Abstract
Background Context:
There are a number of risk factors- from biological, psychological, and social domains- for non-specific chronic low back pain (cLBP). Many cLBP treatments target risk factors on the assumption that the targeted factor is not just associated with cLBP but is also a cause (i.e, a causal risk factor). In most cases this is a strong assumption, primarily due to the possibility of confounding variables. False assumptions about the causal relationships between risk factors and cLBP likely contribute to the generally marginal results from cLBP treatments.
Purpose:
The objectives of this study were to a) using rigorous confounding control compare associations between modifiable causal risk factors identified by Mendelian randomization (MR) studies with associations in a cLBP population and b) estimate the association of these risk factors with cLBP outcomes.
Study Design/Setting:
Cross sectional analysis of a longitudinal, online, observational study.
Patient Sample:
1,376 participants in BACKHOME, a longitudinal observational e-Cohort of U.S. adults with cLBP that is part of the NIH Back Pain Consortium (BACPAC) Research Program.
Outcome Measures:
Pain, Enjoyment of Life, and General Activity (PEG) Scale.
Methods:
Five risk factors were selected based on evidence from MR randomization studies: sleep disturbance, depression, BMI, alcohol use, and smoking status. Confounders were identified using the ESC-DAG approach, a rigorous method for building directed acyclic graphs based on causal criteria. Strong evidence for confounding was found for age, female sex, education, relationship status, financial strain, anxiety, fear avoidance and catastrophizing. These variables were used to determine the adjustment sets for the primary analysis. Potential confounders with weaker evidence were used for a sensitivity analysis.
Results:
Participants had the following characteristics: age 54.9 ± 14.4 years, 67.4% female, 60% never smokers, 29.9% overweight, 39.5% obese, PROMIS sleep disturbance T-score 54.8 ± 8.0, PROMIS depression T-score 52.6 ± 10.1, Fear-avoidance Beliefs Questionnaire 11.6 ± 5.9, Patient Catastrophizing Scale 4.5 ± 2.6, PEG 4.4 ± 2.2. In the adjusted models alcohol use, sleep disturbance, depression, and obesity were associated with PEG, after adjusting for confounding variables identified via a DAG constructed using a rigorous protocol. The adjusted effect estimates- the expected change in the PEG outcome for every standard deviation increase or decrease in the exposure (or category shift for categorical exposures) were the largest for sleep disturbance and obesity. Each SD increase in the PROMIS sleep disturbance T-score resulted in a mean 0.77 (95% CI: 0.66, 0.88) point increase in baseline PEG score. Compared to participants with normal BMI, adjusted mean PEG score was slightly higher by 0.37 points (95% CI: 0.09, 0.65) for overweight participants, about 0.8 to 0.9 points higher for those in obesity classes I and II, and 1.39 (95% CI: 0.98, 1.80) points higher for the most obese participants. Each SD increase in the PROMIS depression T-score was associated with a mean 0.28 (95% CI: 0.17, 0.40) point increase in baseline PEG score, while each SD decrease in number of alcoholic drinks per week resulted in a mean 0.12 (95%CI: 0.01, 0.23) increase in baseline PEG score in the adjusted model.
Conclusions:
Several modifiable causal risk factors for cLBP - alcohol use, sleep disturbance, depression, and obesity- are associated with PEG, after adjusting for confounding variables identified via a DAG constructed using a rigorous protocol. Convergence of our findings for sleep disturbance, depression, and obesity with the results from MR studies, which have different designs and biases, strengthens the evidence for causal relationships between these risk factors and cLBP (1). The estimated effect of change in a risk factors on change in PEG were the largest for sleep disturbance and obesity. Future analyses will evaluate these relationships with longitudinal data.
Keywords: Epidemiology, Methodology/statistics
Introduction
There are a number of risk factors- from biological, psychological, and social domains- for non-specific chronic low back pain (cLBP) (1). Associations between these risk factors and cLBP underlie the widely accepted conceptual model of cLBP, the biopsychosocial model (2). Clinical guidelines for treatment of cLBP recommend a number of different interventions targeted to risk factors associated with the biopsychocial model, most notably therapeutic exercise (3–5), pain neuroscience education (3), manual therapy (3–5), acupuncture (3–5), and cognitive behavioral therapy (CBT) (5). However, the effects from randomized controlled trials (RCT’s) of these treatments are at best modest (6). One reason why cLBP intervention fail may be that the risk factors they target, while they are associated with cLBP, do not cause cLBP. Targeting treatments to causes may lead to new, more effective, therapeutic approaches.
Determining if a risk factor causes cLBP requires studies that minimize confounding bias (i.e., bias due to variables that are a common cause of both a risk factor and an outcome). There are two general approaches for addressing confounding bias: design-based and analysis-based (7). Analysis-based approaches use statistical methods to minimize bias of estimated associations in observational data by adjusting for confounding variables. A common approach for identifying confounders and their corresponding adjustment sets is to construct a directed acyclic graph (DAG), which embeds existing knowledge and theory into a causal graph describing the relationship among risk factors, outcomes, and other important variables (8). Design-based approaches rely on study design, rather than statistical methods, to address confounding bias. The most robust design-based approach is a randomized controlled trial (RCT). While RCT’s are the gold standard for establishing causality (9) for most cLBP risk factors random allocation is either not possible (e.g., obesity) or unethical (e.g. smoking). An alternative design-based approach increasingly used in cLBP research is Mendelian randomization (MR). MR uses germline genetic variants as proxies for risk factors (9). As genetic variants are randomly assigned at conception, they should be independent of confounding factors (9). Therefore, MR attempts to produce comparisons analogous to an RCT, with individuals randomized to a particular genotype, rather than an intervention (9). When specific assumptions are met, the strength of evidence for MR studies lies somewhere between observational studies and randomized controlled trials (RCT’s) (10). While MR and other design-based methods are important tools they have limitations (7). As design-based and analysis-based methods have different underlying assumptions and biases, triangulating the findings from studies done with both approaches provides stronger evidence for causal links than either method independently (7).
Recent MR studies have identified causal links between a variety of risk factors and cLBP (10–36), many of which are modifiable and therefore potential treatment targets. MR cLBP causal effect estimates are based on the association between genetic variants and prevalent cLBP cases in a population, using large publicly available databases (10). The objectives of this study were to a) compare associations between modifiable causal risk factors identified by design-based MR studies with associations defined by an analytic approach in a cLBP population and b) estimate the association of these risk factors with cLBP outcomes. To accomplish these objectives we used data from a unique cLBP cohort study (BACKHOME) (37), which contains measurements of numerous, heterogenous variables from a large number of participants. Variables in this dataset that have been identified in MR studies as modifiable causes of cLBP were selected as exposures (alcohol use, smoking, sleep disturbance, depression, and obesity (10, 23–25, 38) and associations with a composite outcome of pain intensity and interference (PEG score) were determined. Confounding bias was controlled using statistical adjustment based on factors identified via a DAG constructed using a rigorous and structured protocol, and the magnitude and direction of association synthesized with MR results to identify the potential impact of interventions targeted to these risk factors.
Methods
Study Design
Cross sectional analysis of a longitudinal, online, observational study.
Setting
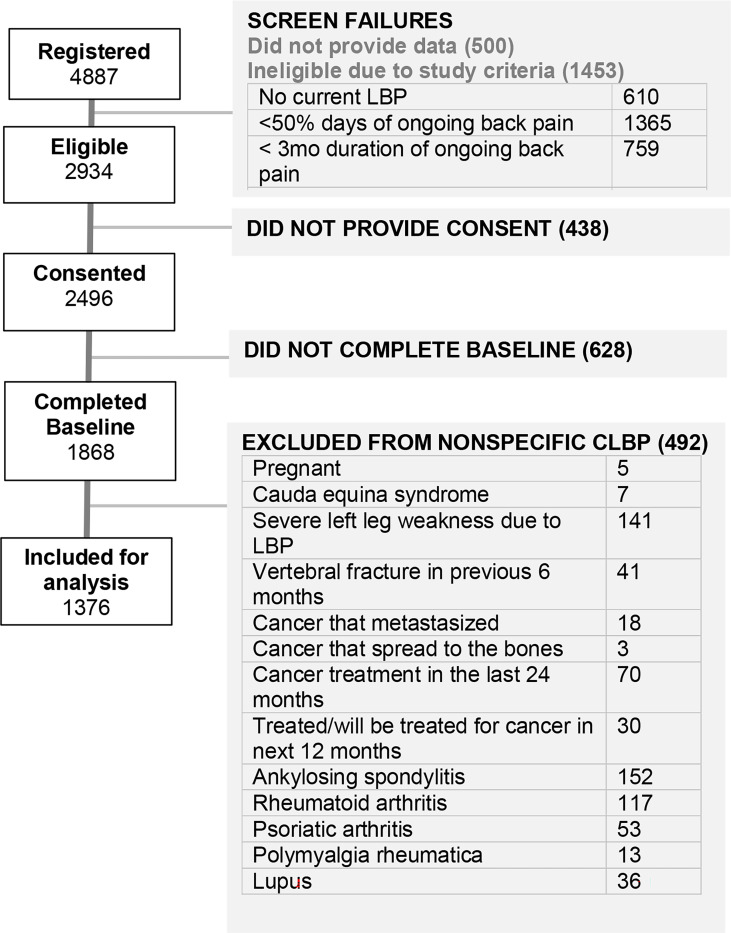
The study was built on the NIH-supported Eureka Research Platform, which allows for the development and hosting of digital clinical studies. It allows completely remote web- and mobile- based recruitment, enrollment, consent, and participation across the United States (39). Enrollment started in July 2021 and will continue until approximately 3,000 participants have been enrolled. Participants will be followed 2 years or more, with surveys completed every 3 months the first year then every 6 months thereafter. This analysis included data from the baseline survey only, using data collected from 1,868 participants who had enrolled in BACKHOME and completed baseline surveys through April 18, 2023. (Figure 1).
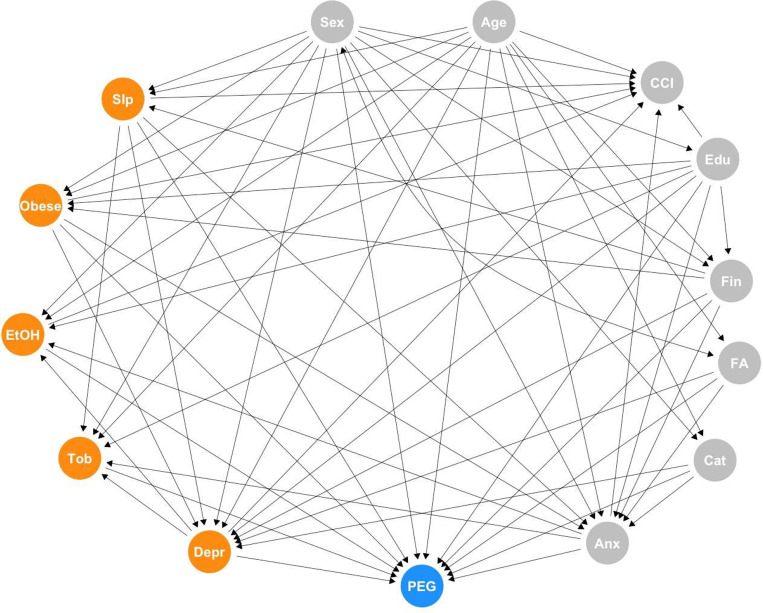
Figure 1.
PEG = three-item scale for assessing pain intensity and interference; FA = Fear Avoidance; Cat = Pain Catastrophization; Depr = Depression; Anx = Anxiety; Slp = Sleep; EtOH = Alcohol use; Tob = Smoking; CCI = Charlson Comorbidity Index; Edu = Education; Fin = Financial strain. Exposures highlighted in orange.
Participants
Participants had to be 18 years of age or older, registered for a Eureka account, currently living in the United States, have an iOS or Android smartphone, have a cell phone number, agree to participate in English, and be able to provide consent to participate in the study. After providing electronic consent to participate in the study, participants were asked to complete a baseline survey about demographics, medical conditions, medications, and behaviors through the study app. Participants could voluntarily provide permission to collect additional data from their smartphones, including geolocation and, among iOS users, HealthKit.
In addition to meeting the requirements for the Eureka platform registration participants had to meet the criteria for cLBP as defined by NIH Pain Consortium Research Task Force (RTF) and BACPAC Minimum Dataset Working Group: current self-report of chronic low back pain (pain between the lower posterior margin of the rib cage and the horizontal gluteal fold), which has persisted for more than the past 3 months AND has resulted in pain on more than half the days in the past 6 months. Participants were recruited through Facebook ads across the United States and targeted emails of prior Eureka participants. As our target population was non-specific cLBP, 492 participants were excluded from the current analysis if they were pregnant, currently diagnosed with cauda equina syndrome, had severe leg weakness due to lower back pain, diagnosed with a vertebral fracture in the previous 6 months, had cancer that metastasized had or spread to bones, had cancer treatment in the last 24 months or planned in the next 12 months, or had a history of autoimmune disorders (ankylosing spondylitis, rheumatoid or psoriatic arthritis, polymyalgia rheumatica, or lupus (Figure 1).
Measurements
The online baseline survey included questions on demographics, back-related pain, back pain treatment, medications, pain impact on quality of life, pain beliefs, medical history, health habits, and traumatic experiences. Detailed methods for all measurements used in this analysis are in Appendix A.
Outcome and Exposures
We selected the baseline PEG score, a three-item scale for assessing pain intensity and interference (40), as the outcome measure. We selected five exposures that have been identified in MR studies as modifiable causes of cLBP that were also measured in our dataset: alcohol use, smoking, sleep disturbance, depression, and obesity (10, 23–25, 38). Sleep disturbance and depression were measured as continuous variables using the PROMIS sleep disturbance 6a T-score (41) and PROMIS depression 4a T-score (41). The number of drinks per week, as a continuous variable, was used to measure alcohol use. Smoking (42) and obesity (43) were both analyzed as categorical variables as detailed in Appendix – Section A.
Confounders
Confounders of the relationships between each exposure of interest and PEG were identified using the rigorous evidence synthesis for constructing directed acyclic graphs (ESC-DAG) approach (44). This is a method for building directed acyclic graphs (DAGs) based on causal criteria which offers a structured protocol for DAG construction and reporting. DAGs are conceptual tools that are widely used to develop analytic strategies (45), especially for controlling for potential confounders in observational data analysis (8). The basic components of a DAG are nodes and edges representing variables and assumptions about their directed interrelationships. Each DAG has an exposure(s), an outcome, and a number of covariates. Differentiating confounders from other covariates, such as mediators, is critical for identifying appropriate adjustment sets to estimate statistics of interest while minimizing bias (46). The ESC-DAG method defines a systematic approach to DAG construction, by incorporating an evidence synthesis protocol into a causal inference framework, specifying how background knowledge is used for determining which variables and connections between variables are included. We followed the three step ESC-DAG method and report our decisions along with relevant literature in Appendix – Section B.
1. Mapping
To begin the graph, a directed edge was drawn from each exposure to the outcome in a single graph. Next, variables collected in the BACKHOME study with a plausible association between at least one of the exposures and/or with PEG, based on the BACPAC theoretical model (1), were added as nodes. Variables that were clearly mediators of pain response (e.g. variables related to neurophysiologic pain mechanism) were excluded as we were primarily interested in estimating the total effect of our exposures. A saturated graph was created by drawing edges from each node to all the other nodes. As the resulting saturated graph was overly complex some nodes were combined if they were conceptually related and had similar inputs and outputs.
2. Translation
Each relationship in the saturated graph was assessed by two authors (PZ and CO) using levels of evidence based on causal criteria (expert opinion, association, temporality, confounding control). The levels of evidence were:
Level 1- Expert opinion only (based on causal models published in the literature)
Level 2- Cross-sectional association
Level 3- Temporal order (longitudinal studies demonstrating that the exposure precedes cLBP)
Level 4- Analysis-based confounding control
Level 5- Design based confounding control (e.g. MR, twin studies)
All edges that included supported by Level 4 or Level 5 evidence were retained. Selected edges with lower levels of evidence that the reviewing authors (PZ and CO) felt were supported by strong theory or expert opinion were also retained. The level of evidence and supporting references for the retained edges in the saturated graph was recorded in a decision log (Appendix – Section B).
3. Integration
Directed edges defined during the translation phase were synthesized into a final DAG that was used to guide the statistical analysis.
The ESC-DAG process resulted in a single, fully specified DAG that considered the five exposures simultaneously along with nodes and directed edges identified via the process of mapping, translation, and integration described above. The nodes retained following the ESC-DAG process are identified in the final DAG (Figure 1). The confounding variables retained in the DAG were age, sex, education, relationship status, financial strain, PROMIS anxiety, fear avoidance, and pain catastrophizing, as defined in the Appendix – Section A. These variables were designated Type A confounders.
BACKHOME variables that did not meet the criteria for Type A confounders, but based on evidence in the literature are plausibly associated with PEG and one or more exposures, were designated Type B confounders. Identifying potential confounders with weaker evidence allowed a sensitivity analysis for each exposure, to determine if the magnitude and direction of the effects we identified would differ substantially if these factors were adjusted for. Including these potential confounders can address one limitation of a DAG-based analysis, which is the omission of important factors needed for adjustment. However, including potential confounders with weaker evidence introduces another potential bias, as the estimate of the total effect of an exposure may be attenuated by mistakenly conditioning on a collider or mediator. The Type B confounding variables included in the sensitivity analysis were current opioid use, expectation of pain relief, post-traumatic stress disorder (PTSD), seeking compensation (lawsuit, worker’s compensation or disability claim), racial/ethnic discrimination, history of low back surgery, pain duration, self-efficacy, cognitive function, fatigue, and social isolation. These variables, with the exposures they are plausibly with, are defined in Appendix – Section A. The sensitivity analysis is reported in Appendix – Section C and described further in the analysis methods below.
Analysis Methods
Baseline characteristics for subjects were reported as means and standard deviations (SDs) for continuous variables and counts and percentages for categorical variables. The association between our primary outcome PEG and each of the five exposure variables was estimated separately using a set of multiple linear regression models (MLRs) via the regression coefficient for the exposure of interest. Based on the comprehensive DAG identified in Figure 1, which includes all exposures along with a set of nodes and directed edges, a minimally sufficient adjustment set (MSAS) was identified for the total effect of each exposure variable separately using the R package daggity (v. 3.1) (47). The MSAS for each exposure set were included as adjustment variables in the MLR to reduce confounding bias in the estimated associations between the PEG outcome and each exposure. The result of this process is a unique adjustment set for each exposure. Two regression coefficients were estimated for each exposure via the MLRs: (1) unadjusted estimates of the exposure regression coefficient which do not control for any confounders, and (2) adjusted estimates of the exposure regression coefficient which control for the MSAS for each exposure. Given our focused examination of exposures with strong evidence in the MR literature and the objective of triangulating evidence, we focus on presenting effect estimates and confidence intervals rather than formal hypothesis testing and thus we refrain from enacting any multiplicity corrections to account for the inspection of multiple exposures. A sensitivity analysis was performed for each exposure-specific model by supplementing the exposure-specific MSAS with an additional set of adjustment factors. Results from the sensitivity analysis are reported in Appendix - Section C. All analyses were performed with SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA).
Results
Table 1 reports the baseline characteristics in the analysis study cohort.
Table 1.
Baseline Characteristics of Vanguard Cohort
| Baseline Characteristic | All participants (n = 1376) |
|---|---|
| Outcome | |
| PEG score (range:0–10, higher=more pain interference) | 4.4 ± 2.2 |
| Exposures | |
| PROMIS sleep disturbance 6a T-score (range:31.7–76.1, higher=more disturbance) | 54.8 ± 8.0 |
| PROMIS depression 4a T-score (range:41.0–79.4, higher=more depressed) | 52.6 ± 10.1 |
| BMI | |
| Underweight (<18.5 kg/m2) | 12 (0.9) |
| Normal weight (18.5–24.9 kg/m2) | 409 (29.8) |
| Overweight (25–29.9 kg/m2) | 410 (29.9) |
| Obesity class I (30–34.9 kg/m2) | 279 (20.3) |
| Obesity class II (35–39.9 kg/m2) | 137 (10.0) |
| Obesity class III (≥40 kg/m2) | 126 (9.2) |
| Number of alcoholic drinks per week | 2.5 ± 4.6 |
| Smoking status | |
| Current smoker | 93 (6.8) |
| Past smoker | 457 (33.2) |
| Never smoked | 826 (60.0) |
| Type A Confounders | |
| Age (years) | 54.9 ± 14.4 |
| Female | 927 (67.4) |
| Education | |
| Some high school | 20 (1.5) |
| High school completed | 237 (17.2) |
| Associates/Technical degree completed | 208 (15.1 |
| College/Baccalaureate degree completed | 434 (31.5) |
| Doctoral/Postgraduate education | 477 (34.7) |
| Relationship status | |
| Married | 788 (57.2) |
| Never married | 210 (15.3) |
| Divorced | 181 (13.2) |
| Domestic partner (living with partner as if married) | 100 (7.3) |
| Widowed | 77 (5.6) |
| Separated | 20 (1.5) |
| High financial strain | 145 (10.6) |
| PROMIS anxiety 4a T-score (range:40.3–81.6, higher=more anxiety) | 54.1 ± 10.1 |
| Fear avoidance score (range:0–24, higher=more avoidance) | 11.6 ± 5.9 |
| Pain catastrophizing score (range:0–12, higher=more catastrophizing) | 4.5 ± 2.6 |
| Type B Confounders | |
| Current opioid use | 156 (11.3) |
| Expectation of pain relief over next 3 months (1=no relief, 10=complete relief) | 4.2 ± 2.2 |
| PTSD diagnosis, childhood and/or adult | 235 (17.1) |
| Filed workers compensation, lawsuit, or disability due to back problem or pain | 217 (15.8) |
| How often treated unfairly due to ethnicity or race | |
| Never | 1002 (72.8) |
| Sometimes | 349 (25.4) |
| Often | 17 (1.2) |
| Always | 8 (0.6) |
| History of low-back operation | |
| None | 1189 (86.4) |
| Decompression | 114 (8.3) |
| Spinal fusion | 73 (5.3) |
| Duration of low-back pain as ongoing problem | |
| 3 – 6 months | 46 (3.4) |
| 6 months – 1 year | 107 (7.8) |
| 1 – 5 years | 463 (33.7) |
| More than 5 years | 759 (55.2) |
| Pain self-efficacy score (range:0–24, higher=more confidence) | 16.2 ± 5.5 |
| PROMIS cognitive function 2a T-score (range:29.5–61.2, higher=more cognitive function) | 49.2 ± 7.4 |
| PROMIS fatigue 4a T-score (range:33.7–75.8, higher=more fatigued) | 57.1 ± 10.5 |
| PROMIS social isolation 4a T-score (range:34.8–74.2, higher=more isolated) | 51.6 ± 8.2 |
Data presented as mean ± SD or n (%)
Table 2 reports the unadjusted and adjusted mean difference in baseline PEG for a given change in baseline exposure levels. Exposure effects are reported as the expected difference in baseline PEG with reference to the baseline reference category for categorical exposures or the expected difference in baseline PEG for each SD shift in the exposure for continuous exposures. The 95% confidence intervals for all of mean differences excluded zero except for the underweight BMI category, in both the unadjusted and adjusted models, and smoking status, in the adjusted model. Each SD increase in the PROMIS sleep disturbance T-score resulted in a mean 0.77 (95% CI: 0.66, 0.88) point increase in baseline PEG score in the adjusted model. In the adjusted model, each SD increase in the PROMIS depression T-score was associated with a mean 0.28 (95% CI: 0.17, 0.40) point increase in baseline PEG score. Compared to participants with normal BMI, adjusted mean PEG score was slightly higher by 0.37 points (95% CI: 0.09, 0.65) for overweight participants, about 0.8 to 0.9 points higher for those in obesity classes I and II, and 1.39 (95% CI: 0.98, 1.80) points higher for the most obese participants. Each SD decrease in number of alcoholic drinks per week resulted in a mean 0.12 (95%CI: 0.01, 0.23) increase in baseline PEG score in the adjusted model. Full results for the sensitivity analysis are presented in Appendix C. The sensitivity analysis adjusted for a wider range of factors, and the results were generally attenuated though the directions of association remained the same, and sleep disturbance and obesity remained the exposures with the strongest associations with PEG.
Table 2.
Mean difference in baseline PEG for given change in baseline exposure.
| Exposure | Unit/referent* | Unadjusted mean difference (95% CI) | Adjusted mean difference (95% CI) |
|---|---|---|---|
| Smoking status | Never smoked | ||
| Current smoker | 1.24 (0.78, 1.70) | 0.36 (−0.07, 0.78)a | |
| Past smoker | 0.10 (−0.15, 0.34) | −0.09 (−0.31, 0.13)a | |
| PROMIS sleep disturbance T-score | +8.0 | 0.87 (0.76, 0.97) | 0.77 (0.66, 0.88)b |
| PROMIS depression T-score | +10.1 | 0.83 (0.72, 0.94) | 0.28 (0.17, 0.40)c |
| BMI | Normal weight (18.5–24.9 kg/m2) | ||
| Underweight (<18.5 kg/m2) | 0.29 (−0.91, 1.50) | 0.29 (−0.86, 1.45)d | |
| Overweight (25–29.9 kg/m2) | 0.36 (0.08, 0.65) | 0.37 (0.09, 0.65)d | |
| Obesity class I (30–34.9 kg/m2) | 1.01 (0.69, 1.33) | 0.86 (0.55, 1.17)d | |
| Obesity class II (35–39.9 kg/m2) | 1.00 (0.60, 1.41) | 0.76 (0.37, 1.15)d | |
| Obesity class III (≥40 kg/m2) | 1.86 (1.45, 2.28) | 1.39 (0.98, 1.80)d | |
| Number of alcoholic drinks per week | −4.6 | 0.29 (0.17, 0.40) | 0.12 (0.01, 0.23)e |
Continuous variable units approximate 1 SD increase or 1 SD decrease; for categorical variables, referent group is listed.
adjusted for age, sex, depression, anxiety, sleep disturbance, education
adjusted for age, sex, financial strain
adjusted for age, sex, BMI, sleep disturbance, education, financial strain, fear avoidance, pain catastrophizing
adjusted for age, sex, education, financial strain
adjusted for age, sex, depression, anxiety, education
Discussion
Our results demonstrate that several modifiable causal risk factors for cLBP identified by MR- alcohol use, sleep disturbance, depression, and obesity- are associated with PEG, after adjusting for confounding variables identified via a DAG constructed using a rigorous protocol. Contrary to MR studies, we did not find an association between smoking and PEG. For alcohol the direction of association was opposite what has been demonstrated in MR studies, as a decrease in alcohol use was associated with an increase, albeit very small, in PEG. For sleep disturbance, depression, and obesity the convergence of our findings with the results from MR studies, which have different designs and biases, strengthen the evidence for causal relationships between these risk factors and cLBP (7). In addition, by analyzing a cLBP cohort and using a continuous variable, PEG, as the outcome we calculated adjusted estimates for the effect of these risk factors on subjects with cLBP. The adjusted effect estimates, presented as the expected change in the PEG outcome for every standard deviation increase or decrease in the exposure (or category shift for categorical exposures) were the largest for sleep disturbance and obesity.
The major strength of our study is the rich BACKHOME dataset, which includes information on multiple risk factors and confounders for a large number of participants. The major weakness is that the validity of the results depends on several assumptions, all of which are common to analysis-based approaches to causal inference (7): no unmeasured confounders, no measurement error in the assessed confounders, and a correctly specified DAG.
While the BACKHOME dataset contains measures of a large number of potential confounders a fundamental limitation of relying on statistical adjustment for confounding variables is that unmeasured confounders can never be excluded (8). There are several potential confounding variables that are not measured in the BACKOME dataset. Some are evident from MR studies; notably, diet (16), systemic inflammation (19), physical activity (32, 36); the microbiome (11), lipids (12), personality traits (13), and blood pressure (27). Structural spinal pathology is another potential unmeasured confounder. Measurement error in the assessed confounders is a much lesser concern, given that all instruments are validated tools widely used in cLBP research.
DAGs depict the assumptions about underlying relationships between variables, which must be true in order for the research conclusions to be valid (48). RCT’s can provide strong evidence for causal relationships, while the evidence from other study designs is necessarily weaker. The Austin Bradford Hill considerations (49), a framework based on inductive reasoning, is commonly used to assess causality, but the only universally agreed upon criterion from that framework is temporality (i.e. cause precedes effect) (49). In the absence of RCT’s there is no consensus on the grading of evidence for causal relationships. As a result, the assumptions underlying DAGs generally rely heavily on judgements by domain experts (8). In fact, a recent review found that only 6% of published DAG’s provided citations supporting one or more edges between nodes (8). A particular strength of our study is that the ESC-DAG method we followed for DAG construction combines methodological rigor- including elements from the Hill considerations and contemporary causal inference methods as well as expert opinion- with detailed documentation. Nevertheless, as with all DAGs, there are built-in assumptions which cannot be proven.
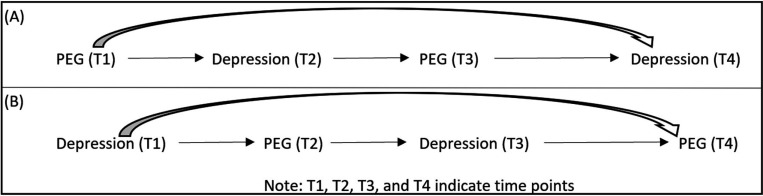
A major limitation of our study is that the data are cross sectional. Therefore, while prior evidence, as documented in decision log in Appendix B, supports the temporal relationships depicted in our DAG, reverse causation cannot be excluded. This is a particular concern for risk factors where bidirectional causal relationships have been demonstrated (13). Although commonly referred to as “feedback loops”, these relationships actually represent co-evolution of variables over time, with the current state of one variable impacting the future state of another variable, which may in turn affect the future state of the original variable (50). A simplified depiction of a bi-directional relationship with depression and PEG is in figure 3.
Figure 3.
Directed acyclic graph (DAG) representations of the bidirectional effects over time. (A)PEG is exposure of interest and depression is outcome of interest; (B) Depression is exposure of interest and PEG is outcome interest.
Adapted from Kunicki EJM, Zach. OSF Preprints As the Wheel Turns: Casual Inference for Feedback Loops and Bidirectional Effects. 2024 (49).
Time varying relationships can also extend to both confounding variables as well as mediators (variables that are in the causal pathway from the exposure to the outcome). As longitudinal data becomes available we will be able to assess the effects of time-varying relationships on our results.
Despite the limitations inherent in our study design, the triangulation of evidence from our analysis-based approach with the results from design-based MR approaches supports causal links between cLBP and three of the risk factors we studied- sleep disturbance, depression, and obesity. The key assumptions about MR studies are that the genetic variant (which serves as an instrumental variable) is robustly associated with the exposure, is not associated with confounders, and is not associated with the outcome other than via its association with the exposure (7). The biases in MR studies, then, are different than the sources of bias in our study. The concordance between MR studies and our findings strengthens the evidence that sleep disturbance, depression, and obesity are causal risk factors for cLBP, because the chance that studies with very different potential sources of bias would align to give similar results is presumably small (7). The evidence from MR studies on the association between alcohol use and cLBP is mixed, with one study showing an association (51), and another not (24). The study by Lv, et al. (24) measured alcohol consumption as the number of drinks per week, as we did, while the study by Williams, et al (38) measured the frequency of alcohol intake, defined as a categorical variable. The different measurements for alcohol consumption may account for the different findings in MR studies. The evidence from MR studies on the association between smoking and cLBP is consistent, although with relative small odds ratios (OR’s), varying between 1.36 (24) and 1.27 (38). While the evidence from MR studies supports a causal association between smoking and cLBP the lack of convergence with our findings suggests further study is needed.
In MR studies causal effect estimates are reported as odds ratios (OR’s), using a case definition of cLBP as the outcome. Our results complement these findings by estimating of the association of change in PEG with reductions in exposures. Estimating the effects of exposures on the absolute scale of PEG, as opposed to a relative measure of association like the OR, is more meaningful for choosing interventions. The effect sizes for the exposures we studied are generally small, below the minimally important difference (MID) for PEG of 1.0 (52). However, these are average effects for the population, and in individual patients the effects of an exposure may be greater. Furthermore, in any one individual there may be a collection of component causes, each of which must be present for an outcome to occur, a concept known as the sufficient cause framework (49). Previously, interventions for the risk factors we studied have focused on each one individually (51, 53–56), with generally disappointing results. A more effective approach may be individualized, multimodal treatment plans that address all causal risk factors. Addressing sleep disturbance and obesity, which have the greatest effect sizes in our study, may be particularly important. There are a number of evidence-based treatments for insomnia, which could be incorporated into a multimodal cLBP treatment program (55). Interventions for obesity in cLBP patients have focused on lifestyle interventions (51), but weight loss drugs for those patients that fit the indications may be a more effective strategy.
In summary, in this study we analyzed baseline data from a unique cLBP cohort, which includes information on multiple risk factors and confounders for a large number of subjects. Using rigorous confounding control we found associations between alcohol use, sleep disturbance, depression, and obesity and PEG. Convergence of our findings for sleep disturbance, depression, and obesity with the results from MR studies, which have different designs and biases, strengthens the evidence that these factors are not just associated with cLBP but cause cLBP. As longitudinal data becomes available from the cohort we will be able to assess the effects of time-varying relationships on our results. The effect of reducing each of these risk factors on PEG was small, with the greatest effects associated with sleep disturbance and obesity. The effect of incorporating treatment of the risk factors we have identified into multimodal cLBP treatment strategies should be a focus of future study.
Figure 2.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number U19AR076737. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Core Center of Patient-centric, Mechanistic Phenotyping in Chronic Low Back (REACH) investigators include the following University of California, San Francisco (unless noted otherwise) personnel in alphabetical order:
Zehra Akkaya, PhD
Prakruthi Amarkumar, PhD
Jeannie Bailey, PhD
Julia Barylak
Sigurd Berven, MD
Andrew Bishara, MD
Dennis M. Black, PhD
Noah Bonnheim, PhD
Atul Butte, MD, PhD
Jennifer Cummings
Karina Del Rosario, MD
Emilia Demarchis, MD
Sibel Demir-Deviren, MD
Susan K. Ewing, MS
Adam Ferguson, PhD
Aaron Fields, PhD
Scott M. Fishman, MD (University of California, Davis)
Sergio Garcia Guerra
Fatemeh Gholi Zadeh Kharrat, PhD
Xiaojie (Summer) Guo
Misung Han, PhD
Trisha Hue, PhD
J. Russell Huie, PhD
C. Anthony Hunt, PhD
Anastasia Keller, PhD
Karim Khattab
Roland Krug, PhD
Gregorji Kurillo, PhD
Feng Lin
Thomas Link, MD, PhD
Jeffrey Lotz, PhD
John Lynch, PhD
Tong Lyu
Rob Matthew, PhD
Wolf Mehling, MD
Esmeralda Mendoza, MPH
Praveen Mummaneni, MD, MBA
Caroline Navy
Conor O’Neill, MD
Jessica Ornowski
Thomas Peterson, PhD
Ananya Rupanagunta (University of California, Berkeley)
Aaron Scheffler, PhD, MS
Shalini Shah, MD (University of California, Irvine)
Irina Strigo, PhD
Naoki Takegami, MD
Abel Torres-Espin, PhD (University of Waterloo)
Salvatore Torrisi, PhD
Sachin Umrao, PhD
Rohit Vashisht, PhD
Joanna Veres
An (Joseph) Vu, PhD
Mark Steven Wallace, MD (University of California, San Diego)
Lucy Ann Wu, MPH
Po-Hung Wu, PhD
Patricia Zheng, MD
Jiamin Zhou, MS
Appendix A: Study measurement details
Outcome measure
The PEG score ranges from 0–10, with higher values indicating more pain interference. It is calculated as the mean of responses to 3 items:
Average pain in past week (0=no pain, 10=worst imaginable pain)
How much pain interfered with enjoyment of life in past week (0=did not interfere, 10=completely interfered)
How much pain interfered with general activity in past week (0=did not interfere, 10=completely interfered)
Exposures
The PROMIS sleep disturbance 6a t-score (range 31.7–76.1, higher=more sleep disturbance) was based on responses to the following 6 questions:
In the past 7 days, my sleep quality was: 1 (=very good) to 5 (=very poor)
In the past 7 days, my sleep was refreshing: 1 (=very much) to 5 (=not at all) Responses to 3–6 ranged from 1 (=not at all) to 5 (=very much) In the past 7 days:
I had a problem with my sleep.
I had difficulty falling asleep.
My sleep was restless.
I tried hard to get to sleep.
The responses to these questions were summed to produce a raw summary score (range 6–30), which was then mapped to a t-score, with 50 representing the mean of a reference population and 10 being the SD of that population.
The PROMIS depression 4a t-score (range 41.0–79.4, higher=more depressed) was based on responses to the following 4 questions, with responses ranging from 1 (=never) to 5 (=always): In the past 7 days…
I felt worthless.
I felt helpless.
I felt depressed.
I felt hopeless.
The responses to these questions were summed to produce a raw summary score (range 4–20), which was then mapped to a t-score, with 50 representing the mean of a reference population and 10 being the SD of that population.
Alcohol use was defined as number of drinks per week as a continuous variable in response to the question: How many alcoholic drinks do you consume per week, on average?
Smoking was measured as a categorical variable in reponse to the question: How would you describe your cigarette smoking?
Never smoked
Current smoker
Used to smoke, but have now quit
Body mass index (BMI) was calculated from self-reported weight and height. Obesity was defined by BMI of 30.0 kg/m2 or higher. Furthermore, underweight was defined as <18.5 kg/m2, normal weight ranges from 18.5–24.9 kg/m2, overweight ranges from 25–29.9 kg/m2, obesity class I ranges from 30–34.9 kg/m2, obesity class II ranges from 35–39.9 kg/m2, and obesity class III constitutes ≥40 kg/m2.
Type A confounding variables
Age was measured as a continuous variable and sex as a binary variable (male/female). Categories for education were some high school, high school completed, associates/technical degree completed, college/baccalaureate degree completed, doctoral/postgraduate education. Categories for relationship status were married, never married, divorced, domestic partner, widowed, separated. Participants were asked how difficult it was to pay for basic necessities; “hard” and “very hard” responses were classified as high financial strain.
The PROMIS anxiety 4a T-score (range:40.3–81.6, higher=more anxiety) was based on responses to the following 4 questions, with responses ranging from 1 (=never) to 5 (=always): In the past 7 day…
I felt fearful
I found it hard to focus on anything other than my anxiety
My worries overwhelmed me
I felt uneasy
The responses to these questions were summed to produce a raw summary score (range 4–20), which was then mapped to a t-score, with 50 representing the mean of a reference population and 10 being the SD of that population.
The Fear Avoidance score (FABQ-PA) (57) (range 0–24, higher=more avoidance) was calculated as the sum of responses (0=completely disagree, 6=completely agree) to the following 4 items:
Physical activity makes my pain worse.
Physical activity might harm my back.
I should not do physical activities which might make my pain worse.
I cannot do physical activities which might make my pain worse.
The Pain Catastrophizing Scale SF (PCS-6) (58) (range 0–12, higher=more catastrophizing) included 3 subscales -- Helplessness, Magnification, and Rumination -- with each subscale having 2 components. The responses to the following 6 statements ranged from 0=not at all to 4=all the time:
When I’m in pain …
It’s awful and I feel that it overwhelms me. (Helplessness subscale)
I feel I can’t stand it anymore. (Helplessness subscale)
I become afraid that the pain will get worse. (Magnification subscale)
I wonder whether something serious may happen. (Magnification subscale)
I keep thinking about how much it hurts. (Rumination subscale)
I keep thinking about how badly I want the pain to stop. (Rumination subscale)
The mean for each subscale was determined, resulting in a 0–4 score. The 3 subscales were then summed to create the total score, ranging from 0–12, with higher scores representing more pain catastrophizing.
Type B confounding variables
Current opioid use was defined as current use for low-back pain or current daily use for any reason. Expectation of pain relief over next 3 months was assessed by the question: Please indicate how much pain relief you expect over the coming three months (range 1 = no relief, and 10 = complete relief). Duration of low back pain was recorded as 3–6 months, 6 months −1 year, 1–5 years or more than 5 years. History of low-back operation was assessed and recorded as none, decompression surgery or spinal fusion surgery. Discrimination was assessed by the question: How often do people treat you unfairly because of your ethnicity or race? 1= never, 2 = sometimes, 3 = often, 4 = always.
Post traumatic stress disorder (PTSD) was noted if participant marked “yes” to having experienced things as a child or as an adult that are unusually or especially frightening, horrible, or traumatic (examples include a serious accident or fire, a physical or sexual assault or abuse, an earthquake or flood, a war, seeing someone be killed or seriously injured, having a loved one die through homicide or suicide) AND participant reported at least 3 of 5 symptoms in past month (nightmares, avoided triggering situations, on guard, felt detached, self- blamed).
Those who marked “yes” to “have you filed or been awarded a worker’s compensation claim related to your back problem,” “are you involved in a lawsuit or legal claim related to your back problem,” or “have you ever applied for, or received, disability insurance for your pain condition” were marked as having filed workers compensation, lawsuit, or disability due to back problem or pain.
Pain self-efficacy score (PSEQ-4) (59) was calculated as the sum of responses to 4 questions on how confident the participant was in doing the following (0=not at all confident, 6=completely confident):
I can cope with my pain in most situations.
I can still do many of the things I enjoy doing, such as hobbies or leisure activity, despite the pain.
I can still accomplish most of my goals in life, despite the pain.
I can live a normal lifestyle, despite the pain.
The total score ranged from 0–24, with higher scores representing greater confidence.
T-scores were calculated for the following PROMIS measures: cognitive function 2a, fatigue 4a, and social isolation 4a (41).
Appendix B: ESC-DAG decision log
Decision Log Type A confounders
| Edge | Level of Evidencea | References |
|---|---|---|
| Anxiety -> Comorbidity (CCI) | 5 | (60–63) |
| Depression -> Comorbidity (CCI) | 5 | (60–68) |
| Sleep -> Comorbidity (CCI) | 5 | (69–72) |
| Financial strain -> Obesity | 3 | (60, 73–75) |
| Obesity -> Comorbidity (CCI) | 5 | (60, 76) |
| Obesity -> Depression | 5 | (60) |
| Obesity -> Anxiety | 5 | (60, 76, 77) |
| Obesity -> PEG | 5 | (10, 33, 60) |
| Anxiety -> Sleep | 5 | (72, 78, 79) |
| Anxiety -> Smoking | 5 | (78, 80, 81) |
| Depression -> Smoking | 5 | (78, 80, 81) |
| Sleep -> Depression | 5 | (72, 78, 82) |
| Depression -> PEG | 5 | (31, 38, 60, 83, 84) |
| Anxiety -> PEG | 5 | (10, 60, 84) |
| Sleep -> PEG | 5 | (25, 31, 78) |
| Education -> Comorbidity (CCI) | 5 | (85–89) |
| Education -> Obesity | 5 | (89, 90) |
| Education -> Anxiety | 5 | (63, 91) |
| Education -> Depression | 5 | (63, 78, 89, 91, 92) |
| Education -> Financial stress | 3 | (93, 94) |
| Education -> Smoking | 5 | (83, 95, 96) |
| Education -> PEG | 5 | (38, 78, 97, 98) |
| Financial strain -> Anxiety | 3 | (99, 100) |
| Financial strain -> Depression | 3 | (99–101) |
| Financial strain -> Sleep | 2 | (102, 103) |
| Financial strain -> PEG | 2 | (78, 97, 104) |
| Sleep -> Smoking | 5 | (72, 78) |
| Smoking -> PEG | 5 | (24, 38, 98) |
| Catastrophization -> Anxiety | 2 | (105, 106) |
| Catastrophization -> Depression | 2 | (105, 106) |
| Catastrophization -> PEG | 4 | (84, 105, 107–111) |
| Fear Avoidance -> Anxiety | 2 | (105, 106, 112) |
| Fear Avoidance -> Depression | 2 | (105, 106, 112) |
| Fear Avoidance -> PEG | 4 | (84, 105, 109–111) |
| Age -> Comorbidity (CCI) | 2 | (60, 113) |
| Age -> Obesity | 3 | (60, 114) |
| Age -> Sleep | 3 | (78, 115) |
| Age -> PEG | 4 | (60, 78, 105, 116, 117) |
| Age -> Smoking | 3 | (78, 118) |
| Age -> Anxiety | 3 | (78, 105, 119–122) |
| Age -> Depression | 3 | (78, 105, 119, 123–125) |
| Age -> Catastrophization | 2 | (105, 112, 126) |
| Age -> Fear Avoidance | 2 | (105, 112, 127) |
| Age -> Financial Strain | 2 | (128) |
| Sex -> Comorbidity (CCI) | 5 | (60, 129) |
| Sex -> Obesity | 5 | (60, 129) |
| Sex -> Sleep | 3 | (130) |
| Sex -> PEG | 4 | (60, 98, 131) |
| Sex -> Smoking | 3 | (132) |
| Sex -> Anxiety | 3 | (60, 133) |
| Sex -> Depression | 3 | (60, 133) |
| Sex -> Catastrophization | 3 | (105, 126, 131) |
| Sex -> Fear Avoidance | 3 | (105, 134) |
| Sex -> Financial strain | 4 | (135) |
| Sex -> Education | 3 | (105, 136, 137) |
| Alcohol -> Sleep | 5 | (138) |
| Anxiety -> Alcohol | 5 | (139–141) |
| Depression -> Alcohol | 5 | (142–145) |
| Age -> Alcohol | 5 | (146–148) |
| Sex -> Alcohol | 5 | (148) |
| Alcohol -> Financial strain | 5 | (144) |
| Education -> Alcohol | 3 | (149) |
| Alcohol -> Comorbidity (CCI) | 3 | (148, 150) |
| Alcohol -> Obesity | 5 | (151) |
Levels of evidence:
Level 1- Expert opinion only (based on causal models published in the literature)
Level 2- Cross-sectional assocation
Level 3- Temporal order (longitudinal studies demonstrating that the exposure precedes cLBP)
Level 4- Analysis-based confounding control
Level 5- Design based confounding control (e.g. MR, twin studies)
Decision Log Type B confounders
| Confounder | Exposures | References |
|---|---|---|
| Expectations | Obesity | (152–157) |
| Self Efficacy | Obesity, Alcohol, Smoking | (84, 110, 158–164) |
| PTSD | Depression, Obesity, Sleep, Smoking, Alcohol | (165–171) |
| Compensation | Obesity, Smoking, Alcohol | (172–182) |
| Mistreatment | Depression, Obesity, Sleep, Smoking, Alcohol | (183–190) |
| Relationship status | Depression, Obesity, Sleep, Smoking, Alcohol | (191–196) |
| Surgery | Depression, Obesity, Sleep, Smoking, Alcohol | (197–204) |
| Opioid use | Depression, Obesity, Sleep, Smoking, Alcohol | (205–210) |
| Duration of LBP | Depression, Obesity, Sleep, Smoking, Alcohol | (57, 172, 211–219) |
| Isolation | Depression, Sleep, Alcohol | (220–224) |
| Fatigue | Depression, Obesity, Sleep, Smoking, Alcohol | (225–233) |
| Cognitive Function | Depression, Obesity, Sleep, Smoking, Alcohol | (234–239) |
Appendix C: Exposure-specific sensitivity analysis
Appendix C Table 1 reports the unadjusted, adjusted, and sensitivity analysis adjusted (based on the combined ESC-DAG adjustment set supplemented with confounders from the sensitivity analysis) mean difference in baseline PEG for a given change in baseline exposure levels. Effect estimates are reported as described in the main paper for each model. Compared to participants who never smoked, the sensitivity analysis adjusted mean baseline PEG score for current smokers was about a quarter of a point higher (mean difference = 0.28; 95% CI: −0.09, 0.64). There was not a significant difference in mean PEG score for past smokers vs. never smokers. Each SD increase in the PROMIS sleep disturbance T-score resulted in a mean 0.36 (95% CI: 0.24, 0.48) point increase in baseline PEG score in the sensitivity analysis adjusted model. In the sensitivity adjusted model, there was no longer an association between depression and PEG: each SD increase in the PROMIS depression T-score was associated with a mean 0.08 (95% CI: −0.05, 0.23) point increase in baseline PEG score. Compared to participants with normal BMI, sensitivity analysis adjusted mean PEG score was slightly higher by 0.24 points (95% CI: 0.02, 0.47) for overweight participants, about 0.3 to 0.4 points higher for those in obesity classes I and II, and 0.55 (95% CI: 0.21, .89) points higher for the most obese participants. There was no association between alcohol use and PEG in the sensitivity analysis. The sensitivity analysis adjusted for a wider range of factors and the results were generally attenuated though the directions of association remained the same, and sleep disturbance and obesity remained the exposures with the strongest associations with PEG.
Appendix C Table 1.
Mean difference in baseline PEG for given change in baseline exposure
| Exposure | Unit/referent* | Unadjusted mean difference (95% CI) | Adjusted mean difference (ESC-DAG adjustment sets) (95% CI) | Adjusted mean difference (95% CI) (ESC-DAG + sensitivity analysis adjustment sets) |
|---|---|---|---|---|
| Smoking status | Never smoked | |||
| Current smoker | 1.24 (0.78, 1.70) | 0.36 (−0.07, 0.78)a | 0.28 (−0.09, 0.64)a | |
| Past smoker | 0.10 (−0.15, 0.34) | −0.09 (−0.31, 0.13)a | −0.07 (−0.26, 0.12)a | |
| PROMIS sleep disturbance T-score | +8.0 | 0.87 (0.76, 0.97) | 0.77 (0.66, 0.88) b | 0.36 (0.24, 0.48) b |
| PROMIS depression T-score | +10.1 | 0.83 (0.72, 0.94) | 0.28 (0.17, 0.40) c | 0.08 (−0.05, 0.23)c |
| BMI | Normal weight (18.5–24.9 kg/m2) | |||
| Underweight (<18.5 kg/m2) | 0.29 (−0.91, 1.50) | 0.29 (−0.86, 1.45)d | −0.33 (−1.26, 0.60)d | |
| Overweight (25–29.9 kg/m2) | 0.36 (0.08, 0.65) | 0.37 (0.09, 0.65) d | 0.24 (0.02, 0.47) d | |
| Obesity class I (30–34.9 kg/m2) | 1.01 (0.69, 1.33) | 0.86 (0.55, 1.17) d | 0.41 (0.16, 0.66) d | |
| Obesity class II (35–39.9 kg/m2) | 1.00 (0.60, 1.41) | 0.76 (0.37, 1.15) d | 0.25 (−0.06, 0.57)d | |
| Obesity class III (≥40 kg/m2) | 1.86 (1.45, 2.28) | 1.39 (0.98, 1.80) d | 0.55 (0.21, 0.89) d | |
| Number of alcoholic drinks per week | −4.6 | 0.29 (0.17, 0.40) | 0.12 (0.01, 0.23) e | 0.01 (−0.08, 0.10)e |
Continuous variable units approximate 1 SD increase or 1 SD decrease; for categorical variables, referent group is listed.
adjusted for age, sex, depression, anxiety, sleep disturbance, education (+pain self-efficacy, PTSD, workers compensation, mistreatment due to race/ethnicity, relationship status, history of low-back operation, current opioid use, duration of low-back pain, fatigue, cognitive function in fully-adjusted model)
adjusted for age, sex, financial strain (+PTSD, mistreatment due to race/ethnicity, relationship status, social isolation, history of low-back operation, current opioid use, duration of low-back pain, fatigue, cognitive function in fully-adjusted model)
adjusted for age, sex, BMI, sleep disturbance, education, financial strain, fear avoidance, pain catastrophizing (+PTSD, mistreatment due to race/ethnicity, relationship status, social isolation, history of low-back operation, current opioid use, duration of low-back pain, fatigue, cognitive function in fully-adjusted model)
adjusted for age, sex, education, financial strain (+expectation of pain relief, pain self-efficacy, PTSD, workers compensation, mistreatment due to race/ethnicity, relationship status, history of low-back operation, current opioid use, duration of low-back pain, fatigue, cognitive function in fully-adjusted model)
adjusted for age, sex, depression, anxiety, education (+pain self-efficacy, PTSD, workers compensation, mistreatment due to race/ethnicity, relationship status, social isolation, history of low-back operation, current opioid use, duration of low-back pain, fatigue, cognitive function in fully-adjusted model)
Work Cited
- 1.Chau A, Steib S, Whitaker E, Kohns D, Quinter A, Craig A, et al. Theoretical Schemas to Guide Back Pain Consortium (BACPAC) Chronic Low Back Pain Clinical Research. Pain Med. 2023;24(Suppl 1):S13–s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJEM, Ostelo RWJG, Guzman J, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ : British Medical Journal. 2015;350:h444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Comparison of Graded Exercise and Graded Exposure Clinical Outcomes for Patients With Chronic Low Back Pain. Journal of Orthopaedic & Sports Physical Therapy. 2010;40(11):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, Chenot JF, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–803. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Annals of Internal Medicine. 2017;166(7):514–30. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al. Noninvasive Treatments for Low Back Pain. Agency for Healthcare Research and Quality (US), Rockville (MD); 2016. [PubMed] [Google Scholar]
- 7.Munafò MR, Higgins JPT, Smith GD. Triangulating Evidence through the Inclusion of Genetically Informed Designs. Cold Spring Harb Perspect Med. 2021;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tennant PWG, Murray EJ, Arnold KF, Berrie L, Fox MP, Gadd SC, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: review and recommendations. International Journal of Epidemiology. 2020;50(2):620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartley AE, Power GM, Sanderson E, Smith GD. A Guide for Understanding and Designing Mendelian Randomization Studies in the Musculoskeletal Field. JBMR Plus. 2022;6(10):e10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elgaeva EE, Tsepilov Y, Freidin MB, Williams FMK, Aulchenko Y, Suri P. ISSLS Prize in Clinical Science 2020. Examining causal effects of body mass index on back pain: a Mendelian randomization study. Eur Spine J. 2020;29(4):686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Han H, Sun X, Zhou G, Zhou Q, Li Z. Causal effects of specific gut microbiota on musculoskeletal diseases: a bidirectional two-sample Mendelian randomization study. Front Microbiol. 2023;14:1238800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai Y, Chen Y, Gu R, Zhang C, Jiang R. Causal association of polyunsaturated fatty acids with chronic pain: a two-sample Mendelian randomization study. Front Nutr. 2023;10:1265928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elgaeva EE, Williams FMK, Zaytseva OO, Freidin MB, Aulchenko YS, Suri P, et al. Bidirectional Mendelian Randomization Study of Personality Traits Reveals a Positive Feedback Loop Between Neuroticism and Back Pain. The Journal of Pain. 2023. [DOI] [PubMed] [Google Scholar]
- 14.Gou L, Zheng Q. How to reduce the risk of cervicalgia and low back pain in obese individuals: A mendelian randomization study. Medicine (Baltimore). 2023;102(18):e33710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan X, Zhang D, Zhang F, Zong Y, Wang H, Shen Z, et al. Causal association of physical activity with low back pain, intervertebral disc degeneration and sciatica: a two-sample mendelian randomization analysis study. Front Cell Dev Biol. 2023;11:1260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Xie ZF. Dried fruit intake causally protects against low back pain: A Mendelian randomization study. Front Nutr. 2023;10:1027481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, Zhou R, He Y, Zhu T, Zhang W. Causal effect of serum 25-hydroxyvitamin D levels on low back pain: A two-sample mendelian randomization study. Front Genet. 2022;13:1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin P, Xing Y, Xiao B, Wei Y, Yan K, Zhao J, et al. Diabetes and intervertebral disc degeneration: A Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1100874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasher M, Williams FMK, Freidin MB, Cherny SS, Malkin I, Livshits G. Insights into the pleiotropic relationships between chronic back pain and inflammation-related musculoskeletal conditions: rheumatoid arthritis and osteoporotic abnormalities. Pain. 2023;164(3):e122–e34. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Karppinen J, Cheah KSE, Chan D, Sham PC, Samartzis D. Integrative analysis of metabolomic, genomic, and imaging-based phenotypes identify very-low-density lipoprotein as a potential risk factor for lumbar Modic changes. Eur Spine J. 2022;31(3):735–45. [DOI] [PubMed] [Google Scholar]
- 21.Liu R, Liu Q, Xu S, Mei R. Mood instability and low back pain: a mendelian randomization study. Front Neurol. 2023;14:1252329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S, Lv X, Deng X, Lai R, Du J, Wang C. Diet and risk of low back pain: a Mendelian randomization analysis. European Spine Journal. 2023. [DOI] [PubMed] [Google Scholar]
- 23.Luo G, Yao Y, Tao J, Wang T, Yan M. Causal association of sleep disturbances and low back pain: A bidirectional two-sample Mendelian randomization study. Front Neurosci. 2022;16:1074605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv Z, Cui J, Zhang J. Smoking, alcohol and coffee consumption and risk of low back pain: a Mendelian randomization study. Eur Spine J. 2022;31(11):2913–9. [DOI] [PubMed] [Google Scholar]
- 25.Shu P, Ji L, Ping Z, Sun Z, Liu W. Association of insomnia and daytime sleepiness with low back pain: A bidirectional mendelian randomization analysis. Front Genet. 2022;13:938334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su M, Tang Y, Kong W, Zhang S, Zhu T. Genetically supported causality between gut microbiota, gut metabolites and low back pain: a two-sample Mendelian randomization study. Front Microbiol. 2023;14:1157451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suri P, Elgaeva EE, Williams FMK, Freidin MB, Zaytseva OO, Aulchenko YS, et al. Evidence of causal effects of blood pressure on back pain and back pain on type II diabetes provided by a bidirectional Mendelian randomization study. Spine J. 2023;23(8):1161–71. [DOI] [PubMed] [Google Scholar]
- 28.Suri P, Elgaeva EE, Williams FMK, Freidin MB, Verzun DA, Tsepilov YA. Repurposing Antihypertensive and Statin Medications for Spinal Pain: A Mendelian Randomization Study. Spine (Phila Pa 1976). 2023;48(22):1568–74. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y, Wu J, Xu M, Zhu T, Sun Y, Chen H, et al. Causal associations of iron status and back pain risk: A Mendelian randomization study. Front Nutr. 2022;9:923590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Feng W, Jin Q. Occupational factors and low back pain: a Mendelian randomization study. Front Public Health. 2023;11:1236331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao C, Zhang Y, Lu P, Xiao B, Sun P, Tao J, et al. Exploring the bidirectional relationship between pain and mental disorders: a comprehensive Mendelian randomization study. J Headache Pain. 2023;24(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X, Yang Y, Yue R, Su C. Potential causal association between leisure sedentary behaviors, physical activity and musculoskeletal health: A Mendelian randomization study. PLoS One. 2023;18(3):e0283014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, Mi J, Peng Y, Han H, Liu Z. Causal Associations of Obesity With the Intervertebral Degeneration, Low Back Pain, and Sciatica: A Two-Sample Mendelian Randomization Study. Front Endocrinol (Lausanne). 2021;12:740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou R, Zhang L, Sun Y, Yan J, Jiang H. Causal Associations between Dietary Habits and Chronic Pain: A Two-Sample Mendelian Randomization Study. Nutrients. 2023;15(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou S, Zhu G, Xu Y, Gao R, Li H, Han G, et al. Mendelian Randomization Study on the Putative Causal Effects of Omega-3 Fatty Acids on Low Back Pain. Front Nutr. 2022;9:819635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Q, Chen L, Shen C. Causal relationship between leisure sedentary behaviors and low back pain risk: a Mendelian randomization study. Eur Spine J. 2023;32(9):3300–8. [DOI] [PubMed] [Google Scholar]
- 37.Hue TF, Lotz JC, Zheng P, Black DM, Bailey J, Ewing SK, et al. Design of the COMEBACK and BACKHOME Studies, Longitudinal Cohorts for Comprehensive Deep Phenotyping of Adults with Chronic Low-Back Pain (cLBP): a part of the BACPAC Research Program. medRxiv. 2024:2024.04.09.24305574. [Google Scholar]
- 38.Williams FMK, Elgaeva EE, Freidin MB, Zaytseva OO, Aulchenko YS, Tsepilov YA, et al. Causal effects of psychosocial factors on chronic back pain: a bidirectional Mendelian randomisation study. Eur Spine J. 2022;31(7):1906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beatty AL, Peyser ND, Butcher XE, Carton TW, Olgin JE, Pletcher MJ, et al. The COVID-19 Citizen Science Study: Protocol for a Longitudinal Digital Health Cohort Study. JMIR Res Protoc. 2021;10(8):e28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauck MC, Lotz J, Psioda MA, Carey TS, Clauw DJ, Majumdar S, et al. The Back Pain Consortium (BACPAC) Research Program: Structure, Research Priorities, and Methods. Pain Med. 2023;24(Suppl 1):S3–s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang H, Haldeman S. Behavior-Related Factors Associated With Low Back Pain in the US Adult Population. Spine. 2018;43(1):28–34. [DOI] [PubMed] [Google Scholar]
- 43.Sendi P, Brunotte R, Potoczna N, Branson R, Horber FF. Health-Related Quality of Life in Patients with Class II and Class III Obesity. Obes Surg. 2005;15(7):1070–6. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson KD, McCann M, Katikireddi SV, Thomson H, Green MJ, Smith DJ, et al. Evidence synthesis for constructing directed acyclic graphs (ESC-DAGs): a novel and systematic method for building directed acyclic graphs. International Journal of Epidemiology. 2019;49(1):322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunicki ZJ, Smith ML, Murray EJ. A Primer on Structural Equation Model Diagrams and Directed Acyclic Graphs: When and How to Use Each in Psychological and Epidemiological Research. Advances in Methods and Practices in Psychological Science. 2023;6(2):25152459231156085. [Google Scholar]
- 46.Velentgas P, Dreyer NA, Wu AW, editors. Outcome Definition and Measurement2013. [Google Scholar]
- 47.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. International Journal of Epidemiology. 2017;45(6):1887–94. [DOI] [PubMed] [Google Scholar]
- 48.Barnard-Mayers R, Kouser H, Cohen JA, Tassiopoulos K, Caniglia EC, Moscicki AB, et al. A case study and proposal for publishing directed acyclic graphs: The effectiveness of the quadrivalent human papillomavirus vaccine in perinatally HIV Infected girls. J Clin Epidemiol. 2022;144:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 50.Kunicki EJM, Zach. OSF Preprints | As the Wheel Turns: Causal Inference for Feedback Loops and Bidirectional Effects. 2024. [Google Scholar]
- 51.Williams A, Lee H, Kamper SJ, O’Brien KM, Wiggers J, Wolfenden L, et al. Causal mechanisms of a healthy lifestyle intervention for patients with musculoskeletal pain who are overweight or obese. Clin Rehabil. 2019;33(6):1088–97. [DOI] [PubMed] [Google Scholar]
- 52.Reed DE 2nd, Stump TE, Monahan PO, Kroenke K. Comparable Minimally Important Differences and Responsiveness of Brief Pain Inventory and PEG Pain Scales across 6 Trials. J Pain. 2024;25(1):142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wai EK, Rodriguez S, Dagenais S, Hall H. Evidence-informed management of chronic low back pain with physical activity, smoking cessation, and weight loss. Spine J. 2008;8(1):195–202. [DOI] [PubMed] [Google Scholar]
- 54.Sanabria-Mazo JP, Colomer-Carbonell A, Borràs X, Castaño-Asins JR, McCracken LM, Montero-Marin J, et al. Efficacy of Videoconference Group Acceptance and Commitment Therapy (ACT) and Behavioral Activation Therapy for Depression (BATD) for Chronic Low Back Pain (CLBP) Plus Comorbid Depressive Symptoms: A Randomized Controlled Trial (IMPACT Study). J Pain. 2023;24(8):1522–40. [DOI] [PubMed] [Google Scholar]
- 55.Wright R, Malec M, Shega JW, Rodriguez E, Kulas J, Morrow L, et al. Deconstructing Chronic Low Back Pain in the Older Adult-Step by Step Evidence and Expert-Based Recommendations for Evaluation and Treatment: Part XI: Dementia. Pain Med. 2016;17(11):1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersson E, Kander T, Werner MU, Cho JH, Kosek E, Bjurström MF. Analgesic efficacy of sleep-promoting pharmacotherapy in patients with chronic pain: a systematic review and meta-analysis. Pain Rep. 2023;8(1):e1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–68. [DOI] [PubMed] [Google Scholar]
- 58.George SZ, Calley D, Valencia C, Beneciuk JM. Clinical Investigation of Pain-related Fear and Pain Catastrophizing for Patients With Low Back Pain. The Clinical Journal of Pain. 2011;27(2):108–15. [DOI] [PubMed] [Google Scholar]
- 59.Chiarotto A, Vanti C, Cedraschi C, Ferrari S, de Lima ESRF, Ostelo RW, et al. Responsiveness and Minimal Important Change of the Pain Self-Efficacy Questionnaire and Short Forms in Patients With Chronic Low Back Pain. J Pain. 2016;17(6):707–18. [DOI] [PubMed] [Google Scholar]
- 60.Tarabeih N, Kalinkovich A, Shalata A, Cherny SS, Livshits G. Deciphering the Causal Relationships Between Low Back Pain Complications, Metabolic Factors, and Comorbidities. J Pain Res. 2022;15:215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhattacharya R, Shen C, Sambamoorthi U. Excess risk of chronic physical conditions associated with depression and anxiety. BMC Psychiatry. 2014;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li GH, Cheung CL, Chung AK, Cheung BM, Wong IC, Fok MLY, et al. Evaluation of bi-directional causal association between depression and cardiovascular diseases: a Mendelian randomization study. Psychol Med. 2022;52(9):1765–76. [DOI] [PubMed] [Google Scholar]
- 63.Jones DP, Wootton RE, Gill D, Carter AR, Gunnell D, Munafò MR, et al. Mental Health as a Mediator of the Association Between Educational Inequality and Cardiovascular Disease: A Mendelian Randomization Study . J Am Heart Assoc. 2021;10(17):e019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Read JR, Sharpe L, Modini M, Dear BF. Multimorbidity and depression: A systematic review and meta-analysis. J Affect Disord. 2017;221:36–46. [DOI] [PubMed] [Google Scholar]
- 65.Tang B, Yuan S, Xiong Y, He Q, Larsson SC. Major depressive disorder and cardiometabolic diseases: a bidirectional Mendelian randomisation study. Diabetologia. 2020;63(7):1305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bergstedt J, Pasman JA, Ma Z, Harder A, Yao S, Parker N, et al. Distinct genomic signatures and modifiable risk factors underly the comorbidity between major depressive disorder and cardiovascular disease. medRxiv. 2024. [Google Scholar]
- 67.Berk M, Köhler-Forsberg O, Turner M, Penninx B, Wrobel A, Firth J, et al. Comorbidity between major depressive disorder and physical diseases: a comprehensive review of epidemiology, mechanisms and management. World Psychiatry. 2023;22(3):366–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu T, Yang F, He K, Ying J, Cui H. Association of mental health with the risk of coronary artery disease in patients with diabetes: A mendelian randomization study. Nutrition, Metabolism and Cardiovascular Diseases. 2022;32(3):703–9. [DOI] [PubMed] [Google Scholar]
- 69.von Känel R, Meister-Langraf RE, Zuccarella-Hackl C, Schiebler SLF, Znoj H, Pazhenkottil AP, et al. Sleep disturbance after acute coronary syndrome: A longitudinal study over 12 months. PLoS One. 2022;17(6):e0269545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liao L-z, Li W-d, Liu Y, Li J-p, Zhuang X-d, Liao X-x. Causal assessment of sleep on coronary heart disease. Sleep Medicine. 2020;67:232–6. [DOI] [PubMed] [Google Scholar]
- 71.Gao XL, Jia ZM, Zhao FF, An DD, Wang B, Cheng EJ, et al. Obstructive sleep apnea syndrome and causal relationship with female breast cancer: a mendelian randomization study. Aging (Albany NY). 2020;12(5):4082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibson MJ, Lawlor DA, Millard LAC. Identifying the potential causal role of insomnia symptoms on 11,409 health-related outcomes: a phenome-wide Mendelian randomisation analysis in UK Biobank. BMC Med. 2023;21(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pearson-Stuttard J, Banerji T, Capucci S, de Laguiche E, Faurby MD, Haase CL, et al. Real-world costs of obesity-related complications over eight years: a US retrospective cohort study in 28,500 individuals. International Journal of Obesity. 2023;47(12):1239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larsson SC, Spyrou N, Mantzoros CS. Body fatness associations with cancer: evidence from recent epidemiological studies and future directions. Metabolism. 2022;137:155326. [DOI] [PubMed] [Google Scholar]
- 75.Farmer RE, Mathur R, Schmidt AF, Bhaskaran K, Fatemifar G, Eastwood SV, et al. Associations Between Measures of Sarcopenic Obesity and Risk of Cardiovascular Disease and Mortality: A Cohort Study and Mendelian Randomization Analysis Using the UK Biobank. J Am Heart Assoc. 2019;8(13):e011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Casanova F, O’Loughlin J, Martin S, Beaumont RN, Wood AR, Watkins ER, et al. Higher adiposity and mental health: causal inference using Mendelian randomization. Hum Mol Genet. 2021;30(24):2371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amiri S, Behnezhad S. Obesity and anxiety symptoms: a systematic review and meta-analysis. Neuropsychiatr. 2019;33(2):72–89. [DOI] [PubMed] [Google Scholar]
- 78.O’Hagan ET, Cashin AG, Hübscher M, Mohammad Alsaadi S, Gustin S, McAuley JH. Does poor sleep quality lead to increased low back pain the following day? Scand J Pain. 2023;23(2):333–40. [DOI] [PubMed] [Google Scholar]
- 79.Zhou F, Li S, Xu H. Insomnia, sleep duration, and risk of anxiety: A two-sample Mendelian randomization study. J Psychiatr Res. 2022;155:219–25. [DOI] [PubMed] [Google Scholar]
- 80.Fluharty M, Taylor AE, Grabski M, Munafò MR. The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nicotine Tob Res. 2017;19(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Treur JL, Munafò MR, Logtenberg E, Wiers RW, Verweij KJH. Using Mendelian randomization analysis to better understand the relationship between mental health and substance use: a systematic review. Psychol Med. 2021;51(10):1593–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai L, Bao Y, Fu X, Cao H, Baranova A, Zhang X, et al. Causal links between major depressive disorder and insomnia: A Mendelian randomisation study. Gene. 2021;768:145271. [DOI] [PubMed] [Google Scholar]
- 83.Zhao SS, Holmes MV, Zheng J, Sanderson E, Carter AR. The impact of education inequality on rheumatoid arthritis risk is mediated by smoking and body mass index: Mendelian randomization study. Rheumatology (Oxford). 2022;61(5):2167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee H, Hübscher M, Moseley GL, Kamper SJ, Traeger AC, Mansell G, et al. How does pain lead to disability? A systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain. 2015;156(6):988–97. [DOI] [PubMed] [Google Scholar]
- 85.Jones DP, Wootton RE, Gill D, Carter AR, Gunnell D, Munafò MR, et al. Mental Health as a Mediator of the Association Between Educational Inequality and Cardiovascular Disease: A Mendelian Randomization Study. Journal of the American Heart Association. 2021;10(17):e019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lyons A, Yilmazer T. Health and Financial Strain: Evidence from the Survey of Consumer Finances. Southern Economic Journal. 2005;71:873–90. [Google Scholar]
- 87.Tillmann T, Vaucher J, Okbay A, Pikhart H, Peasey A, Kubinova R, et al. Education and coronary heart disease: mendelian randomisation study. Bmj. 2017;358:j3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou H, Zhang Y, Liu J, Yang Y, Fang W, Hong S, et al. Education and lung cancer: a Mendelian randomization study. Int J Epidemiol. 2019;48(3):743–50. [DOI] [PubMed] [Google Scholar]
- 89.Lee JO, Kosterman R, Jones TM, Herrenkohl TI, Rhew IC, Catalano RF, et al. Mechanisms linking high school graduation to health disparities in young adulthood: a longitudinal analysis of the role of health behaviours, psychosocial stressors, and health insurance. Public Health. 2016;139:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Böckerman P, Viinikainen J, Pulkki-Råback L, Hakulinen C, Pitkänen N, Lehtimäki T, et al. Does higher education protect against obesity? Evidence using Mendelian randomization. Prev Med. 2017;101:195–8. [DOI] [PubMed] [Google Scholar]
- 91.Demange PA, Boomsma DI, van Bergen E, Nivard MG. Evaluating the causal relationship between educational attainment and mental health. medRxiv. 2023. [Google Scholar]
- 92.Viinikainen J, Bryson A, Böckerman P, Elovainio M, Pitkänen N, Pulkki-Råback L, et al. Does education protect against depression? Evidence from the Young Finns Study using Mendelian randomization. Prev Med. 2018;115:134–9. [DOI] [PubMed] [Google Scholar]
- 93.Miech RA, Hauser RM. Socioeconomic status and health at midlife. A comparison of educational attainment with occupation-based indicators. Ann Epidemiol. 2001;11(2):75–84. [DOI] [PubMed] [Google Scholar]
- 94.Statistics USBoL. 2022. [Google Scholar]
- 95.Siahpush M, Singh GK, Jones PR, Timsina LR. Racial/ethnic and socioeconomic variations in duration of smoking: results from 2003, 2006 and 2007 Tobacco Use Supplement of the Current Population Survey. J Public Health (Oxf). 2010;32(2):210–8. [DOI] [PubMed] [Google Scholar]
- 96.Assari S. The Benefits of Higher Income in Protecting against Chronic Medical Conditions Are Smaller for African Americans than Whites. Healthcare (Basel). 2018;6(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Latza U, Kohlmann T, Deck R, Raspe H. Can health care utilization explain the association between socioeconomic status and back pain? Spine (Phila Pa 1976). 2004;29(14):1561–6. [DOI] [PubMed] [Google Scholar]
- 98.Jarvik JG, Comstock BA, Heagerty PJ, Turner JA, Sullivan SD, Shi X, et al. Back pain in seniors: the Back pain Outcomes using Longitudinal Data (BOLD) cohort baseline data. BMC Musculoskelet Disord. 2014;15:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berkovic D, Ayton D, Briggs AM, Ackerman IN. “The Financial Impact Is Depressing and Anxiety Inducing”: A Qualitative Exploration of the Personal Financial Toll of Arthritis. Arthritis Care Res (Hoboken). 2021;73(5):671–9. [DOI] [PubMed] [Google Scholar]
- 100.Sahle BW, Chen W, Melaku YA, Akombi BJ, Rawal LB, Renzaho AMN. Association of Psychosocial Factors With Risk of Chronic Diseases: A Nationwide Longitudinal Study. American Journal of Preventive Medicine. 2020;58(2):e39–e50. [DOI] [PubMed] [Google Scholar]
- 101.Guan N, Guariglia A, Moore P, Xu F, Al-Janabi H. Financial stress and depression in adults: A systematic review. PLoS One. 2022;17(2):e0264041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gaston SA, Strassle PD, Alhasan DM, Pérez-Stable EJ, Nápoles AM, Jackson CL. Financial hardship, sleep disturbances, and their relationship among men and women in the United States during the COVID-19 pandemic. Sleep Health. 2023;9(4):551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morin CM, Vézina-Im LA, Ivers H, Micoulaud-Franchi JA, Philip P, Lamy M, et al. Prevalent, incident, and persistent insomnia in a population-based cohort tested before (2018) and during the first-wave of COVID-19 pandemic (2020). Sleep. 2022;45(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marshall GL, Baker TA, Song C, Miller DB. Pain and Hardship Among Older Men: Examining the Buffering Effect of Medicare Insurance Coverage. American Journal of Men’s Health. 2018;12(5):1439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wood L, Bejarano G, Csiernik B, Miyamoto GC, Mansell G, Hayden JA, et al. Pain catastrophising and kinesiophobia mediate pain and physical function improvements with Pilates exercise in chronic low back pain: a mediation analysis of a randomised controlled trial. J Physiother. 2023;69(3):168–74. [DOI] [PubMed] [Google Scholar]
- 106.Rogers AH, Farris SG. A meta-analysis of the associations of elements of the fear-avoidance model of chronic pain with negative affect, depression, anxiety, pain-related disability and pain intensity. Eur J Pain. 2022;26(8):1611–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hall AM, Kamper SJ, Emsley R, Maher CG. Does pain-catastrophising mediate the effect of tai chi on treatment outcomes for people with low back pain? Complement Ther Med. 2016;25:61–6. [DOI] [PubMed] [Google Scholar]
- 108.Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7(4):261–71. [DOI] [PubMed] [Google Scholar]
- 109.Buer N, Linton SJ. Fear-avoidance beliefs and catastrophizing: occurrence and risk factor in back pain and ADL in the general population. Pain. 2002;99(3):485–91. [DOI] [PubMed] [Google Scholar]
- 110.Ryum T, Stiles TC. Changes in pain catastrophizing, fear-avoidance beliefs, and pain self-efficacy mediate changes in pain intensity on disability in the treatment of chronic low back pain. Pain Rep. 2023;8(5):e1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Joyce CT, Chernofsky A, Lodi S, Sherman KJ, Saper RB, Roseen EJ. Do Physical Therapy and Yoga Improve Pain and Disability through Psychological Mechanisms? A Causal Mediation Analysis of Adults with Chronic Low Back Pain. J Orthop Sports Phys Ther. 2022;52(7):470–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morton L, de Bruin M, Krajewska M, Whibley D, Macfarlane GJ. Beliefs about back pain and pain management behaviours, and their associations in the general population: A systematic review. Eur J Pain. 2019;23(1):15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. [DOI] [PubMed] [Google Scholar]
- 114.Jura M, Kozak LP. Obesity and related consequences to ageing. Age (Dordr). 2016;38(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Endomba FT, Tchebegna PY, Chiabi E, Angong Wouna DL, Guillet C, Chauvet-Gélinier JC. Epidemiology of insomnia disorder in older persons according to the Diagnostic and Statistical Manual of Mental Disorders: a systematic review and meta-analysis. Eur Geriatr Med. 2023;14(6):1261–72. [DOI] [PubMed] [Google Scholar]
- 116.Wettstein M, Eich W, Bieber C, Tesarz J. Pain Intensity, Disability, and Quality of Life in Patients with Chronic Low Back Pain: Does Age Matter? Pain Med. 2019;20(3):464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jarvik JG, Comstock BA, Bresnahan BW, Nedeljkovic SS, Nerenz DR, Bauer Z, et al. Study protocol: the Back Pain Outcomes using Longitudinal Data (BOLD) registry. BMC Musculoskelet Disord. 2012;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maxwell CJ, Hirdes JP. The prevalence of smoking and implications for quality of life among the community-based elderly. Am J Prev Med. 1993;9(6):338–45. [PubMed] [Google Scholar]
- 119.Byrne GJ, Pachana NA. Anxiety and depression in the elderly: do we know any more? Curr Opin Psychiatry. 2010;23(6):504–9. [DOI] [PubMed] [Google Scholar]
- 120.Flint AJ. Epidemiology and comorbidity of anxiety disorders in the elderly. Am J Psychiatry. 1994;151(5):640–9. [DOI] [PubMed] [Google Scholar]
- 121.Flint AJ. Generalised anxiety disorder in elderly patients : epidemiology, diagnosis and treatment options. Drugs Aging. 2005;22(2):101–14. [DOI] [PubMed] [Google Scholar]
- 122.Lenze EJ, Mulsant BH, Shear MK, Schulberg HC, Dew MA, Begley AE, et al. Comorbid anxiety disorders in depressed elderly patients. Am J Psychiatry. 2000;157(5):722–8. [DOI] [PubMed] [Google Scholar]
- 123.Murphy RA, Hagaman AK, Reinders I, Steeves JA, Newman AB, Rubin SM, et al. Depressive Trajectories and Risk of Disability and Mortality in Older Adults: Longitudinal Findings From the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2016;71(2):228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Diegelmann M, Schilling OK, Wahl HW. Feeling blue at the end of life: Trajectories of depressive symptoms from a distance-to-death perspective. Psychol Aging. 2016;31(7):672–86. [DOI] [PubMed] [Google Scholar]
- 125.Almeida OP. Prevention of depression in older age. Maturitas. 2014;79(2):136–41. [DOI] [PubMed] [Google Scholar]
- 126.Wheeler CHB, Williams ACC, Morley SJ. Meta-analysis of the psychometric properties of the Pain Catastrophizing Scale and associations with participant characteristics. Pain. 2019;160(9):1946–53. [DOI] [PubMed] [Google Scholar]
- 127.Markfelder T, Pauli P. Fear of pain and pain intensity: Meta-analysis and systematic review. Psychol Bull. 2020;146(5):411–50. [DOI] [PubMed] [Google Scholar]
- 128.FRB: Insights into the Financial Experiences of Older Adults: A Forum Briefing Paper 2024. [Available from: https://www.federalreserve.gov/econresdata/older-adults-survey/July-2013-Introduction.htm.
- 129.Pott J, Horn K, Zeidler R, Kirsten H, Ahnert P, Kratzsch J, et al. Sex-Specific Causal Relations between Steroid Hormones and Obesity-A Mendelian Randomization Study. Metabolites. 2021;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suh S, Cho N, Zhang J. Sex Differences in Insomnia: from Epidemiology and Etiology to Intervention. Curr Psychiatry Rep. 2018;20(9):69. [DOI] [PubMed] [Google Scholar]
- 131.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB. Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratory-induced pain. Pain. 2004;111(3):335–41. [DOI] [PubMed] [Google Scholar]
- 132.@NIDAnews. Are there gender differences in tobacco smoking? | National Institute on Drug Abuse. −−.
- 133.Faravelli C, Alessandra Scarpato M, Castellini G, Lo Sauro C. Gender differences in depression and anxiety: the role of age. Psychiatry Res. 2013;210(3):1301–3. [DOI] [PubMed] [Google Scholar]
- 134.Waardenburg S, Visseren L, van Daal E, Brouwer B, van Zundert J, van Kuijk SMJ, et al. Do Men and Women Have a Different Association between Fear-Avoidance and Pain Intensity in Chronic Pain? An Experience Sampling Method Cohort-Study. J Clin Med. 2022;11(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bank FR. Gender Disparities in Financial Well-Being from the Survey of Household Economics and Decisionmaking. 2020. [Google Scholar]
- 136.Hadjar A, Krolak-Schwerdt S, Priem K, Glock S. Gender and educational achievement. Educational Research. 2014;56(2):117–25. [Google Scholar]
- 137.Richard Reeves SK. Racial disparities in the high school graduation gender gap. Brookings. 2023. [Google Scholar]
- 138.Zheng JW, Ai SZ, Chang SH, Meng SQ, Shi L, Deng JH, et al. Association between alcohol consumption and sleep traits: observational and mendelian randomization studies in the UK biobank. Mol Psychiatry. 2024. [DOI] [PubMed] [Google Scholar]
- 139.Bowen MT, George O, Muskiewicz DE, Hall FS. FACTORS CONTRIBUTING TO THE ESCALATION OF ALCOHOL CONSUMPTION. Neurosci Biobehav Rev. 2022;132:730–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miloyan B, Van Doorn G. Longitudinal association between social anxiety disorder and incident alcohol use disorder: results from two national samples of US adults. Soc Psychiatry Psychiatr Epidemiol. 2019;54(4):469–75. [DOI] [PubMed] [Google Scholar]
- 141.Torvik FA, Rosenström TH, Gustavson K, Ystrom E, Kendler KS, Bramness JG, et al. Explaining the association between anxiety disorders and alcohol use disorder: A twin study. Depress Anxiety. 2019;36(6):522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Köhler CA, Evangelou E, Stubbs B, Solmi M, Veronese N, Belbasis L, et al. Mapping risk factors for depression across the lifespan: An umbrella review of evidence from meta-analyses and Mendelian randomization studies. J Psychiatr Res. 2018;103:189–207. [DOI] [PubMed] [Google Scholar]
- 143.Zhu C, Chen Q, Si W, Li Y, Chen G, Zhao Q. Alcohol Use and Depression: A Mendelian Randomization Study From China. Front Genet. 2020;11:585351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harrison S, Davies AR, Dickson M, Tyrrell J, Green MJ, Katikireddi SV, et al. The causal effects of health conditions and risk factors on social and socioeconomic outcomes: Mendelian randomization in UK Biobank. Int J Epidemiol. 2020;49(5):1661–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Polimanti R, Peterson RE, Ong JS, MacGregor S, Edwards AC, Clarke TK, et al. Evidence of causal effect of major depression on alcohol dependence: findings from the psychiatric genomics consortium. Psychol Med. 2019;49(7):1218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.@samhsagov. 2021 NSDUH Annual National Report. 2024.
- 147.Stewart SA, Copeland AL, Cherry KE. Risk Factors for Substance Use across the Lifespan. J Genet Psychol. 2023;184(2):145–62. [DOI] [PubMed] [Google Scholar]
- 148.Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet. 2022;400(10347):185–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mulia N, Witbrodt J, Karriker-Jaffe KJ, Li L, Lui CK, Zapolski T. Education matters: longitudinal pathways to mid-life heavy drinking in a national cohort of black Americans. Addiction. 2022;117(8):2225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barbería-Latasa M, Gea A, Martínez-González MA. Alcohol, Drinking Pattern, and Chronic Disease. Nutrients. 2022;14(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu T, Nakanishi T, Yoshiji S, Butler-Laporte G, Greenwood CMT, Richards JB. Dose-dependent Association of Alcohol Consumption With Obesity and Type 2 Diabetes: Mendelian Randomization Analyses. J Clin Endocrinol Metab. 2023;108(12):3320–9. [DOI] [PubMed] [Google Scholar]
- 152.Sullivan MJ, Rodgers WM, Kirsch I. Catastrophizing, depression and expectancies for pain and emotional distress. Pain. 2001;91(1–2):147–54. [DOI] [PubMed] [Google Scholar]
- 153.Crombez G, Eccleston C, Vlaeyen JW, Vansteenwegen D, Lysens R, Eelen P. Exposure to physical movements in low back pain patients: restricted effects of generalization. Health Psychol. 2002;21(6):573–8. [PubMed] [Google Scholar]
- 154.Trost Z, France CR, Thomas JS. Exposure to movement in chronic back pain: evidence of successful generalization across a reaching task. Pain. 2008;137(1):26–33. [DOI] [PubMed] [Google Scholar]
- 155.Urban-Baeza A, Zárate-Kalfópulos B, Romero-Vargas S, Obil-Chavarría C, Brenes-Rojas L, Reyes-Sánchez A. Influence of depression symptoms on patient expectations and clinical outcomes in the surgical management of spinal stenosis. J Neurosurg Spine. 2015;22(1):75–9. [DOI] [PubMed] [Google Scholar]
- 156.Cormier S, Lavigne GL, Choinière M, Rainville P. Expectations predict chronic pain treatment outcomes. Pain. 2016;157(2):329–38. [DOI] [PubMed] [Google Scholar]
- 157.Perrot S, Allaert FA, Concas V, Laroche F. “When will I recover?” A national survey on patients’ and physicians’ expectations concerning the recovery time for acute back pain. Eur Spine J. 2009;18(3):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Martinez-Calderon J, Zamora-Campos C, Navarro-Ledesma S, Luque-Suarez A. The Role of Self-Efficacy on the Prognosis of Chronic Musculoskeletal Pain: A Systematic Review. J Pain. 2018;19(1):10–34. [DOI] [PubMed] [Google Scholar]
- 159.Cheng ST, Leung CMC, Chan KL, Chen PP, Chow YF, Chung JWY, et al. The relationship of self-efficacy to catastrophizing and depressive symptoms in community-dwelling older adults with chronic pain: A moderated mediation model. PLoS One. 2018;13(9):e0203964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Montag LT, Salomons TV, Wilson R, Duggan S, Bisson EJ. Examining the roles of depression, pain catastrophizing, and self-efficacy in quality of life changes following chronic pain treatment. Can J Pain. 2023;7(1):2156330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roseen EJ, Gerlovin H, Felson DT, Delitto A, Sherman KJ, Saper RB. Which Chronic Low Back Pain Patients Respond Favorably to Yoga, Physical Therapy, and a Self-care Book? Responder Analyses from a Randomized Controlled Trial. Pain Med. 2021;22(1):165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Riley SP, Bialosky J, Coronado RA. Are Changes in Fear-Avoidance Beliefs and Self-efficacy Mediators of Function and Pain at Discharge in Patients With Acute and Chronic Low Back Pain? J Orthop Sports Phys Ther. 2020;50(6):301–8. [DOI] [PubMed] [Google Scholar]
- 163.Curran F, Davis ME, Murphy K, Tersigni N, King A, Ngo N, et al. Correlates of physical activity and sedentary behavior in adults living with overweight and obesity: A systematic review. Obes Rev. 2023;24(11):e13615. [DOI] [PubMed] [Google Scholar]
- 164.Kruger ES, Serier KN, Pfund RA, McKay JR, Witkiewitz K. Integrative data analysis of self-efficacy in 4 clinical trials for alcohol use disorder. Alcohol Clin Exp Res. 2021;45(11):2347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Suri P, Boyko EJ, Smith NL, Jarvik JG, Jarvik GP, Williams FMK, et al. Post-traumatic Stress Disorder Symptoms are Associated With Incident Chronic Back Pain: A Longitudinal Twin Study of Older Male Veterans. Spine (Phila Pa 1976). 2019;44(17):1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pimentel SD, Adams H, Ellis T, Clark R, Sully C, Paré C, et al. The Sequential Relation Between Changes in Catastrophizing and Changes in Posttraumatic Stress Disorder Symptom Severity. J Trauma Stress. 2020;33(5):731–40. [DOI] [PubMed] [Google Scholar]
- 167.López-Martínez AE, Ramírez-Maestre C, Esteve R. An examination of the structural link between post-traumatic stress symptoms and chronic pain in the framework of fear-avoidance models. Eur J Pain. 2014;18(8):1129–38. [DOI] [PubMed] [Google Scholar]
- 168.Fung HW, Chien WT, Lam SKK, Ross CA. Investigating post-traumatic stress disorder (PTSD) and complex PTSD among people with self-reported depressive symptoms. Front Psychiatry. 2022;13:953001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.van den Berk-Clark C, Secrest S, Walls J, Hallberg E, Lustman PJ, Schneider FD, et al. Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring smoking: A systematic review and meta-analysis. Health Psychol. 2018;37(5):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Maher AR, Apaydin EA, Hilton L, Chen C, Troxel W, Hall O, et al. Sleep management in posttraumatic stress disorder: a systematic review and meta-analysis. Sleep Med. 2021;87:203–19. [DOI] [PubMed] [Google Scholar]
- 171.Palmisano AN, Fogle BM, Tsai J, Petrakis IL, Pietrzak RH. Disentangling the association between PTSD symptom heterogeneity and alcohol use disorder: Results from the 2019–2020 National Health and Resilience in Veterans Study. J Psychiatr Res. 2021;142:179–87. [DOI] [PubMed] [Google Scholar]
- 172.Hayden JA, Dunn KM, van der Windt DA, Shaw WS. What is the prognosis of back pain? Best Pract Res Clin Rheumatol. 2010;24(2):167–79. [DOI] [PubMed] [Google Scholar]
- 173.Hayden JA, Chou R, Hogg-Johnson S, Bombardier C. Systematic reviews of low back pain prognosis had variable methods and results: guidance for future prognosis reviews. J Clin Epidemiol. 2009;62(8):781–96.e1. [DOI] [PubMed] [Google Scholar]
- 174.Besen E, Gaines B, Linton SJ, Shaw WS. The role of pain catastrophizing as a mediator in the work disability process following acute low back pain. Journal of Applied Biobehavioral Research. 2017;22(1):e12085. [Google Scholar]
- 175.Steenstra IA, Munhall C, Irvin E, Oranye N, Passmore S, Van Eerd D, et al. Systematic Review of Prognostic Factors for Return to Work in Workers with Sub Acute and Chronic Low Back Pain. J Occup Rehabil. 2017;27(3):369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fujii T, Matsudaira K, Oka H. Factors associated with fear-avoidance beliefs about low back pain. J Orthop Sci. 2013;18(6):909–15. [DOI] [PubMed] [Google Scholar]
- 177.Corbière M, Sullivan MJL, Stanish WD, Adams H. Pain and depression in injured workers and their return to work: A longitudinal study. Canadian Journal of Behavioural Science / Revue canadienne des sciences du comportement. 2007;39(1):23–31. [Google Scholar]
- 178.Wong JJ, Tricco AC, Côté P, Liang CY, Lewis JA, Bouck Z, et al. Association Between Depressive Symptoms or Depression and Health Outcomes for Low Back Pain: a Systematic Review and Meta-analysis. J Gen Intern Med. 2022;37(5):1233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ostbye T, Dement JM, Krause KM. Obesity and workers’ compensation: results from the Duke Health and Safety Surveillance System. Arch Intern Med. 2007;167(8):766–73. [DOI] [PubMed] [Google Scholar]
- 180.Choi EB, Sang D. Obesity and the risk for occupational injuries: A literature review. Journal of Environmental and Occupational Health. 2015;4(3):163–70. [Google Scholar]
- 181.Gallagher RM, Williams RA, Skelly J, Haugh LD, Rauh V, Milhous R, et al. Workers’ Compensation and return-to-work in low back pain. Pain. 1995;61(2):299–307. [DOI] [PubMed] [Google Scholar]
- 182.Chin WS, Liao SC, Pan SC, Guo YL. Occupational and Non-occupational Injuries Can Result in Prolonged Augmentation of Psychiatric Disorders. J Epidemiol. 2022;32(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ziadni MS, Sturgeon JA, Bissell D, Guck A, Martin KJ, Scott W, et al. Injustice Appraisal, but not Pain Catastrophizing, Mediates the Relationship Between Perceived Ethnic Discrimination and Depression and Disability in Low Back Pain. J Pain. 2020;21(5–6):582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Simmons A, Vasquez A, Green K, Christopher M, Colgan DD. The impact of ethnic discrimination on chronic pain: the role of sex and depression. Ethn Health. 2023;28(7):1053–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dickens H, Bruehl S, Rao U, Myers H, Goodin B, Huber FA, et al. Cognitive-Affective-Behavioral Pathways Linking Adversity and Discrimination to Daily Pain in African-American Adults. J Racial Ethn Health Disparities. 2023;10(6):2718–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hammett PJ, Eliacin J, Saenger M, Allen KD, Meis LA, Krein SL, et al. The Association Between Racialized Discrimination in Health Care and Pain Among Black Patients With Mental Health Diagnoses. J Pain. 2024;25(1):217–27. [DOI] [PubMed] [Google Scholar]
- 187.Agbonlahor O, DeJarnett N, Hart JL, Bhatnagar A, McLeish AC, Walker KL. Racial/Ethnic Discrimination and Cardiometabolic Diseases: A Systematic Review. J Racial Ethn Health Disparities. 2023:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cheng P, Cuellar R, Johnson DA, Kalmbach DA, Joseph CL, Cuamatzi Castelan A, et al. Racial discrimination as a mediator of racial disparities in insomnia disorder. Sleep Health. 2020;6(5):543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hicks MR, Kogan SM. The influence of racial discrimination on smoking among young black men: A prospective analysis. J Ethn Subst Abuse. 2020;19(2):311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Glass JE, Williams EC, Oh H. Racial/ethnic discrimination and alcohol use disorder severity among United States adults. Drug Alcohol Depend. 2020;216:108203. [DOI] [PubMed] [Google Scholar]
- 191.Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):173–83. [DOI] [PubMed] [Google Scholar]
- 192.Bulloch AG, Williams JV, Lavorato DH, Patten SB. The relationship between major depression and marital disruption is bidirectional. Depress Anxiety. 2009;26(12):1172–7. [DOI] [PubMed] [Google Scholar]
- 193.Sobal J, Hanson KL, Frongillo EA. Gender, ethnicity, marital status, and body weight in the United States. Obesity (Silver Spring). 2009;17(12):2223–31. [DOI] [PubMed] [Google Scholar]
- 194.Patel NP, Grandner MA, Xie D, Branas CC, Gooneratne N. “Sleep disparity” in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health. 2010;10:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kahn EB, Ramsey LT, Brownson RC, Heath GW, Howze EH, Powell KE, et al. The effectiveness of interventions to increase physical activity: A systematic review1,2. American Journal of Preventive Medicine. 2002;22(4, Supplement 1):73–107. [DOI] [PubMed] [Google Scholar]
- 196.Metsä-Simola N, Moustgaard H, Martikainen P. Time patterns of external and alcohol-related mortality after marital and non-marital separation: the contribution of psychiatric morbidity. J Epidemiol Community Health. 2020;74(6):510–8. [DOI] [PubMed] [Google Scholar]
- 197.Stanton E, Fresquez Z, Muehlbauer EJ, Wang JC, Buser Z. Onset of mental disorders in patients who developed failed back surgery syndrome. Eur Spine J. 2022;31(10):2612–8. [DOI] [PubMed] [Google Scholar]
- 198.Wu Q, Cui X, Guan LC, Zhang C, Liu J, Ford NC, et al. Chronic pain after spine surgery: Insights into pathogenesis, new treatment, and preventive therapy. J Orthop Translat. 2023;42:147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yun SY, Kim DH, Do HY, Kim SH. Clinical insomnia and associated factors in failed back surgery syndrome: a retrospective cross-sectional study. Int J Med Sci. 2017;14(6):536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bekeris J, Wilson LA, Fiasconaro M, Poeran J, Liu J, Girardi F, et al. New Onset Depression and Anxiety After Spinal Fusion Surgery: Incidence and Risk Factors. Spine (Phila Pa 1976). 2020;45(16):1161–9. [DOI] [PubMed] [Google Scholar]
- 201.Havakeshian S, Mannion AF. Negative beliefs and psychological disturbance in spine surgery patients: a cause or consequence of a poor treatment outcome? Eur Spine J. 2013;22(12):2827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nakajima K, Miyahara J, Ohtomo N, Nagata K, Kato S, Doi T, et al. Impact of body mass index on outcomes after lumbar spine surgery. Sci Rep. 2023;13(1):7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nunna RS, Ostrov PB, Ansari D, Dettori JR, Godolias P, Elias E, et al. The Risk of Nonunion in Smokers Revisited: A Systematic Review and Meta-Analysis. Global Spine J. 2022;12(3):526–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Han L, Han H, Liu H, Wang C, Wei X, He J, et al. Alcohol Abuse and Alcohol Withdrawal Are Associated with Adverse Perioperative Outcomes Following Elective Spine Fusion Surgery. Spine (Phila Pa 1976). 2021;46(9):588–95. [DOI] [PubMed] [Google Scholar]
- 205.Ibrahim AR, Elgamal ME, Moursi MO, Shraim BA, Shraim MA, Shraim M, et al. The Association between Early Opioids Prescribing and the Length of Disability in Acute Lower Back Pain: A Systematic Review and Narrative Synthesis. Int J Environ Res Public Health. 2022;19(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Burns JW, Bruehl S, France CR, Schuster E, Orlowska D, Buvanendran A, et al. Psychosocial factors predict opioid analgesia through endogenous opioid function. Pain. 2017;158(3):391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Young-Wolff KC, Klebaner D, Weisner C, Von Korff M, Campbell CI. Smoking Status and Opioid-related Problems and Concerns Among Men and Women on Chronic Opioid Therapy. Clin J Pain. 2017;33(8):730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Stokes A, Berry KM, Collins JM, Hsiao CW, Waggoner JR, Johnston SS, et al. The contribution of obesity to prescription opioid use in the United States. Pain. 2019;160(10):2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Serdarevic M, Osborne V, Striley CW, Cottler LB. The association between insomnia and prescription opioid use: results from a community sample in Northeast Florida. Sleep Health. 2017;3(5):368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.LaRowe LR, Powers JM, Garey L, Rogers AH, Zvolensky MJ, Ditre JW. Pain-related anxiety, sex, and co-use of alcohol and prescription opioids among adults with chronic low back pain. Drug Alcohol Depend. 2020;214:108171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jess MA, Ryan C, Hamilton S, Wellburn S, Atkinson G, Greenough C, et al. Does Duration of Pain at Baseline Influence Longer-term Clinical Outcomes of Low Back Pain Patients Managed on an Evidence-Based Pathway? Spine (Phila Pa 1976). 2021;46(3):191–7. [DOI] [PubMed] [Google Scholar]
- 212.Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, Weiser S. Catastrophizing-a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J. 2014;14(11):2639–57. [DOI] [PubMed] [Google Scholar]
- 213.Herr KA, Mobily PR, Smith C. Depression and the experience of chronic back pain: a study of related variables and age differences. Clin J Pain. 1993;9(2):104–14. [DOI] [PubMed] [Google Scholar]
- 214.Dunn KM, Croft PR. The importance of symptom duration in determining prognosis. Pain. 2006;121(1–2):126–32. [DOI] [PubMed] [Google Scholar]
- 215.Leboeuf-Yde C, Kyvik KO, Bruun NH. Low back pain and lifestyle. Part II--Obesity. Information from a population-based sample of 29,424 twin subjects. Spine (Phila Pa 1976). 1999;24(8):779–83; discussion 83–4. [DOI] [PubMed] [Google Scholar]
- 216.Alsaadi SM, McAuley JH, Hush JM, Maher CG. Prevalence of sleep disturbance in patients with low back pain. Eur Spine J. 2011;20(5):737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Leboeuf-Yde C, Kyvik KO, Bruun NH. Low back pain and lifestyle. Part I: Smoking. Information from a population-based sample of 29,424 twins. Spine (Phila Pa 1976). 1998;23(20):2207–13; discussion 14. [DOI] [PubMed] [Google Scholar]
- 218.Scott SC, Goldberg MS, Mayo NE, Stock SR, Poîtras B. The association between cigarette smoking and back pain in adults. Spine (Phila Pa 1976). 1999;24(11):1090–8. [DOI] [PubMed] [Google Scholar]
- 219.Skillgate E, Pico-Espinosa OJ, Hallqvist J, Bohman T, Holm LW. Healthy lifestyle behavior and risk of long duration troublesome neck pain or low back pain among men and women: results from the Stockholm Public Health Cohort. Clin Epidemiol. 2017;9:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Oliveira VC, Ferreira ML, Morso L, Albert HB, Refshauge KM, Ferreira PH. Patients’ perceived level of social isolation affects the prognosis of low back pain. Eur J Pain. 2015;19(4):538–45. [DOI] [PubMed] [Google Scholar]
- 221.Hajek A, Kretzler B, König HH. The Association Between Obesity and Social Isolation as Well as Loneliness in the Adult Population: A Systematic Review. Diabetes Metab Syndr Obes. 2021;14:2765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Griffin SC, Williams AB, Ravyts SG, Mladen SN, Rybarczyk BD. Loneliness and sleep: A systematic review and meta-analysis. Health Psychol Open. 2020;7(1):2055102920913235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dyal SR, Valente TW. A Systematic Review of Loneliness and Smoking: Small Effects, Big Implications. Subst Use Misuse. 2015;50(13):1697–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Le TM, Wang W, Zhornitsky S, Dhingra I, Chen Y, Zhang S, et al. The Neural Processes Interlinking Social Isolation, Social Support, and Problem Alcohol Use. Int J Neuropsychopharmacol. 2021;24(4):333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Adnan R, Van Oosterwijck J, Danneels L, Willems T, Meeus M, Crombez G, et al. Differences in psychological factors, disability and fatigue according to the grade of chronification in non-specific low back pain patients: A cross-sectional study. J Back Musculoskelet Rehabil. 2020;33(6):919–30. [DOI] [PubMed] [Google Scholar]
- 226.Lukkahatai N, Saligan LN. Association of catastrophizing and fatigue: a systematic review. J Psychosom Res. 2013;74(2):100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.de Moraes Vieira EB, de Góes Salvetti M, Damiani LP, de Mattos Pimenta CA. Self-efficacy and fear avoidance beliefs in chronic low back pain patients: coexistence and associated factors. Pain Manag Nurs. 2014;15(3):593–602. [DOI] [PubMed] [Google Scholar]
- 228.Jacobsen HB, Kallestad H, Landrø NI, Borchgrevink PC, Stiles TC. Processes in acceptance and commitment therapy and the rehabilitation of chronic fatigue. Scand J Psychol. 2017;58(3):211–20. [DOI] [PubMed] [Google Scholar]
- 229.Corfield EC, Martin NG, Nyholt DR. Co-occurrence and symptomatology of fatigue and depression. Compr Psychiatry. 2016;71:1–10. [DOI] [PubMed] [Google Scholar]
- 230.Lim W, Hong S, Nelesen R, Dimsdale JE. The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects. Arch Intern Med. 2005;165(8):910–5. [DOI] [PubMed] [Google Scholar]
- 231.Kim SJ, Kim S, Jeon S, Leary EB, Barwick F, Mignot E. Factors associated with fatigue in patients with insomnia. J Psychiatr Res. 2019;117:24–30. [DOI] [PubMed] [Google Scholar]
- 232.McCallum SM, Batterham PJ, Calear AL, Sunderland M, Carragher N, Kazan D. Associations of fatigue and sleep disturbance with nine common mental disorders. J Psychosom Res. 2019;123:109727. [DOI] [PubMed] [Google Scholar]
- 233.Wüst RC, Morse CI, de Haan A, Rittweger J, Jones DA, Degens H. Skeletal muscle properties and fatigue resistance in relation to smoking history. Eur J Appl Physiol. 2008;104(1):103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pereira Nery ECH, Rocha NP, Cruz VT, Silva AG. Systematic review and meta-analysis on the association between chronic low back pain and cognitive function. Pain Pract. 2023;23(4):399–408. [DOI] [PubMed] [Google Scholar]
- 235.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–40. [DOI] [PubMed] [Google Scholar]
- 236.Prickett C, Brennan L, Stolwyk R. Examining the relationship between obesity and cognitive function: a systematic literature review. Obes Res Clin Pract. 2015;9(2):93–113. [DOI] [PubMed] [Google Scholar]
- 237.Wardle-Pinkston S, Slavish DC, Taylor DJ. Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Med Rev. 2019;48:101205. [DOI] [PubMed] [Google Scholar]
- 238.Conti AA, McLean L, Tolomeo S, Steele JD, Baldacchino A. Chronic tobacco smoking and neuropsychological impairments: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;96:143–54. [DOI] [PubMed] [Google Scholar]
- 239.Wang G, Li DY, Vance DE, Li W. Alcohol Use Disorder as a Risk Factor for Cognitive Impairment. J Alzheimers Dis. 2023;94(3):899–907. [DOI] [PubMed] [Google Scholar]