Abstract
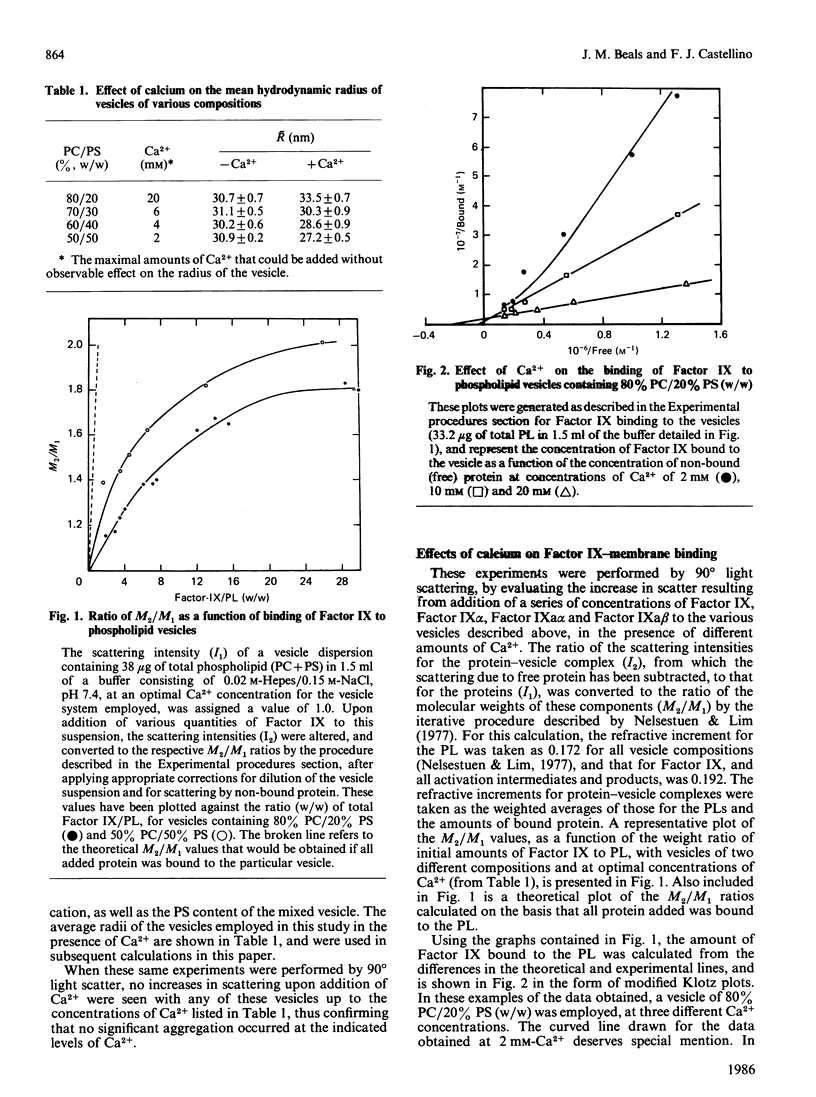
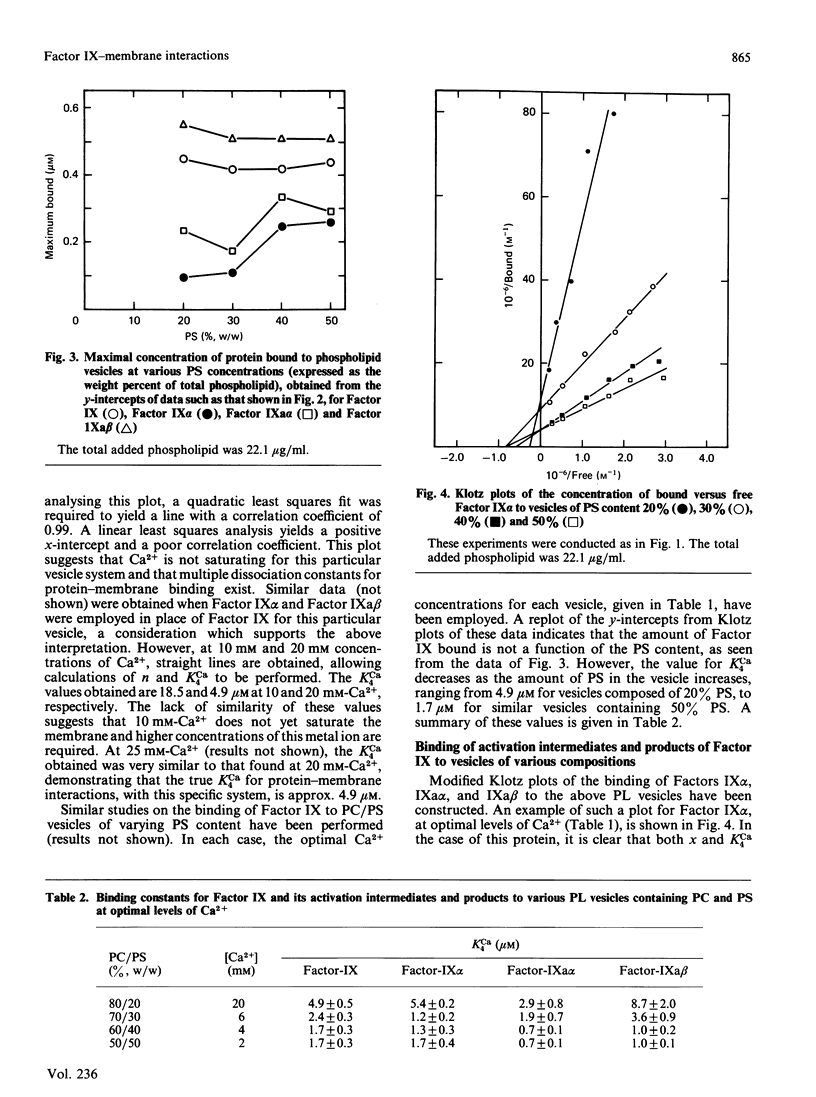
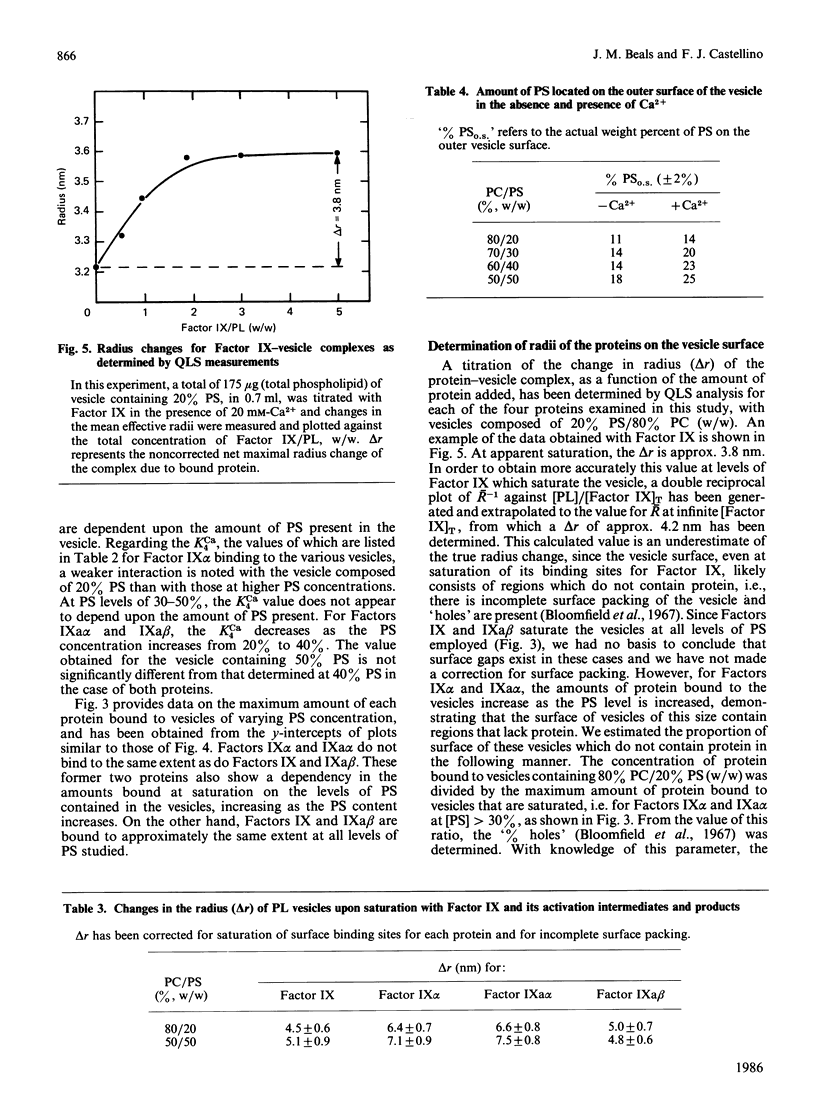
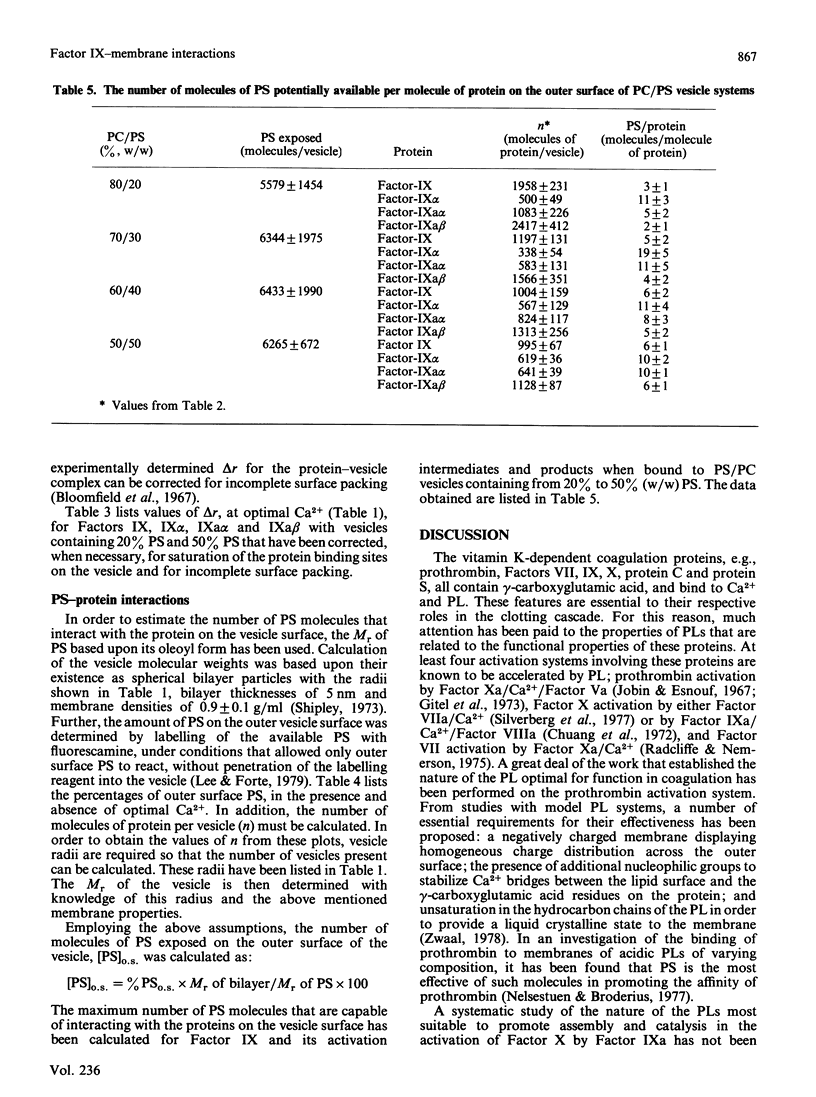
The interactions of bovine factor IX, its activation intermediate, Factor IX alpha, and its activation products, Factor IXa alpha and Factor IXa beta, with phospholipid vesicles, of mean radius of approx. 30 nm, containing various amounts of phosphatidylserine (PS) and phosphatidylcholine (PC), have been examined. For Factor IX, Factor IX alpha, Factor IXa alpha and Factor IXa beta, the dissociation constants, at saturating levels of Ca2+, are independent of the PS concentration in the vesicle after levels of 20-30% (w/w) have been reached, and attain minimum values of approx. 1.7, 1.7, 0.7 and 1.0 microM, respectively, with vesicles containing 50% PS. The amount of protein bound per vesicle particle is independent of the PS content, above 20% PS, for Factor IX and Factor IXa beta, with values of approx. 995-1197 and 1128-1566 molecules/vesicle, respectively. With Factor IX alpha, a dependence on the amount of protein bound with the content of PS is seen, which ranges from 338 to 619 molecules/vesicle with membranes containing 30-50% PS. For Factor IXa alpha, no regularity is noted and a range of 583-1083 molecules of protein/vesicle is observed with the systems employed. Examination of the radii of the proteins on the vesicle demonstrates that Factors IX alpha and IXa alpha occupy considerably more of the surface than do Factors IX and IXa beta, suggesting that a reason for the decreased number of binding sites for the former two proteins on the vesicle may be related to their greater surface spatial requirements.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Byrne R., Castellino F. J. Cation binding properties of the multiple subforms of RVV-X, the coagulant protein from Vipera russelli. Biochemistry. 1982 Jan 5;21(1):125–132. doi: 10.1021/bi00530a022. [DOI] [PubMed] [Google Scholar]
- Amphlett G. W., Byrne R., Castellino F. J. The binding of calcium to the activation products of bovine factor IX. J Biol Chem. 1979 Jul 25;254(14):6333–6336. [PubMed] [Google Scholar]
- BANGHAM A. D. A correlation between surface charge and coagulant action of phospholipids. Nature. 1961 Dec 23;192:1197–1198. doi: 10.1038/1921197a0. [DOI] [PubMed] [Google Scholar]
- Bajaj S. P. Cooperative Ca2+ binding to human factor IX. Effects of Ca2+ on the kinetic parameters of the activation of factor IX by factor XIa. J Biol Chem. 1982 Apr 25;257(8):4127–4132. [PubMed] [Google Scholar]
- Barenholz Y., Gibbes D., Litman B. J., Goll J., Thompson T. E., Carlson R. D. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 1977 Jun 14;16(12):2806–2810. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- Bloomfield V., Dalton W. O., Van Holde K. E. Frictional coefficients of multisubunit structures. I. Theory. Biopolymers. 1967 Feb;5(2):135–148. doi: 10.1002/bip.1967.360050202. [DOI] [PubMed] [Google Scholar]
- Byrne R., Castellino F. J. The influence of metal ions in the activation of bovine factor IX by the coagulant protein of Russell's viper venom. Arch Biochem Biophys. 1978 Oct;190(2):687–692. doi: 10.1016/0003-9861(78)90327-2. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Kou A. Y. Improved procedure for the separation of phospholipids by high-performance liquid chromatography. J Chromatogr. 1982 Jan 8;227(1):25–31. doi: 10.1016/s0378-4347(00)80352-7. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Gould K. G., Rees D. J., Brownlee G. G. Molecular cloning of the gene for human anti-haemophilic factor IX. Nature. 1982 Sep 9;299(5879):178–180. doi: 10.1038/299178a0. [DOI] [PubMed] [Google Scholar]
- Chuang T. F., Sargeant R. B., Hougie C. The intrinsic activation of factor X in blood coagulation. Biochim Biophys Acta. 1972 Jul 19;273(2):287–291. doi: 10.1016/0304-4165(72)90219-x. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Drakenberg T., Fernlund P., Roepstorff P., Stenflo J. beta-Hydroxyaspartic acid in vitamin K-dependent protein C. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1802–1806. doi: 10.1073/pnas.80.7.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Kato H., Davie E. W. The mechanism of activation of bovine factor IX (Christmas factor) by bovine factor XIa (activated plasma thromboplastin antecedent). Biochemistry. 1974 Oct 22;13(22):4508–4516. doi: 10.1021/bi00719a006. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Thompson A. R., Legaz M. E., Meyer R. G., Davie E. W. Isolation and characterization of bovine factor IX (Christmas factor). Biochemistry. 1973 Nov 20;12(24):4938–4945. doi: 10.1021/bi00748a019. [DOI] [PubMed] [Google Scholar]
- Gitel S. N., Owen W. G., Esmon C. T., Jackson C. M. A polypeptide region of bovine prothrombin specific for binding to phospholipids. Proc Natl Acad Sci U S A. 1973 May;70(5):1344–1348. doi: 10.1073/pnas.70.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobin F., Esnouf M. P. Studies on the formation of the prothrombin-converting complex. Biochem J. 1967 Mar;102(3):666–674. doi: 10.1042/bj1020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama K., Ericsson L. H., Enfield D. L., Walsh K. A., Neurath H., Davie E. W., Titani K. Comparison of amino acid sequence of bovine coagulation Factor IX (Christmas Factor) with that of other vitamin K-dependent plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4990–4994. doi: 10.1073/pnas.76.10.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D., McConnell H. M. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 1971 Mar 30;10(7):1111–1120. doi: 10.1021/bi00783a003. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C., Forte J. G. Asymmetric labeling of amino lipids in liposomes. Biochim Biophys Acta. 1979 Jul 5;554(2):375–387. doi: 10.1016/0005-2736(79)90378-x. [DOI] [PubMed] [Google Scholar]
- Lim T. K., Bloomfield V. A., Nelsestuen G. L. Structure of the prothrombin- and blood clotting factor X-membrane complexes. Biochemistry. 1977 Sep 20;16(19):4177–4181. doi: 10.1021/bi00638a007. [DOI] [PubMed] [Google Scholar]
- Lindquist P. A., Fujikawa K., Davie E. W. Activation of bovine factor IX (Christmas factor) by factor XIa (activated plasma thromboplastin antecedent) and a protease from Russell's viper venom. J Biol Chem. 1978 Mar 25;253(6):1902–1909. [PubMed] [Google Scholar]
- Link R. P., Castellino F. J. Kinetic comparison of bovine blood coagulation factors IXa alpha and IXa beta toward bovine factor X. Biochemistry. 1983 Aug 16;22(17):4033–4041. doi: 10.1021/bi00286a007. [DOI] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K., Kisiel W. The occurrence of beta-hydroxyaspartic acid in the vitamin K-dependent blood coagulation zymogens. Biochem Biophys Res Commun. 1983 Aug 30;115(1):8–14. doi: 10.1016/0006-291x(83)90961-0. [DOI] [PubMed] [Google Scholar]
- Mertens K., Cupers R., Van Wijngaarden A., Bertina R. M. Binding of human blood-coagulation Factors IXa and X to phospholipid membranes. Biochem J. 1984 Nov 1;223(3):599–605. doi: 10.1042/bj2230599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi T., Taniguchi T., Fujikawa K., Titani K., Kobata A. The structures of the carbohydrate moieties of bovine blood coagulation factor IX (Christmas factor). J Biol Chem. 1983 May 25;258(10):6020–6024. [PubMed] [Google Scholar]
- Nelsestuen G. L., Broderius M. Interaction of prothrombin and blood-clotting factor X with membranes of varying composition. Biochemistry. 1977 Sep 20;16(19):4172–4177. doi: 10.1021/bi00638a006. [DOI] [PubMed] [Google Scholar]
- Nelsestuen G. L., Broderius M., Martin G. Role of gamma-carboxyglutamic acid. Cation specificity of prothrombin and factor X-phospholipid binding. J Biol Chem. 1976 Nov 25;251(22):6886–6893. [PubMed] [Google Scholar]
- Nelsestuen G. L., Kisiel W., Di Scipio R. G. Interaction of vitamin K dependent proteins with membranes. Biochemistry. 1978 May 30;17(11):2134–2138. doi: 10.1021/bi00604a017. [DOI] [PubMed] [Google Scholar]
- Nelsestuen G. L., Lim T. K. Equilibria involved in prothrombin- and blood-clotting factor X-membrane binding. Biochemistry. 1977 Sep 20;16(19):4164–4171. doi: 10.1021/bi00638a005. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Pangborn W. A., Poste G. Studies on membrane fusion. II. Induction of fusion in pure phospholipid membranes by calcium ions and other divalent metals. Biochim Biophys Acta. 1976 Oct 5;448(2):265–283. doi: 10.1016/0005-2736(76)90241-8. [DOI] [PubMed] [Google Scholar]
- Radcliffe R., Nemerson Y. Activation and control of factor VII by activated factor X and thrombin. Isolation and characterization of a single chain form of factor VII. J Biol Chem. 1975 Jan 25;250(2):388–395. [PubMed] [Google Scholar]
- SCHIFFMAN S., RAPAPORT S. I., PATCH M. J. THE IDENTIFICATION AND SYNTHESIS OF ACTIVATED PLASMA THROMBOPLASTIN COMPONENT (PTC'). Blood. 1963 Dec;22:733–749. [PubMed] [Google Scholar]
- Silverberg S. A., Nemerson Y., Zur M. Kinetics of the activation of bovine coagulation factor X by components of the extrinsic pathway. Kinetic behavior of two-chain factor VII in the presence and absence of tissue factor. J Biol Chem. 1977 Dec 10;252(23):8481–8488. [PubMed] [Google Scholar]
- Zur M., Nemerson Y. Kinetics of factor IX activation via the extrinsic pathway. Dependence of Km on tissue factor. J Biol Chem. 1980 Jun 25;255(12):5703–5707. [PubMed] [Google Scholar]
- Zwaal R. F. Membrane and lipid involvement in blood coagulation. Biochim Biophys Acta. 1978 Jul 31;515(2):163–205. doi: 10.1016/0304-4157(78)90003-5. [DOI] [PubMed] [Google Scholar]


