Abstract
Penetration and propagation of herpesviruses in the nervous system require the action of several glycoproteins. To assay for a function of glycoproteins gC, gK, and gL in the neuroinvasiveness of pseudorabies virus (PrV), deletion mutants lacking one of these glycoproteins and corresponding rescuants were inoculated in the nasal cavity of adult mice. We demonstrate that the lack of gL almost prevented the virus from penetrating and propagating in trigeminal, sympathetic, and parasympathetic tracks innervating the nasal cavity, while the lack of gC and gK only slowed the invasion of the nervous system. The conclusion of this and previous studies is that only gB, gD, gH, and gL are indispensable for penetration into neurons, while gB, gH, and gL (and, in some categories of neurons, also gE and gI) are necessary for transneuronal transfer in the mouse model. The deletion of other glycoprotein genes has little effect on PrV neuroinvasiveness although it may affect the dissemination of the virus.
Penetration and propagation of herpesviruses in the nervous system necessitate the presence of several viral glycoproteins involved in entry and/or viral egress. Of the 10 structural glycoproteins of pseudorabies virus (PrV), five (gC, gE, gI, gM, and gN) are dispensable for growth in cell culture (reviewed in reference 19). These proteins are also dispensable for penetration into neurons, although gE and gI are required for transneuronal transfer in some categories of neurons (3, 5, 6, 10, 12, 16, 17, 20, 25, 27, 28, 29; also reviewed in reference 9). Five glycoproteins (gB, gD, gH, gK, and gL) are necessary for multiplication in cell culture (19). gB, gD, and gH are required for penetration into host cells and into peripheral neurons (1, 4, 21, 22, 23). The presence of gB and gH, but not of gD, is an absolute requirement for cell-to-cell spread and transneuronal transfer. The role of gK and gL in the viral neuroinvasiveness remained to be investigated. After use of a mutant named PrV-gKβ, in which the UL53 open reading frame was interrupted by a lacZ cassette, it was reported that gK was involved in virus release (13). In its absence, the production of infectious particles is reduced by a factor of 30, unless the mutant is propagated on complementing cells. The presence of gK seems to prevent the reinfection of already infected cells by progeny virions, thus favoring the dissemination of the infection. Glycoprotein gL has been shown to form a complex with gH (14). In contrast to what has been observed with herpes simplex virus type 1 (24), the presence of gL is not required for the maturation of PrV gH, which is incorporated normally in the envelope of a gL deletion mutant. This mutant, named PrV-ΔgLβ, is defective in cell entry and is drastically impaired in cell-to-cell spread. However, a reversion mutant, designated PrV-ΔgLPass, could be selected from a PrV-ΔgLβ population by coseeding cells infected with gL-transcomplemented PrV-ΔgLβ and normal Vero cells (15). This mutant carries a duplication of sequences in the Us genomic region encompassing parts of the gG and gD genes, as well as a translocation of gH-encoding sequences. The rearrangement resulted in an in-frame fusion of the 5′ part of the gD gene to the 3′ part of the gH gene, which gives rise to a chimeric gDH protein. The presence of the fusion protein is sufficient to restore the ability of the mutant to grow in noncomplementing Vero, MDBK, or PK15 cells. PrV-ΔgLPass also carries a deletion in the gC gene which seems to favor multiplication of the mutant in cell culture, since a gC-restored PrV-ΔgLPass had a tendency to lose gC during passages (B. G. Klupp, personal communication). Here we compared the neuroinvasiveness of PrV-gKβ and PrV-ΔgLβ after intranasal inoculation of adult mice to that of rescued viruses in which a wild-type glycoprotein gene has been restored. We also analyzed the neuroinvasiveness of PrV-ΔgLPass. For comparison, we have included in this study a mutant named PrV-ΔgC which contains a lacZ expression cassette inserted into the partially deleted gC gene of mutant Kag111− (16; T. C. Mettenleiter, unpublished results) (16). Our results demonstrate that gL, but not gC or gK, is essential for penetration into and propagation in neurons and that the gDH fusion protein can replace gL in this respect.
MATERIALS AND METHODS
Cells and viruses.
Mutants are based on the wild-type PrV strain Kaplan (PrV-Ka). The engineering of PrV-Kag111−, PrV-gKβ, PrV-ΔgLβ, PrV-ΔgLPass, and rescuants in which the corresponding wild-type gene was restored has been described elsewhere (13, 14, 15, 16). PrV-ΔgC, PrV-ΔgLβ, and PrV-ΔgLPass carry a deletion in one of the glycoprotein genes and an insertion of the lacZ gene. In PrV-gKβ, the lacZ gene was inserted in the middle of the gK gene and interrupted the gK open reading frame. The rescuants do not express β-galactosidase (β-Gal). PrV-ΔgC and PrV-ΔgLPass and gC, gK, and gL rescuants were propagated on Vero cells. PrV-gKβ and PrV-ΔgLβ were propagated on complementing Vero cells expressing either gK or gL, as already described (13, 14). It is known that recombination between viral DNA and viral sequences present in complementing cells can result in the formation of revertant viruses. To keep the frequency of revertants in mutant populations as low as possible, several single plaques of PrV-gKβ or PrV-ΔgLβ were picked from complementing Vero cells, multiplied on complementing cells, and titrated on complementing and normal Vero cells by plaque assay under methyl cellulose. After 3 days at 37°C, cells were incubated for 4 h in 330-μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, 2 mM MgCl2, and 0.1% Triton X-100 in phosphate-buffered saline (PBS) and were counterstained with crystal violet. Both mutants formed wild-type-sized plaques on complementing Vero cells with blue cells on the edge. As already described, only small foci of blue cells (or isolated blue cells), but no plaques, were observed on normal Vero cells inoculated with PrV-gKβ (13), indicating that the clones did not contain detectable amount of revertants. In contrast, most cloned harvests of PrV-ΔgLβ titrated on normal Vero cells gave few plaques which were not stained with X-Gal among thousands of isolated (or small foci of) blue cells, indicating the presence of revertants. A clone of PrV-ΔgLβ which contained fewer than 10−4 revertants was selected for amplification on complementing Vero cells. Mutant and rescuant working stocks were obtained as follows: normal or complementing Vero cells (depending on the virus) were infected at a multiplicity of infection (MOI) of 0.01 PFU/cell and were incubated at 37°C in a CO2 atmosphere. After development of a complete cytopathic effect, cells were harvested and intra- and extracellular virus was concentrated and purified by centrifugation through a glycerol cushion (2). Resuspended viruses were titrated by plaque assay on Vero cells (and on complementing cells for PrV-gKβ and PrV-ΔgLβ) under methyl cellulose. Depending on the mutant, titers ranged between 2 × 107 PFU/ml (PrV-ΔgLβ) and 6 × 108 PFU/ml (PrV-gKβ and gC rescuant; Table 1). After amplification, the PrV-gKβ stock was still devoid of revertants, while the PrV-ΔgLβ stock contained around 0.3% of viruses which no longer expressed the lacZ gene and could therefore be considered revertants. In view of the frequency of recombination of PrV-ΔgLβ in complementing Vero cells, no further attempt was made to obtain a better stock. Viral suspensions were stored in aliquots at −70°C until use.
TABLE 1.
Health status of mice after intranasal inoculation of mutant or rescuant virusesa
| Virus mutant or rescuant | Titer of inoculumb (104 PFU) | No. of mice inoculated | Health status of mice | No. of healthy, sick, or dead mice at various times p.i.
|
|||
|---|---|---|---|---|---|---|---|
| 48 h | 72 h | 90 h | 114 h | ||||
| PrV-ΔgC | 100 | 5 | Sick | 1/5 (S1) | |||
| Dead | 4/5 | ||||||
| PrV-gKβ | 200 | 12 | Healthy | 12/12 (SP3) | |||
| Sick | 9/9 (SP3) | ||||||
| PrV-ΔgLβ | 6 | 9 | Healthy | 9/9 (SP3) | 5/6 (SP1) | ||
| Sick | 1/6 (SP1) | 2/4 (SP2) | |||||
| Dead | 2/4 | ||||||
| ΔgLpass | 43 | 10 | Healthy | 10/10 | 10/10 (SP5) | 3/5 | |
| Sick | 1/5 (S1) | 3/3 (S3) | |||||
| Dead | 1/5 | ||||||
| PrV-gCr | 200 | 3 | Sick | 2/3 (SP2) | |||
| Dead | 1/3 | ||||||
| PrV-gKr | 50 | 3 | Sick | 1/3 (SP1) | |||
| Dead | 2/3 | ||||||
| PrV-gLr | 60 | 3 | Sick | 3/3 (SP3) | |||
The number of mice sacrificed (S) and perfused for neuroanatomical studies (P) is given in parentheses.
Three microliters.
Verification of genotypes of mutant viruses.
We verified that our mutant stocks were not contaminated with viruses of unexpected genotypes. To this end, 106 Vero cells were infected at various MOIs with mutants or corresponding rescuants and were incubated at 37°C for 3 days under methylcellulose. Cells were then treated with X-Gal as described above.
Restriction maps of PrV-gKβ, PrV-ΔgLβ, and rescuants were also analyzed. DNA was extracted from infected Vero cells after lysis in 1% NP-40, 0.1% sodium deoxycholate, 10 mM Tris, and 1 mM EDTA, pH 8. After removal of the nuclei by centrifugation, sodium dodecyl sulfate and proteinase K were added to the supernatant to final concentrations of 0.5% and 200 μg/ml, followed by incubation at 65°C for 1 h. DNA was purified according to standard procedures (24a). Purified DNA was digested with BamHI, and fragments were separated on a 0.6% agarose gel. Southern blot analysis of the PrV-gKβ and rescuant DNA was also performed. Digested DNA was transferred onto a positively charged nylon membrane and was hybridized with a digoxigenin-labeled gK probe prepared by primer extension with the Klenow DNA polymerase from a linearized pGC plasmid in which the gK gene was introduced at a BamHI site. The primer was situated immediately upstream of the gK gene.
Finally, the absence of gL sequences in PrV-ΔgLPass was controlled by PCR, using two 21-mer primers (L1 and L2), one localized within the deletion and the other outside in the UL1 gene coding for gL. Another set of primers situated in the UL49.5 gene coding for gN (described in reference 18), which should give an amplification product with both the mutant DNA and rescued DNA, was used as a positive control.
Sequences of the primers are as follows: L1, 5′-CGC TTC GCC ACG CTC CAG TTG-3′; L2, 5′-GCT CGT GAC GGC GGT CAT GTC-3′; N1, 5′-GGC CAC GAC GAG CAC CGC CAG-3′; and N2, 5′-CTC GCA CAC ACC AGG ATG GTC-3′.
L1 and L2 start at positions 1060 and 1463, respectively, of the published gL sequence. N1 and N2 start at positions 135 and 360, respectively, of the published gN sequence (GenBank accession nos. U02513 and U38547). PCR amplifications were done with around 20 ng of wild-type or mutant DNA, 1 pmol of each primer, 2.5 U of Taq DNA polymerase (Appligene), a 50 μM concentration of each deoxynucleoside triphosphate, and 10% dimethyl sulfoxide per reaction mixture. Thirty cycles of amplification at 95°C for 60 s, 50°C for 40 s, and 72°C for 120 s were performed. PCR products were separated in 2% agarose gels. A pBR322 DNA-MspI digest was used as markers. PCR could not be used to control the quality of the PrV-gKβ and PrV-ΔgLβ DNAs, because viral DNA was contaminated with a low amount of DNA from the complementing Vero cells.
Animal experiments.
Our research on mice complied with all relevant institutional policies. Intranasal inoculation of the mutants into adult mice was performed as described earlier (2). Briefly, 3 μl of undiluted viral suspension was instilled into the right nostril of 6-week-old Swiss mice under deep anesthesia using a Hamilton syringe connected to a catheter. All animals developed typical symptoms of pseudorabies, e.g., hunched posture and itching, and died or were sacrificed as soon as possible for ethical reasons. To study viral penetration and propagation in the nervous system, mice were euthanatized at various times postinfection (p.i.) with pentobarbital and were transcardially perfused (flow rate, 10 ml/min) with 20 ml of PBS (150 mM NaCl, 7.4 mM Na2HPO4, and 2.4 mM KH2PO4) followed by 4% paraformaldehyde in PBS (150 ml) and 20% sucrose in PBS (60 ml). The head was skinned, and the lower jaw and teeth were removed. The spinal cord was dissected. Both head and spinal cord (usually still in vertebral column) were decalcified in PBS containing 0.1 M EDTA for 10 days at 4°C and were then kept in 20% sucrose in PBS for 24 h at 4°C. All tissues were frozen at −70°C and cut into transverse sections (30 μm thick) that were collected in two parallel series on gelatin-coated slides.
For detection of β-Gal activity in the tissues of mutant-infected mice, one series of sections was incubated for 4 h in 330-μg/ml X-Gal, 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, 2 mM MgCl2, and 0.1% Triton X-100 in PBS (18). After counterstaining with neutral red (0.01%), the sections were mounted with Entellan (Merck). For detection of rescuant viruses (or revertants) which no longer expressed the lacZ gene, tissue sections were permeabilized in PBS–0.1% Triton X-100 for 30 min at room temperature, washed three times in PBS, and incubated overnight at 4°C with rabbit anti-PrV serum diluted 1:1,000 in PBS. After three washes with PBS, the sections were incubated for 1 h at 4°C with biotinylated anti-rabbit antibodies, avidin, and biotinylated β-Gal. The β-Gal activity was then revealed as explained above. Observation was through a Zeiss microscope with a 4× objective.
RESULTS
Control of genotype of mutant viruses.
Vero cells were infected with mutant or rescuant viruses at various MOIs, incubated at 37°C for 3 days under methylcellulose, and stained with X-Gal. PrV-ΔgC and PrV-ΔgLPass gave plaques with blue cells on the edge. As already reported, only isolated (or small foci of 2 to 10) blue cells but no plaques were observed after infection with PrV-gKβ, while PrV-ΔgLβ gave occasional white plaques representing revertants in a background of isolated (or small foci of) blue cells. Rescuants gave wild-type plaques which did not stain blue after X-Gal treatment.
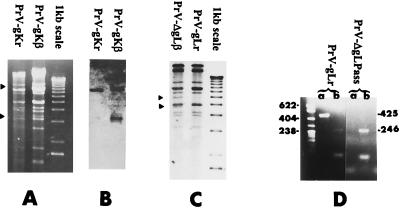
Restriction map analysis of PrV-gKβ, PrV-ΔgLβ, and rescuants confirmed the expected genotype of the mutants. The insertion of a lacZ cassette in the gK gene introduced a new BamHI site within the 5′ BamHI fragment. After BamHI digestion, the 5′ fragment was cleaved into 8- and 3-kbp subfragments (arrowheads in Fig. 1A). The 3-kbp fragment could be seen between bands 10 and 11, but the 8-kbp fragment comigrated with fragments 5, 5′, and 6. It could be visualized on the Southern blot shown in Fig. 1B, together with the 3-kbp fragment at the expected position. In the case of PrV-ΔgLβ DNA, the new BamHI site introduced with the lacZ cassette in the gL gene resulted in the cleavage of fragment 6 into 4- and 5-kbp subfragments (arrowheads in Fig. 1C).
FIG. 1.
Genotype assessment of viral stocks. BamHI restriction map of PrV-gKβ and PrV-gKr DNA (A) or of PrV-ΔgLβ and PrV-gLr (C). (B) Southern blot analysis of PrV-gKβ and PrV-gKr DNA. Digested DNA shown in panel A was transferred onto a cellulose membrane and hybridized with a digoxigenin-labeled gK probe prepared from a linearized pGC-gK plasmid by primer extension performed with the Klenow DNA polymerase. (D) PCR amplifications with PrV-ΔgLPass or rescuant DNA, L1 and L2 primers (lanes a) or N1 and N2 primers (lanes b), Taq DNA polymerase, deoxynucleoside triphosphate, and dimethyl sulfoxide, as explained in Materials and Methods. PCR products were separated in 2% agarose gels. Their size is indicated on the right. A pBR322 DNA-MspI digest was used as marker (the size of the bands [in nucleotides] is indicated on the left).
The lack of contamination of PrV-ΔgLPass with gL-expressing viruses (whether rescuant or mutants of unexpected genotype) was verified by PCR, using suitable primers (see Materials and Methods). These primers yielded an amplification product of 425 nucleotides with the rescuant DNA but yielded nothing with PrV-ΔgLPass DNA, while two primers, N1 and N2, designed to amplify part of the gN gene (18), gave the expected amplification product of 246 nucleotides on both DNAs (Fig. 1D). Therefore, PrV-ΔgLPass, as expected, did not contain gL sequences.
Survival of mice inoculated with mutant or rescuant viruses.
Series of female Swiss mice (aged 6 to 8 weeks) were inoculated in the right nostril with 3 μl of the virus suspension, representing between 6 × 104 and 2 × 106 PFU. Mice infected with rescuant viruses were sick or dead at 2 days p.i. with typical symptoms of PrV, i.e., mad itching and hunched posture (Table 1). Since death rapidly follows the appearance of the symptoms (usually less than 2 to 3 h), sick animals were euthanatized as rapidly as possible for ethical reasons. In mice inoculated with PrV-ΔgC or complemented PrV-gKβ, symptoms and death occurred 1 day later. Animals inoculated with complemented PrV-ΔgLβ or with PrV-ΔgLPass developed symptoms from 3 to 5 days p.i. (Table 1).
Penetration and propagation of mutant and rescuant viruses in nervous system.
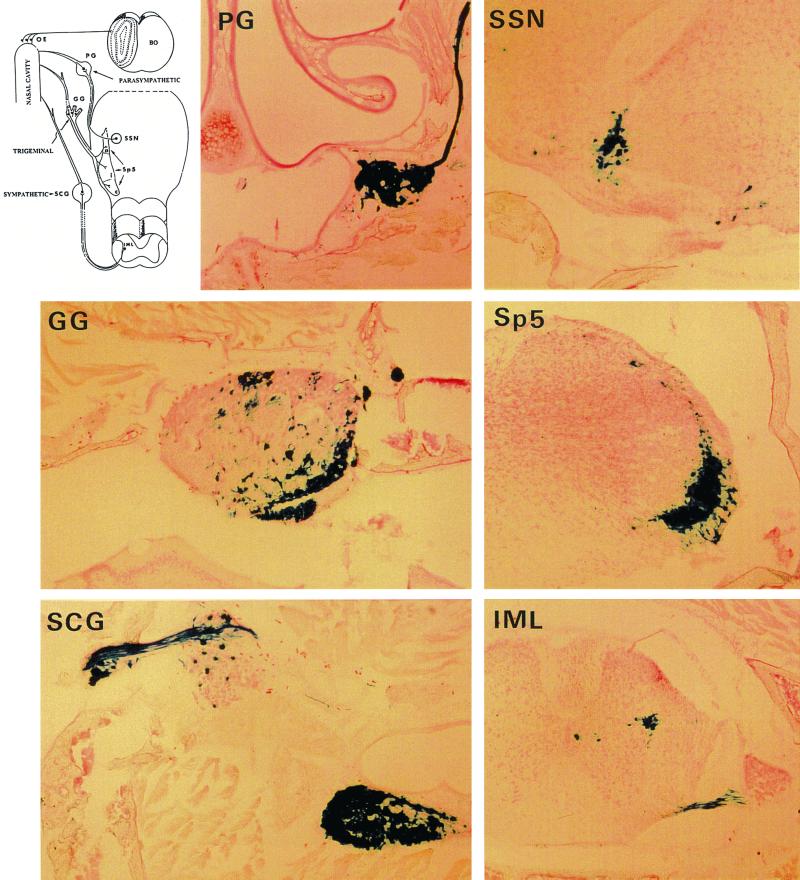
In each series of experiments, several animals were perfused under deep anesthesia. Their head and spinal cords were dissected, decalcified in 1 mM EDTA, frozen, and cut in transverse serial sections (thickness, 30 μm). The number of animals sacrificed and perfused for examination of their tissues is recorded in Table 1, except for PrV-ΔgC, where another series of inoculation was performed. In this case, animals were sacrificed and perfused at 24, 48, and 79 h. At the later time animals were moribund. As explained later, the extent of infection of the nervous system of sick animals was very similar whatever the mutant or rescuant inoculated (except in the case of PrV-ΔgLβ), and very little individual variation was observed. The nervous system of animals sacrificed before the occurrence of the symptoms showed fewer infected neurons, but conclusions were similar. Only results obtained when animals became sick are given in this paper. At the onset of the symptoms, the right nasal cavity of mice inoculated with PrV-ΔgC and PrV-gKβ was heavily infected. Many infected foci could be found in the respiratory and in the vomeronasal organ. Clusters of infected olfactory neurons were also observed in the olfactory epithelium (OE) (Table 2). In PrV-ΔgC-inoculated animals, the left nasal cavity was also infected, though less heavily, while it was absolutely free of infection in PrV-gKβ-inoculated animals (not shown). This last finding was unusual. The olfactory bulb did not contain infected neurons, thus confirming that PrV could not be transmitted in mice from olfactory neurons to second-order neurons in this structure. The pterygopalatine ganglia (PG), Gasser ganglia (GG), and superior cervical ganglia (SCG) contain cell bodies of parasympathetic, trigeminal, or sympathetic neurons innervating the nasal cavity (first-order neurons). The three types of ganglia were heavily infected on the right (Fig. 2 and Table 2). Left ganglia also contained few infected neurons (not shown). First-order parasympathetic, trigeminal, and sympathetic neurons connect with second-order neurons in the superior salivatory nucleus (SSN) and spinal trigeminal nucleus (Sp5) regions of the brain and the intermediolateral nucleus (IML) in the spinal cord, respectively (Fig. 2). These regions of the nervous system were infected, indicating that viruses with a deletion of gC or gK could also be transferred through synapses in the three categories of neurons (Fig. 2 and Table 2). Animals infected with rescued viruses were sick or dead 2 days p.i. Their tissues were examined after immunocytochemical staining with an anti-PrV serum. In every aspects, the extent of infection in the nasal cavity (right or left) and in the nervous system was similar to that observed in wild-type-PrV- or gG-deleted PrV-inoculated mice (2) (Table 2). It was also similar to what is described above concerning PrV-ΔgC- or PrV-gKβ-infected animals at 79 or 72 h, respectively (Fig. 2 and Table 2), except the lack of infection of the left nasal cavity, which remains characteristic of PrV-gKβ-inoculated mice.
TABLE 2.
Viral invasion of the nasal cavity and nervous system in mice inoculated intranasally with mutant or rescuant PrVa
| Mutant (PrV) | Incubation time (h) | Extent of infection in:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Epithelia
|
First-order neurons
|
Second-order neurons
|
|||||||
| RE | OE | PG | GG | SCG | SSN | Sp5 | IML | ||
| gCr | 48 | ++ | ++ | NE | ++ | ++ | ++ | ++ | ++ |
| gKr | 48 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| gLr | 48 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| ΔgC | 79 | ++ | ++ | NE | ++ | ++ | ++ | ++ | ++ |
| gKβ | 72 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| ΔgL | 90 | − (+) | +/− (+) | + (+) | + (++) | + (++) | − (++) | +/− (+) | − (−) |
| gLpass | 72 | ++ | ++ | + | + | ++ | ++ | +/− | + |
The extent of infection is compared to that observed at 48 h in wild-type-PrV-and gG-deleted PrV-inoculated mice. Mice were sacrificed and perfused when moribund, except that mice inoculated with PrV-ΔgLPass were perfused at 72 h, a few hours before the onset of symptoms. In most cases, infection was similar to that in wild-type-PrV-inoculated mice (++). In PrV-ΔgLβ-inoculated animals, the extent of infection by a β-Gal-expressing virus was lower (+), much lower (+/−), or absent (−). In this case, tissues were also infected by a revertant no longer expressing β-Gal. The extent of infection by the revertant is indicated in parentheses (see also Fig. 3). NE, not examined.
FIG. 2.
Penetration and propagation of PrV-gKβ in the nervous system of adult mice after intranasal inoculation. Mice inoculated with PrV-gKβ were sacrificed and perfused when moribund. The head (minus skin, lower jaw, and teeth) was decalcified in EDTA, frozen, and entirely cut in 30-μm-thick sections. All other sections were treated with X-Gal. Massive infection is present in the nasal cavity (not shown); in PG, GG, and SCG containing first-order neurons of the parasympathetic, trigeminal, and sympathetic routes; and in regions of brain and spinal cord containing second-order neurons of the three routes: SSN, Sp5, and IML. Note that there is another ganglion close to the SCG which contains infected neurons and probably belongs to the same chain of sympathetic ganglia. Schematic representation of the innervation of the nasal cavity is shown on the upper left for clarity (BO, olfactory bulb). Magnification, ×30.
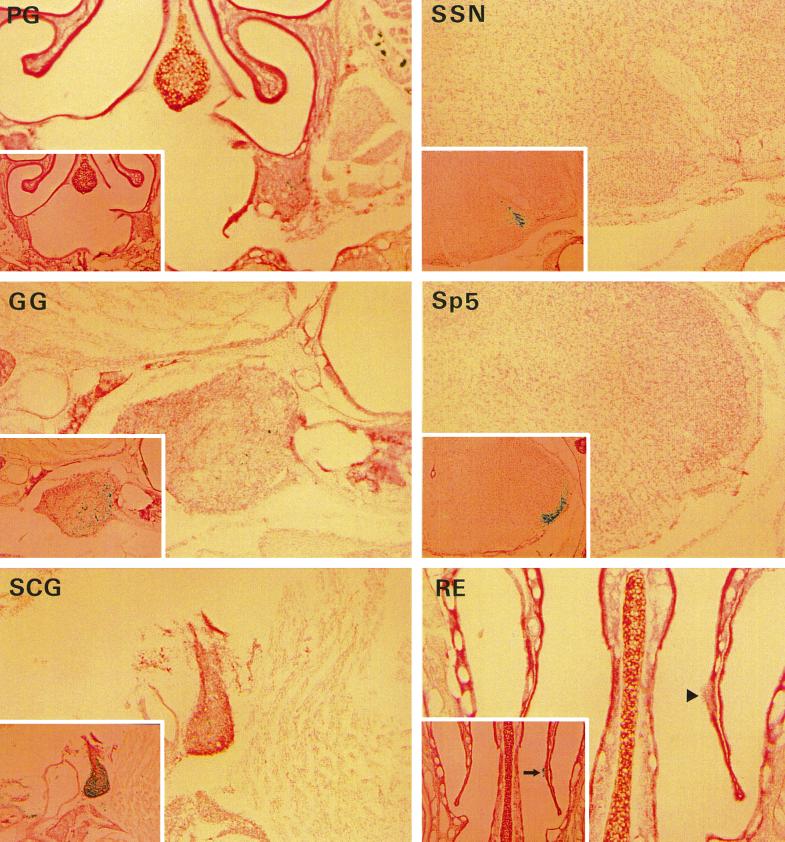
On the contrary, the lack of glycoprotein gL clearly restricted the capability of the virus to propagate in infected animals. At 90 h p.i., the respiratory epithelium (RE) was devoid of β-Gal-expressing cells but showed few necrotic lesions (arrowhead in panel RE [Fig. 3]). When the RE of animals sacrificed earlier (2 and 3 days p.i.) was examined, a few dozen blue cells were found (not shown). Neurons staining blue after X-Gal treatment were present at 90 h p.i. in the OE (Table 2). The mutant was also able to penetrate in first-order parasympathetic, trigeminal, and sympathetic neurons, though less efficiently than the wild type, and did not propagate (or propagated very poorly) to second-order neurons (Fig. 3 and Table 2). At 90 h p.i., only a few dozen blue neurons were found in the PG, GG, and SCG, fewer than a dozen in the Sp5, and none in the SSN and IML. Since the extent of infection was not sufficient to explain the occurrence of severe PrV symptoms, immunostaining with anti-PrV immune serum was performed on the second series of sections in order to detect the eventual presence of β-Gal-negative revertants. Clearly, the few spontaneous revertants which were present in the inoculum multiplied in the nasal cavity and by 90 h p.i. had invaded the parasympathetic and trigeminal tracks and the SCG to an extent similar to that for rescued viruses (gCr, gKr, or gLr) after 2 days (Fig. 3 and Table 2). Second-order neurons in the IML were not infected (not shown). Necrotic lesions in the RE mentioned above correspond to regions which were heavily infected by revertant virus (arrow in panel RE [Fig. 3]).
FIG. 3.
The neuroinvasiveness of PrV-ΔgLβ is severely impaired. One series of 30-μm-thick sections was treated with X-Gal in order to visualize cells and neurons infected with PrV-ΔgLβ. In order to reveal the eventual presence of spontaneous revertants (no longer expressing β-Gal), the other series was treated with rabbit anti-PrV antibodies and then with a biotinylated anti-rabbit antibody, followed by avidin and biotinylated β-Gal. The final X-Gal treatment stained in blue cells or neurons infected with mutant or revertant PrV. Here we show typical sections of the RE, ganglia, and brain which are either not infected or are poorly infected with the mutant. The same region in adjacent sections after immunostaining with anti-PrV antibodies is shown in the inset. Heavy infection with revertant PrV (no longer expressing β-Gal) is obvious except in the case of the PG, where the extent of infection was similar with or without treatment with anti-PrV antibodies. Extensive infection of this ganglion with revertant viruses is noticed on the two following sections (not shown). Magnification, ×30 (×15 for the insets). Notice the presence of a lesion in the RE, which does not contain β-Gal-expressing cells (arrowhead) but is stained after treatment with an anti-PrV immune serum (arrow in inset).
Finally, 3 days after infection of mice with PrV-ΔgLPass, we found infected first- and second-order neurons in parasympathetic, trigeminal, and sympathetic routes, indicating that the mutant was able to penetrate and propagate in the nervous system, although less rapidly than wild-type or rescuant viruses (Table 2).
DISCUSSION
Using intranasal inoculation of adult mice, we compared the neuroinvasiveness of deletion mutants lacking glycoprotein gC, gK, or gL to that of their respective rescuants in which the deleted sequences were reintroduced by recombination. As expected, the virulence and neuroinvasiveness of rescuants, as illustrated by the survival time of inoculated animals and the extent of infection of the nervous system, were indistinguishable from that of the wild-type or gG-deleted PrV (2). The virulence of the mutants was reduced, as illustrated by the finding that mutant-inoculated mice were still healthy at 48 h p.i. while rescuant-infected animals were all very sick or dead. Mutant-inoculated mice remained healthy for an additional 24 h (in the case of PrV-ΔgC and PrV-gKβ) or 40 to 48 h (in the case of PrV-ΔgLβ and PrV-ΔgLPass). Thus, symptom onset and death were delayed in mutant-infected animals.
When the nervous system of moribund PrV-ΔgC- and PrV-gKβ-inoculated mice was examined, we observed no significant difference in the nature and the number of infected neurons from those for animals inoculated with wild-type or rescued viruses, indicating that gC and gK are not essential for viral neuroinvasiveness. In all cases we observed that first- and second-order neurons on the trigeminal, sympathetic, and parasympathetic tracks were infected. As already reported, the infection propagated transneuronally at synapses or locally between adjacent, unconnected neurons (1, 2, 18). For instance, local transfer of the mutants was exemplified by the fact that a ganglion like the SCG, containing neurons which innervate other regions of the head besides those which extend to the nasal cavity, is massively infected (Fig. 2). gC promotes adsorption of free virions to target cells by interacting with cell surface proteoglycans which carry heparan sulfate moieties (11, 19). This function is not essential in vitro probably because other glycoproteins like or gD can also mediate attachment. Our results confirm that gC is also not essential for infection of animals in an experimental system in which high amounts of virus are introduced deeply into the nasal cavity, i.e., in close contact with target cells. However, even under these favorable conditions, the neuroinvasion of a virus from which gC has been deleted is slower than that of wild-type PrV. Thus, we postulate than a PrV lacking the gC gene would be selected against under natural conditions. In addition, it would probably be less transmissible from one animal to the other.
Glycoprotein gK is considered essential in tissue culture. In its absence, progeny viral titers are reduced by a factor of 30 (13) and the mutant needs to be grown in complementing cells. More specifically, the presence of gK in cellular and viral membranes seems to prevent readsorption on already infected cells, thus favoring the spread of the infection. The absence of gK also reduces but does not totally abolish cell-to-cell spread in noncomplementing cells, since after 2 days at 37°C under agarose, small foci of infected cells can be observed. That the mutant could not spread at distance was also found in mice, as shown by the fact that controlateral nostrils remained free of infection, even late in the incubation period, an unusual observation. Transneuronal and local transfer of the infection was also slower, but finally, 3 days p.i., the same categories and approximately the same number of neurons were infected. We were surprised to observe that the left ganglia contained a small number of infected neurons even though the left nasal cavity was free of infection. Either these ganglia contain a minority of neurons extending to the controlateral nasal cavity, or left and right fibers of the trigeminal, sympathetic, and parasympathetic tracks come in proximity somewhere in the central nervous system (CNS), thus allowing local transfer of the virus to occur. Although gK, like gC, is not essential sensu stricto for viral neuroinvasiveness, it must be important for viral propagation under natural conditions because viral spread is reduced in the absence of this protein.
The extent of infection of the nervous system at the onset of the symptoms was remarkably comparable, whether the mice were inoculated with wild-type PrV; mutants deleted from glycoproteins gG, gM, gN (2, 18), gC, or gK (this study); or rescuants from these deletion mutants. In all cases, it was indistinguishable from what is shown in Fig. 2. Symptoms which were rapidly followed by death invariably occurred when the GG, PG, and SCG (containing first-order neurons innervating the nasal cavity) as well as the SP5, SSN, and IML in the CNS and spinal cord (containing second-order neurons of the trigeminal, parasympathetic, and sympathetic routes) were heavily infected. The infection of the CNS is mostly limited to these regions. The olfactory bulb is not infected, showing that in the mouse model the olfactory route is not the route of entry into the CNS. Death occurs so rapidly, especially in mice inoculated with wild-type virus and rescuants, that there is not much time for the virus to propagate in the CNS. Our estimation is that there is time for four or five cycles of multiplication first in the RE and then in the nervous system. There is no time either to mount an immune response, and this may partly explain why results obtained with mice or rats sometimes differ from those obtained with pigs.
Results obtained with PrV-ΔgLβ-infected animals need to be interpreted in the light of these observations. The survival time of the mice was doubled compared to that of wild-type-PrV-infected animals. The nasal cavity of mutant-infected mice contained only few β-Gal-expressing cells, essentially in the OE. The TG, SCG, and PG contained infected neurons, although much less than observed at 48 h in ganglia from rescuant-infected mice, and the infection did not propagate efficiently to second-order neurons in the CNS, as shown by the absence of X-Gal staining in the SSN and IML and the weak staining observed in the trigeminal track (Sp5). In this case, the extent of infection with the mutant did not correlate with the severity of the symptoms. To explain this apparent discrepancy, a second series of sections was stained with anti-PrV antibodies which revealed the presence of viruses no longer expressing β-Gal in the nasal cavity and in the nervous system. The degree of infection with this revertant virus population 4 days p.i. was similar to that observed 2 days p.i. with wild-type PrV or rescuants in the TG, SCG, and SSN, although it was lower in the PG, Sp5, and IML. Therefore, we think that the death of the animals was due to the presence of spontaneous revertants in the inoculum which multiplied in the nasal cavity and finally invaded the nervous system. We estimate that 200 or 300 neurons in the PG, GG, and SCG were infected by PrV-ΔgLβ (and less than a dozen in the SP5). Thus, the mutant was able to penetrate in the CNS, although less efficiently than wild-type PrV or rescuant viruses, but did not propagate at all or only very poorly. The relatively high number of first-order neurons found infected with the mutant was surprising. gL forms a complex with gH, and both are essential for the fusion between the viral envelope and the cellular cytoplasmic membrane during penetration and also for direct cell-to-cell spread from infected to adjacent noninfected cells (14). We know from previous work that a virus with a deletion of gH is unable to penetrate and propagate in the CNS of adult mice (4). We have shown that 106 PFU of a complemented gH mutant is able to infect at most three or four dozen trigeminal and sympathetic neurons directly from the nasal cavity and that the progeny of complemented mutant viruses was unable to propagate. As a consequence, the number of infected neurons decreased with time. Why did we find more infected first-order neurons with a complemented inoculum of PrV-ΔgLβ, the titer of which was 30 times lower? It is quite possible that the difference is due to the presence of revertants in the PrV-ΔgLβ inoculum. A few epithelial cells and neurons were probably coinfected by mutant and revertant, giving rise to a mixed progeny of transcomplemented mutants and revertants, both being infectious. That the presence of wild-type PrV favors the neuroinvasiveness of a mutant with a deletion of a glycoprotein essential for penetration in certain categories of neurons has been documented (8). Taken together, we postulate that not only is PrV-ΔgLβ affected in propagation but also in penetration into the CNS.
In PrV-ΔgLPass, the loss of gL is compensated by the formation of a gDH chimeric protein. Besides this genomic rearrangement, it also carries a deletion of gC-encoding sequences which probably resulted from extensive passaging of the mutant virus in cell culture. Here we show that not only cell culture infectivity but also neuroinvasiveness is restored by the gDH hybrid protein, since the virus mutant with a deletion of gL finally invades the same categories of neurons. However, neuroinvasion is slower than in mice infected with wild-type PrV or rescuants. Since it is also slower than with a virus mutant with a deletion of gC, the delay does not appear to be solely due to the absence of gC.
In summary, with this report we have finally completed our analysis of the role of all known glycoproteins in the neuroinvasiveness of PrV after intranasal inoculation of adult mice (1, 2, 3, 4, 18). This represents the most complete study of the involvement of herpesvirus glycoproteins in neuroinvasion under identical conditions, in an isogenic viral background. The neuropathogenicity and the neuroinvasiveness of mutants with deletions of gB, gC, gD, gE, or gI have also been analyzed by others in mice or rats and less exhaustively in the natural host, the pig (5, 6, 7, 8, 10, 12, 16, 17, 20, 21, 22, 25, 26, 27, 28, 29). Due to the size of the pig, it is obviously difficult to perform an exhaustive study of the viral neuroinvasiveness in this animal. Investigations have been mostly restricted to the olfactory bulb and the GG. Our study in the mouse certainly helps to delineate areas of the porcine nervous system which could be investigated more thoroughly in the future. For instance, in addition to the GG we would recommend studying viral invasion in the SCG, which could be easily located along the carotid artery, and in the PG, as well as in sections of the brain which contain second-order neurons of the trigeminal and parasympathetic tracks. A few sections of the spinal cord where sympathetic first-order neurons make connections could be profitably studied as well. The conclusion of these studies is that glycoproteins gB, gD, gH, and gL are essential for penetration into neurons but that only three of them, gB, gH, and gL, are necessary for transneuronal transfer. Thus, the basic requirements for entry into and spread in the nervous system parallel those in cultured cells. In addition gE and gI, which form heterodimers on the virion surface, are also essential for transneuronal transfer in certain categories of neurons, while they are generally considered dispensable for multiplication in cell cultures (reviewed in references 9 and 19). On the contrary, gK, which is required for cell-to-cell spread in culture, seems to be less important in vivo. The lack of other nonessential glycoproteins, gC, gG, gM, and gN, delayed the appearance of the symptoms to a variable extent but did not abolish the neuroinvasiveness of PrV in adult mice. More limited results obtained in the pig suggest that the neuroinvasiveness of a mutant with a deletion of gC is normal, while animals inoculated with a mutant with a deletion of gM survived longer and exhibited symptoms which differed from those induced by wild-type PrV.
ACKNOWLEDGMENTS
This study was supported by the CNRS through the UPR A9053, the MENRT through the PRFMMIP program, the European Community (EEC contract BMH4-CT97-2573), and the Deutsche Forschungsgemeinschaft (Me 854/4-1).
We thank Félix Rey and Françoise Bras for critical reading of the manuscript; Sybille Dezélée, whose expertise in molecular biology was greatly appreciated; and Marc Labétoulle for his help in localizing brain structures.
REFERENCES
- 1.Babic N, Mettenleiter T C, Flamand A, Ugolini G. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J Virol. 1993;67:4421–4426. doi: 10.1128/jvi.67.7.4421-4426.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babic N, Mettenleiter T C, Ugolini G, Flamand A, Coulon P. Propagation of pseudorabies virus in the nervous system of the mouse after intranasal inoculation. Virology. 1994;204:616–625. doi: 10.1006/viro.1994.1576. [DOI] [PubMed] [Google Scholar]
- 3.Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. doi: 10.1006/viro.1996.0247. [DOI] [PubMed] [Google Scholar]
- 4.Babic N, Klupp B G, Makoschey B, Karger A, Flamand A, Mettenleiter T C. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J Gen Virol. 1996;77:2277–2285. doi: 10.1099/0022-1317-77-9-2277. [DOI] [PubMed] [Google Scholar]
- 5.Card J P, Rinaman L, Lynn R B, Lee B H, Meade R P, Miselis R R, Enquist L W. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijkstra J M, Gerdts V, Klupp B G, Mettenleiter T C. Deletion of glycoprotein gM of pseudorabies virus results in attenuation for the natural host. J Gen Virol. 1997;78:2147–2151. doi: 10.1099/0022-1317-78-9-2147. [DOI] [PubMed] [Google Scholar]
- 8.Enquist L W, Dubin J, Whealy M E, Card J P. Complementation analysis of pseudorabies virus gE and gI mutants in retinal ganglion cell neurotropism. J Virol. 1994;68:5275–5279. doi: 10.1128/jvi.68.8.5275-5279.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enquist L W, Husak P J, Banfield B W, Smith G A. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs L, Mulder W A M, Oirschot J T V, Gielkens A L J, Kimman T J. Deleting two amino acids in glycoprotein gI of pseudorabies virus decreases virulence and neurotropism for pigs, but does not affect immunogenicity. J Gen Virol. 1993;74:2201–2206. doi: 10.1099/0022-1317-74-10-2201. [DOI] [PubMed] [Google Scholar]
- 11.Karger A, Saalmüller A, Tufaro F, Banfield B W, Mettenleiter T C. Cell surface proteoglycans are not essential for infection by pseudorabies virus. J Virol. 1995;69:3482–3489. doi: 10.1128/jvi.69.6.3482-3489.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimman T G, de Wind N, Oei-Lie N, Pol J M A, Berns A J M, Gielkens A L J. Contribution of single genes within the unique short region of Aujeszky's disease virus (suid herpesvirus type 1) to virulence, pathogenesis and immunogenicity. J Gen Virol. 1992;73:243–251. doi: 10.1099/0022-1317-73-2-243. [DOI] [PubMed] [Google Scholar]
- 13.Klupp B G, Baumeister J, Dietz P, Granzow H, Mettenleiter T C. Pseudorabies virus glycoprotein gK is a virion structural component involved in virus release but is not required for entry. J Virol. 1998;72:1949–1958. doi: 10.1128/jvi.72.3.1949-1958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klupp B G, Fuchs W, Weiland E, Mettenleiter T C. Pseudorabies virus glycoprotein L is necessary for virus infectivity but dispensable for virion localization of glycoprotein H. J Virol. 1997;71:7687–7695. doi: 10.1128/jvi.71.10.7687-7695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klupp B G, Mettenleiter T C. Glycoprotein gL-independent infectivity of pseudorabies virus is mediated by a gD-gH fusion protein. J Virol. 1999;73:3014–3022. doi: 10.1128/jvi.73.4.3014-3022.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kritas S K, Pensaert M B, Mettenleiter T C. Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky's disease virus in the olfactory nervous pathway of the pig. J Gen Virol. 1994;75:2319–2327. doi: 10.1099/0022-1317-75-9-2319. [DOI] [PubMed] [Google Scholar]
- 17.Levine J D, Zhao X-S, Miselis R R. Direct and indirect retino-hypothalamic projections to the supraoptic nucleus in the female albino rat. J Comp Neurol. 1994;341:214–224. doi: 10.1002/cne.903410207. [DOI] [PubMed] [Google Scholar]
- 18.Masse M J, Jöns A, Dijkstra J M, Mettenleiter T C, Flamand A. Glycoproteins gM and gN of pseudorabies virus are dispensable for penetration and propagation in the nervous system of adult mice. J Virol. 1999;73:10503–10507. doi: 10.1128/jvi.73.12.10503-10507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mettenleiter T C. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet Res. 2000;31:99–115. doi: 10.1051/vetres:2000110. [DOI] [PubMed] [Google Scholar]
- 20.Mulder W A M, Jacobs L, Priem J, Kok G L, Wagenaar F, Kimman T G, Pol J M A. Glycoprotein gE-negative pseudorabies virus has a reduced capability to infect second- and third-order neurons of the olfactory and trigeminal routes in the porcine central nervous system. J Gen Virol. 1994;75:3095–3106. doi: 10.1099/0022-1317-75-11-3095. [DOI] [PubMed] [Google Scholar]
- 21.Mulder W A M, Pol J, Kimman T, Kok G, Priem J, Peters B. Glycoprotein gD-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI. J Virol. 1996;70:2191–2200. doi: 10.1128/jvi.70.4.2191-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peeters B, de Wind N, Hooisma M, Wagenaar F, Gielkens A, Moormann R. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J Virol. 1992;66:894–905. doi: 10.1128/jvi.66.2.894-905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauh I, Mettenleiter T C. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J Virol. 1991;65:5348–5356. doi: 10.1128/jvi.65.10.5348-5356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 25.Sams J M, Jansen A S P, Mettenleiter T C, Loewy A D. Pseudorabies virus mutants as transneuronal markers. Brain Res. 1995;687:182–190. doi: 10.1016/0006-8993(95)00484-8. [DOI] [PubMed] [Google Scholar]
- 26.Standish A, Enquist L W, Escardo J A, Schwaber J S. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci. 1995;15:1998–2012. doi: 10.1523/JNEUROSCI.15-03-01998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H-J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang M, Card J P, Tirabassi R S, Miselis R R, Enquist L W. Retrograde, transneuronal spread of pseudorabies virus in defined neuronal circuits in the rat brain is facilitated by gE mutations that reduce virulence. J Virol. 1999;73:4350–4359. doi: 10.1128/jvi.73.5.4350-4359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]