Abstract
Aim:
There is a need to elucidate intermittent Theta burst stimulation (iTBS) as a novel treatment in persistent somatoform pain disorder (PSPD).
Methods:
Twenty patients were randomly allocated to active iTBS (n = 11) and sham iTBS (n = 9) and received 10 iTBS sessions, 2 sessions per day, sequentially to primary motor and dorsolateral prefrontal cortices for 5 days in a week. Each iTBS session comprised of 2 sec. per train of 10 bursts (3 pulses per burst at 50 Hz; total 30 pulses) and were given with a gap of 5 Hz, total of 20 trains, and 600 pulses. Visual Analogue Scale, Brief Pain Inventory and Global Pain Scale (GPS), Montgomery and Asberg Depression Rating Scale, Hamilton Anxiety Rating Scale - Anxiety, World Health Organization Quality-of-Life Scale-brief, and Pittsburgh Sleep Quality Index were applied at baseline, after last session, and at 2 weeks after last TBS session. Intention to treat analysis was conducted.
Results:
Both groups were comparable for baseline psychopathology scores including clinical variables like age (t = 0.865; P = 0.398), duration of illness (t = 1.600; P = 0.127), and motor threshold (t = 0.304; P = 0.765). On repeated measures ANOVA, a significant within-group time effect for VAS, BPI-Severity, BPI-Interference, BDI – II, MADRS, HAM-A, and WHOQOL- BREF was found for active and sham TBS groups, respectively. GPS scores had significant within-group (active) * time interaction (F = 11.651; P = .001; ηp2 = 0.538) and between-group * time interaction (F = 3.407; P = 0.044; ηp2 = 0.159). However, between-group * time effect interaction was lost after covariance (F = 1.726; P = 0.196; ηp2 = 0.110).
Conclusion:
No major adverse effects were reported. Our pilot trial concludes that safe therapeutic efficacy of iTBS in PSPD is inconclusive. Lower total number of sessions along with small sample size may limit the study findings.
Keywords: Excitatory, iTBS, medically unexplained pain, prefrontal cortex, transcranial magnetic stimulation
INTRODUCTION
Persistent somatoform pain disorder (PSPD) is a chronic, severe, and distressing pain condition. Challenges like inadequate treatment approach, resistance to analgesic drugs, and high risk of drug misuse support the need of novel treatment options in PSPD.[1]
Transcranial magnetic stimulation (TMS) is a noninvasive and safe procedure to modulate brain activity.[2] TMS has been approved for varied psychiatric conditions that are highly comorbid with PSPD.[2] TBS is found be noninferior to the rTMS but has advantage in terms of reduced administration time.[2]
Available evidence of exclusive use of rTMS in PSPD is limited to case series reporting benefits.[3,4] Studies have used TMS with promising effects in refractory depression but with pain as an allied outcome.[5,6,7,8,9] Importantly, most of the studies have chosen dorso-lateral prefrontal cortex (DLPFC) as the site for the pain modulation.[10,11] Via preclinical studies, neuromodulation of primary motor cortex via has shown to strengthen connections for evoking muscle contractions (MEPs), produce physiological plasticity resulting in complex adaptive physiological and anatomical changes.[12] Moreso, clinical studies have shown that excitatory neuromodulation of primary motor cortex has shown efficacy in chronic neuropathic pains.[10] In addition, recent Indian clinical practice guidelines have suggested primary motor cortex (M1) as the putative site for neuromodulation in chronic functional pain syndromes.[2] Interestingly, TBS when compared to rTMS has shown to have better analgesic effects while stimulating M1 for cold pain threshold.[10]
With this background, we aim to study the effects of sequential iTBS over left primary motor cortex (C3) and left dorso-lateral prefrontal cortex (LDLPFC) on pain scores in PSPD and allied psychopathology scores.
METHODS
The study was conducted at the department of psychiatry of a tertiary care hospital in north India. Recruitment of participants was dated from December 5, 2022, and until August 5, 2023. The study protocol was registered with Clinical Trials Registry, India (Reference number CTRI/2022/12/047819) and approved by the ethical committee of the institute (reference number SGRR/IEC/03/22; IEC Registration No. ECR/710/Inst/UK/2015/RR-21).
Participants
A sample of 20 right-handed participants in the age range of 18–59 years, meeting the diagnostic criteria for persistent somatoform pain disorder as per diagnostic criteria for research of International Classification of Diseases-tenth edition (ICD-10 DCR), were enrolled.
We excluded patients with any form of comorbid psychiatric disorder including substance-induced mental and behavioral disorder of psychoactive substances (excluding nicotine and caffeine) either harmful use or dependence. Patients with the history suggestive of any seizures, brain injury/surgery, and neoplasm were not considered for the study. Furthermore, exclusion criteria included the presence of any metal part in the body (e.g., cardiac pacemaker, stents in neck, or brain)[13] along with exposure to ECT in the last 6 months.[14]
Study overview
Before enrolment, a written and duly signed informed consent was taken from the participants and their caregivers (as needed). A total of 25 patients were screened for the study, and a total of 20 patients were enrolled for the study (desired sample size could not be achieved citing time bound recruitment phase of the dissertation). Out of 20, 15 patients completed the treatment (9 patients in the active group and 6 patients in the sham group). The enrolment flow for the current trial is based on Consolidated Standards of Reporting Trials (CONSORT) statement and checklist (see Figure 1: Enrolment flow “CONSORT”).
Figure 1.
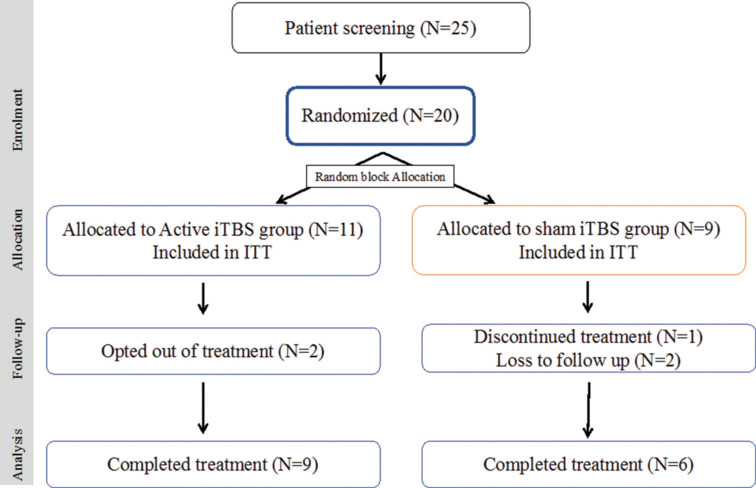
Enrolment Flow - intermittent Theta Burst Stimulation (iTBS); ITT: intention-to-treat analysis
All participants continued the pharmacological treatment in a naturalistic manner. None of the patients were given psychotherapeutic support.
The patients were assessed at baseline, after completion of 10 sessions, and after 2 weeks of iTBS treatment and on outpatient basis.
rTMS treatment parameters
A MagVenture - MagPro-R30 device with B65 butterfly coils (figure of 8 shaped coil) was used to provide iTBS sessions. The placement of TMS coil was done using the standardized international 10-20 EEG system. The stimulation site of first session was left motor cortex (M1, i.e. C3 as per the international 10-20 EEG system) and for second session was left dorsolateral prefrontal cortex (DLPFC, F3 location as per the international 10-20 EEG system). Sequential site stimulation of M1 and left DLPFC were conducted in line with the protocols suggested by the recent meta-analysis by Toh and colleagues.[15]
A total of 10 sessions of iTBS were given to all the participants, 2 sessions per day[8,9] with half an hour gap for 5 days in a week.[15] The participants were given 1200 pulses per day (600 pulses in 1st session at C3 and 600 pulses in 2nd session at F3) [see Supplementary Figure 1 (175.2KB, tif) ]. Details of the iTBS sessions are described as per Chauhan and colleagues.[14] The stimulation parameters for both the groups were decided based on existing TBS stimulation studies.[7,9]
RMT was calculated using Rossini–Rothwell algorithm and at 80% relative to participant’s RMT.[2] For sham iTBS, B-65 sham coil surface was used; however, all the participants had similar experience in terms of sound and scalp contact similar to active groups.
Clinical measures
The primary outcome measure was severity of persistent somatoform pain, in terms of pain intensity, related emotional changes, and pain’s impact on daily functioning. Secondary outcome measures were depression, anxiety, quality of life, mental wellbeing, sleep quality, and sexual functioning.
The pain intensity was measured using Visual Analogue Scale (VAS), Brief Pain Inventory-Short form (BPI-SF), and Global Pain Scale (GPS). VAS uses a 10 cm line (no pain to worst pain) representing a subjective measure for acute and chronic pain.[16,17,18] To measure symptoms of depression and its severity, Montgomery and Asberg Depression Rating Scale (MADRS) was used. Hamilton Anxiety Rating Scale - Anxiety (HAM-A) is a 21-item self-report inventory was used to assess the concomitant of anxiety in patients.[19] Quality of life and sleep quality were assessed using World Health Organization Quality-of-Life Scale-brief (WHOQOL – BREF)-Hindi version[20] and global score of Pittsburgh Sleep Quality Index (PSQI).[21] For allocation and blinding details, kindly refer the supplementary material.
The enrolled participants were randomly allocated to treatment and placebo groups using blocks (fixed block size of 4) of random number sequences. These numbers were sealed in the opaque envelopes. For each participant, the envelopes were open by the clinician imparting iTBS just before administration of the first session. The participants and the accompanying caregivers were blind to the arm of the treatment (see supplementary file for details). The rater was blinded to the overall randomization process.
Statistical analysis
SPSS version 28 for Windows (IBM Corp., Armonk, New York, USA) software was used. The Kolmogorov–Smirnov test were used to check normality. The sample characteristics were measured using the independent Chi-square test and t-test. All participants were included in the intention-to-treat (ITT) analysis. The last observation carried forward (LOCF) was used for the drop outs. The overall effect of treatment using the restricted maximum likelihood (REML) model analysis (using growth curve) with allocation order as “subjects,” treatment (active/sham) as the between-subject factor, and time (pre-treatment, immediately post 10th iTBS session, and 2 weeks post-iTBS) as within-subject factor.
As estimated a priori sample size could not be reached due to inadequate number of patients visiting within the time limited for the study, post hoc power estimation (using G*Power software) was done. Cohen’s f was calculated from the results of ANOVA. With the resultant statistics, i.e. Cohen’s f and P values, and the sample size used, number of groups, and time points, post hoc power was calculated for each of the studied variable.
RESULTS
For sample characteristics see Table 1.
Table 1.
Comparison of the sociodemographic profile and clinical variables as intention to treat analysis (ITT) across two groups (n=20)
| Variables | Active (n=11) Mean±SD/n (%) | Sham (n=9) Mean±SD/n (%) | χ2/t | df | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (in years) | 45.73±14.29 | 40.00±15.26 | 0.87 | 18 | 0.398 | |||||||
| Duration of illness (in years) | 9.82±4.51 | 6.89±3.44 | 1.60 | 18 | 0.127 | |||||||
| Motor Threshold | 49.91±3.70 | 50.44±4.19 | 0.03 | 18 | 0.765 | |||||||
| Gender | Male | 2 (18.2) | 2 (22.2) | 0.05& | 1 | 1.000 | ||||||
| Female | 9 (81.8) | 7 (77.8) | ||||||||||
| Marital Status | Unmarried | 1 (9.1) | 2 (22.2) | 1.42& | 2 | 0.770 | ||||||
| Married | 9 (81.8) | 7 (77.8) | ||||||||||
| Widowed | 1 (9.1) | 0 (0.0) | ||||||||||
| Religion | Hindu | 8 (72.7) | 7 (77.8) | 0.07& | 1 | 1.000 | ||||||
| Muslim | 3 (27.3) | 2 (22.2) | ||||||||||
| Residence | Rural | 6 (54.5) | 6 (66.7) | 0.75& | 2 | 0.811 | ||||||
| Semi – Urban | 4 (36.4) | 2 (22.2) | ||||||||||
| Urban | 1 (9.1) | 1 (11.1) | ||||||||||
| Family type | Nuclear | 6 (54.5) | 4 (44.4) | 0.20& | 1 | 1.000 | ||||||
| Joint | 5 (45.5) | 5 (55.6) | ||||||||||
| Socioeconomic status | Low | 3 (27.3) | 5 (55.6) | 1.65& | 1 | 0.362 | ||||||
| Medium | 8 (72.7) | 4 (44.4) | ||||||||||
| High | 0 (0.0) | 0 (0.0) | ||||||||||
| Education | Not at all | 2 (18.2) | 2 (22.2) | 1.65& | 1 | 0.362 | ||||||
| Primary | 2 (18.2) | 3 (33.3) | ||||||||||
| Secondary | 5 (45.5) | 1 (11.1) | ||||||||||
| Graduation | 2 (18.2) | 3 (33.3) | ||||||||||
| Occupation | Never Employed | 7 (63.6) | 7 (77.8) | 2.65& | 3 | 0.663 | ||||||
| Unemployed | 3 (27.3) | 1 (11.1) | ||||||||||
| Full time | 0 (0.0) | 1 (11.1) | ||||||||||
| Self Employed | 1 (9.1) | 0 (0.0) | ||||||||||
| Past Medical History | Absent | 7 (63.6) | 7 (77.8) | 0.47& | 1 | 0.642 | ||||||
| Present | 4 (36.4) | 2 (22.2) | ||||||||||
| Family Psychiatric History | Absent | 10 (90.9) | 9 (100) | 0.86& | 1 | 1.000 | ||||||
| Present | 1 (9.1) | 0 (0.0) | ||||||||||
| History of Hospitalization (Psychiatric) | Absent | 5 (45.5) | 7 (77.8) | 2.16& | 1 | 0.197 | ||||||
| Present | 6 (54.5) | 2 (22.2) | ||||||||||
| Medical Comorbidity | Absent | 8 (72.7) | 4 (44.4) | 1.65& | 1 | 0.362 | ||||||
| Present | 3 (27.3) | 5 (55.6) | ||||||||||
| Pain Reliver used | No | 6 (54.5) | 6 (66.7) | 0.30& | 1 | 0.670 | ||||||
| Yes | 5 (45.5) | 3 (33.3) | ||||||||||
| Antidepressant used | No | 1 (9.1) | 4 (44.4) | 5.34& | 4 | 0.254 | ||||||
| SSRI | 3 (27.3) | 3 (33.3) | ||||||||||
| SNRI | 3 (27.3) | 0 (0.0) | ||||||||||
| NaSSA | 3 (27.3) | 1 (11.1) | ||||||||||
| More than one antidepressant | 1 (9.1) | 1 (11.1) | ||||||||||
| Benzodiazepine used | Nil | 1 (9.1) | 2 (22.2) | 1.42& | 2 | 0.770 | ||||||
| Lorazepam | 1 (9.1) | 0 (0.0) | ||||||||||
| Clonazepam | 9 (81.8) | 7 (77.8) | ||||||||||
| Mood Stabilizers used | Nil | 5 (45.5) | 6 (66.7) | 1.76& | 2 | 0.554 | ||||||
| Anticonvulsants | 4 (36.4) | 3 (33.3) | ||||||||||
| Lithium | 2 (18.2) | 0 (0.0) | ||||||||||
SSRIs: Selective Serotonin Reuptake Inhibitors; SNRI: Serotonin/Norepinephrine Reuptake inhibitors; TCA: Tricyclic Antidepressants; NaSSA: Noradrenaline and specific serotonergic antidepressant; P<0.05 levels (2 tailed), &Fisher exact value, df: degree of freedom
For baseline differences of both the groups, see supplementary file [Supplementary Table 1]. No patient discontinued the treatment due to major side effects of iTBS. Two patients in the active group discontinued the treatment after the 1st session of iTBS due to scalp discomfort and associated apprehension. Rest, none of the patients reported any adverse effects.
Supplementary Table 1.
Comparison at base line characteristics for continuous variable across two groups as per Intention to treat (n=20)
| Variables | Group A (n=11) Mean±SD | Group B (n=9) Mean±SD | t/U | Df | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (in years) | 45.73±14.29 | 40.00±15.26 | 0.865 | 18 | 0.398 | |||||||
| Duration of illness | 9.82±4.51 | 6.89±3.44 | 1.600 | 18 | 0.127 | |||||||
| CPZ Dose | 12.50±0.00 | 10.84±10.87 | 0.256 | 6 | 0.806 | |||||||
| Motor Threshold | 49.91±3.70 | 50.44±4.19 | -0.304 | 18 | 0.765 | |||||||
| VAS | 74.55±6.88 | 67.78±8.33 | 1.992 | 18 | 0.062 | |||||||
| BPI – SF | Severity | 5.89±1.58 | 5.72±1.72 | 0.229 | 18 | 0.822 | ||||||
| Interference | 6.25±0.99 | 5.72±1.06 | 1.145 | 18 | 0.267 | |||||||
| GPS | 86.32±12.27 | 73.67±19.32 | 1.781 | 18 | 0.092 | |||||||
| MADRAS | 21.36±6.96 | 17.67±8.94 | 1.041 | 18 | 0.312 | |||||||
| HAM-A | 25.64±6.98 | 21.44±3.91 | 31.500# | - | 0.170 | |||||||
| WHOQOL- BREF | Physical | 34.18±7.19 | 41.67±13.71 | -1571 | 18 | 0.134 | ||||||
| Psychological | 40.45±11.26 | 39.67±18.91 | 0.116 | 18 | 0.909 | |||||||
| Social | 56.82±10.25 | 56.89±22.88 | 49.500# | - | 1.000 | |||||||
| Environmental | 62.64±8.50 | 61.33±7.67 | 44.000# | - | 0.665 | |||||||
| PSQI | 14.18±3.49 | 12.89±3.72 | 0.800 | 18 | 0.434 | |||||||
P<0.05 levels (2 tailed), #Mann – Whitney U; CPZ: Chlorpromazine equivalent dose of antipsychotics; VAS (Visual Analogue Scale), BPI - SF (Brief Pain Inventory – Short form), GPS (Global Pain Scale), MADRAS (Montgomery and Asberg Depression Rating Scale), HAM-A (Hamilton Anxiety Rating Scale - Anxiety), WHOQOL-BREF (World Health Organization Quality-of-Life Scale), PSQI (Pittsburgh Sleep Quality Index)
Within-group * time (active and sham) effects of the ITT analysis using the repeated-measures ANOVA have been described [see Supplementary File, Tables 2 and 4].
Supplementary Table 2.
Interaction effect of intervention withing ACTIVE (iTBS) group (Intention to treat) analysis across pre-treatment phase, end of 10th Session, and 2 weeks post-TBS treatment for (n=11)
| Variables | A Mean±SD |
B Mean±SD |
C Mean±SD |
F | P | Partial eta2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS | 74.55±6.88 | 49.09±22.12 | 40.00±20.49 | 23.951$ | 0.001 | 0.705 | ||||||||
| BPI – SF | Severity | 5.89±1.58 | 3.42±1.86 | 2.92±1.97 | 27.407$ | 0.001 | 0.733 | |||||||
| Interference | 6.25±0.994 | 3.909±1.21 | 3.496±1.06 | 48.289$ | 0.001 | 0.828 | ||||||||
| GPS | 86.32±12.27 | 75.00±15.58 | 70.68±16.33 | 11.651 | 0.001 | 0.538 | ||||||||
| MADRS | 21.36±6.96 | 11.91±3.81 | 8.00±5.37 | 24.339 | 0.001 | 0.709 | ||||||||
| HAM-A | 25.64±6.98 | 14.55±5.97 | 11.63±6.58 | 33.734 | 0.001 | 0.771 | ||||||||
| WHOQOL-BREF | Physical | 34.18±7.19 | 44.27±10.96 | 55.64±9.63 | 24.945$ | 0.001 | 0.714 | |||||||
| Psychological | 40.45±11.26 | 47.27±13.84 | 62.72±15.27 | 14.952 | 0.001 | 0.599 | ||||||||
| Social | 56.82±10.25 | 58.00±10.56 | 64.18±11.91 | 1.853$ | 0.183 | 0.156 | ||||||||
| Environmental | 62.63±8.50 | 67.82±6.65 | 75.18±8.35 | 9.130$ | 0.002 | 0.477 | ||||||||
| PSQI | 14.18±3.49 | 13.09±3.11 | 12.27±3.101 | 2.891$ | 0.079 | 0.224 | ||||||||
$Sphericity assumed values; VAS (Visual Analogue Scale), BPI - SF (Brief Pain Inventory – Short form), GPS (Global Pain Scale), MADRAS (Montgomery and Asberg Depression Rating Scale), HAM-A (Hamilton Anxiety Rating Scale - Anxiety), WHOQOL-BREF (World Health Organization Quality-of-Life Scale), PSQI (Pittsburgh Sleep Quality Index)
Supplementary Table 4.
Comparison of the socio-demographic profile and clinical variables as per protocol across two groups (n=15)
| Variables | Active (n=9) Mean±SD/n (%) | Sham (n=6) Mean±SD/n (%) | χ 2 /t/U | df | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (in years) | 45.73±14.290 | 40.00±15.256 | 1.480 | 13 | 0.163 | |||||||
| Duration of illness (in years) | 9.82±4.512 | 6.89±3.444 | 1.107 | 13 | 0.288 | |||||||
| Motor Threshold | 49.90±3.700 | 50.44±4.186 | 33.000# | - | 0.529 | |||||||
| Gender | Male | 2 (22.2) | 2 (33.3) | 0.227& | 1 | 1.000 | ||||||
| Female | 7 (77.8) | 4 (66.7) | ||||||||||
| Marital Status | Unmarried | 0 (0.0) | 2 (33.3) | 3.336& | 2 | 0.143 | ||||||
| Married | 8 (88.9) | 4 (66.7) | ||||||||||
| Separated | 0 (0.0) | 0 (0.0) | ||||||||||
| Divorced | 0 (0.0) | 0 (0.0) | ||||||||||
| Widowed | 1 (11.1) | 0 (0.0) | ||||||||||
| Religion | Hindu | 7 (77.8) | 4 (66.7) | 0.227& | 1 | 1.000 | ||||||
| Muslim | 2 (22.2) | 2 (33.3) | ||||||||||
| Sikh | 0 (0.0) | 0 (0.0) | ||||||||||
| Christian | 0 (0.0) | 0 (0.0) | ||||||||||
| Other | 0 (0.0) | 0 (0.0) | ||||||||||
| Residence | Rural | 6 (66.7) | 6 (100) | 2.500& | 1 | 0.229 | ||||||
| Semi - Urban | 3 (33.3) | 0 (0.0) | ||||||||||
| Urban | 0 (0.0) | 0 (0.0) | ||||||||||
| Family type | Nuclear | 5 (55.6) | 3 (50) | 0.45& | 1 | 1.000 | ||||||
| Joint | 4 (44.4) | 3 (50) | ||||||||||
| Socioeconomic status | Low | 2 (22.2) | 5 (83.3) | 5.402& | 1 | 0.041 | ||||||
| Medium | 7 (77.8) | 1 (16.7) | ||||||||||
| High | 0 (0.0) | 0 (0.0) | ||||||||||
| Education | Not at all | 2 (22.2) | 2 (33.3) | 1.665& | 3 | 0.808 | ||||||
| Primary | 1 (11.1) | 1 (16.7) | ||||||||||
| Secondary | 4 (44.4) | 1 (16.7) | ||||||||||
| Graduation | 2 (22.2) | 2 (33.3) | ||||||||||
| Post-Graduation | 0 (0.0) | 0 (0.0) | ||||||||||
| Occupation | Never Employed | 6 (66.7) | 4 (66.7) | 2.220& | 3 | 0.849 | ||||||
| Unemployed | 2 (22.2) | 1 (16.7) | ||||||||||
| Full time | 0 (0.0) | 1 (16.7) | ||||||||||
| Part time | 0 (0.0) | 0 (0.0) | ||||||||||
| Self Employed | 1 (11.1) | 0 (0.0) | ||||||||||
| Past Medical History | Not Significant | 7 (77.8) | 5 (83.3) | 0.069& | 1 | 1.000 | ||||||
| Significant | 2 (22.2) | 1 (16.7) | ||||||||||
| Family Psychiatric History | Not Significant | 8 (88.9) | 6 (100) | 0.714& | 1 | 1.000 | ||||||
| Significant | 1 (11.1) | 0 (0.0) | ||||||||||
| History of Hospitalization (Psychiatric) | Not Significant | 4 (44.4) | 5 (83.3) | 2.269& | 1 | 0.287 | ||||||
| Significant | 5 (55.6) | 1 (16.7) | ||||||||||
| Comorbidity | Not Significant | 6 (66.7) | 2 (33.3) | 1.607& | 1 | 0.315 | ||||||
| Significant | 3 (33.3) | 4 (66.7) | ||||||||||
| Pain Reliver used | No | 5 (55.6) | 4 (66.7) | 0.185& | 1 | 1.000 | ||||||
| Yes | 4 (44.4) | 2 (33.3) | ||||||||||
| Antidepressant used | NO | 1 (11.1) | 3 (50) | 3.715& | 4 | 0.595 | ||||||
| SSRIs | 3 (33.3) | 1 (16.7) | ||||||||||
| SNRIs | 2 (22.2) | 0 (0.0) | ||||||||||
| TCAs | 0 (0.0) | 0 (0.0) | ||||||||||
| MAQIs | 0 (0.0) | 0 (0.0) | ||||||||||
| NaSSAs | 2 (22.2) | 1 (16.7) | ||||||||||
| Combination | 1 (11.1) | 1 (16.7) | ||||||||||
| Benzodiazepine used | No | 0 (0.0) | 2 (33.3) | 3.336& | 2 | 0.143 | ||||||
| Lorazepam | 0 (0.0) | 0 (0.0) | ||||||||||
| Clonazepam | 8 (88.9) | 4 (66.7) | ||||||||||
| Combination | 1 (11.1) | 0 (0.0) | ||||||||||
| Alprazolam | 0 (0.0) | 0 (0.0) | ||||||||||
| Diazepam | 0 (0.0) | 0 (0.0) | ||||||||||
| Mood Stabilizers used | No | 3 (33.3) | 3 (50) | 1.390& | 2 | 0.622 | ||||||
| Anticonvulsants | 4 (44.4) | 3 (50) | ||||||||||
| Lithium | 2 (22.2) | 0 (0.0) | ||||||||||
| CPZ Dose | 12.50±0.000 | 10.84±10.869 | -1.516 | 4 | 0.204 | |||||||
P<0.05 levels (2 tailed), #Mann - Whitney U, &Fisher exact value, df: degree of freedom, CPZ: Chlorpromazine equivalent dose of antipsychotics
The intention-to-treat analysis using the mixed-model ANOVA showed no significant group * time effect of any variable except GPS. However, the significant score of GPS could not be retained when covariates like age, duration of illness, and HAM-A scores values as were considered as covariates [GPS (F = 1.726; P = 0.196)]. [see Table 2] [see Table 1]. Post hoc power estimates for clinical measures across the groups and time points ranged between 0.613 and 0.971, except for one variable (BPI-SF-interference) [see Table 1]. Three variables- WHOQOL-BREF- Social and Environmental, and PSQI showed the power of >0.8.
Table 2.
Group (active and sham) *time (pretreatment phase, end of 10th session, and 2 weeks post-TBS) interaction using mixed model ANOVA intention to treat analysis (n=20)
| Variables | Active (n=11) | Sham (n=9) | F | P | Partial eta2 | Cohen’s f (Power) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS | A Mean±SD |
74.55±6.876 | 67.78±8.333 | 1.453 | 0.248 | 0.075 | 0.284 (0.648) | |||||||||||
| B Mean±SD |
49.09±22.115 | 51.11±12.693 | ||||||||||||||||
| C Mean±SD |
40.00±20.493 | 45.56±15.092 | ||||||||||||||||
| BPI – SF | Severity | A Mean±SD |
5.89±1.580 | 5.72±1.716 | 0.923 | 0.407 | 0.049 | 0.226 (0.674) | ||||||||||
| B Mean±SD |
3.42±1.856 | 3.69±1.782 | ||||||||||||||||
| C Mean±SD |
2.92±1.969 | 3.69±1.887 | ||||||||||||||||
| Interference | A Mean±SD |
6.25±0.994 | 5.72±1.064 | 2.732$ | 0.100 | 0.132 | 0.389 (0.181) |
|||||||||||
| B Mean±SD |
3.91±1.210 | 4.42±1.281 | ||||||||||||||||
| C Mean±SD |
3.50±1.058 | 4.14±1.516 | ||||||||||||||||
| GPS | A Mean±SD |
86.32±12.275 | 73.67±19.330 | 3.407$ 1.726* |
0.044 0.196* |
0.159 0.110* |
0.434 (0.59) 0.351 (0.721) |
|||||||||||
| B Mean±SD |
75.00±15.576 | 74.61±13.70 | ||||||||||||||||
| C Mean±SD |
70.68±16.330 | 67.50±17.561 | ||||||||||||||||
| MADRS | A Mean±SD |
21.36±6.961 | 17.67±8.944 | 1.427 | 0.251 | 0.073 | 0.280 (0.644) | |||||||||||
| B Mean±SD |
11.91±3.807 | 11.11±5.645 | ||||||||||||||||
| C Mean±SD |
8.00±5.367 | 9.67±6.423 | ||||||||||||||||
| HAM-A | A Mean±SD |
25.64±6.975 | 21.44±3.909 | 3.414 | 0.074 | 0.159 | 0.438 (0.693) | |||||||||||
| B Mean±SD |
14.55±5.973 | 15.11±6.451 | ||||||||||||||||
| C Mean±SD |
11.64±6.577 | 14.00±6.671 | ||||||||||||||||
| WHOQOL- BREF | Physical | A Mean±SD |
34.18±7.195 | 41.67±13.711 | 2.637$ | 0.085 | 0.128 | 0.383 (0.613) | ||||||||||
| B Mean±SD |
44.27±10.964 | 45.22±14.087 | ||||||||||||||||
| C Mean±SD |
55.64±9.626 | 52.22±15.619 | ||||||||||||||||
| Psychological | A Mean±SD |
40.45±11.264 | 39.67±18.908 | 0.395 | 0.608 | 0.021 | 0.146 (0.707) | |||||||||||
| B Mean±SD |
47.27±13.835 | 47.22±15.180 | ||||||||||||||||
| C Mean±SD |
62.73±15.271 | 57.67±17.029 | ||||||||||||||||
| Social | A Mean±SD |
56.82±10.255 | 56.89±26.883 | 0.011 | 0.971 | 0.001 | 0.031 (0.971) | |||||||||||
| B Mean±SD |
58.00±10.545 | 59.00±15.716 | ||||||||||||||||
| C Mean±SD |
64.18±11.906 | 64.56±15.084 | ||||||||||||||||
| Environmental | A Mean±SD |
62.64±8.500 | 61.33±7.664 | 0.275$ | 0.761 | 0.015 | 0.123 (0.808) | |||||||||||
| B Mean±SD |
67.82±6.646 | 65.56±5.503 | ||||||||||||||||
| C Mean±SD |
75.18±8.352 | 70.89±8.162 | ||||||||||||||||
| PSQI | A Mean±SD |
14.18±3.488 | 12.89±3.723 | 0.167$ | 0.847 | 0.009 | 0.095 (0.866) | |||||||||||
| B Mean±SD |
13.09±3.113 | 12.22±3.667 | ||||||||||||||||
| C Mean±SD |
12.27±3.101 | 11.56±3.877 | ||||||||||||||||
A: pretreatment, B: post 10th session, C: 2 week post – iTBS treatment; VAS (Visual Analogue Scale), BPI - SF (Brief Pain Inventory – Short form), GPS (Global Pain Scale), MADRS (Montgomery and Asberg Depression Rating Scale), HAM-A (Hamilton Anxiety Rating Scale - Anxiety), WHOQOL-BREF (World Health Organization Quality-of-Life Scale), PSQI (Pittsburgh Sleep Quality Index),; $Sphericity assumed values; *GPS values computed in respect to age, duration of illness, HAM-A scores (pre – post scores (C -A)) and BDI – II scores (pre – post scores (C -A)) values as covariates
An additional per-protocol analysis with similar findings was done [Supplementary Tables 4 and 5].
Supplementary Table 5.
Interaction effect of intervention between active and sham groups (per protocol analysis) across pre-treatment phase, end of 10th Session, and 2 weeks post-TBS treatment for (n=15)
| Variables | A Mean±SD |
B Mean±SD |
C Mean±SD |
F | P | Partial eta2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS | Active (n=11) | 74.55±6.876 | 49.09±22.115 | 40.00±20.493 | 0.893$ | 0.421 | 0.064 | |||||||||
| Sham (n=9) | 67.78±8.333 | 51.11±12.693 | 45.56±15.092 | |||||||||||||
| BPI – SF | Severity | Active (n=11) | 5.89±1.580 | 3.42±1.856 | 2.92±1.969 | 0.356$ | 0.704 | 0.027 | ||||||||
| Severity | Sham (n=9) | 5.72±1.716 | 3.69±1.782 | 3.69±1.887 | ||||||||||||
| Interference | Active (n=11) | 6.25±0.994 | 3.91±1.210 | 3.50±1.058 | 1.228$ | 0.309 | 0.086 | |||||||||
| Interference | Sham (n=9) | 5.72±1.064 | 4.42±1.281 | 4.14±1.516 | ||||||||||||
| GPS | Active (n=11) | 86.32±12.275 | 75.00±15.576 | 70.68±16.330 | 2.876$ | 0.074 | 0.181 | |||||||||
| Sham (n=9) | 73.67±19.330 | 74.61±13.700 | 67.50±17.561 | |||||||||||||
| MADRAS | Active (n=11) | 21.36±6.961 | 11.91±3.807 | 8.00±5.367 | 0.647 | 0.532 | 0.047 | |||||||||
| Sham (n=9) | 17.67±8.944 | 11.11±5.645 | 9.67±6.423 | |||||||||||||
| HAM-A | Active (n=11) | 25.64±6.975 | 14.55±5.973 | 11.64±6.577 | 1.874 | 0.191 | 0.126 | |||||||||
| Sham (n=9) | 21.44±3.909 | 15.11±6.451 | 14.00±6.671 | |||||||||||||
| WHOQOL-BREF | Physical | Active (n=11) | 34.18±7.195 | 44.27±10.964 | 55.64±9.626 | 1.163$ | 0.328 | 0.082 | ||||||||
| Sham (n=9) | 41.67±13.711 | 45.22±14.087 | 52.22±15.619 | |||||||||||||
| Psychological | Active (n=11) | 40.45±11.264 | 47.27±13.835 | 62.73±15.271 | 0.157$ | 0.856 | 0.012 | |||||||||
| Sham (n=9) | 39.67±18.908 | 47.22±15.180 | 57.67±17.029 | |||||||||||||
| Social | Active (n=11) | 56.82±10.255 | 58.00±10.545 | 64.18±11.906 | 0.011$ | 0.989 | 0.001 | |||||||||
| Sham (n=9) | 56.89±26.883 | 59.00±15.716 | 64.56±15.084 | |||||||||||||
| Environmental | Active (n=11) | 62.64±8.500 | 67.82±6.646 | 75.18±8.352 | 0.028$ | 0.962 | 0.002 | |||||||||
| Sham (n=9) | 61.33±7.664 | 65.56±5.503 | 70.89±8.162 | |||||||||||||
| PSQI | Active (n=11) | 14.18±3.488 | 13.09±3.113 | 12.27±3.101 | 0.004$ | 0.996 | 0.000 | |||||||||
| Sham (n=9) | 12.89±3.723 | 12.22±3.667 | 11.56±3.877 | |||||||||||||
A: pre -treatment, B: post 10th session, C: 2 week post – iTBS treatment; VAS (Visual Analogue Scale), BPI - SF (Brief Pain Inventory – Short form), GPS (Global Pain Scale), MADRAS (Montgomery and Asberg Depression Rating Scale), HAM-A (Hamilton Anxiety Rating Scale - Anxiety), WHOQOL-BREF (World Health Organization Quality-of-Life Scale), PSQI (Pittsburgh Sleep Quality Index), $Sphericity assumed values
DISCUSSION
Our study is among the first to sequentially stimulate the left primary motor cortex and DLPFC to modulate pain in PSPD. We found that iTBS showed statistically significant improvement in global pain. However, benefits could not sustain after considering the confounding factors (age, duration of illness, anxiety, and depression) implying that the improvement in pain scores was secondary to advancing age, duration of illness, and psychopathology. Other RCT’s have addressed neuromodulation in somatic pains but as a secondary outcome, primary being refractory depression.[8,9]
iTBS was well tolerated, and none of the concerning side effects as per safety guidelines by Rossi and colleagues[13] were reported by the patients. Interestingly, two patient in the index study dropped out of the trial due to trivial scalp discomfort and allied apprehension. This could be due to central sensitization seen in chronic pain conditions and enhanced noci-plasticity.[22]
Post hoc power estimates for most of the variables, especially for the primary variable, were less than desirable (i.e., >0.8). This is a significant limitation of the study. Other limitations of the study are small sample size, short duration of follow-up (2 weeks), relatively high dropout rate, and protocol having a modest intensity (80% RMT; 1200 pulses per day). Moreso, some of the quantitative clinical measures like BPI-SF, GPS, and PSQI were applied with the help of translator, but without any formal translation procedure is also among the important limitation. For future studies, biophysical models and neuronavigation may be used to counter optimal angles of stimulation and personalized delivery of the stimulus.[13]
Existing rTMS studies support the active role of LDLPFC modulation to ameliorate pain. Improvements in depression can be explained by the activation of DLPFC (approved site for clinical depression).[4]
Our pilot trial concludes that although safe and well tolerated, the therapeutic efficacy of intermittent TBS in persistent somatoform pain disorder is inconclusive. Choice of lower total number of sessions along with small sample size may limit the study findings.
Ethical approval and/or institutional review board (IRB)
Ethical approval was taken. The protocol was registered with Clinical Trials Registry, India (Reference number CTRI/2022/12/047819) and approved by the ethical committee of the institute (reference number SGRR/IEC/03/22; IEC Registration No. ECR/710/Inst/UK/2015/RR-21).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY FILE
Methodology
Clinical measures
The pain intensity was measured using Visual Analogue Scale (VAS), Brief Pain Inventory-Short form (BPI-SF) and Global Pain Scale (GPS). VAS uses a 10 cm line (no pain to worst pain) representing a subjective measure for acute and chronic pain. BPI-SF is a self – report inventory to assess the severity of pain and its impact on daily functioning. GPS is a brief yet thorough tool (Numeric rating of 0 – no pain, 10 – severe pain) to monitor change in acute or chronic pain over time. GPS also includes items that include related emotional changes and pain’s impact on day to day functioning. To measure symptoms of depression and its severity, and Montgomery and Asberg Depression Rating Scale (MADRS) were used. Whereas, MADRS has 10 items based on clinical interviews moving from broadly phased questions about symptoms to more detailed ones. Hamilton Anxiety Rating Scale - Anxiety (HAM-A) is a 21-items self-report inventory with each item scored on a scale of 0 (not present) to 4 (severe) used to assess the concomitant of anxiety in patients. All the participants were also assessed on quality of life using World Health Organization Quality-of-Life Scale-brief (WHOQOL – BREF)-Hindi version and sleep quality was assessed using the global score of Pittsburgh Sleep Quality Index (PSQI).
Allocation and blinding
The enrolled participants were sequentially and randomly allocated to treatment and placebo groups using blocks (fixed block size of 4) of random number sequences. These numbers were sealed in the opaque envelopes. For each participant, the envelopes were open by the clinician imparting iTBS just before administration of the first session. The participants and the accompanying caregivers were blind to the arm of the treatment. To ensure this, each participant was asked to guess the type of treatment.
All the subjects were rated at baseline, post 10 iTBS session and after 2 weeks post iTBS treatment by an independent rater. The rater was blinded to the overall randomization process. The good integrity of blind was reflected by the guess matrix (Cohen’s Kappa coefficient = -0.05, ‘no agreement’).
Sample characteristics
Both control and treatment groups were commensurate to each other in common sociodemographic characteristics such as gender, marital status, religion, residence, family type, socioeconomic status, education and employment status. Both group subjects were proportionate in terms of past medical history, family psychiatric history, history of hospitalization, medical comorbidity, pain reliver used, antidepressant used, benzodiazepine used, and mood stabilizers used. The subjects allotted to active and sham group did not reflect any significant difference in terms of age (t (20) = 0.865, P = 0.398), duration of illness (t (20) = 1.600, P = 0.127), motor threshold (t (20) = 0.034, P = 0.765) (see supplementary table 1).
The intention to treat analysis using the repeated measures ANOVA showed significant effect of time (from pre-treatment to post 10th session of iTBS and 2 weeks post iTBS treatment) in active group over clinical variables like VAS (F = 23.951; p = 0.001), BPI-Severity (F=27.407; P=.001), BPI-Interference (F= 48.289; P=.001), GPS (F=11.651; P=.001), MADRS (F=24.33; P=.001), HAM-A (F=33.73; P=.001) and WHOQOL- BREF (psychological: F=27.407; P=.001; environmental: F = 9.130; p = 0.002) (supplementary table 2).
The intention to treat analysis using the repeated measures ANOVA showed significant effect of time (from pre-treatment to post 10th session of iTBS and 2 weeks post iTBS treatment) in sham group clinical variables like VAS (F = 9.665; p = 0.002), BPI-Severity (F=9.115; P=.011), BPI-Interference (F= 6.157; P=.029), MADRS (F=5.528; P=.041), HAM-A (F=9.556; P=.013) and WHOQOL- BREF (physical: F=4.082; P=.037; psychological: F=8.483; P=.003; environmental: F = 5.975; p = 0.012) (supplementary table 3).
Supplementary Table 3.
Interaction effect of intervention withing SHAM (iTBS control-group) (Intention to treat analysis) across pre-treatment phase, end of 10th Session, and 2 weeks post-TBS treatment for (n=9)
| Variables | A Mean±SD |
B Mean±SD |
C Mean±SD |
F | P | Partial eta2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAS | 67.78±8.33 | 51.11±12.69 | 45.56±15.09 | 9.665$ | 0.002 | 0.547 | ||||||||
| BPI – SF | Severity | 5.72±1.72 | 3.69±1.78 | 3.68±1.89 | 9.115 | 0.011 | 0.533 | |||||||
| Interference | 5.72±1.06 | 4.41±1.28 | 4.14±1.52 | 6.157 | 0.029 | 0.435 | ||||||||
| GPS | 73.67±19.33 | 74.61±13.699 | 67.50±17.560 | 2.312$ | 0.131 | 0.224 | ||||||||
| MADRAS | 17.67±8.944 | 11.11±5.645 | 9.67±6.423 | 5.528 | 0.041 | 0.409 | ||||||||
| HAM-A | 21.44±3.908 | 15.11±6.451 | 14.00±6.671 | 9.556 | 0.013 | 0.544 | ||||||||
| WHOQOL-BREF | Physical | 41.67±13.71 | 45.22±14.087 | 52.22±15.619 | 4.082$ | 0.037 | 0.338 | |||||||
| Psychological | 39.67±18.91 | 47.22±15.180 | 57.67±17.029 | 8.483$ | 0.003 | 0.515 | ||||||||
| Social | 56.88±22.883 | 59.00±15.716 | 64.56±15.084 | 1.376$ | 0.281 | 0.147 | ||||||||
| Environmental | 61.33±7.664 | 65.00±5.502 | 70.89±8.162 | 5.975$ | 0.012 | 0.428 | ||||||||
| PSQI | 12.89±3.723 | 12.22±3.67 | 11.56±3.877 | 2.526 | 0.143 | 0.240 | ||||||||
$Sphericity assumed values; VAS (Visual Analogue Scale), BPI - SF (Brief Pain Inventory – Short form), GPS (Global Pain Scale), MADRAS (Montgomery and Asberg Depression Rating Scale), HAM-A (Hamilton Anxiety Rating Scale - Anxiety), WHOQOL-BREF (World Health Organization Quality-of-Life Scale), PSQI (Pittsburgh Sleep Quality Index)
Schema of study design showing timeline, group division, number of iTBS sessions and stimulation sites (C3 and F3) as per international 10-20 system EEG
REFERENCES
- 1.Grover S, Aneja J, Sharma A, Malhotra R, Varma S, Basu D, et al. Explanatory models of somatoform disorder patients attending a psychiatry outpatient clinic: A study from North India. Int J Soc Psychiatry. 2014;60:492–8. doi: 10.1177/0020764013501484. [DOI] [PubMed] [Google Scholar]
- 2.Tikka SK, Siddiqui MA, Garg S, Pattojoshi A, Gautam M. Clinical practice guidelines for the therapeutic use of repetitive transcranial magnetic stimulation in neuropsychiatric disorders. Indian J Psychiatry. 2023;65:270–88. doi: 10.4103/indianjpsychiatry.indianjpsychiatry_492_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh SM, Prakash V, Choudhary S, Avasthi A. The effectiveness of high-frequency repetitive transcranial magnetic stimulation in persistent somatoform pain disorder: A case series. Cureus. 2018;10:e2729. doi: 10.7759/cureus.2729. doi: 10.7759/cureus.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar N, Singal P, Chakladar A. Novel intervention of high-frequency repetitive transcranial magnetic stimulation in patients with somatic symptom disorder and its safety and outcome. Indian J Psychiatry. 2023;65:887–91. doi: 10.4103/indianjpsychiatry.indianjpsychiatry_65_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery DH, Holtzheimer PE, 3rd, Fawaz W, Russo J, Neumaier J, Dunner DL, et al. Transcranial magnetic stimulation reduces pain in patients with major depression: A sham-controlled study. J Nerv Ment Dis. 2007;195:378–81. doi: 10.1097/NMD.0b013e31802f58d1. [DOI] [PubMed] [Google Scholar]
- 6.Phillips AL, Burr RL, Dunner DL. rTMS effects in patients with co-morbid somatic pain and depressive mood disorders. J Affect Disord. 2018;241:411–6. doi: 10.1016/j.jad.2018.08.065. [DOI] [PubMed] [Google Scholar]
- 7.Corlier J, Tadayonnejad R, Wilson AC, Lee JC, Marder KG, Ginder ND, et al. Repetitive transcranial magnetic stimulation treatment of major depressive disorder and comorbid chronic pain: Response rates and neurophysiologic biomarkers. Psychol Med. 2023;53:823–32. doi: 10.1017/S0033291721002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Ruhaili I, Al-Huseini S, Al-Kaabi S, Mahadevan S, Al-Sibani N, Al Balushi N, et al. An evaluation of the effectiveness of repetitive transcranial magnetic stimulation (rTMS) for the management of treatment-resistant depression with somatic attributes: A hospital-based study in Oman. Brain Sci. 2023;13:1289. doi: 10.3390/brainsci13091289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Lei F, Zou K, Zheng Z. Rapid improvements and subsequent effects in major depressive disorder patients with somatic pain using rTMS combined with sertraline. Sci Rep. 2023;13:17973. doi: 10.1038/s41598-023-44887-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993;78:393–401. doi: 10.3171/jns.1993.78.3.0393. [DOI] [PubMed] [Google Scholar]
- 11.Cardenas-Rojas A, Pacheco-Barrios K, Giannoni-Luza S, Rivera-Torrejon O, Fregni F. Noninvasive brain stimulation combined with exercise in chronic pain: A systematic review and meta-analysis. Expert Rev Neurother. 2020;20:401–12. doi: 10.1080/14737175.2020.1738927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Martin JH. Effects of motor cortex neuromodulation on the specificity of corticospinal tract spinal axon outgrowth and targeting in rats. Brain Stimul. 2023;16:759–71. doi: 10.1016/j.brs.2023.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauhan P, Garg S, Tikka SK, Khattri S. Efficacy of intensive cerebellar intermittent theta burst stimulation (iCiTBS) in treatment-resistant schizophrenia: A randomized placebo-controlled study. Cerebellum. 2021;20:116–23. doi: 10.1007/s12311-020-01193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toh EYJ, Ng JSP, McIntyre RS, Tran BX, Ho RC, Ho CSH, et al. Repetitive transcranial magnetic stimulation for fibromyalgia: An updated systematic review and meta-analysis. Psychosom Med. 2022;84:400–9. doi: 10.1097/PSY.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 16.Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, et al. Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev. 2018;2:e088. doi: 10.5435/JAAOSGlobal-D-17-00088. doi: 10.5435/JAAOSGlobal-D-17-00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–38. [PubMed] [Google Scholar]
- 18.Gentile DA, Woodhouse J, Lynch P, Maier J, McJunkin T. Reliability and validity of the Global Pain Scale with chronic pain sufferers. Pain Physician. 2011;14:61–70. [PubMed] [Google Scholar]
- 19.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 20.“WHOQOL–BREF,”. 2016 Available from: https://www.who.int/tools/whoqol/whoqol-bref/docs/default-source/publishing-policies/whoqol-bref/hindi-whoqol-bref . [Last accessed on 2021 May 02] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Nijs J, Malfliet A, Nishigami T. Nociplastic pain and central sensitization in patients with chronic pain conditions: A terminology update for clinicians. Braz J Phys Ther. 2023;27:100518. doi: 10.1016/j.bjpt.2023.100518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schema of study design showing timeline, group division, number of iTBS sessions and stimulation sites (C3 and F3) as per international 10-20 system EEG


