Abstract
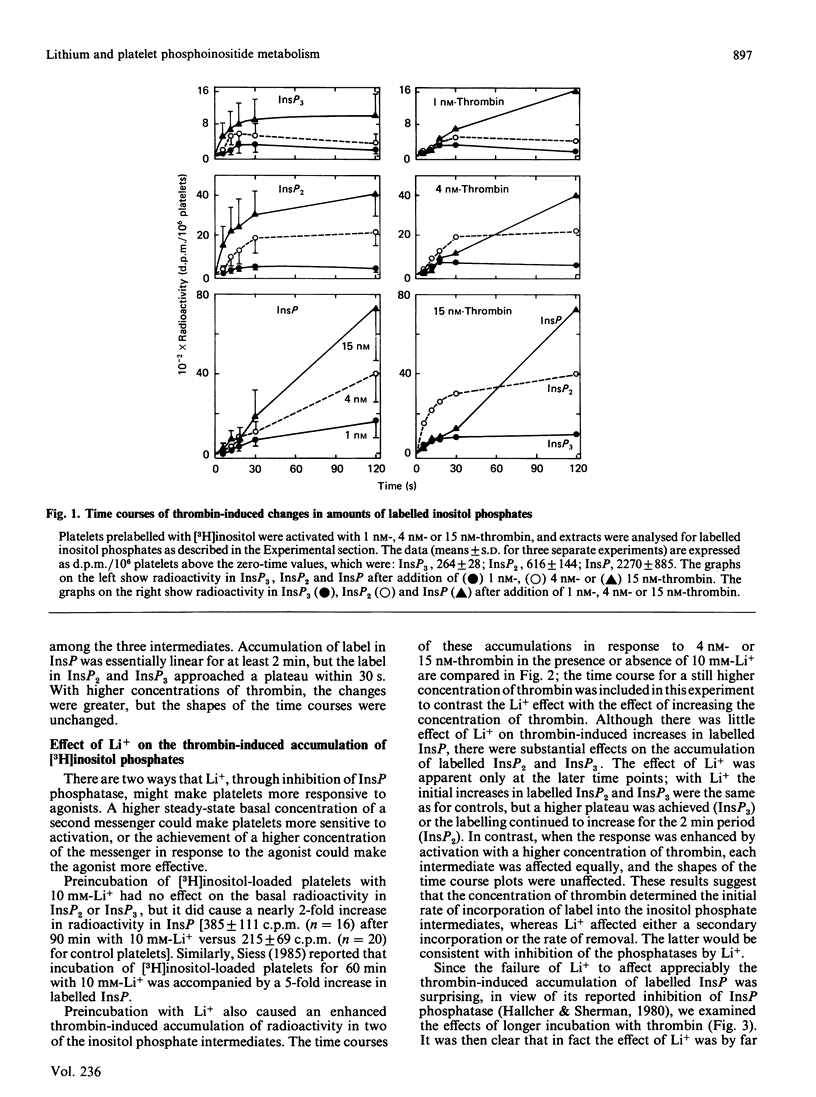
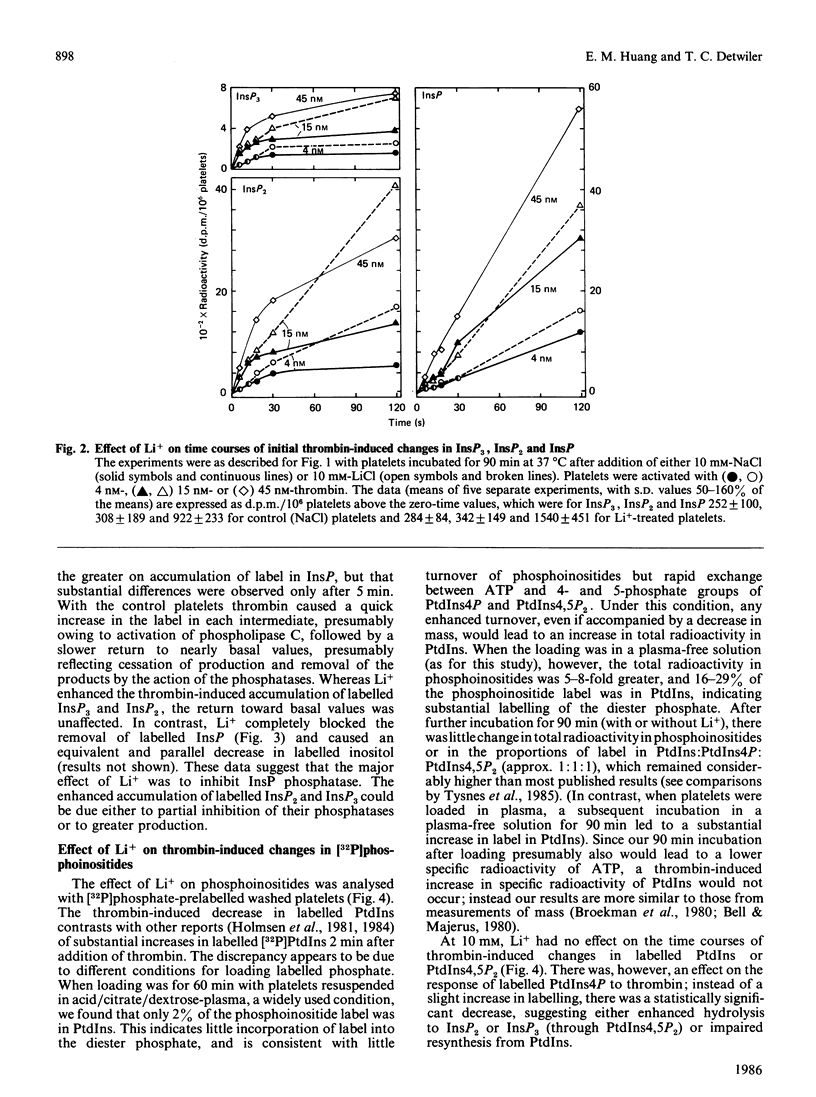
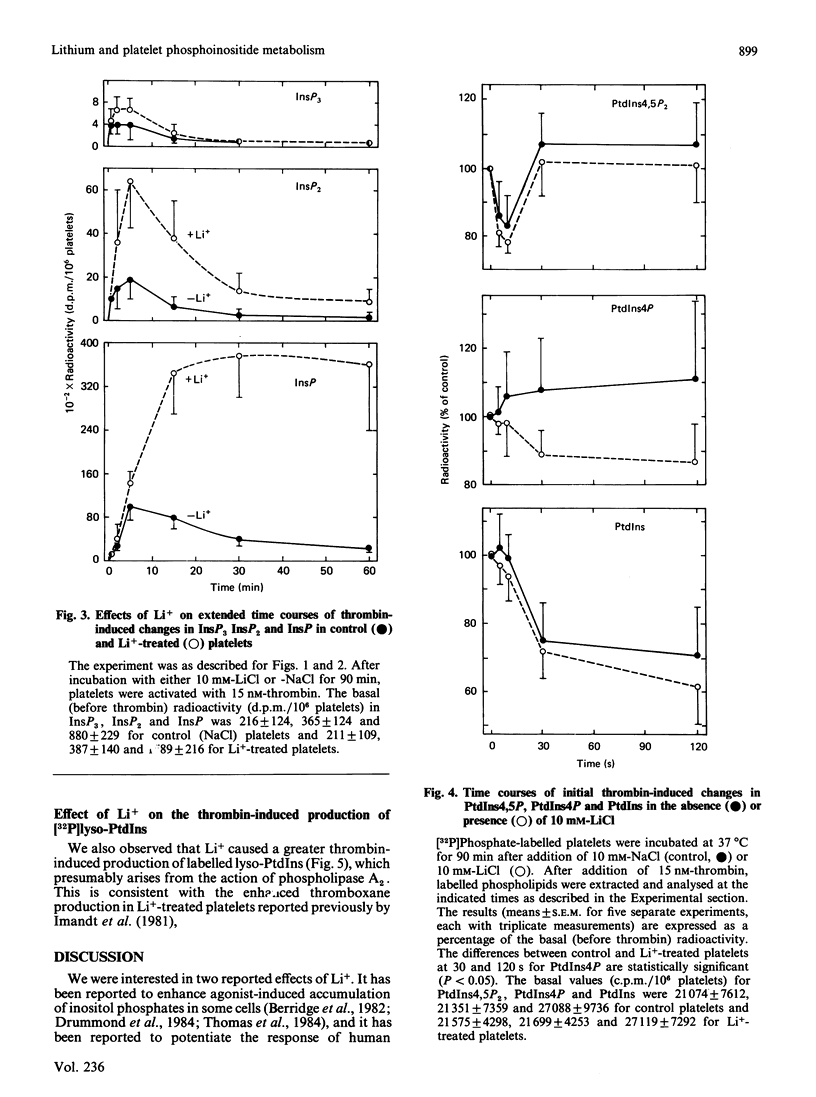
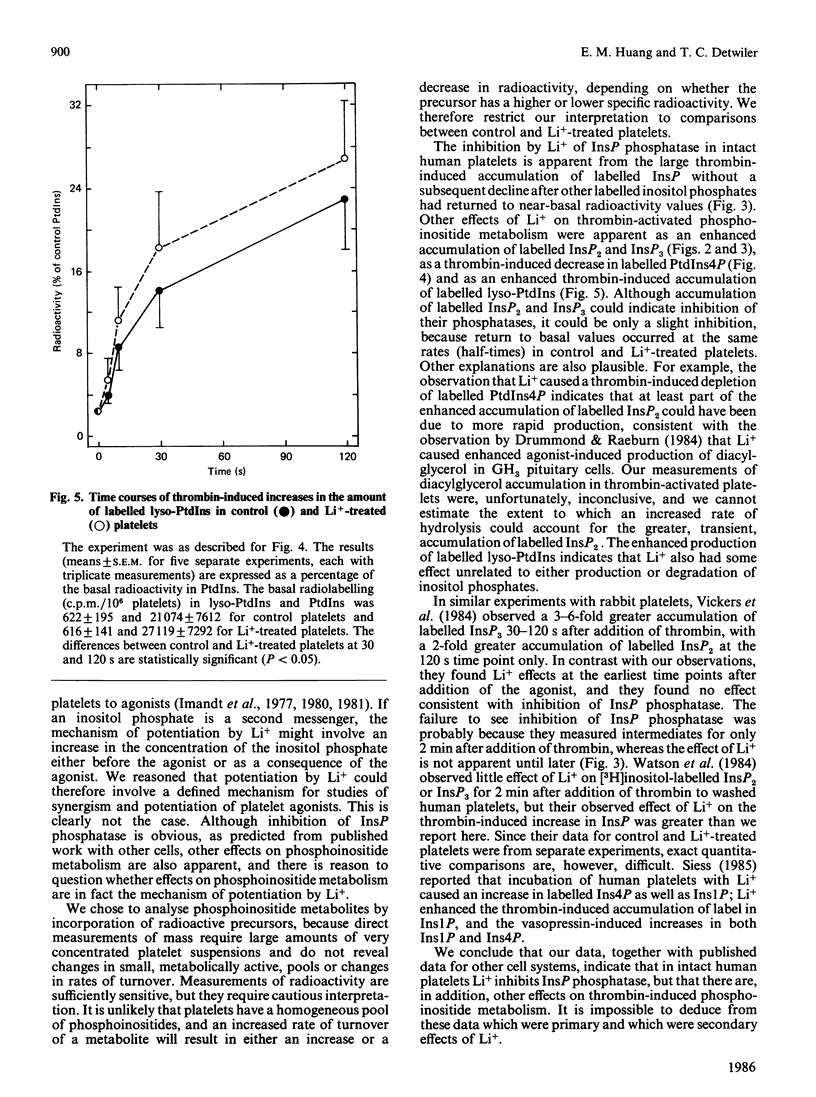
The effects on phosphoinositide metabolism of preincubation of platelets for 90 min with 10 mM-Li+ were studied. Measurements were made of [32P]phosphate-labelled phosphoinositides and of [3H]inositol-labelled inositol mono-, bis- and tris-phosphate (InsP, InsP2 and InsP3). Li+ had no effect on the basal radioactivity in the phosphoinositides or in InsP2 or InsP3, but it caused a 1.8-fold increase in the basal radioactivity in InsP. Li+ caused a 4-, 3- and 2-fold enhanced thrombin-induced accumulation of label in InsP, InsP2 and InsP3 respectively. Although the elevated labelling of InsP2 and InsP3 returned to near-basal values within 30-60 min, the high labelling of InsP did not decline over a period of 60 min after addition of thrombin to Li+-treated platelets, consistent with inhibition of InsP phosphatase by Li+. The effect of Li+ was not due to a shift in the thrombin dose-response relationship; increasing concentrations of thrombin enhanced the initial rate of production of radiolabelled inositol phosphates, whereas Li+ affected either a secondary production or the rate of their removal. The only observed effect of Li+ on phosphoinositide metabolism was a thrombin-induced decrease (P less than 0.05) in labelled phosphatidylinositol 4-phosphate in Li+-treated platelets; this suggests an effect on phospholipase C. Li+ enhanced (P less than 0.05) the thrombin-induced increase in labelled lysophosphatidylinositol, suggesting an effect on phospholipase A2. It is concluded that Li+ inhibits InsP phosphatase and has other effects on phosphoinositide metabolism in activated platelets. The observed effects occur too slowly to be the mechanism by which Li+ potentiates agonist-induced platelet activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. II. IMMUNOLOGICAL AND CLINICAL STUDIES. J Clin Invest. 1964 May;43:856–869. doi: 10.1172/JCI104971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J. H., Blisner M. E., Holland W. H., Hipps P. P., Sherman W. R. Increased brain myo-inositol 1-phosphate in lithium-treated rats. Biochem Biophys Res Commun. 1976 Jul 26;71(2):664–670. doi: 10.1016/0006-291x(76)90839-1. [DOI] [PubMed] [Google Scholar]
- Allison J. H., Stewart M. A. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971 Oct 27;233(43):267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Majerus P. W. Thrombin-induced hydrolysis of phosphatidylinositol in human platelets. J Biol Chem. 1980 Mar 10;255(5):1790–1792. [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Broekman M. J., Ward J. W., Marcus A. J. Phospholipid metabolism in stimulated human platelets. Changes in phosphatidylinositol, phosphatidic acid, and lysophospholipids. J Clin Invest. 1980 Aug;66(2):275–283. doi: 10.1172/JCI109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Bushfield M., Macphee C. H. Thyrotropin-releasing hormone-stimulated [3H]inositol metabolism in GH3 pituitary tumor cells. Studies with lithium. Mol Pharmacol. 1984 Mar;25(2):201–208. [PubMed] [Google Scholar]
- Drummond A. H., Raeburn C. A. The interaction of lithium with thyrotropin-releasing hormone-stimulated lipid metabolism in GH3 pituitary tumour cells. Enhancement of stimulated 1,2-diacylglycerol formation. Biochem J. 1984 Nov 15;224(1):129–136. doi: 10.1042/bj2240129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Holmsen H., Dangelmaier C. A., Holmsen H. K. Thrombin-induced platelet responses differ in requirement for receptor occupancy. Evidence for tight coupling of occupancy and compartmentalized phosphatidic acid formation. J Biol Chem. 1981 Sep 25;256(18):9393–9396. [PubMed] [Google Scholar]
- Holmsen H., Dangelmaier C. A., Rongved S. Tight coupling of thrombin-induced acid hydrolase secretion and phosphatidate synthesis to receptor occupancy in human platelets. Biochem J. 1984 Aug 15;222(1):157–167. doi: 10.1042/bj2220157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imandt L., Genders T., Wessels H., Haanen C. The effect of lithium on platelet aggregation and platelet release reaction. Thromb Res. 1977 Sep;11(3):297–308. doi: 10.1016/0049-3848(77)90183-9. [DOI] [PubMed] [Google Scholar]
- Imandt L., Tijhuis D., Wessels H., Haanen C., van den Berg R., Thomas C. Lithium stimulates thromboxane B2 formation in human platelets. Prostaglandins Med. 1981 Nov;7(5):421–429. doi: 10.1016/0161-4630(81)90031-8. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E., Sasson J. P. Mass changes in myoinositol trisphosphate in human platelets stimulated by thrombin. Inhibitory effects of phorbol ester. J Biol Chem. 1985 Jul 25;260(15):8657–8660. [PubMed] [Google Scholar]
- Seyfred M. A., Farrell L. E., Wells W. W. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat liver plasma membranes. J Biol Chem. 1984 Nov 10;259(21):13204–13208. [PubMed] [Google Scholar]
- Sherman W. R., Leavitt A. L., Honchar M. P., Hallcher L. M., Phillips B. E. Evidence that lithium alters phosphoinositide metabolism: chronic administration elevates primarily D-myo-inositol-1-phosphate in cerebral cortex of the rat. J Neurochem. 1981 Jun;36(6):1947–1951. doi: 10.1111/j.1471-4159.1981.tb10819.x. [DOI] [PubMed] [Google Scholar]
- Siess W. Evidence for the formation of inositol 4-monophosphate in stimulated human platelets. FEBS Lett. 1985 Jun 3;185(1):151–156. doi: 10.1016/0014-5793(85)80760-2. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Alexander J., Williamson J. R. Relationship between inositol polyphosphate production and the increase of cytosolic free Ca2+ induced by vasopressin in isolated hepatocytes. J Biol Chem. 1984 May 10;259(9):5574–5584. [PubMed] [Google Scholar]
- Tysnes O. B., Aarbakke G. M., Verhoeven A. J., Holmsen H. Thin-layer chromatography of polyphosphoinositides from platelet extracts: interference by an unknown phospholipid. Thromb Res. 1985 Nov 1;40(3):329–338. doi: 10.1016/0049-3848(85)90268-3. [DOI] [PubMed] [Google Scholar]
- Vickers J. D., Kinlough-Rathbone R. L., Mustard J. F. Accumulation of the inositol phosphates in thrombin-stimulated, washed rabbit platelets in the presence of lithium. Biochem J. 1984 Dec 1;224(2):399–405. doi: 10.1042/bj2240399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]


