Abstract
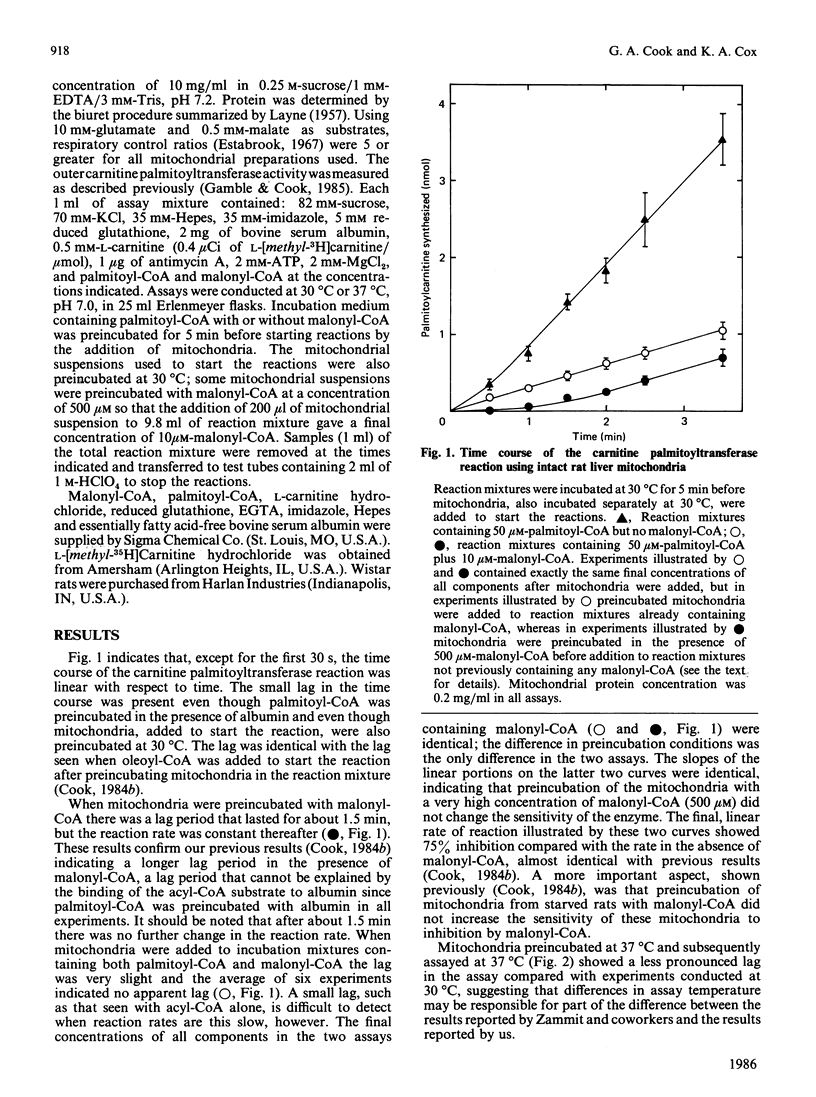
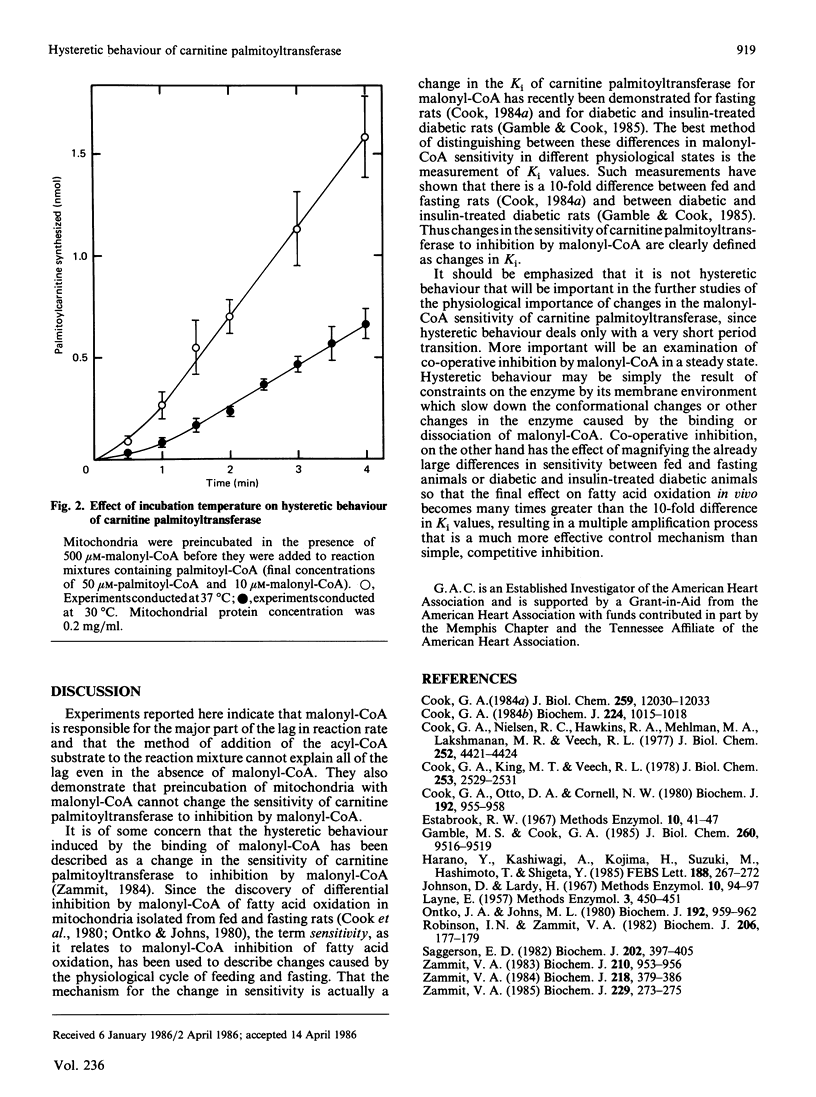
Continuous assays of carnitine palmitoyltransferase were used to study the hysteretic behaviour of the enzyme. When reactions were started by adding mitochondria to complete reaction mixtures, there was a lag in the assay even in the absence of malonyl-CoA. When mitochondria were preincubated with malonyl-CoA in the absence of palmitoyl-CoA, there was a greater lag period in the assay of carnitine palmitoyltransferase, but this lag was less prominent at 37 degrees C than at 30 degrees C. Preincubation of mitochondria with malonyl-CoA did not change the sensitivity of the enzyme to inhibition by malonyl-CoA.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Cook G. A. Involvement of hysteretic effects in the inhibition of carnitine palmitoyltransferase by malonyl-CoA. Biochem J. 1984 Dec 15;224(3):1015–1018. doi: 10.1042/bj2241015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A., King M. T., Veech R. L. Ketogenesis and malonyl coenzyme A content of isolated rat hepatocytes. J Biol Chem. 1978 Apr 25;253(8):2529–2531. [PubMed] [Google Scholar]
- Cook G. A., Nielsen R. C., Hawkins R. A., Mehlman M. A., Lakshmanan M. R., Veech R. L. Effect of glucagon on hepatic malonyl coenzyme A concentration and on lipid synthesis. J Biol Chem. 1977 Jun 25;252(12):4421–4424. [PubMed] [Google Scholar]
- Cook G. A., Otto D. A., Cornell N. W. Differential inhibition of ketogenesis by malonyl-CoA in mitochondria from fed and starved rats. Biochem J. 1980 Dec 15;192(3):955–958. doi: 10.1042/bj1920955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble M. S., Cook G. A. Alteration of the apparent Ki of carnitine palmitoyltransferase for malonyl-CoA by the diabetic state and reversal by insulin. J Biol Chem. 1985 Aug 15;260(17):9516–9519. [PubMed] [Google Scholar]
- Harano Y., Kashiwagi A., Kojima H., Suzuki M., Hashimoto T., Shigeta Y. Phosphorylation of carnitine palmitoyltransferase and activation by glucagon in isolated rat hepatocytes. FEBS Lett. 1985 Sep 2;188(2):267–272. doi: 10.1016/0014-5793(85)80385-9. [DOI] [PubMed] [Google Scholar]
- Ontko J. A., Johns M. L. Evaluation of malonyl-CoA in the regulation of long-chain fatty acid oxidation in the liver. Evidence for an unidentified regulatory component of the system. Biochem J. 1980 Dec 15;192(3):959–962. doi: 10.1042/bj1920959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Carnitine acyltransferase activities in rat liver and heart measured with palmitoyl-CoA and octanoyl-CoA. Latency, effects of K+, bivalent metal ions and malonyl-CoA. Biochem J. 1982 Feb 15;202(2):397–405. doi: 10.1042/bj2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Effects of the mode of addition of acyl-CoA on the initial rate of formation of acylcarnitine in the presence of carnitine by intact rat liver mitochondria in vitro. Biochem J. 1985 Jul 1;229(1):273–275. doi: 10.1042/bj2290273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Increased sensitivity of carnitine palmitoyltransferase I activity to malonyl-CoA inhibition after preincubation of intact rat liver mitochondria with micromolar concentrations of malonyl-CoA in vitro. Biochem J. 1983 Mar 15;210(3):953–956. doi: 10.1042/bj2100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Time-dependence of inhibition of carnitine palmitoyltransferase I by malonyl-CoA in mitochondria isolated from livers of fed or starved rats. Evidence for transition of the enzyme between states of low and high affinity for malonyl-CoA. Biochem J. 1984 Mar 1;218(2):379–386. doi: 10.1042/bj2180379. [DOI] [PMC free article] [PubMed] [Google Scholar]


