Abstract
Background.
Despite the importance of the transition to fatherhood as a critical life stage among young adult men, much remains unknown about the factors predictive of ideal cardiovascular health (CVH) and how CVH is impacted as young men face new roles and responsibilities associated with fatherhood.
Methods.
To address this gap, the Dad Bod Study is a prospective, longitudinal and observational study designed to examine how fatherhood affects young men’s CVH. A total of 125, first-time prospective fathers (men, 19–39 years) will be enrolled and followed over 1.5 years. Metrics of the American Heart Association’s “Life’s Essential 8” as well as demographic, social, and psychosocial factors will be collected at four time points ((baseline (during the pregnant partner’s 2nd trimester) 1-month postpartum, 6-months postpartum, and 1-year postpartum). The primary aims are to measure predictors of CVH among first-time fathers and describe longitudinal changes in CVH. A secondary aim is to identify best practices for recruitment, retention, and remote data collection in this population.
Summary.
The Dad Bod Study offers a novel examination of CVH among first-time fathers, exploring how new paternal roles and responsibilities impact cardiovascular health. Findings may provide key insights into critical CVH behaviors and risk factors to monitor, preserve, and improve as young men transition to fatherhood.
Keywords: men’s health, cardiovascular health, paternal, fatherhood
Introduction
Cardiovascular disease (CVD) is a leading cause of death among men in the United States, despite >80% of all cardiovascular events being preventable through healthy lifestyles and management of known CVD risk factors1,2. Maintaining health promoting behaviors and avoiding the development of adverse risk behaviors earlier in life (primordial prevention) can improve cardiovascular health (CVH) and reduce the future burden of CVD3–5.
Young adulthood encompasses an age range between 18 and 39 years old6. During this life stage many critical decisions and goal pursuits occur that can have enduring ramifications – young adults often complete their education, enter the workforce, establish social networks and romantic relationships, and potentially start a family. Recent trends indicate a steady or slightly increasing incidence of CVD in young adults contrasting with the generally decreasing rates observed in middle-aged and older adults2,7. Exacerbating this trend, recent nationally representative data have shown that CVH in young adults has not improved in the last decade8. These findings portend a potential future burden of CVD and reversal of decreasing rates of CVD in later developmental periods as current young adults age.
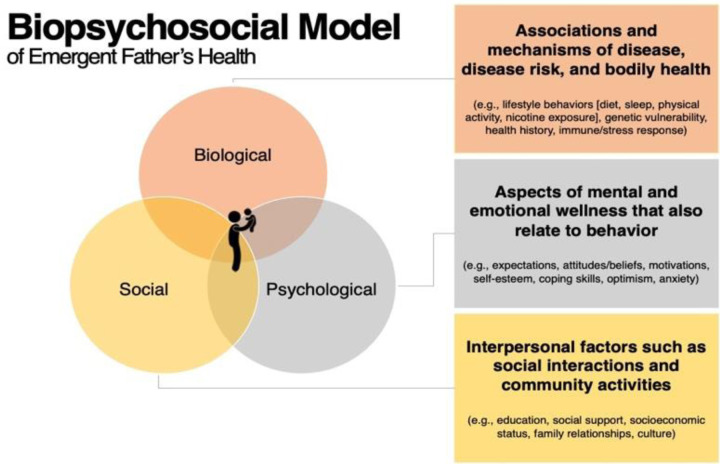
The transition to fatherhood among young men represents a major, discrete life event in which substantial biological, psychological, and social changes can occur 9–11 (Figure 1). Some men may be motivated to improve their health during the transition to fatherhood, while others are challenged to improve or maintain health as they experience disruption to their lifestyle routines10–12. A new father’s ability to choose a healthy lifestyle may also be influenced by social or socioeconomic and structural elements13–15. Further, the transition to fatherhood can significantly impact men’s health, as the stress and anxiety associated with new parental responsibilities may exacerbate or trigger various physical and mental health issues, including depression, which has been well documented among men during the perinatal and postpartum period16,17.
Figure 1.
Biopsychosocial Model of the overlapping interplay of biological, psychological, and social factors that may impact emergent father’s health.
As men transition into fatherhood and throughout the postpartum period, several undesirable CVH behaviors have been observed. These include declines in physical activity and increased sedentary behavior18,19, reduced sleep20–22, poor diet quality20,23, and increases in body weight19,20,22,24–27. However, the impact of fatherhood on health behaviors is not uniformly negative. Some studies have also noted positive associations such as greater physical activity20 and lower rates of smoking19 among new fathers. Evidence also suggests that the relationship of fatherhood with CVH may also differ by age in which men enter into fatherhood and by race/ethnicity28. Despite the importance of the transition to fatherhood as a critical life stage among young men, much remains unknown about the factors predictive of ideal CVH and how CVH is impacted as young men face new roles and responsibilities associated with fatherhood.
The current study, titled ‘The Dad Bod Study’ was designed as a prospective, longitudinal and observational study designed to examine how fatherhood affects young men’s CVH. Specifically, the study aims to (1) examine demographic, social, and psychosocial factors that are predictive of CVH among first-time fathers, (2) examine longitudinal changes to CVH in first-time fathers using the American Heart Association’s “Life’s Essential 8” metric and (3) identify best practices for recruitment, retention, and remote data collection among first-time fathers. This article describes the design, methodology, and rationale for the Dad Bod Study.
Methods
Ethics Statement and Data Availability
The study was approved by the University of California, Irvine Institutional Review Board (IRB #4907, approved May 1, 2024). After study completion, the anonymized data and material will be made publicly available.
Community Engagement Studios
In the Spring of 2024, our research team conducted a series of Community Engagement Studios (CE Studios) in partnership with the Institute for Clinical and Translational Science at the University of California, Irvine. CE Studios are a consultative model meant to provide research teams with rapid feedback from individuals representative of the population of interest on specific aspects of their existing study design before a research project is implemented29,30. During each CE Studio, we presented a brief overview of the research project then provided prompts regarding aspects of this project. CE Studio participants participated in a facilitated discussion and provided their opinions and recommendations. Each CE Studios focused on a different aspect of the project allowing us to gain relevant insights on recruitment methods, number of timepoints and perceptions on participant burden, data collection methods, and strategies for participant retention. Insights gathered from the CE Studios were used by the research team to refine and strengthen the methodological approach of this study described in subsequent sections.
Participant Recruitment
When designing the study, the research team carefully considered known barriers and facilitators for recruiting and retaining male participants in longitudinal health research31. Recruitment for the study is expected to begin in Fall 2024. We will recruit 125 young adult (age 19–39), male individuals. Participants will be recruited from across the United States during their pregnant partner’s 2nd trimester and will be allowed to enroll in the study up until the pregnant partner’s 35th week of pregnancy during the 3rd trimester. First-time fathers are defined as a biological father who has not yet experienced the live birth of his own child32.
Multiple methods of participant recruitment will be used. Participants will be recruited using Native Health Research33, a participant recruitment platform that uses social media and digital advertisements to connect interested participants with research studies. Recruitment will also take place through online support groups for prospective fathers. Printed advertisements will be displayed on notice boards at various community venues in Orange County, CA, including workplaces, community group meeting locations, sports complexes, golf clubs, and local library branches. Additionally, local obstetrician-gynecologists within the university and local hospital systems will be informed about the study and provided with flyers to distribute during prenatal care visits. Interested men will complete a brief online screener, via URL link or QR code on study advertisements, to determine eligibility. As part of the screener, participants will be asked to indicate how they heard about the study during the initial enrollment process, allowing us to track and analyze the effectiveness of various recruitment methods.
Inclusion and Exclusion Criteria
Inclusion and exclusion criteria are intended to maximize both eligibility and generalizability (Table 1). We will recruit only males, as the study aims seek to answer the question of how fatherhood affects men’s health. Additionally, we will exclude men aged ≥40, given our focus on management of lifestyle behaviors and cardiovascular risk factors before onset of cardiovascular disease. There will be no exclusion based on racial or ethnic status. Due to budget limitations, study materials will be provided only in English. Additionally, recognizing the diverse nature of modern families, the study adopts an inclusive approach with no eligibility restrictions on fathers’ living arrangements or planned involvement in infant care. However, this information will be collected for analytical purposes.
Table 1.
Inclusion and Exclusion Criteria
Inclusion Criteria
|
Exclusion Criteria
|
Data Collection
Eligible participants will attend a study orientation session, conducted via video call. After being informed about the study design interested participants will provide written informed consent. Enrolled men will complete four longitudinal assessments (baseline (during the pregnant partner’s 2nd trimester) 1-month postpartum, 6-months postpartum, and 1-year postpartum) over the course of the study. The baseline data collection timepoint will occur immediately after consenting to participate in the study. Time points were chosen based on feedback gathered in CE Studios and to be frequent enough to allow for comprehensive assessment of trends in CVH without being onerous on participants.
Given a general disengagement with traditional preventive care among young men34,35, this study utilizes novel approaches to recruit, retain, and collect all data remotely. Because of the remote nature, participants can be recruited anywhere in the United States, potentially allowing for a more diverse sample36. Additionally, participants will complete all aspects of the study from their home and will not have to come to any university or clinical site, therefore decreasing participant burden. Participants will be provided with instructional videos demonstrating the proper procedures for collecting at-home metrics, (e.g., blood pressure using a study-provided blood pressure cuff and at-home dried blood spot sample collection kits). Study staff will be available to offer additional assistance via email or video call, as needed. To maintain the study’s focus on the transition to fatherhood, participants will be compassionately withdrawn from the study in the event of pregnancy loss, stillbirth, or other circumstances resulting in an unsuccessful completion of the pregnancy.
Collection of Life’s Essential 8 Cardiovascular Health Metrics
In 2022, the American Heart Association released its new iteration of quantifying CVH – “Life’s Essential 8”37. Life’s Essential 8 consists of 4 modifiable health behaviors four behaviors (diet, physical activity, sleep health, and smoking) and four modifiable risk factors (body weight, blood lipids, blood glucose, and blood pressure). At each of the four data collection time points, participants will complete assessments of each of the Life’s Essential 8 metrics (see Table 2). Each metric is designed to take <5 minutes to assess, easing participant burden. To facilitate remote data collection, participants will be mailed study-provided equipment including a digital body weight scale and blood pressure cuff at the onset of the study. Furthermore, at-home dried blood spot sample collection kits will be mailed to participant’s homes at each of the four assessment time points. Participants will be instructed to collect the sample while fasting (at least 8 hours since the prior meal) and will be encouraged to complete the sample collection at the same time of day for each of the four time points. Following the collection protocol, participants will seal their dried blood spot samples and return them for laboratory processing using the provided prepaid envelopes. Each metric will be scored on an ordinal point scoring scale of 0 to 100 points according to the American Heart Association scoring algorithm37. A higher score typically indicates better adherence to optimal health behaviors or risk factor management in that particular area. An overall CVH score will be calculated by summing the scores for each of the 8 metrics and dividing the total by 8, to provide a Life’s Essential 8 score ranging from 0 to 100.
Table 2.
Life’s Essential 8 Metrics and Method of Measurementa
| Metric | Method of Measurement |
|---|---|
| 1.Diet | Self-administrated dietary recall using diet quality photo navigation (DietID)51–53, from which a Healthy Eating Index-202054 (HEI-2020) score will be calculated. |
| 2.Physical Activity | Self-reported minutes of moderate or vigorous physical activity via International Physical Activity Questionnaire (IPAQ)55. |
| 3.Nicotine Exposure | Self-reported use of cigarettes or products containing nicotine via Behavioral Risk Factor Surveillance System Tobacco Use core questions56. |
| 4.Sleep Health | Self-reported average hours of sleep per night via Pittsburgh Sleep Quality Index (PSQI) item57. |
| 5.Body Mass Index (BMI) | Objective measurement of weight from study-provided scale (Tanita HS-302 Light Powered Digital Scale) and self-reported height. BMI calculated as kg/m2. |
| 6.Blood Lipids | Objective measurement of plasma total and HDL-cholesterol with calculation of non-HDL-cholesterol and fasting blood glucose from at-home dried blood spot sample collection58 (Molecular Testing Labs). |
| 7.Blood Glucose | |
| 8.Blood Pressure | Objective measurement of systolic and diastolic blood pressure from study-provided blood pressure cuff (Omron Bronze Upper Arm Monitor). |
All eight metrics will be collected at four assessment time points: BL: baseline (during the pregnant partner’s 2nd trimester); 1M: 1-month postpartum; 6M: 6-months postpartum; and 12M: 12-month postpartum)
Questionnaires and Other Assessment Measures
The constructs, measures used, and time points at which questionnaire data collection will occur are outlined in Table 3. Some questionnaires are collected only once as they are relevant to only one phase of the study. Other measures are collected at multiple time points to observe change over time. Because the 1-month postpartum time point is anticipated to be a more onerous time for participants, questionnaire data collection is intentionally limited.
Participant Incentives
Participants will be compensated for participation in the study at each timepoint with a $40 gift card (up to $160 total for completion of all timepoints). Compensation is one method being used to help maximize high retention rates throughout the study. As research volunteers, participants can stop taking part in the study at any point during the study period. In that scenario, participants will be compensated based on the study timepoints they have completed. Following the study’s completion, participants will be allowed to keep the study-provided weight scale and blood pressure cuff.
Data Management
An electronic software program (RedCap software) will be used to enter and store data and will only be accessible to the authorized members of the research team38. All data will be stored in a de-identified format, with personal identifiers removed and replaced with unique study IDs to protect participant privacy.
Sample Size
Sample size was based on the power to detect the trend regression coefficient in simulation of the Generalized Estimating Equation model (Aim 2). The power to detect a non-null moderate trend effect (−0.05 points in CVH per month39) with a sample size of 125 first-time fathers is 0.87 (see Table 4).
Table 4.
Power to Detect a Non-Null Trend Effect for Various Sample Size and Proposed Interaction Effect Sizes
| Effect Size | N =100 | N =125 | N =150 |
|---|---|---|---|
| −0.1 | 0.99 | 1 | 0.999 |
| −0.05 | 0.769 | 0.867 | 0.924 |
| −0.01 | 0.279 | 0.306 | 0.390 |
Statistical Analysis
For all analyses, baseline data will be described using standard descriptive statistics including frequency distributions, means and SDs, and medians and interquartile ranges. The primary outcome is change in Life’s Essential 8 total score. Analysis plans specific to reach of the three research aims are described in detail below.
Aim 1 Analysis
Data will be split into training and testing sets with 80% of the sample allocated to training data and the remaining 20% left for testing data. Descriptive statistics of the baseline covariates (means and standard deviations) will be generated to check whether marginal distributions of predictive variables between training and test sets are relatively similar. Should this not be the case, we will regenerate the assignment of observations to the training set until distributions are similar. We will use XGBoost regression as the predictive algorithm with Mean Squared Error (MSE) as the primary loss function40. We select model parameters using grid search over the number of splits, training speed, observation subsampling rate, and feature sampling rate optimizing the 5-fold cross-validation MSE. Once the final model parameters are chosen, we will train the model a final time using all of the training data, after which we will report the predictive metrics on the testing data
Once the final model is chosen, SHAP scores (Shapley Additive exPlanations) will be computed41. For each covariate, Mean Absolute SHAP will be computed to rank each predictor’s importance toward predicting 1-year post-partum CVH. Among predictors with a Mean Absolute SHAP >5 (meaning, on average, the predictor influences the final prediction at least 5 points on the Life’s Essential 8 composite score), we will plot each observation SHAP versus the predictor value. This will give a more in-depth view of how predictor values influence model outputs. Mean absolute SHAP scores and plots will be reported and serve as hypothesis generation tools for future study of targeted interventions aimed at specific population subgroups of young adult men.
Aim 2 Analysis
Generalized Estimating Equations will be used to model the trajectory of Life’s Essential 8 composite score among first time fathers and will be based on the model described below. Let be the mean LE8 composite score of a participant at observation .
Where is the number of months to/since birth on observation for participant . is the set of confounding predictors for CVH trajectories. We assume an AR (1) correlation structure between successive measurements on the same participant, under the assumption that CVH measurements taken closer together in time will be more correlated than those taken further apart. Based on the group trends observed, we will consider the use of interaction terms between and a grouping variable that can explain differences in trend.
There are 2 main parameters of interest:: comparing two subpopulations of first-time fathers who are similar with respect to and differing in time till/since birth by 1 month, is the mean difference in Life’s Essential 8 composite score. Parameters will be estimated using a GEE, and the corresponding estimates and 95% sandwich-based confidence intervals will be reported 42.
Aim 3 Analysis
Throughout the study, we will collect data that can be analyzed to identify best practices for recruitment, retention, and remote data collection among first-time fathers for future research. After completing the fourth data collection timepoint, participants will be invited to complete a study feedback questionnaire43. Additionally, they will have the option to participate in an exit interview with a study coordinator to discuss their experiences in the study. These data will be reported with exploratory summary statistics.
Missing Data
We expect there to be missing data, which will naturally affect the inference in all 3 aims. We will use information from staff feedback and participant study feedback questionnaire to inform the appropriate missingness paradigm. If it is reasonable to assume that missing observations are completely unrelated to cardiovascular health, we will proceed with a complete case analysis for all aims. If the missing observations are related to our observed quantities, we will implement a multiple imputation strategy and amend the inference (p-values, confidence intervals, mean absolute SHAP) to account for the uncertainty in the imputation procedure. If there is non-ignorable missingness, we will present our complete case results with an accompanying worst-case sensitivity analysis over reasonable ranges of what could have been observed.
Discussion
The Dad Bod Study is a prospective, longitudinal and observational study of young men who are prospective first-time fathers. Research during the perinatal period has predominantly focused on maternal and child health44,45, often overlooking the significant biological, social, and psychosocial changes that may be experienced by fathers (Figure 1). This bias has left a substantial gap in our understanding of paternal health during this transformative life stage. A better understanding of paternal CVH during the perinatal period is critical to meet new fathers’ needs during this significant life transition. This understanding has implications for both paternal health and the overall wellbeing of the mother-father-infant triad11.
The Dad Bod Study brings several innovations in its study design and builds on prior studies examining the impact of fatherhood on men’s health. This study’s longitudinal design, spanning from mid-pregnancy through the first year of fatherhood, allows for a comprehensive assessment of CVH trajectories, capturing both acute and long-term changes associated with new paternal roles and responsibilities. By utilizing the American Heart Association’s Life’s Essential 8 metrics, the study provides a standardized and comprehensive evaluation of paternal CVH, encompassing both behavioral and biological factors. This is compared to other widely used risk assessment tools such as the Pooled Cohort ASCVD Risk Equations46 or the more recent American Heart Association Predicting Risk of CVD EVENTs (PREVENT) equations47. However, while this study includes several objective metrics of CVH (e.g., dried blood spot for LDL cholesterol), several Life’s Essential 8 metrics are collected from validated self-report measures (e.g., sleep via the Pittsburgh Sleep Quality). This was largely done for feasibility and cost; however, future studies could use objective measures when possible (e.g., accelerometer for measurement of physical activity and sleep). Another notable strength of the study lies in its utilization of comprehensive questionnaires to assess a wide range of social and psychosocial constructs. The data derived from these questionnaires are crucial, as they provide essential context for understanding the factors that influence the potential for improving or maintaining CVH37. Lastly, the study’s remote data collection protocol was specifically designed to minimize participant burden by enabling fathers to complete all study components from their home environment. This approach acknowledges the time constraints often experienced by new fathers and may potentially enhance recruitment and retention rates, as well as data quality, by accommodating the participants’ busy schedules and reducing logistical barriers to participation.
This study has limitations that also warrant discussion. First, participants may modify aspects of their behaviors in response to participation in the study (i.e., Hawthorne effect48), which may introduce bias into the collection of self-reported health metrics. Additionally, a potential limitation is participation bias, wherein individuals who are already health-conscious may be more likely to participate, potentially affecting the generalizability of the results. However, this study utilizes validated, widely used measures, which can account for some level of potential self-report bias. Generalizability may also be impacted by restricting the sample to English speakers49. In Aim 1 we seek to build a machine learning predictive model for estimating 1-year post-partum CVH from baseline features. Sample size limitations likely inhibit building a truly generalizable model; however, it will be adequate for hypotheses generation for discriminating features of first-time fathers who will decline in CVH. In Aim 2, as our sample is purely composed of first-time fathers, it is difficult to discriminate with precision whether the change in CVH health among first time fathers is due to the effect of simply aging, or whether the birth of their first child resulted in some accelerative effect on CVH. While this study provides critical preliminary data on the transition to fatherhood, in a subsequent study, it would be useful to recruit both first-time fathers and age-matched non-expecting men and follow them over similar amounts of time. Following baseline data collection, this study requires follow-up at three postpartum timepoints. While data collection strategies that minimize participant burden are used, there may be incomplete data among men who are lost to follow-up. Multiple reminder strategies and participant incentives will be implemented across the duration of the study to minimize potential participant attrition. Due to the remote data collection methods50, which decrease participant burden, anticipated participant attrition is expected to be low. Any additional challenges to recruitment and retention (Aim 3) will provide important learning opportunities that would strengthen a future study’s design and execution.
Summary
This prospective, longitudinal and observational study represents a novel examination of CVH among first-time fathers and how CVH may change as young men face new roles and responsibilities associated with fatherhood. The identification of specific factors that are predictive of CVH allow for hypotheses generation of discriminating features of first-time fathers who will decline in CVH. This in turn could set the stage for public health interventions and clinical applications targeted towards these factors. Additionally, outcomes provide novel insights into CVH behaviors and risk factors that would be most critical to monitor, preserve, and improve among young men.
Table 2.
Dad Bod Study Questionnaire Constructs, Measures, and Assessment Schedule
| Construct | Measure | Time Pointsa | |||
|---|---|---|---|---|---|
| BL | 1M | 6M | 12M | ||
| Individual and Interpersonal Factors | |||||
| Demographics | Race and ethnicity59, health insurance56, marital status56, educational attainment56, employment status56, household income56, individuals within the household56. | ♂ | ♂ | ♂ | |
| Neighborhood Characteristics | Area Deprivation Index (ADI)60 | ♂ | ♂ | ♂ | |
| Food Security | U.S Household Food Security Survey Module: Six-Item Short Form Security Scale61 | ♂ | ♂ | ♂ | |
| Other Pillars of Food Security | Center for Nutrition & Health Impact-developed measure (includes availability, utilization and stability)62 | ♂ | ♂ | ♂ | |
| Household Resilience | Center for Nutrition & Health Impact-developed measure (includes absorptive capacity, adaptive capacity, and transformative capacity)63 | ♂ | ♂ | ♂ | |
| Nutrition Security | Center for Nutrition & Health Impact-developed measure (includes nutrition security, healthfulness choice, and dietary choice)64 | ♂ | ♂ | ♂ | |
| Substance Use | Alcohol (abbreviated from the National Epidemiologic Survey on Alcohol and Related Conditions and the Alcohol Use Disorders Identification Test (AUDIT))65,66 | ♂ | ♂ | ♂ | |
| Cannabis (abbreviated National Institute on Drug Abuse (NIDA) Quick Screen V1.0)67 | ♂ | ♂ | ♂ | ||
| Vaping (abbreviated Population Assessment of Tobacco and Health (PATH) questionnaire)68 | ♂ | ♂ | ♂ | ||
| Psychosocial Factors | |||||
| Self-Esteem | Rosenberg Self-Esteem Scale69 | ♂ | ♂ | ♂ | |
| Well-Being (Affect) | Bradburn Scale of Psychological Well-Being70 | ♂ | ♂ | ♂ | |
| Stress | Perceived Stress Scale (PSS-10)71 | ♂ | ♂ | ♂ | |
| Social Support | Duke-UNC Functional Social Support Questionnaire72 | ♂ | ♂ | ♂ | |
| Social and Psychosocial Factors Relevant to the Transition to Fatherhood | |||||
| Paternal Adaptation | Paternal Adaptation Questionnaire73 | ♂ | |||
| Depression | Edinburgh Postnatal Depression Scale (EPDS)74 | ♂ | ♂ | ♂ | |
| Experiences of First Childbirth | Father’s Experiences of First Childbirth (FTFQ)75 | ♂ | |||
| Involvement of Care | Baby Care Scale – Antenatal76 | ♂ | |||
| Baby Care Scale – Postnatal76 | ♂ | ||||
| Attachment | Paternal Antenatal Attachment Scale77 | ♂ | |||
| Parenting Practices | Baby Care Questionnaires (BCQ)78 | ♂ | ♂ | ||
Four assessment time points: BL: baseline (during the pregnant partner’s 2nd trimester); 1M: 1-month postpartum; 6M: 6-months postpartum; and 12M: 12-month postpartum)
Sources of Funding
This work was supported by the American Heart Association Career Development Award (https://doi.org/10.58275/AHA.24CDA1258755.pc.gr.193626). The development of this project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grants UL1 TR001414 and 1UM1TR004927. Partial funding for publishing this article open access was provided by the University of California Libraries under a transformative open access agreement with the publisher.
Non-Standard Abbreviation and Acronyms
- CE Studio
Community Engagement Studio
- CVD
Cardiovascular Disease
- CVH
Cardiovascular Health
- SHAP
Shapley Additive exPlanations
Funding Statement
This work was supported by the American Heart Association Career Development Award (https://doi.org/10.58275/AHA.24CDA1258755.pc.gr.193626). The development of this project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grants UL1 TR001414 and 1UM1TR004927. Partial funding for publishing this article open access was provided by the University of California Libraries under a transformative open access agreement with the publisher.
Footnotes
Competing Interests Disclosure
The authors declare that they have no competing interests.
Data Sharing
Data sharing is not applicable to this article as no datasets were generated or analyzed. After study completion, the anonymized data and material will be made publicly available.
References
- 1.Ioachimescu OC. From Seven Sweethearts to Life Begins at Eight Thirty: A Journey From Life’s Simple 7 to Life’s Essential 8 and Beyond. J Am Heart Assoc. 2022;11:e027658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin SS, Aday AW, Almarzooq ZI, Anderson CA, Arora P, Avery CL, Baker-Smith CM, Barone Gibbs B, Beaton AZ, Boehme AK. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation. 2024;149:e347–e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooding HC, Shay CM, Ning H, Gillman MW, Chiuve SE, Reis JP, Allen NB, Lloyd◻Jones DM. Optimal lifestyle components in young adulthood are associated with maintaining the ideal cardiovascular health profile into middle age. J Am Heart Assoc. 2015;4:e002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu K, Daviglus ML, Loria CM, Colangelo LA, Spring B, Moller AC, Lloyd-Jones DM. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation. 2012;125:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2014;130:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. Am Psychol. 2000;55:469. [PubMed] [Google Scholar]
- 7.Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–240. doi: 10.1038/nrcardio.2017.154 [DOI] [PubMed] [Google Scholar]
- 8.Shetty NS, Parcha V, Patel N, Yadav I, Basetty C, Li C, Pandey A, Kalra R, Li P, Arora G. AHA Life’s essential 8 and ideal cardiovascular health among young adults. Am J Prev Cardiology. 2023;13:100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrell-Carrió F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. The Annals of Family Medicine. 2004;2:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garfield CF. Supporting fatherhood before and after it happens. Pediatrics. 2015;135:e528–e530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garfield CF, Clark-Kauffman E, Davis MM. Fatherhood as a component of men’s health. JAMA. 2006;296:2365–2368. [DOI] [PubMed] [Google Scholar]
- 12.Yogman M, Garfield CF, Bauer NS, Gambon TB, Lavin A, Lemmon KM, Mattson G, Rafferty JR, Wissow LS, Child CoPAo, et al. Fathers’ roles in the care and development of their children: The role of pediatricians. Pediatrics. 2016;138. [DOI] [PubMed] [Google Scholar]
- 13.Churchwell K, Elkind MS, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. [DOI] [PubMed] [Google Scholar]
- 14.Bell CN, Thorpe RJ Jr, Bowie JV, LaVeist TA. Race disparities in cardiovascular disease risk factors within socioeconomic status strata. Ann Epidemiol. 2018;28:147–152. [DOI] [PubMed] [Google Scholar]
- 15.Whitaker KM, Jacobs DR Jr, Kershaw KN, Demmer RT, Booth III JN, Carson AP, Lewis CE, Goff DC Jr, Lloyd-Jones DM, Gordon-Larsen P. Racial disparities in cardiovascular health behaviors: the coronary artery risk development in young adults study. Am J Prev Med. 2018;55:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhabra J, McDermott B, Li W. Risk factors for paternal perinatal depression and anxiety: A systematic review and meta-analysis. Psychology of Men & Masculinities. 2020;21:593. [Google Scholar]
- 17.Garfield CF, Duncan G, Rutsohn J, McDade TW, Adam EK, Coley RL, Chase-Lansdale PL. A longitudinal study of paternal mental health during transition to fatherhood as young adults. Pediatrics. 2014;133:836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corder K, Winpenny EM, Foubister C, Guagliano JM, Hartwig XM, Love R, Clifford Astbury C, van Sluijs EMF. Becoming a parent: A systematic review and meta-analysis of changes in BMI, diet, and physical activity. Obes Rev. 2020;21:e12959. doi: 10.1111/obr.12959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neshteruk CD, Norman K, Armstrong SC, Cholera R, D’Agostino E, Skinner AC. Association between parenthood and cardiovascular disease risk: Analysis from NHANES 2011–2016. Prev Med Rep. 2022;27:101820. doi: 10.1016/j.pmedr.2022.101820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whooten RC, Kotelchuck M, Gonzalez AVC, Johnson N, Kwete G, Luo M, Muir HF, Barth EA, Smith N, Taveras EM. Expectant fathers’ health behaviors, infant care intentions, and social-emotional wellbeing in the perinatal period: A latent class analysis and comparison to mothers. Prev Med Rep. 2023;36:102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wynter K, Francis LM, Fletcher R, McBride N, Dowse E, Wilson N, Di Manno L, Teague S, Macdonald JA, Consortium AFR. Sleep, mental health and wellbeing among fathers of infants up to one year postpartum: A scoping review. Midwifery. 2020;88:102738. [DOI] [PubMed] [Google Scholar]
- 22.Lo BK, Kang AW, Haneuse S, Yu X, Ash Tv, Redline S, Taveras EM, Davison KK. Changes in Fathers’ Body Mass Index, Sleep, and Diet From Prebirth to 12 Months Postbirth: Exploring the Moderating Roles of Parenthood Experience and Coparenting Support. Ann Behav Med. 2021;55:1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Versele V, Stok FM, Aerenhouts D, Deforche B, Bogaerts A, Devlieger R, Clarys P, Deliens T. Determinants of changes in women’s and men’s eating behavior across the transition to parenthood: A focus group study. Int J Behav Nutr Phys Act. 2021;18:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garfield CF, Duncan G, Gutina A, Rutsohn J, McDade TW, Adam EK, Coley RL, Chase-Lansdale PL. Longitudinal Study of Body Mass Index in Young Males and the Transition to Fatherhood. Am J Mens Health. 2016;10:Np158–np167. doi: 10.1177/1557988315596224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Versele V, Stas L, Aerenhouts D, Deliens T, Clarys P, Gucciardo L, Bogaerts A, Devlieger R. Changes in maternal and paternal body composition during the transition to parenthood (TRANSPARENTS). Obesity (Silver Spring). 2023;31:225–233. [DOI] [PubMed] [Google Scholar]
- 26.Saxbe D, Corner GW, Khaled M, Horton K, Wu B, Khoddam HL. The weight of fatherhood: identifying mechanisms to explain paternal perinatal weight gain. Health Psychol Rev. 2018;12:294–311. doi: 10.1080/17437199.2018.1463166 [DOI] [PubMed] [Google Scholar]
- 27.Brown DM, Abrams B, Cohen AK, Rehkopf DH. Motherhood, fatherhood and midlife weight gain in a US cohort: Associations differ by race/ethnicity and socioeconomic position. SSM-population health. 2017;3:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker JJF, Garfield CF, Simon CD, Colangelo LA, Bancks MP, Allen NB. Fatherhood and Cardiovascular Health, Disease, and Mortality: Associations From the Multi-Ethnic Study of Atherosclerosis. AJPM focus. 2024;3:100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joosten YA, Israel TL, Head A, Vaughn Y, Villalta Gil V, Mouton C, Wilkins CH. Enhancing translational researchers’ ability to collaborate with community stakeholders: Lessons from the Community Engagement Studio. J Clin Transl Sci. 2018;2:201–207. doi: 10.1017/cts.2018.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joosten YA, Israel TL, Williams NA, Boone LR, Schlundt DG, Mouton CP, Dittus RS, Bernard GR, Wilkins CH. Community Engagement Studios: A Structured Approach to Obtaining Meaningful Input From Stakeholders to Inform Research. Acad Med. 2015;90:1646–1650. doi: 10.1097/acm.0000000000000794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borg DJ, Haritopoulou-Sinanidou M, Gabrovska P, Tseng H-W, Honeyman D, Schweitzer D, Rae KM. Barriers and facilitators for recruiting and retaining male participants into longitudinal health research: a systematic review. BMC Med Res Methodol. 2024;24:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandler S, Field PA. Becoming a father: First◻time fathers’ experience of labor and delivery. J Nurse Midwifery. 1997;42:17–24. [DOI] [PubMed] [Google Scholar]
- 33.Nativve Health Research. Ads For Clinical Research Recruitment. https://www.healthresearch.study/. 2024. Accessed August 22.
- 34.Gooding HC, Gidding SS, Moran AE, Redmond N, Allen NB, Bacha F, Burns TL, Catov JM, Grandner MA, Harris KM. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: report from a National Heart, Lung, and Blood Institute Working Group. J Am Heart Assoc. 2020;9:e016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mursa R, Patterson C, Halcomb E. Men’s help◻seeking and engagement with general practice: An integrative review. J Adv Nurs. 2022;78:1938–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart J, Krows ML, Schaafsma TT, Heller KB, Brown ER, Boonyaratanakornkit J, Brown CE, Leingang H, Liou C, Bershteyn A. Comparison of racial, ethnic, and geographic location diversity of participants enrolled in clinic-based vs 2 remote COVID-19 clinical trials. JAMA Netw Open. 2022;5:e2148325–e2148325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd-Jones DM, Allen NB, Anderson CA, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation. 2022;146:e18–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C, Li Y, Zhao M, Zhang C, Bovet P, Xi B. Using the New “Life’s Essential 8” Metrics to Evaluate Trends in Cardiovascular Health Among US Adults From 2005 to 2018: Analysis of Serial Cross-sectional Studies. JMIR Public Health and Surveillance. 2023;9:e45521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen T, Guestrin C. Xgboost: A scalable tree boosting system. Paper/Poster presented at: Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining; 2016; [Google Scholar]
- 41.Lundberg SM, Lee S-I. A unified approach to interpreting model predictions. Adv Neural Inf Process Syst. 2017;30. [Google Scholar]
- 42.Schober P, Vetter TR. Repeated measures designs and analysis of longitudinal data: If at first you do not succeed—try, try again. Anesth Analg. 2018;127:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yessis JL, Kost RG, Lee LM, Coller BS, Henderson DK. Development of a research participants’ perception survey to improve clinical research. Clin Transl Sci. 2012;5:452–460. doi: 10.1111/j.1752-8062.2012.00443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotelchuck M, Lu M. Father’s role in preconception health. Matern Child Health J. 2017;21:2025–2039. [DOI] [PubMed] [Google Scholar]
- 45.Hodgson S, Painter J, Kilby L, Hirst J. The experiences of first-time fathers in perinatal services: Present but invisible. Paper/Poster presented at: Healthcare; 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’donnell CJ. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 47.Khan SS, Matsushita K, Sang Y, Ballew SH, Grams ME, Surapaneni A, Blaha MJ, Carson AP, Chang AR, Ciemins E, et al. Development and Validation of the American Heart Association’s PREVENT Equations. Circulation. 2024;149:430–449. doi: 10.1161/circulationaha.123.067626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sedgwick P, Greenwood N. Understanding the Hawthorne effect. BMJ. 2015;351. [DOI] [PubMed] [Google Scholar]
- 49.Muthukumar AV, Morrell W, Bierer BE. Evaluating the frequency of English language requirements in clinical trial eligibility criteria: A systematic analysis using ClinicalTrials.gov. PLoS Med. 2021;18:e1003758. doi: 10.1371/journal.pmed.1003758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hensen B, Mackworth-Young C, Simwinga M, Abdelmagid N, Banda J, Mavodza C, Doyle A, Bonell C, Weiss H. Remote data collection for public health research in a COVID-19 era: ethical implications, challenges and opportunities. Health Policy Plan. 2021;36:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner-McGrievy G, Hutto B, Bernhart JA, Wilson MJ. Comparison of the diet ID platform to the automated self-administered 24-hour (ASA24) dietary assessment tool for assessment of dietary intake. J Am Nutr Assoc. 2022;41:360–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dansinger ML, Breton GL, Joly JE, Rhee LQ, Katz DL. Rapid, digital dietary assessment in association with cardiometabolic biomarkers. Am J Health Promot. 2023:08901171231156513. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein AM, Rhee LQ, Njike VY, Katz DL. Dietary Assessment by Pattern Recognition: a comparative analysis. Curr Dev Nutr. 2023;7:101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shams-White MM, Pannucci TE, Lerman JL, Herrick KA, Zimmer M, Mathieu KM, Stoody EE, Reedy J. Healthy Eating Index-2020: Review and Update Process to Reflect the Dietary Guidelines for Americans, 2020–2025. J Acad Nutr Diet. 2023;123:1280–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.Mss.0000078924.61453.Fb [DOI] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention (CDC) [database online]. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2022. [Google Scholar]
- 57.Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 58.Kapur S, Kapur S, Zava D. Cardiometabolic risk factors assessed by a finger stick dried blood spot method. J Diabetes Sci Technol. 2008;2:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mathews K, Phelan J, Jones NA, Konya S, Marks R, Pratt BM, Coombs J, Bentley M. National Content Test: Race and ethnicity analysis report. US Department of Commerce, Economics and Statistics Administration, US Census Bureau. 2015. [Google Scholar]
- 60.Kind AJ, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. The New England journal of medicine. 2018;378:2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blumberg SJ, Bialostosky K, Hamilton WL, Briefel RR. The effectiveness of a short form of the Household Food Security Scale. Am J Public Health. 1999;89:1231–1234. doi: 10.2105/ajph.89.8.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calloway EE, Carpenter LR, Gargano T, Sharp JL, Yaroch AL. New measures to assess the “Other” three pillars of food security–availability, utilization, and stability. Int J Behav Nutr Phys Act. 2023;20:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calloway EE, Carpenter LR, Gargano T, Sharp JL, Yaroch AL. Development of three new multidimensional measures to assess household food insecurity resilience in the United States. Front Public Health. 2022;10:1048501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calloway EE, Carpenter LR, Gargano T, Sharp JL, Yaroch AL. Development of new measures to assess household nutrition security, and choice in dietary characteristics. Appetite. 2022;179:106288. [DOI] [PubMed] [Google Scholar]
- 65.Grant BF, Dawson DA. Introduction to the national epidemiologic survey on alcohol and related conditions. Alcohol Research & Health. 2006;29:74. [Google Scholar]
- 66.Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, Maynard C, Burman ML, Kivlahan DR. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Arch Intern Med. 2003;163:821–829. [DOI] [PubMed] [Google Scholar]
- 67.The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x [DOI] [PubMed] [Google Scholar]
- 68.Hyland A, Ambrose BK, Conway KP, Borek N, Lambert E, Carusi C, Taylor K, Crosse S, Fong GT, Cummings KM. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tobacco control. 2017;26:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenberg M. Rosenberg self-esteem scale. Journal of Religion and Health. 1965. [Google Scholar]
- 70.McDOWELL I, Praught E. On the measurement of happiness: an examination of the Bradburn Scale in the Canada Health Survey. Am J Epidemiol. 1982;116:949–958. [DOI] [PubMed] [Google Scholar]
- 71.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983:385–396. [PubMed] [Google Scholar]
- 72.Broadhead W, Gehlbach SH, De Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire: Measurement of social support in family medicine patients. Med Care. 1988:709–723. [DOI] [PubMed] [Google Scholar]
- 73.Eskandari N, Simbar M, Vadadhir A, Baghestani AR. Design and evaluation of the psychometric properties of a paternal adaptation questionnaire. Am J Mens Health. 2018;12:2018–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. [DOI] [PubMed] [Google Scholar]
- 75.Premberg Å, Taft C, Hellström A-L, Berg M. Father for the first time-development and validation of a questionnaire to assess fathers’ experiences of first childbirth (FTFQ). BMC Pregnancy Childbirth. 2012;12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pinto TM, Nunes-Costa R, Figueiredo B. The Baby Care Scale: A psychometric study with fathers during pregnancy and the postpartum period. Front Psychol. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Condon JT. The assessment of antenatal emotional attachment: development of a questionnaire instrument. Br J Med Psychol. 1993;66:167–183. [DOI] [PubMed] [Google Scholar]
- 78.Winstanley A, Gattis M. The Baby Care Questionnaire: A measure of parenting principles and practices during infancy. Infant Behav Dev. 2013;36:762–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed. After study completion, the anonymized data and material will be made publicly available.