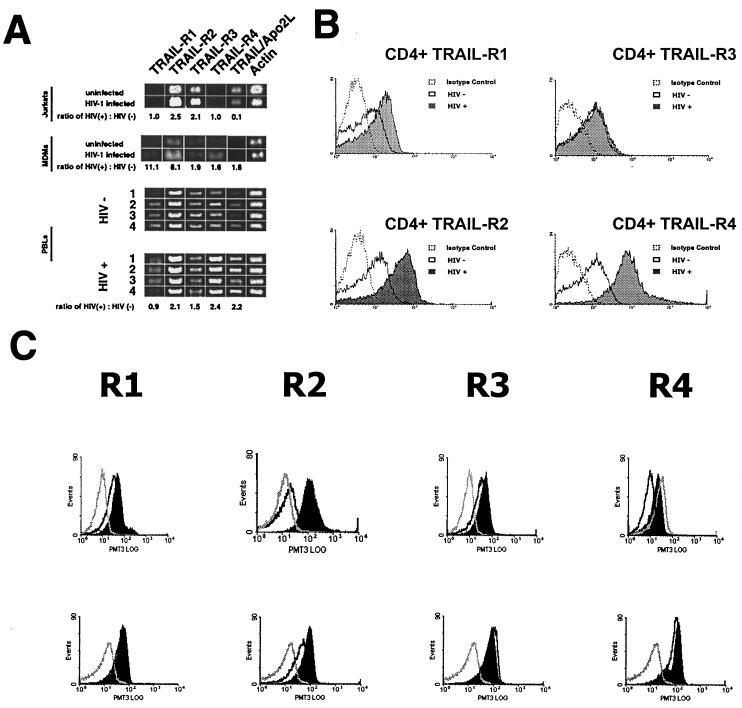
To evaluate whether HIV-infected T cells demonstrate changes in TRAIL/Apo2L and/or TRAIL receptor expression, Jurkat T cells were infected with HIVIIIB (2) and expression of mRNAs specific for TRAIL receptors 1, 2, 3, and 4 and for TRAIL/Apo2L was determined by RT-PCR (24, 47). The relative band intensities of amplified products for each receptor and TRAIL were measured and normalized to the intensity of amplified β-actin message. The relative band intensities of TRAIL-R2 (P < 0.04; n = 3) and -R3 (P < 0.04; n = 3) mRNAs were significantly increased (2.1- to 2.5-fold) in infected cells over those in mock-infected cells, while those of TRAIL-R1 (P = 0.1; n = 3) and TRAIL-R4 (P < 0.1; n = 3) mRNAs were unchanged (Fig. 1A, top panel). No differences in TRAIL/Apo2L mRNA expression were observed between infected and uninfected cells.
FIG. 1.
(A) Analysis of TRAIL-R1, TRAIL-R2, TRAIL-R3, TRAIL-R4, and TRAIL mRNA expression by RT-PCR. (Top) HIV-infected Jurkat T cells show increased message for TRAIL-R2, TRAIL-R3, and TRAIL/Apo2L compared to mock-infected controls (normalized to β-actin). (Center) HIV-1Bal-infected MDM show increased message for all TRAIL receptors and TRAIL/Apo2L compared to mock-infected controls. (Bottom) PBL from four HIV-1-positive individuals show increased message for TRAIL-R2, -R3, and -R4 or TRAIL/Apo2L compared to PBL from four HIV-1-negative individuals. (B) Cell surface expression of TRAIL-R1, -R2, -R3, and -R4 in CD4+ T cells from HIV-infected patients and healthy controls. Open histograms with dotted lines, cells from HIV-infected patients stained with an isotype control MAb; shaded histograms, cells from HIV-1-infected patients stained with anti-TRAIL receptor MAbs; open histograms with solid lines, cells from healthy controls stained with anti-TRAIL receptor MAbs. (C) Jurkat T cells (top) or PBL (bottom) from HIV-negative patients were treated with gp120 or a control (BSA) as indicated and analyzed by flow cytometry for TRAIL receptor expression. Open histograms with dotted lines, isotype control staining; open histograms with solid lines, cells treated with BSA and stained with the indicated anti-TRAIL receptor antibody; shaded histograms, cells treated with gp120 and stained with the indicated anti-TRAIL receptor antibody.
Next, using RT-PCR, we examined TRAIL/Apo2L and TRAIL receptor expression in PBL from patients infected with HIV. RNAs for all four receptors as well as TRAIL were detected, and significant differences were found between HIV-positive and HIV-negative samples. Densitometric analysis of the PCR products from PBL of four HIV-infected donors compared to those for four uninfected individuals revealed a consistent 2.0- to 2.4-fold increase in the mRNA expression level of TRAIL-R2 (P = 0.001), TRAIL-R4 (P = 0.01), and TRAIL/Apo2L (P = 0.02) (Fig. 1A, bottom panels). However, levels of TRAIL-R1 (P = 0.690) and TRAIL-R3 (P = 0.286) mRNAs in HIV-infected and uninfected individuals were similar. We also analyzed mRNA expression in MDM that were mock infected or infected with HIVBal in vitro. In comparison to mock-infected MDM, HIV-infected MDM had increased expression of all four TRAIL receptors and TRAIL/Apo2L (n = 3; P < 0.01) (Fig. 1A, center panel).
The cell surface expression of all four TRAIL receptors was also evaluated by flow cytometry using TRAIL receptor-specific antibodies (23). In accordance with the RT-PCR results, the cell surface expression of TRAIL-R2 (mean channel fluorescence [MCF], 3.0 for uninfected and 9.6 for infected CD4+ T cells [P < 0.001] and 0.6 for uninfected and 1.8 for infected CD8+ T cells [P < 0.001]) and TRAIL-R4 (MCF, 1.7 for uninfected and 11.2 for infected CD4+ T cells [P < 0.001] and 0.2 for uninfected and 2.1 for infected CD8+ T cells [P < 0.001]) was increased on both CD4+ T cells (Fig. 1B) and CD8+ T cells (data not shown) from 7 HIV-infected patients compared to those from 19 HIV-negative donors tested, while levels of TRAIL-R1 and TRAIL-R3 were not significantly altered. In mock- or HIVBal-infected MDM (data not shown), in vitro infection was associated with increased levels of TRAIL-R1 (n = 3; MCF, 2.4 for uninfected and 5.8 for infected cells [P = 0.01]), TRAIL-R3 (n = 3; MCF, 4.1 for uninfected and 13.2 for infected cells [P < 0.004]), and TRAIL-R4 (n = 3; MCF, 2.7 for uninfected and 9.6 for infected cells [P = 0.006]), while TRAIL-R2 expression was not significantly changed (n = 3; P < 0.1).
To investigate potential mechanisms involved in TRAIL-R2 upregulation, we treated both Jurkat T cells and primary T cells from uninfected donors with either HIV gp120 or a control (BSA). Following 16 h of treatment, cells were analyzed by flow cytometry. Significant increases in TRAIL-R2 expression were observed following gp120 treatments, but not control treatments, in both Jurkat T cells and primary T cells (Fig. 1C).
These data demonstrate significant dysregulation of TRAIL receptor expression in cells from patients infected with HIV and following HIV infection or gp120 treatment in vitro, and they suggest that both HIV-infected and uninfected cells from HIV-infected patients are sensitive to TRAIL receptor ligation.
Cells from HIV-positive patients undergo cell death following in vitro treatment with LZhuTRAIL and agonistic TRAIL receptor antibodies.
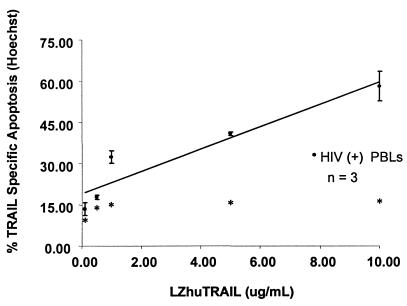
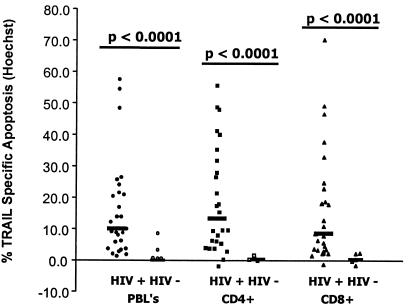
Previous studies have shown that surface expression of TRAIL receptors is insufficient to predict the sensitivity of cells to TRAIL-induced killing (21, 30). Therefore, PBL from HIV-infected individuals with various treatment histories and viral loads were cultured in vitro with LZhuTRAIL and analyzed for TRAIL-mediated killing. Dose-dependent death of PBL (Fig. 2) and CD4+ T cells (data not shown) was observed in cells from HIV-positive patients treated with increasing amounts of LZhuTRAIL but not in cells from HIV-negative donors (data not shown). Similarly, cells treated with gp120, but not cells treated with a control protein, developed a dose-dependent sensitivity to TRAIL-mediated killing (data not shown). By Hoechst staining, the median TSA in PBL from HIV-infected patients was 10.0% (n = 26), compared to 0.70% (n = 5) in PBL from healthy donors (P < 0.0001) (Fig. 3). The median TSA observed in CD4+ T cells from HIV-infected patients was 13.3% (n = 26), compared to 0.3% for healthy donors (n = 5; P = 0.0001) (Fig. 3), while the median TSA in CD8+ T cells was 8.5% (n = 26), compared to 0.3% (n = 5; P < 0.0001) (Fig. 3) for controls. To confirm the results obtained by Hoechst staining, we also analyzed apoptosis following TRAIL/Apo2L treatment in specimens obtained from an additional 10 HIV-infected and 5 uninfected patients using TUNEL. In confirmation of the results obtained by Hoechst staining, the median TSA in HIV-positive PBL was 4.65% (n = 10), whereas that for HIV-negative donors was only 0.7% (n = 5; P < 0.0001) (data not shown). The median TSA in HIV-positive CD4+ cells was 8.3% (n = 5), while the median TSA for healthy controls was 0.3% (n = 5; P = 0.001); the median TSA in HIV-positive CD8+ cells was 4.7% (n = 5), in contrast to controls, where the median TSA was 0.3% (n = 5; P = 0.004). Further, agonistic MAbs directed against TRAIL-R1 (clone M271) and TRAIL-R2 (clones M412 and M413) (23) induced apoptosis in PBL (the median TSA was 38.3% for controls [n = 7], 64.3% with M271 [n = 7; P = 0.004], 46.6% with M412 [n = 7; P = 0.03], and 66.6% with M413 [n = 7; P = 0.03]), CD4+ (median TSA, 3.2% for controls [n = 7], 16.2% with M271 [n = 7; P = 0.02], 6.4% with M412 [n = 7; P = 0.01], and 13.7% with M413 [n = 7; P = 0.02]), and CD8+ (median TSA, 3.0% for controls [n = 7], 17.2% with M271 [n = 7; P = 0.004], 6.5% with M412 [n = 7; P = 0.01], and 14.5% with M413 [n = 7; P = 0.02]) T-cell subsets, similar to that observed with LZhuTRAIL.
FIG. 2.
Sensitivity of PBL from HIV-1-infected donors to titrated doses of LZhuTRAIL. Cells were isolated from HIV-1-infected donors and incubated with increasing concentrations of LZhuTRAIL as indicated. Cell death was measured by Hoechst staining. Data are representative of three independent experiments. Spontaneous levels of apoptosis are indicated by asterisks.
FIG. 3.
LZhuTRAIL induces apoptosis in cells from HIV-1-infected patients. PBL from 26 randomly selected HIV-1-infected patients or 5 uninfected controls were treated with 1 μg of LZhuTRAIL and analyzed for apoptosis by Hoechst staining, as were CD4+ T cells and CD8+ T cells.
Together, these data indicate that LZhuTRAIL and agonistic MAbs to TRAIL-R1 and -R2 specifically and selectively induce apoptosis in CD4+ and CD8+ T cells from HIV-infected patients, but not in cells from uninfected controls.
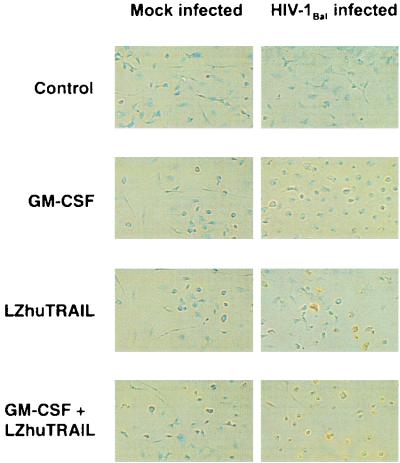
LZhuTRAIL induces selective apoptosis of MDM infected with HIVBal.
In view of the important role played by macrophages in the pathophysiology of HIV disease (37, 43), we investigated whether TRAIL/Apo2L induces apoptosis in macrophages. MDM from 11 donors were mock or HIVBal infected and 14 days later were treated with 1 μg of LZhuTRAIL/ml for 12 h and assessed for apoptosis by TUNEL staining. LZhuTRAIL induced significant apoptosis in HIVBal cultures (the median TSA was 20.47%, versus 7.96% in mock-infected cultures [P < 0.001]), indicating that TRAIL/Apo2L triggers cell death in in vitro-infected macrophages (Fig. 4). Since it has been previously reported that GM-CSF increases the sensitivity of MDM to TRAIL/Apo2L-induced cell death (47), we also treated MDM with GM-CSF and assessed them for apoptosis induced by TRAIL/Apo2L. Addition of GM-CSF did not significantly alter the sensitivity of HIV-positive or -negative MDM to TRAIL/Apo2L (Fig. 4).
FIG. 4.
Induction of apoptosis in macrophages by LZhuTRAIL. MDM from 11 HIV-1-negative donors were mock or HIV-1Bal infected with or without GM-CSF. Fourteen days following infection, cells were treated with LZhuTRAIL and analyzed by TUNEL to determine the levels of apoptosis.
LZhuTRAIL induces apoptosis of resting memory CD4 T cells from patients infected with HIV.
To determine whether TRAIL/Apo2L has cytotoxic effects on those cells that represent the principal HIV reservoir in vivo, we treated PBL from 14 HIV-positive patients receiving highly active antiretroviral therapy (HAART) who had suppressed viral replication (<50 copies of viral RNA/ml for more than 12 months) with 1 μg of LZhuTRAIL/ml and assessed cell death in CD4 T cells with phenotypic markers which predict the latently infected CD4 T cell pool. Several groups have established that latently infected T cells have a CD4+ HLA-DR− CD45RO+ or CD4+ HLA-DR− CD62L+ phenotype; however, only a small fraction of these cells are latently infected (6, 9, 15, 17, 42). The median TSA for LZhuTRAIL-treated cells with a CD4+HLA-DR− CD62L+ phenotype was 3.6%, compared to 1.2% for untreated cell cultures (n = 14; P = 0.01). CD4+ HLA-DR− CD45RO+ cells from HIV-infected patients had a median TSA of 3.41% (n = 14) in cultures treated with LZhuTRAIL, in contrast to 1.4% for untreated cultures (n = 14; P = 0.005). These findings suggest that LZhuTRAIL induces apoptosis in a variety of cell types, including latently HIV infected CD4+ T cells.
In vitro treatment of cells from HIV-infected patients reduces HIV production and reduces the proportion of latently infected cells.
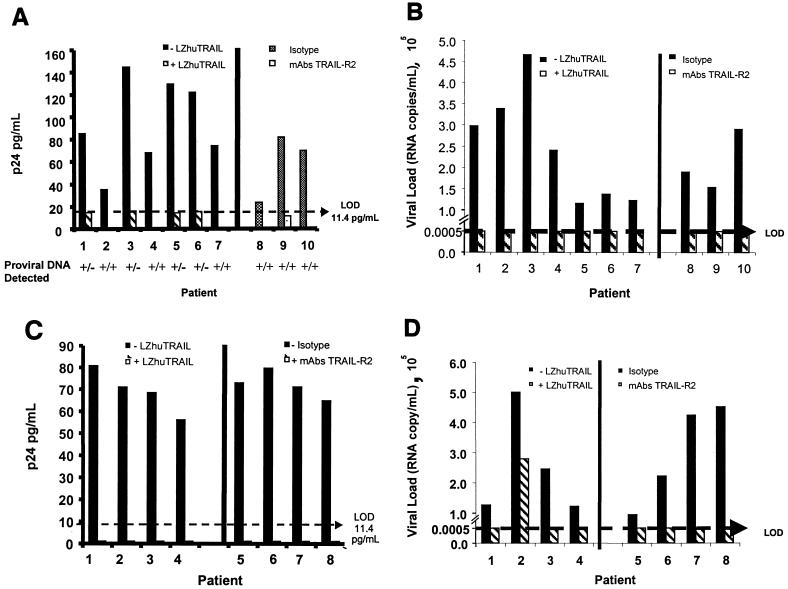
Isolated CD4+ HLA-DR− CD45RO+ cells from seven HIV-infected patients receiving HAART whose plasma viral load remained below the level of detection (<50 copies/ml) for more than 12 months were assessed for detectable HIV following treatment with TRAIL. By micrococulture, untreated cultures produced a mean of 94.35 pg of p24 antigen/ml (standard deviation [SD] = 39.28 pg/ml). In contrast, we could not detect p24 antigen production from six of seven cultures treated with LZhuTRAIL (P < 0.001), where the limit of detection in this assay is 11.4 pg/ml (Fig. 5A, patients 1 to 7). We also measured viral RNA levels in the culture supernatants and found similar results: untreated cultures had significant levels of HIV RNA (mean, 2.45 × 105 copies/ml), whereas HIV RNA was undetectable in all seven culture supernatants from cells treated with LZhuTRAIL (P = < 0.002) (Fig. 5B, patients 1 to 7). Amplification of HIV DNA was also performed, using a commercial assay specific for the gag region of HIV with a detection limit of 2 copies of DNA. By this measure, HIV DNA could not be detected in four of seven treated samples. To eliminate the possibility of a false-negative result from the commercial assay, the four samples which tested negative were retested using a nested PCR with a different set of primers specific for the pol region of HIV (18). This assay has a detection limit of 1 copy of DNA (31). These samples also tested consistently negative for HIV DNA (Fig. 5A). Treatment and control groups were also compared using maximum-likelihood estimates of IUPM. By this estimate LZhuTRAIL significantly reduced the HIV burden of resting memory cells (P = 0.03) (Table 1). Similar experiments were conducted using agonistic MAbs to TRAIL-R2. All three PBL cultures treated with a TRAIL-R2-specific MAb had undetectable levels of viral p24 antigen production (P = 0.04) (Fig. 5A, patients 8 to 10) and undetectable levels (<50 copies/ml) of supernatant HIV-specific RNA (P < 0.001) (Fig. 5B, patients 8 to 10), whereas untreated cultures produced high levels of virus as measured by p24 antigen production (mean ± SD, 58.15 ± 4.97 pg/ml) and supernatant viral RNA (mean ± SD, 2.05 × 105 ± 0.58 × 105 copies/ml) (P < 0.001). In contrast to treatments with LZhuTRAIL (where viral DNA was undetectable in four of seven samples), HIV DNA was detected in three of three samples treated with agonistic TRAIL-R2 antibodies, and IUPM in treated and untreated cells were not significantly different (Table 1).
FIG. 5.
LZhuTRAIL reduces viral gene expression in infected cells. CD4/DR− RO+ cells were isolated from seven HIV-1-infected patients, treated with LZhuTRAIL or agonistic TRAIL receptor antibodies (or isotype controls), and analyzed for p24 antigen production (A) and for the presence of integrated proviral DNA. (B) Viral RNA in culture supernatants. (C and D) Unfractionated cells from four HIV-1-infected patients with suppressed plasma viremia for more than 12 months were treated with or without LZhuTRAIL or agonistic MAbs to TRAIL-R2 (or isotype controls) and tested for p24 antigen (C) or viral RNA in culture supernatants (D).
TABLE 1.
Effect of treatment with LZhuTRAIL or agonistic anti-R2 antibodies on IUPM
| Treatment and cell type | Patient | IUPM in:
|
|
|---|---|---|---|
| Control cells | Treated cells | ||
| LZhuTRAIL, resting memory cellsa | 1 | 0.41 | 0.02 |
| 2 | 2.29 | 0.02 | |
| 3 | 0.08 | 0.01 | |
| 4 | 2.29 | 0.08 | |
| 5 | 0.41 | 0.01 | |
| 6 | 0.41 | 0.01 | |
| 7 | 0.41 | 0.01 | |
| LZhuTRAIL, PBLb | 1 | 0.41 | 0.41 |
| 2 | 0.41 | 0.01 | |
| 3 | 0.41 | 0.01 | |
| 4 | 0.41 | 0.01 | |
| Anti-TRAIL-R2, resting memory cellsc | 1 | 2.29 | 2.29 |
| 2 | 2.29 | 2.29 | |
| 3 | 7.18 | 2.29 | |
| 4 | 7.18 | 2.29 | |
| Anti-TRAIL-R2, PBLd | 1 | 2.29 | 0.41 |
| 2 | 0.41 | 0.01 | |
| 3 | 2.29 | 2.29 | |
| 4 | 2.29 | 2.29 | |
P = 0.03 for the difference between control and treated cells.
P = 0.02 for the difference between control and treated cells.
P = 0.13 for the difference between control and treated cells.
P = 0.49 for the difference between control and treated cells.
Finally, to determine whether all cell types capable of producing virus that were present in the peripheral blood of infected patients were killed by TRAIL/Apo2L treatment, we subjected unfractionated PBL to microculture following treatment with either LZhuTRAIL or a MAb to TRAIL-R2 (M412). In PBL cultures treated with LZhuTRAIL, p24 antigen production was undetectable in four of four samples, whereas untreated samples produced a mean of 69.13 pg of p24 antigen/ml (SD = 10.17 pg/ml; P < 0.001) (Fig. 5C, patients 1 to 4). Among the same samples, supernatant viral RNA levels were 2.65 × 105 copies/ml in untreated samples versus 280 copies/ml in LZhuTRAIL-treated samples (P = < 0.001). Although viral DNA was still detectable in all samples, IUPM were significantly reduced in LZhuTRAIL-treated samples (P = 0.02) (Table 1). Agonistic TRAIL-R2 antibodies had a similar effect in PBL cultures: untreated cultures produced significant amounts of p24 antigen (mean ± SD, 72.31 ± 6.06 pg/ml; n = 4) (Fig. 5C, patients 5 to 8) and of viral RNA (mean ± SD, 2.97 × 105 ± 1.7 × 105 copies/ml) (Fig. 5D, patients 5 to 8), whereas treated samples had undetectable levels of p24 antigen production (P < 0.001) and a mean viral load of <50 copies/ml (P = 0.01), but viral DNA was still detectable. In these assays using an agonistic anti-TRAIL R2 antibody, IUPM did not differ between treatment groups (Table 1).
DISCUSSION
Despite successful control of HIV replication in patients receiving HAART, the prolonged life span and slow rate of decay of latently infected CD4+ T cells provide a long-lasting cellular reservoir for HIV (8, 10, 15, 45). Indeed, independent projections estimate the time required to fully eliminate HIV in patients on completely suppressive HAART alone to be 10 to 60 years (9, 15, 16, 52). Latently infected resting memory CD4+ T cells contain integrated DNA provirus yet are transcriptionally inactive and may therefore escape both immune recognition and the antiviral effects of HAART regimes which affect only viral RNA species (10). Since current therapies for HIV are ineffective in eradicating latently infected cell populations, control of these populations depends on the interplay of cellular half-life and the ability to suppress viral replication in order to prevent repopulation of the latent reservoir (25). The importance of this reservoir (17) is underscored by observations of viral rebound following withdrawal of HAART (11). Thus, in order to eradicate HIV, it is critical to develop strategies to eliminate latently infected cells. Here we demonstrate that TRAIL/Apo2L treatment in vitro induces death of cells including the relevant latently infected cell populations that are principal HIV reservoirs in vivo.
While both FasL and TNF have been shown to induce apoptosis of cells from HIV-infected patients (3, 35), the nonselective induction of apoptosis and toxicity related to activation limit their clinical utility as potential therapies for HIV infection. By contrast, systemic administration of TRAIL/Apo2L to healthy mice and nonhuman primates has been shown to be safe and to lack cytotoxic effects (1, 5, 19, 20, 23, 40, 50). In models utilizing animals engrafted with human tumors, treatment with TRAIL/Apo2L induces significant tumor-specific apoptosis, tumor regression, and improved survival (1, 50), with no identifiable toxicity.
The first goal of this study was to determine whether TRAIL receptor expression changes during HIV infection. Jurkat T cells and MDM infected with HIV in vitro and both CD4+ and CD8+ T cells from HIV-positive patients are associated with dysregulation of TRAIL and TRAIL receptor expression. Importantly, these effects are seen with HIVIIIB and HIVBal as well as clinical isolates. In addition, LZhuTRAIL kills HIV-infected cells as well as bulk PBL, CD4 T cells, CD8 T cells, CD4+ CD62L+ HLA-DR− cells, and CD4+ HLA-DR− CD45RO+ cells from HIV-infected patients. Together these data lay the foundation for the hypothesis that TRAIL/Apo2L may induce apoptosis of a variety of cells (including latently infected cells) from HIV-infected patients and may therefore be of clinical utility.
In order to explore this hypothesis, we evaluated the ability of TRAIL/Apo2L to eradicate cells capable of producing HIV by analyzing (i) p24 antigen production, (ii) viral RNA production and (iii) HIV viral DNA production (i.e., both provirus and unintegrated DNA) in cells treated with LZhuTRAIL, and (iv) IUPM. Following LZhuTRAIL treatment, cells were extensively washed, cocultured for 14 days with irradiated feeder cells, and assayed for viral production. In six of the seven cultures of CD4+ HLA-DR− CD45RO+ cells treated with LZhuTRAIL and in three of three cultures treated with agonistic MAbs to TRAIL-R2, p24 antigen production was not detected. In all cultures of CD4+ HLA-DR− CD45RO+ cells treated with LZhuTRAIL or with the agonistic antibody, no viral RNA was detected, thus demonstrating the antiviral effects of TRAIL/Apo2L on these cells. Of particular interest, HIV DNA was not detected in four of seven cultures of CD4+ CD45RO+ HLA-DR− cells treated with LZhuTRAIL, suggesting an ability to eradicate latently infected cells. Further, in these experiments LZhuTRAIL reduced IUPM in both sorted resting memory cells and bulk PBL. Together these findings indicate that TRAIL/Apo2L in vitro can induce significant apoptosis in cells from HIV patients, including latently infected CD4 T cells and HIV-infected macrophages.
It is noteworthy that our cumulative data suggest that both infected and uninfected cells die following TRAIL receptor stimulation. In bulk assays, up to 20% of cells die following LZhuTRAIL treatment, which is significantly more cells than are physically infected by the virus. Further, limiting-dilution coculture assays demonstrate that infected cells are killed by such treatments. Thus, uninfected cells and latently infected cells (which do not express HIV proteins) die following treatment. Insight into how cells that do not express HIV proteins can have altered TRAIL receptor expression as well as altered sensitivity to TRAIL-mediated killing is provided by our data demonstrating that soluble gp120 (or whole inactivated HIV) can exert these effects. While gp120 induced changes in TRAIL receptor regulation, it is likely not the only possible mechanism for rendering an uninfected (or latently infected) cell susceptible to TRAIL. These data demonstrate that a soluble mediator(s) is involved.
Thus, in addition to the evidence that TRAIL/Apo2L is cytotoxic to transformed or cytomegalovirus-infected human cells, we have shown that cells from HIV-infected patients are similarly sensitive to TRAIL-mediated apoptosis. These data provide a basis for future evaluation of TRAIL/Apo2L as therapy for humans, particularly in view of the encouraging safety results of in vivo administration of TRAIL/Apo2L to mice and nonhuman primates. A note of caution has been raised by a recent study, using human hepatocytes from livers harvested but not used for transplantation, that demonstrated TRAIL-induced apoptosis in these cells (33). However, not all of the different TRAIL/Apo2L preparations or agonists possess this activity (46), and whether TRAIL induces apoptosis of freshly isolated hepatocytes or hepatocytes in vivo remains to be determined.
Our findings are the first to demonstrate that TRAIL/Apo2L selectively induces cell death in cells from HIV patients, including latently infected CD4+ T cells and macrophages, without deleterious effects on cells from uninfected patients. Based on these findings, we propose that patients with prolonged viral suppression be treated with TRAIL agonists. In such patients, prolonged viral suppression has been associated with eradication of >99.9% of lymphoid (and thus macrophage-associated) virus (4, 26, 27, 41, 44), and persistence of HIV chiefly within CD4+ CD45RO+ HLA-DR− T cells (6, 8, 9, 16, 51, 52), some of which are latently infected. Further, as single-dose in vitro therapy with LZhuTRAIL can eradicate provirus and inducible virus production in slightly more than half of the cultures tested, multiple cycles of therapy must be considered. Further studies on LZhuTRAIL are therefore warranted to address such issues as safety, tolerability, and in vivo effects on viral reservoirs and viral turnover.
ACKNOWLEDGMENTS
This work was supported by grants from The Medical Research Council of Canada, the Canadian Foundation for AIDS Research (CANFAR), and the Doris Duke Foundation (to A.D.B.). A.D.B. is the recipient of a Career Scientist Award from the Ontario HIV Treatment Network (OHTN) and a Premiers Research Excellence Award from the Province of Ontario. J.J.L. and B.N.P. have received Studentship Awards from the OHTN, and A.A.P. has received a Postdoctoral Fellowship Award from the OHTN.
We gratefully acknowledge the excellent administrative assistance of Ann Carisse and the efforts of Michael Bazant and André Lauzière in recruiting blood donors, and we thank B. W. D. Badley for critical review of the manuscript. The statistical expertise of Bharati Sanghvi is also greatly appreciated.
REFERENCES
- 1.Ashkenazi A, Pai R C, Fong S, Leung S, Lawrence D A, Marsters S A, Blackie C, Chang L, McMurtrey A E, Hebert A, DeForge L, Koumenis I L, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall R H. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Investig. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badley A D, McElhinny J A, Leibson P J, Lynch D H, Alderson M R, Paya C V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badley A D, Pilon A A, Landay A, Lynch D H. Mechanisms of HIV associated lymphocyte apoptosis. Blood. 2000;96:2951–2964. [PubMed] [Google Scholar]
- 4.Cavert W, Notermans D W, Staskus K, Wietgrefe S W, Zupancic M, Gebhard K, Henry K, Zhang Z-Q, Mills R, McDade H, Schuwirth C M, Goudsmit J, Danner S A, Haase A T. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 5.Chinnaiyan A M, Prasad U, Shankar S, Hamstra D A, Shanaiah M, Chenevert T L, Ross B D, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000;97:1754–1759. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun T W, Engel D, Berrey M M, Shea T, Corey L, Fauci A S. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun T W, Engel D, Mizell S B, Hallahan C W, Fischette M, Park S, Davey R T, Jr, Dybul M, Kovacs J A, Metcalf J A, Mican J M, Berrey M M, Corey L, Lane H C, Fauci A S. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 8.Chun T W, Fauci A S. Latent reservoirs of HIV: obstacles to the eradication of virus. Proc Natl Acad Sci USA. 1999;96:10958–10961. doi: 10.1073/pnas.96.20.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper D A, Emery S. Latent reservoirs of HIV infection: flushing with IL-2? Nat Med. 1999;5:611–612. doi: 10.1038/9454. [DOI] [PubMed] [Google Scholar]
- 11.Davey R T, Jr, Bhat N, Yoder C, Chun T W, Metcalf J A, Dewar R, Natarajan V, Lempicki R A, Adelsberger J W, Miller K D, Kovacs J A, Polis M A, Walker R E, Falloon J, Masur H, Gee D, Baseler M, Dimitrov D S, Fauci A S, Lane H C. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degli-Esposti M. To die or not to die—the quest of the TRAIL receptors. J Leukoc Biol. 1999;65:535–542. doi: 10.1002/jlb.65.5.535. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich H, et al. HLA DNA typing. In: Innis M, editor. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press Inc.; 1990. pp. 261–271. [Google Scholar]
- 14.Emery J G, McDonnell P, Burke M B, Deen K C, Lyn S, Silverman C, Dul E, Appelbaum E R, Eichman C, DiPrinzio R, Dodds R A, James I E, Rosenberg M, Lee J C, Young P R. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 15.Finzi D, Blankson J, Siliciano J D, Margolick J B, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn T C, Chaisson R E, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano R F. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 16.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 17.Finzi D, Siliciano R F. Viral dynamics in HIV-1 infection. Cell. 1998;93:665–671. doi: 10.1016/s0092-8674(00)81427-0. [DOI] [PubMed] [Google Scholar]
- 18.Fransen K, et al. Design and evaluation of new, highly sensitive and specific primers for polymerase chain reaction detection of HIV-1-infected primary lymphocytes. Mol Cell Probes. 1994;8:317–322. doi: 10.1006/mcpr.1994.1043. [DOI] [PubMed] [Google Scholar]
- 19.Giovarelli M, Musiani P, Garotta G, Ebner R, Di Carlo E, Kim Y, Cappello P, Rigamonti L, Bernabei P, Novelli F, Modesti A, Coletti A, Ferrie A K, Lollini P L, Ruben S, Salcedo T, Forni G. A “stealth effect”: adenocarcinoma cells engineered to express TRAIL elude tumor-specific and allogeneic T cell reactions. J Immunol. 1999;163:4886–4893. [PubMed] [Google Scholar]
- 20.Gliniak B, Le T. Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res. 1999;59:6153–6158. [PubMed] [Google Scholar]
- 21.Griffith T S, Chin W A, Jackson G C, Lynch D H, Kubin M Z. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 22.Griffith T S, Lynch D H. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 23.Griffith T S, Rauch C T, Smolak P J, Waugh J Y, Boiani N, Lynch D H, Smith C A, Goodwin R G, Kubin M Z. Functional analysis of TRAIL receptors using monoclonal antibodies. J Immunol. 1999;162:2597–2605. [PubMed] [Google Scholar]
- 24.Griffith T S, Wiley S R, Kubin M Z, Sedger L M, Maliszewski C R, Fanger N A. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grossman Z, Polis M, Feinberg M B, Grossman Z, Levi I, Jankelevich S, Yarchoan R, Boon J, De Wolf F, Lange J M A, Goudsmit J, Dimitrov D S, Paul W E. Ongoing HIV dissemination during HAART. Nat Med. 1999;5:1099–1104. doi: 10.1038/13410. [DOI] [PubMed] [Google Scholar]
- 26.Gunthard H F, Wong J K, Ignacio C C, Guatelli J C, Riggs N L, Havlir D V, Richman D D. Human immunodeficiency virus replication and genotypic resistance in blood and lymph nodes after a year of potent antiretroviral therapy. J Virol. 1998;72:2422–2428. doi: 10.1128/jvi.72.3.2422-2428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haase A T, Henry K, Zupancic M, Sedgewick G, Faust R A, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang Z-Q, Dailey P J, Balfour H H, Erice A, Perelson A S. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 28.Han X, Becker K, Degen H J, Jablonowski H, Strohmeyer G. Synergistic stimulatory effects of tumour necrosis factor alpha and interferon gamma on replication of human immunodeficiency virus type 1 and on apoptosis of HIV-1-infected host cells. Eur J Clin Investig. 1996;26:286–292. doi: 10.1046/j.1365-2362.1996.116271.x. [DOI] [PubMed] [Google Scholar]
- 29.Herbein G, Van Lint C, Lovett J L, Verdin E. Distinct mechanisms trigger apoptosis in human immunodeficiency virus type 1-infected and in uninfected bystander T lymphocytes. J Virol. 1998;72:660–670. doi: 10.1128/jvi.72.1.660-670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer J L, Schroter M, Burns K, Mattmann C, Rimoldi D, French L E, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 31.Janssens W, Fransen K, Loussert-Ajaka I, Heyndrickx L, Ivens T, Eberle J, Nkengasong J. Diagnosis of HIV-1 group O infection by polymerase chain reaction. Lancet. 1995;346:451–452. doi: 10.1016/s0140-6736(95)92828-6. [DOI] [PubMed] [Google Scholar]
- 32.Jeremias I, Herr I, Boehler T, Debatin K M. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur J Immunol. 1998;28:143–152. doi: 10.1002/(SICI)1521-4141(199801)28:01<143::AID-IMMU143>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Jo M, Kim T-H, Seol D-W, Esplen J E, Dorko K, Billiar T R, Strom S C. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 34.Katsikis P D, Garcia-Ojeda M E, Torres-Roca J F, Tijoe I M, Smith C A, Herzenberg L A. Interleukin-1β converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurence J, Mitra D, Steiner M, Lynch D H, Siegal F P, Staiano-Coico L. Apoptotic depletion of CD4+ T cells in idiopathic CD4+ T lymphocytopenia. J Clin Investig. 1996;97:672–680. doi: 10.1172/JCI118464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCoig C, Van Dyke G, Chou C S, Picker L, Ramilo O, Vitetta E. An anti-CD45RO immunotoxin eliminates T cells latently infected with HIV-1 in vitro. Proc Natl Acad Sci USA. 1999;96:11482–11485. doi: 10.1073/pnas.96.20.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meltzer M S, Skillman D R, Gomatos P J, Kalter D C, Gendelman H E. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- 38.Miura Y, Misawa N, Maeda N, Inagaki Y, Tanaka Y, Ito M, Kayagaki N, Yamamoto N, Yagita H, Mizusawa H, Koyanagi Y. Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J Exp Med. 2001;193:651–660. doi: 10.1084/jem.193.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers L E, McQuay L J, Hollinger F B. Dilution assay statistics. J Clin Microbiol. 1994;32:732–739. doi: 10.1128/jcm.32.3.732-739.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagane M, Pan G, Weddle J J, Dixit V M, Cavenee W K, Huang H J. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847–853. [PubMed] [Google Scholar]
- 41.Orenstein J M, Feinberg M, Yoder C, Schrager L, Mican J M, Schwartzentruber D J, Davey R T, Walker R E, Falloon J, Kovacs J, Miller K D, Fox C, Metcalf J A, Masur H, Polis M A. Lymph node architecture preceding and following 6 months of potent antiviral therapy: follicular hyperplasia persists in parallel with p24 antigen restoration after involution and CD4 cell depletion in an AIDS patient. AIDS. 1999;13:2219–2229. doi: 10.1097/00002030-199911120-00004. [DOI] [PubMed] [Google Scholar]
- 42.Ostrowski M A, Chun T W, Justement S J, Motola I, Spinelli M A, Adelsberger J, Ehler L A, Mizell S B, Hallahan C W, Fauci A S. Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol. 1999;73:6430–6435. doi: 10.1128/jvi.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poli G, Fauci A S. The role of monocyte/macrophages and cytokines in the pathogenesis of HIV infection. Pathobiology. 1992;60:246–251. doi: 10.1159/000163729. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz L, van Lunzen J, Arno A, Stellbrink H-J, Schneider C, Rull M, Castella E, Ojanguren I, Richman D D, Clotet B, Tenner-Racz K, Racz P. Protease inhibitor-containing regimens compared with nucleoside analogues alone in the suppression of persistent HIV-1 replication in lymphoid tissue. AIDS. 1999;13:F1–F8. doi: 10.1097/00002030-199901140-00001. [DOI] [PubMed] [Google Scholar]
- 45.Saag M S, Kilby J M. HIV-1 and HAART: a time to cure, a time to kill. Nat Med. 1999;5:609–611. doi: 10.1038/9452. [DOI] [PubMed] [Google Scholar]
- 46.Saiura A, Sata M, Hirata Y, Nagai R, Makuuchi M. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–384. [Google Scholar]
- 47.Sedger L M, Shows D M, Blanton R A, Peschon J J, Goodwin R G, Cosman D, Wiley S R. IFN-γ mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol. 1999;163:920–926. [PubMed] [Google Scholar]
- 48.Vidalain P O, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walczak H, Degli-Esposti M A, Johnson R S, Smolak P J, Waugh J Y, Boiani N, Timour M S, Gerhart M J, Schooley K A, Smith C A, Goodwin R G, Rauch C T. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walczak H, Miller R E, Ariail K, Gliniak B, Griffith T S, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin R G, Rauch C T, Schuh J C, Lynch D H. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 51.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, Markowitz M, Ho D D. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X D, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59:2747–2753. [PubMed] [Google Scholar]