Abstract
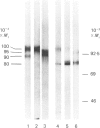
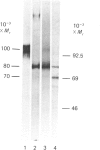
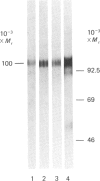
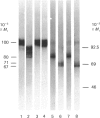
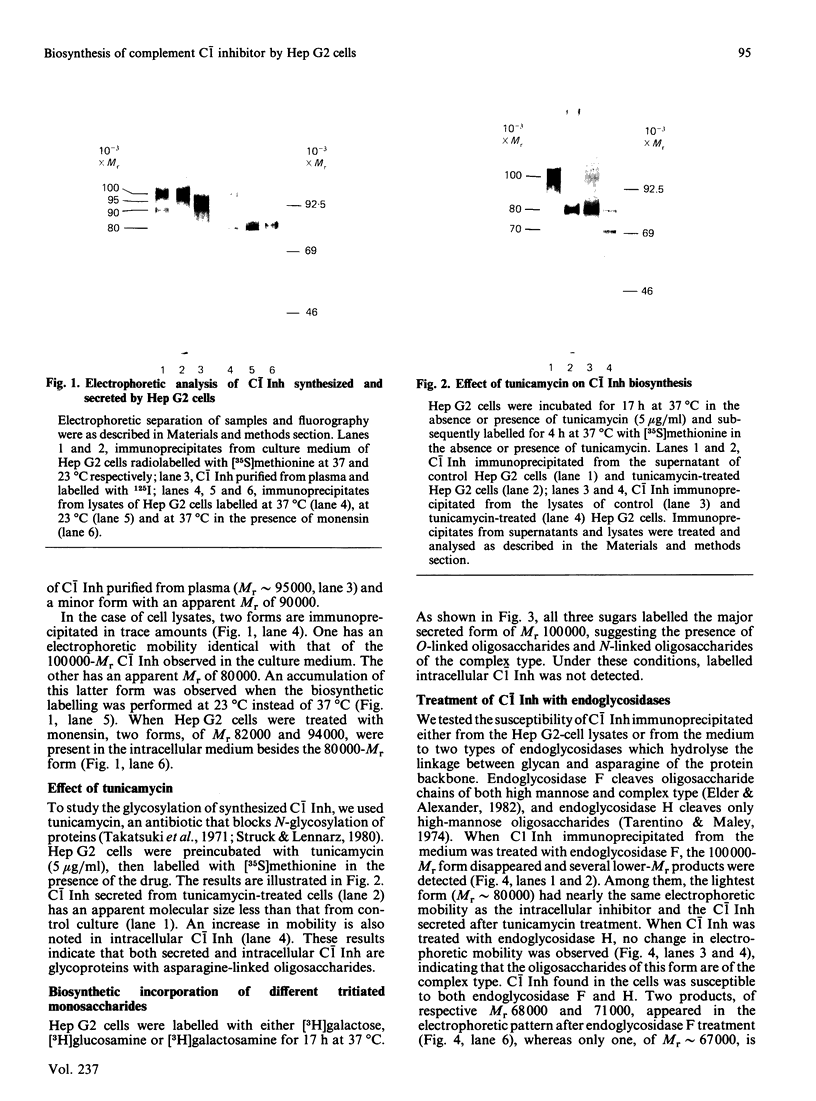
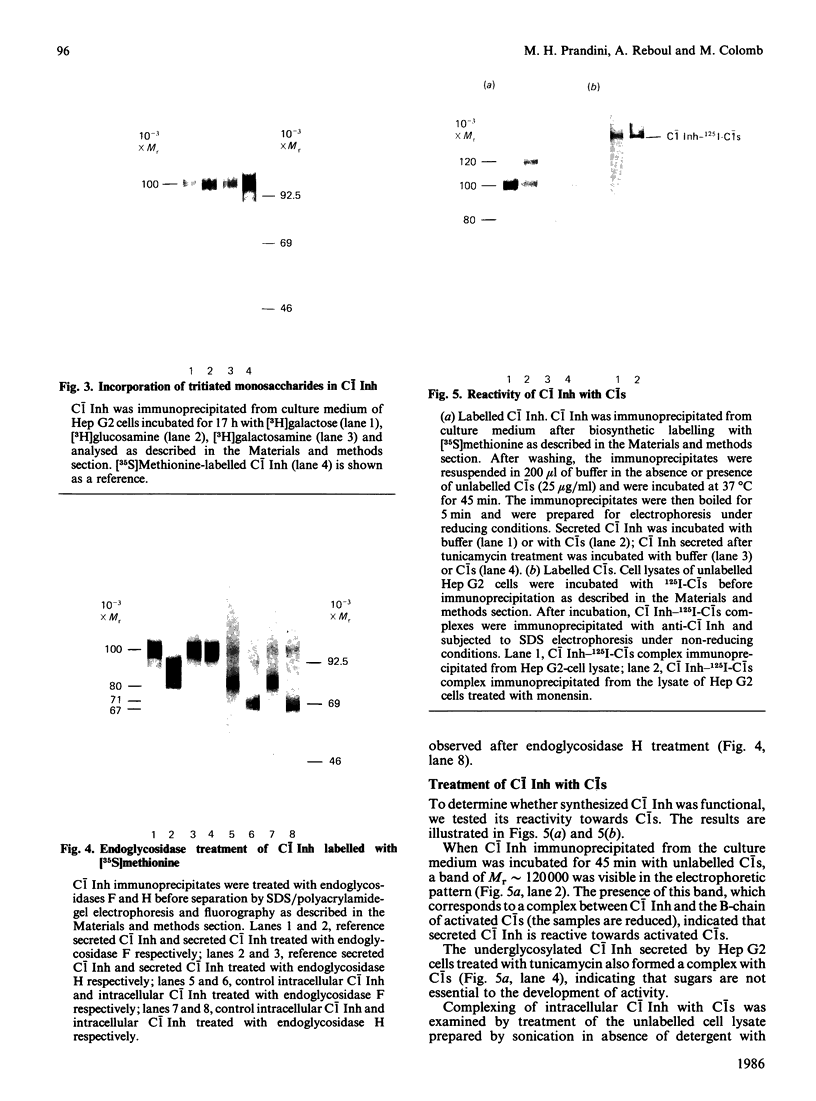
The biosynthesis of C1 Inh (C1 inhibitor) was studied in a human hepatoma cell line (Hep G2) by metabolic labelling, immunoprecipitation with anti-(C1 Inh) serum, analysis on SDS/polyacrylamide gel slabs and fluorography. Two forms of C1 Inh are secreted by Hep G2: a minor form of Mr 90,000 and a major form of Mr approximately 100,000. The latter form is also found in small amounts intracellularly in co-existence with an 80,000-Mr form. Accumulation of the 80,000-Mr C1 Inh is favoured when the cells are labelled at 23 degrees C instead of 37 degrees C or when they are treated with monensin. In the presence of tunicamycin, a compound that blocks the formation of N-asparagine-linked oligosaccharide chains, a decrease in Mr of both secreted and intracellular major forms is observed, indicating that secreted and intracellular C1 Inh contain N-linked oligosaccharide units. The 100,000 Mr secreted C1 Inh is sensitive to endoglycosidase F but resistant to endoglycosidase H, and it incorporates [3H]galactose, [3H]glucosamine and [3H]galactosamine, indicating the presence of both N-linked oligosaccharides of the complex type and O-linked oligosaccharides. The intracellular C1 Inh contains N-linked oligosaccharide units of the high-mannose type as demonstrated by endoglycosidase H-sensitivity. The functional activity of C1 Inh during its biosynthesis was tested by studying its reactivity towards C1s. Both secreted and intracellular C1 Inh form covalent-like complexes with purified plasma C1s. The underglycosylated C1 Inh secreted in presence of tunicamycin is still reactive with purified C1s. These results clearly show that sugars are not essential for this inhibitory activity of C1 Inh.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlaud G. J., Sim R. B., Duplaa A. M., Colomb M. G. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979 Jul;16(7):445–450. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- Bensa J. C., Reboul A., Colomb M. G. Biosynthesis in vitro of complement subcomponents C1q, C1s and C1 inhibitor by resting and stimulated human monocytes. Biochem J. 1983 Nov 15;216(2):385–392. doi: 10.1042/bj2160385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomb M. G., Arlaud G. J., Villiers C. L. Structure and activation of C1: current concepts. Complement. 1984;1(2):69–80. doi: 10.1159/000467818. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes C. D., Pensky J., Ratnoff O. D. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified serum C1 inactivator. J Lab Clin Med. 1970 Nov;76(5):809–815. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gigli I., Mason J. W., Colman R. W., Austen K. F. Interaction of plasma kallikrein with the C1 inhibitor. J Immunol. 1970 Mar;104(3):574–581. [PubMed] [Google Scholar]
- Harpel P. C., Cooper N. R. Studies on human plasma C1 inactivator-enzyme interactions. I. Mechanisms of interaction with C1s, plasmin, and trypsin. J Clin Invest. 1975 Mar;55(3):593–604. doi: 10.1172/JCI107967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A. Human C1 inhibitor: improved isolation and preliminary structural characterization. Biochemistry. 1983 Oct 11;22(21):5001–5007. doi: 10.1021/bi00290a019. [DOI] [PubMed] [Google Scholar]
- Haupt H., Heimburger N., Kranz T., Schwick H. G. Ein Beitrag zur Isolierung und Charakterisierung des Cl-Inaktivators aus Humanplasma. Eur J Biochem. 1970 Dec;17(2):254–261. doi: 10.1111/j.1432-1033.1970.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Johnson A. M., Alper C. A., Rosen F. S., Craig J. M. C1 inhibitor: evidence for decreased hepatic synthesis in hereditary angioneurotic edema. Science. 1971 Aug 6;173(3996):553–554. doi: 10.1126/science.173.3996.553. [DOI] [PubMed] [Google Scholar]
- LEVY L. R., LEPOW I. H. Assay and properties of serum inhibitor of C'l-esterase. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:608–611. doi: 10.3181/00379727-101-25034. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Minta J. O. The role of sialic acid in the functional activity and the hepatic clearance of C1-INH. J Immunol. 1981 Jan;126(1):245–249. [PubMed] [Google Scholar]
- Morris K. M., Aden D. P., Knowles B. B., Colten H. R. Complement biosynthesis by the human hepatoma-derived cell line HepG2. J Clin Invest. 1982 Oct;70(4):906–913. doi: 10.1172/JCI110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Sjöholm I., Wiman B. Structural and circular-dichroism studies on the interaction between human C1-esterase inhibitor and C1s. Biochem J. 1983 Sep 1;213(3):617–624. doi: 10.1042/bj2130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt E., Berger H., Sano Y. Size and shape of human C1-inhibitor. FEBS Lett. 1981 Aug 31;131(2):283–285. doi: 10.1016/0014-5793(81)80385-7. [DOI] [PubMed] [Google Scholar]
- Pensky J., Schwick H. G. Human serum inhibitor of C'1 esterase: identity with alpha-2-neuraminoglycoprotein. Science. 1969 Feb 14;163(3868):698–699. doi: 10.1126/science.163.3868.698. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., LEPOW I. H. Some properties of an esterase derived from preparations of the first component of complement. J Exp Med. 1957 Aug 1;106(2):327–343. doi: 10.1084/jem.106.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo B. P., Dattwyler R. J., Kaplan A. P., Ghebrehiwet B. Synthesis of C1 inhibitor (C1-INA) by a human monocyte-like cell line, U937. J Immunol. 1985 Aug;135(2):1313–1319. [PubMed] [Google Scholar]
- Reboul A., Arlaud G. J., Sim R. B., Colomb M. G. A simplified procedure for the purification of C1-inactivator from human plasma. Interaction with complement subcomponents C1r and C1s. FEBS Lett. 1977 Jul 1;79(1):45–50. doi: 10.1016/0014-5793(77)80347-5. [DOI] [PubMed] [Google Scholar]
- Reboul A., Bensa J. C., Colomb M. G. Characteristics of complement subcomponents C1r and C1s synthesized by Hep G2 cells. Biochem J. 1986 Jan 15;233(2):559–564. doi: 10.1042/bj2330559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul A., Prandini M. H., Bensa J. C., Colomb M. G. Characterization of C1q, C1s and C-1 Inh synthesized by stimulated human monocytes in vitro. FEBS Lett. 1985 Oct 7;190(1):65–68. doi: 10.1016/0014-5793(85)80428-2. [DOI] [PubMed] [Google Scholar]
- Salvesen G. S., Catanese J. J., Kress L. F., Travis J. Primary structure of the reactive site of human C1-inhibitor. J Biol Chem. 1985 Feb 25;260(4):2432–2436. [PubMed] [Google Scholar]
- Sim R. B., Reboul A., Arlaud G. J., Villiers C. L., Colomb M. G. Interaction of 125I-labelled complement subcomponents C-1r and C-1s with protease inhibitors in plasma. FEBS Lett. 1979 Jan 1;97(1):111–115. doi: 10.1016/0014-5793(79)80063-0. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Arima K., Tamura G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot (Tokyo) 1971 Apr;24(4):215–223. doi: 10.7164/antibiotics.24.215. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Weiss V., Engel J. Heparin-stimulated modification of C1-inhibitor by subcomponent C1s of human complement. Hoppe Seylers Z Physiol Chem. 1983 Mar;364(3):295–301. [PubMed] [Google Scholar]
- Yeung Laiwah A. C., Jones L., Hamilton A. O., Whaley K. Complement-subcomponent-C1-inhibitor synthesis by human monocytes. Biochem J. 1985 Feb 15;226(1):199–205. doi: 10.1042/bj2260199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziccardi R. J. A new role for C-1-inhibitor in homeostasis: control of activation of the first component of human complement. J Immunol. 1982 Jun;128(6):2505–2508. [PubMed] [Google Scholar]
- de Agostini A., Schapira M., Wachtfogel Y. T., Colman R. W., Carrel S. Human plasma kallikrein and C1 inhibitor form a complex possessing an epitope that is not detectable on the parent molecules: demonstration using a monoclonal antibody. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5190–5193. doi: 10.1073/pnas.82.15.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]