Abstract
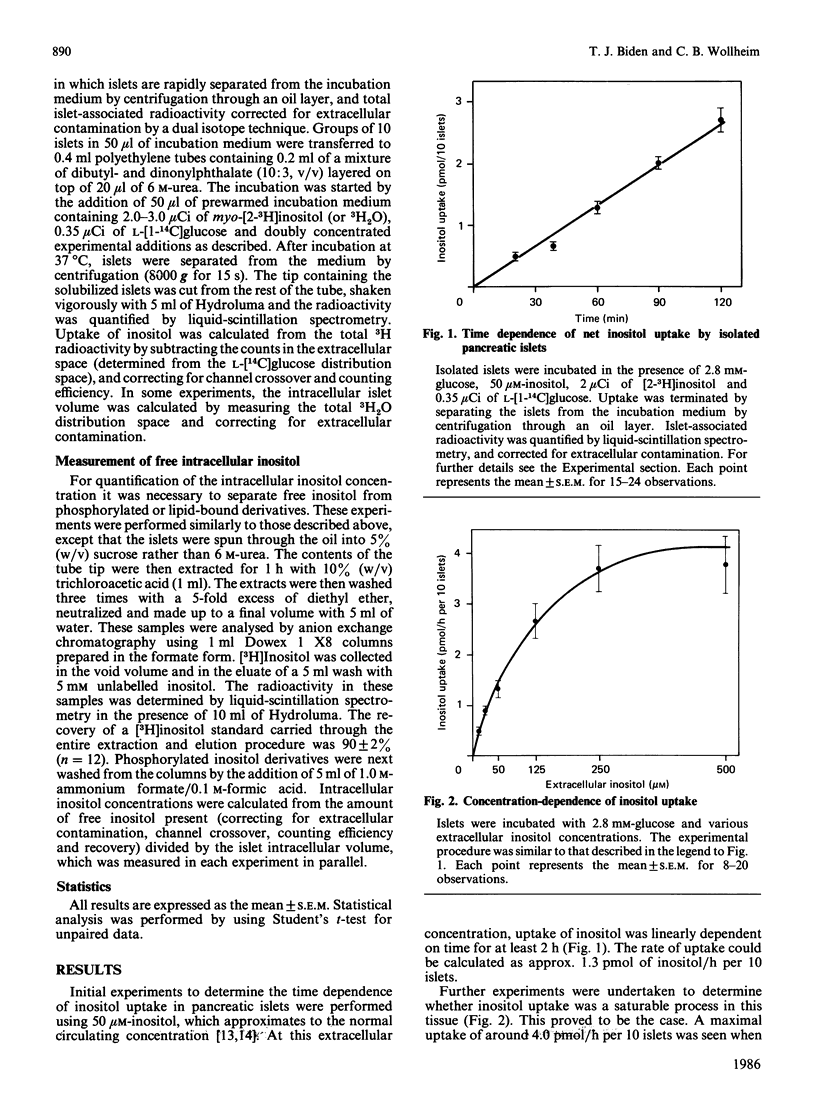
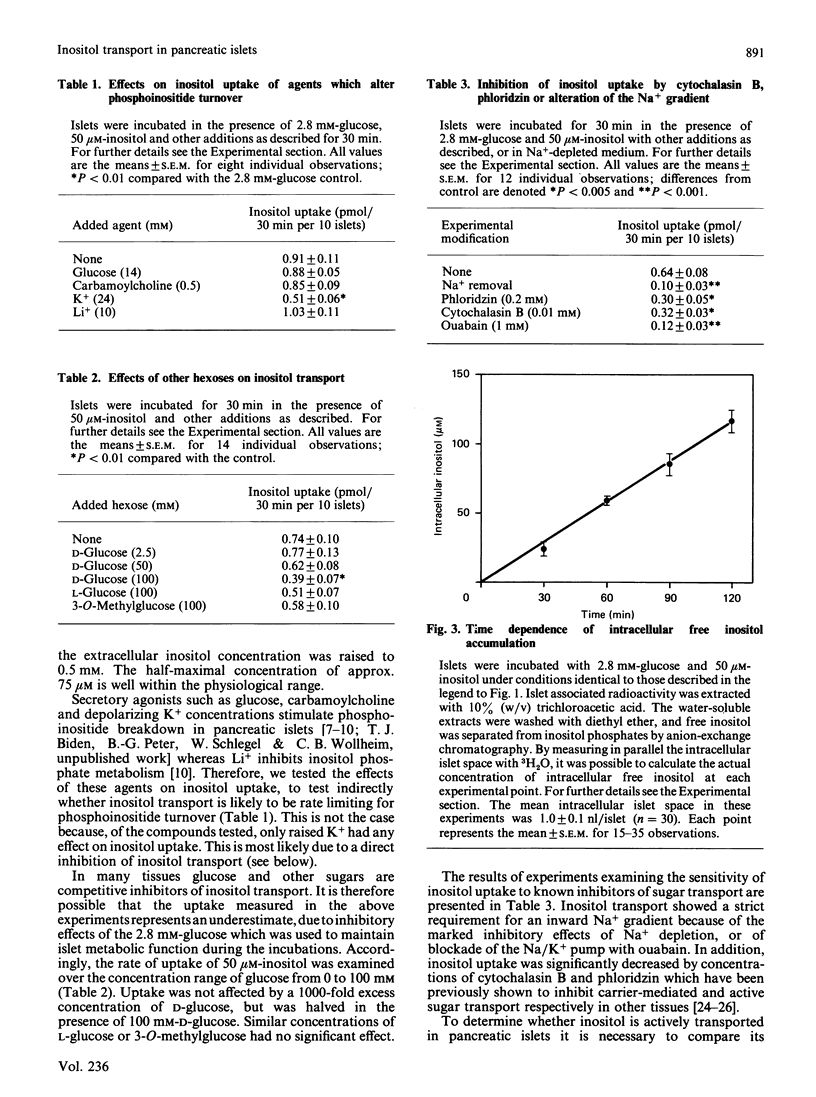
myo-Inositol transport by isolated pancreatic islets was measured with a dual isotope technique. Uptake was saturable with a half-maximal response at approx. 75 microM. With 50 microM-inositol, uptake was linear for at least 2 h during which time the free intracellular concentration rose to double that of the incubation medium. Inositol transport is therefore active and probably energized by electrogenic co-transport of Na+ down its concentration gradient as uptake was inhibited by ouabain, Na+ removal or depolarizing K+ concentrations. Inositol transport was abolished by cytochalasin B which binds to hexose carriers, but not by carbamoylcholine or Li+ which respectively stimulate or inhibit phosphoinositide turnover. Uptake of inositol was not affected by 3-O-methylglucose or L-glucose (both 100 mM) nor by physiological concentrations of D-glucose. The results suggest that most intracellular inositol in pancreatic islets would be derived from the extracellular medium. Since the transport mechanism is distinct from that of glucose, inositol uptake would not be inhibited during periods of hyperglycaemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. H., Jr, Blackard W. G. Effect of lithium on pancreatic islet insulin release. Endocrinology. 1978 Jan;102(1):291–295. doi: 10.1210/endo-102-1-291. [DOI] [PubMed] [Google Scholar]
- Axen K. V., Schubart U. K., Blake A. D., Fleischer N. Role of Ca2+ in secretagogue-stimulated breakdown of phosphatidylinositol in rat pancreatic islets. J Clin Invest. 1983 Jul;72(1):13–21. doi: 10.1172/JCI110951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin R., Lavau M. Effects of high-fat diet on glucose metabolism in isolated pancreatic acini of rats. Am J Physiol. 1982 Dec;243(6):G448–G454. doi: 10.1152/ajpgi.1982.243.6.G448. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Best L., Dunlop M., Malaisse W. J. Phospholipid metabolism in pancreatic islets. Experientia. 1984 Oct 15;40(10):1085–1091. doi: 10.1007/BF01971455. [DOI] [PubMed] [Google Scholar]
- Best L., Malaisse W. J. Nutrient and hormone-neurotransmitter stimuli induce hydrolysis of polyphosphoinositides in rat pancreatic islets. Endocrinology. 1984 Nov;115(5):1814–1820. doi: 10.1210/endo-115-5-1814. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers A. Sugar transport in animal cells: the passive hexose transfer system. Prog Biophys Mol Biol. 1984;43(1):33–69. doi: 10.1016/0079-6107(84)90003-8. [DOI] [PubMed] [Google Scholar]
- Caspary W. F., Crane R. K. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim Biophys Acta. 1970 Apr 21;203(2):308–316. doi: 10.1016/0005-2736(70)90145-8. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Rhoten W. B. Phosphoinositide metabolism and insulin secretion from isolated rat pancreatic islets. J Clin Invest. 1976 Mar;57(3):684–691. doi: 10.1172/JCI108325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., FREINKEL N. The distribution of free mesoinositol in mammalian tissues, including some observations on the lactating rat. Biochem J. 1961 Mar;78:606–610. doi: 10.1042/bj0780606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg F., Jr D-myoinositol 1-phosphate as product of cyclization of glucose 6-phosphate and substrate for a specific phosphatase in rat testis. J Biol Chem. 1967 Apr 10;242(7):1375–1382. [PubMed] [Google Scholar]
- Elbrink J., Bihler I. Characteristics of the membrane transport of sugars in the lens of the eye. Biochim Biophys Acta. 1972 Sep 1;282(1):337–351. doi: 10.1016/0005-2736(72)90339-2. [DOI] [PubMed] [Google Scholar]
- Freinkel N., El Younsi C., Dawson M. C. Inter-relations between the phospholipids of rat pancreatic islets during glucose stimulation, and their response to medium inositol and tetracaine. Eur J Biochem. 1975 Nov 1;59(1):245–252. doi: 10.1111/j.1432-1033.1975.tb02448.x. [DOI] [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N. Transport of myo-inositol into endoneurial preparations of sciatic nerve from normal and streptozotocin-diabetic rats. Biochem J. 1983 Mar 15;210(3):775–781. doi: 10.1042/bj2100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner A. M., Schultz S. G., Curran P. F. Sodium and sugar fluxes across the mucosal border of rabbit ileum. J Gen Physiol. 1969 Mar;53(3):362–383. doi: 10.1085/jgp.53.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goresky C. A., Nadeau B. E. Uptake of materials by the intact liver. The exchange of glucose across the cell membranes. J Clin Invest. 1974 Feb;53(2):634–646. doi: 10.1172/JCI107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J Clin Invest. 1982 Nov;70(5):1009–1018. doi: 10.1172/JCI110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Winegrad A. I., Carpentier J. L., Brown M. J., Fukuma M., Orci L. Rabbit sciatic nerve fascicle and 'endoneurial' preparations for in vitro studies of peripheral nerve glucose metabolism. J Neurochem. 1979 Nov;33(5):1007–1018. doi: 10.1111/j.1471-4159.1979.tb05237.x. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Sacktor B., Daughaday W. H. myo-Inositol transport in renal brush border vesicles and it inhibition by D-glucose. Am J Physiol. 1980 Aug;239(2):F113–F120. doi: 10.1152/ajprenal.1980.239.2.F113. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues. Effects of calcium and sodium on glucose metabolism and insulin release. Biochem J. 1974 Jan;138(1):33–45. doi: 10.1042/bj1380033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Evidence for mediated transport of glucose in mammalian pancreatic -cells. Biochim Biophys Acta. 1971 Jul 6;241(1):147–154. doi: 10.1016/0005-2736(71)90312-9. [DOI] [PubMed] [Google Scholar]
- Kasahara M., Inui K., Takano M., Hori R. Distinction of three types of D-glucose transport systems in animal cells. Biochem Biophys Res Commun. 1985 Oct 30;132(2):490–496. doi: 10.1016/0006-291x(85)91160-x. [DOI] [PubMed] [Google Scholar]
- Laychock S. G. Identification and metabolism of polyphosphoinositides in isolated islets of Langerhans. Biochem J. 1983 Oct 15;216(1):101–106. doi: 10.1042/bj2160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. An enzymatic fluorimetric assay for myo-inositol. Anal Biochem. 1984 Sep;141(2):382–389. doi: 10.1016/0003-2697(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Karl I. E., Daughaday W. H. Concentration of myo-inositol in skeletal muscle of the rat occurs without active transport. J Clin Invest. 1980 Apr;65(4):783–788. doi: 10.1172/JCI109728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague W., Morgan N. G., Rumford G. M., Prince C. A. Effect of glucose on polyphosphoinositide metabolism in isolated rat islets of Langerhans. Biochem J. 1985 Apr 15;227(2):483–489. doi: 10.1042/bj2270483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Rumford G. M., Montague W. Studies on the role of inositol trisphosphate in the regulation of insulin secretion from isolated rat islets of Langerhans. Biochem J. 1985 Jun 15;228(3):713–718. doi: 10.1042/bj2280713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pace C. S., Clements R. S. Myo-inositol and the maintenance of beta-cell function in cultured rat pancreatic islets. Diabetes. 1981 Aug;30(8):621–625. doi: 10.2337/diab.30.8.621. [DOI] [PubMed] [Google Scholar]
- Palmano K. P., Whiting P. H., Hawthorne J. N. Free and lipid myo-inositol in tissues from rats with acute and less severe streptozotocin-induced diabetes. Biochem J. 1977 Oct 1;167(1):229–235. doi: 10.1042/bj1670229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prpić V., Blackmore P. F., Exton J. H. myo-Inositol uptake and metabolism in isolated rat liver cells. J Biol Chem. 1982 Oct 10;257(19):11315–11322. [PubMed] [Google Scholar]
- Reddy V. N., Varma S. D., Chakrapani B. Intraocular transport of myoinositol. I. Accumulation in the rabbit ciliary body. Invest Ophthalmol. 1970 Oct;9(10):785–793. [PubMed] [Google Scholar]
- Silverman M. Glucose transport in the kidney. Biochim Biophys Acta. 1976 Dec 14;457(3-4):303–351. doi: 10.1016/0304-4157(76)90003-4. [DOI] [PubMed] [Google Scholar]
- Slaby F., Bryan J. High uptake of myo-inositol by rat pancreatic tissue in vitro stimulates secretion. J Biol Chem. 1976 Aug 25;251(16):5078–5086. [PubMed] [Google Scholar]
- Whiting P. H., Palmano K. P., Hawthorne J. N. Enzymes of myo-inositol and inositol lipid metabolism in rats with streptozotocin-induced diabetes. Biochem J. 1979 Jun 1;179(3):549–553. doi: 10.1042/bj1790549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Kikuchi M., Renold A. E., Sharp G. W. Somatostatin- and epinephrine-induced modifications of 45Ca++ fluxes and insulin release in rat pancreatic islets maintained in tissue culture. J Clin Invest. 1977 Nov;60(5):1165–1173. doi: 10.1172/JCI108869. [DOI] [PMC free article] [PubMed] [Google Scholar]


