Abstract
Phosphorus (P) additives may be deleterious for health. We measured the P content of key foods, and associations of P intake with biomarkers in the Boston Puerto Rican Health Study (BPRHS). Direct chemical analysis of 92 foods was done with the molybdenum blue spectrophotometric method and inductively coupled plasma mass spectrometry (ICP-MS). A novel algorithm was used to determine bioavailable, natural, and added P. We estimated P intakes from foods in 1323 participants, aged 45–75 y, and associations of these with serum P, fibroblast growth factor 23 (FGF23), parathyroid hormone (PTH), and Klotho. Relationships between intakes and status markers were assessed with Pearson’s correlations and t-tests. Our food analyses generally support P values in the USDA nutrient database, with the exceptions of American and cheddar cheese, which had more P than in the database. Women had higher added P intake than men, and younger participants had higher added P than those older. Total P intake tended to be positively associated with serum P and klotho, and inversely associated with PTH, but relationships were not strong. Puerto Rican adults have high intake of additive P. Culturally sensitive interventions that highlight dietary quality are needed.
Keywords: Phosphorus intake, Food additives, Biomarkers, Food samples, Chemical analysis, Hispanic, Aging, Health Disparities, Bioavailability, Epidemiology, Nutrition, Diet
1. Introduction
Compounds with added phosphorus (P) are used in diverse applications, making them among the most used additives in the world (Cooke, 2017; Gutiérrez, 2013). Added P is found in foods in the form of phosphoric acid, calcium phosphate, monocalcium phosphate, sodium phosphate, sodium acid pyrophosphate, sodium aluminum phosphate, sodium tripolyphosphate, potassium triphosphate, and others (Calvo et al., 2014; Sullivan et al., 2007). Added P serves several functions in food manufacturing and processing, such as leavening, anti-caking, acidulant, emulsification, and stabilization (Calvo and Uribarri, 2013; Molins, 1990; Moore et al., 2015). On average, individuals are estimated to consume 8–10 pounds of food additives per year (Assembly Standing Committee on Mental Health Mental Retardation and Developmental Disabilities, 2007), as processed foods are commonly consumed. In the U.S, about 23 % of foods contain added P (Moore et al., 2015), and P intake among adults is estimated at 180 % of the recommended value, without fully accounting for added P (Suki and Moore, 2016). A recent study using national data (1988–1994–2001–2016) showed that mean intake of total and natural P increased, but added P intake decreased (from 14.6 % to 11.6 %) over the past few decades, which may be due to, in part to misclassification of foods (e.g., lack of separation of frozen and fresh meat) and measurement error in nutrient databases and calculations of added P (Fulgoni and Fulgoni, 2021).
Importantly, added P has greater bioavailability than natural P, contributing to greater bodily exposure (Gutiérrez et al., 2015). Early findings (from the pre-1970s) suggested that added P appeared to be safe in amounts up to 30–70 mg/kg/d (Onufrak et al., 2008; Yoon et al., 2017), and these additives were classified as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA) (Carrigan et al., 2014). Phosphorus content in the food supply is increasing, with the constant need to improve food stability, taste, and preparation (Calvo et al., 2014). Despite growing concerns, the P content of foods is not required to be reported on food labels (Uribarri and Calvo, 2003), and there are currently no regulations on the amount that foods can contain in the U.S. Prior studies showed underestimation of total dietary P in nutrient databases, compared with direct chemical analysis, (Benini et al., 2011; Carrigan et al., 2014; Sherman and Mehta, 2009; Sullivan et al., 2007). This measurement error may obscure important relationships between dietary P intake and health outcomes.
There is currently no distinction between P intake from additives and natural P in food composition databases. One recent study quantified total, added, and natural P from food, but was not comprehensive (Fulgoni and Fulgoni, 2021). The assumption used in that study exempted added P in frozen meat products and mixed dishes, which are likely important sources of added P. Our group previously developed new P metrics that capture natural and added P from different food sources, and their bioavailability, in an African American cohort (Duong et al., 2022). Serum P, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), and Klotho protein are known P biomarkers. It is important to examine how our newly developed P metrics relate to these biomarkers among aging adults, considering their important role in P biochemistry (Kuro-o, 2019). Added phosphate salts are more rapidly and efficiently absorbed (> 90 %) than natural forms (20–60 %), and plant sources have lower bioavailability than animal sources (Calvo et al., 2014; Cupisti and Kalantar-Zadeh, 2013; Nouri et al., 2010; Takeda et al., 2017; Williams et al., 2014; Winger et al., 2012).
This study aimed to measure the P content of several key foods frequently consumed by Puerto Ricans that may be a potential source of added P, and to update the P measures used in our previously developed algorithm. This is important, as foods consumed differ between populations based on cultural preference and other demographic factors. Second, we aimed to describe P intake, its bioavailability, and P biochemistry status, using a panel of functional P biomarkers – serum P, FGF23, PTH, and Klotho protein – in the Boston Puerto Rican Health Study (BPRHS).
2. Materials and methods
2.1. Selection of food samples
Food sampling was carried out following the United States Department of Agriculture (USDA) national food sampling plan (Pehrsson et al., 2000), but focused on local foods. To ensure equality in the food sample distribution, counties of participants represented in the BPRHS were ranked in descending order, based on population density, and stratified into high, medium, or low population size. Grocery store outlets were selected based on availability of foods consumed by the Puerto Rican population. Six to eight samples of each food product (name brands and generic brands) representing canned legumes, meat, bread, cereal, cheese, and sweets were purchased between October 2021 to May 2022.
2.2. Reagents and supplies
All reagents used were analytical grade. Double deionized water (Milli-Q system water; Millipore, Bedford, MA, USA), nitric acid (70 % HNO3, Fisher Scientific, Fair Lawn, NJ, USA), hydrogen peroxide (50 % H2O2, Fisher Scientific), phosphorus standard solution for colorimetric (1000 mg/L, Hach, Loveland, CO, USA), P standard for Inductively coupled plasma mass spectrometry (ICP-MS) (1 mg/L, Sigma-Aldrich, St. Louis, MO, USA), hydroquinone (Sigma-Aldrich), ammonium molybdate tetrahydrate (Sigma-Aldrich), and concentrated sulfuric acid (Sigma-Aldrich), anhydrous sodium sulfite (EMD Millipore, Billerica, MA) were used. A certified reference material SRM 1546a, meat homogenate from the National Institute of Standards and Technology (NIST) was used for independent validation of analytical results.
2.3. Chemical analysis
2.3.1. Sample homogenization
A half or quarter of a serving size (based on the food label) of each food product was weighed, minced, and placed in polypropylene containers. An appropriate amount of water was added to heterogenous food products and homogenized with a tissue homogenizer (model PRO250; PRO Scientific Inc., Oxford, CT, USA). A 1 g aliquot from the homogenized sample was taken for P analysis and was stored at −20°C until further processing. The remainder of the homogenized sample was stored at −20°C for future use.
2.3.2. Digestion protocol
For sample digestion, one gram of each analytical sample was weighed directly into a 110 mL polytetrafluoroethylene (PTFE) Mars6 Xpress Plus reaction vessel. Eight milliliters of HNO3 and 2 mL of H2O2 were added into each tube, then closed. This mixture was subjected to a P digestion program at 180 °C with 15 mins ramp to temperature, at maximum power of 1000 W over 30 mins. Upon digestion program execution and cooling at room temperature, digested samples were adjusted to 100 mL using deionized water. A 15 mL aliquot of the dilute solution was then stored in clean 15 mL VWR polypropylene centrifuge tubes and stored at 4 °C until further analysis. Due to the wide range of P content in foods, colorimetric P analysis was first performed on all food samples as a quick check of P content and as a means of determining an optimal sample dilution factor for subsequent ICP-MS analyses.
2.3.3. Colorimetric analysis
The well-established molybdenum blue colorimetric method, described previously (Gliszczyńska-Świgło and Rybicka, 2021), was used for colorimetric analysis of P in foods. Anhydrous ammonium molybdate (5 g) was dissolved in 60 mL de-ionized water and mixed with a solution of 15 mL concentrated sulfuric acid diluted in 40 mL deionized water to make a 5 % molybdate sulfuric (VI) acid solution. This mixture was stored in a beaker wrapped with aluminum foil at 4 °C, which is stable for up to 4 weeks. Another 0.5 g hydroquinone (benzene-1,4-diol) was dissolved in 100 mL deionized water with concentrated sulfuric acid (10 μL) to make a 0.5 % hydroquinone solution. This, also stored at 4 °C in a beaker wrapped with aluminum foil and has been shown to be stable for 4 weeks. A fresh 20 % sodium sulfite (Na2SO3) solution was prepared daily by dissolving 20 g Na2SO3 in 100 mL deionized water. To measure the total P in each sample, a flat bottom 48-well Costar cluster plate was used. Each well except the blank contained 0.08 mL of sample, 0.08 mL of de-ionized water (or 0.16 mL de-ionized water as a blank), 0.08 mL of 5 % molybdate sulfuric (VI) acid solution, 0.08 mL of 0.5 % hydroquinone solution, and 0.08 mL of 20 % sodium sulfite. The plate containing the mixture, as described above, was left in the dark for 30 mins and absorbance was read in a spectrophotometer (TECAN Infinite Pro M200, Männedorf, Switzerland) for each sample in triplicate at wavelength of 823 nm. The absorbance of all sample solutions, including control samples, were read and P was estimated using the calibration curve for P.
Quantitation of P in samples was based on a nine point external standard calibration in the range 0.39–100 μg/mL. A linear calibration curve, y = 0.0074 × x + 0.1034, was obtained for P, with a correlation coefficient of 0.9993. The limit of detection (LOD) and limit of quantitation (LOQ) values were calculated as 3.40 and 10.4 μg/mL, respectively.
2.3.4. ICP-MS analysis
ICP-MS analysis of P was conducted in an Agilent 7900 ICP-MS (Agilent Technologies, Tokyo, Japan). Diluted digested samples (digest solutions) were further diluted (1:100 or 1:10 v/v) to fit the optimal calibration range of ICP-MS analysis (1–1000 ppb). Digest solutions with undetectable P concentration from the colorimetric method were analyzed with ICP-MS without further dilution. Instrument parameters were as follows: RF power, 1550 W; Nebulizer gas flow 1.03 L/min; nebulizer mode: Micro mist; and RF power 1550 W. A nine-point calibration curve in the linear range of 3.91–1000 ng/mL was generated for P quantitation. The linear equation for P was: y = 4.588 × x + 178.9 (R2 > 0.999). The method LOD was estimated to be 0.04–0.4 μg/g sample for 10× and 100× dilution, respectively. The ICP-MS instrument LOD was estimated to be ~0.2 ppb (ng/mL). All samples were above method LOQ (~0.6 ppb).
The colorimetric analysis was used for triaging purposes and to calculate dilution factors for ICP-MS to ensure P in samples for ICP-MS analysis was within the linear range of the calibration curve (above). This effort would avoid contamination of the instrument from very concentrated samples and eliminate the need for multiple analyses of samples at different dilutions. For these reasons P data by the colorimetric method are not reported. Strong correlation (r = 0.91) was found between P values by the ICP-MS and colorimetric method (excluding 12 samples that were below the colorimetric method’s LOD).
2.3.5. Quality assurance
For chemical analysis, several quality assurance elements were incorporated, including several procedural blanks, instrument blanks, NIST SRM 1546a, phosphorus standard solution, random true duplicate sample analyses for 35 % of all samples, and data acquisition in triplicates. Procedural blanks and the NIST SRM 1546a were inserted into the same testing process as the samples. P standards for colorimetric and ICP-MS were diluted serially with de-ionized water at various concentrations to fit the standard curve of the spectrophotometer (9 data points, range: 0.39–100μg/mL P) and ICP-MS (9 data points, 3.91–1000 ng P/mL). The solutions were vortexed to ensure complete mixing.
The relative standard deviation from independent replicate analysis of food samples had an arithmetic mean of 7.7 % and standard deviation of the mean of 0.38 % (range 0.5 %– 25 %). Sample homogeneity is likely the major component of this variability. The quantitation accuracy was confirmed with the SRM 1564a. The P content in the SRM 1546a was 1640 μg/mg, which falls within the reported range of 1651 +/− 32 μg/mg (1619 – 1683). The coefficient of variation of independent replicates was under 6.9 %.
2.4. BPRHS study design
2.4.1. Study population
These analyses were conducted using data from the BPRHS and the Boston Puerto Rican Osteoporosis Study (BPROS), an ancillary study to the BPRHS. The BPRHS is a longitudinal cohort study designed to determine factors contributing to health disparities experienced by U.S. mainland Puerto Rican adults. As previously described (Tucker et al., 2010), the recruitment of participants was from areas of high Hispanic density in the Greater Boston area selected using data from the year 2000 Census. Households with Puerto Rican adults aged 45 – 75 y were identified and one eligible adult from each qualified household was randomly selected. Recruitment of participants was through door-to-door approaches and community activities. Exclusion criteria were inability to answer questions due to a serious health condition, plans to move from the area within 2 y, or Mini-Mental State Exam (MMSE) score ≤ 10. Of 1504 participants with complete baseline interviews, participants with missing: FFQ data (n = 19), body mass index (n = 17), educational status (n = 2), or poverty status (n = 82); or with reported energy intakes < 600 or > 4800 kcal/d (n =61) were excluded; therefore 1323 were included in final analyses (Fig. 1). To examine associations with biochemical markers, additional analysis was conducted for a subset of participants with serum P, FGF23 and Klotho (n = 418), and PTH (n = 701) measures. Another subset, of 353 participants with complete PTH and other P status biomarkers, was further examined to test the correlation between the biomarkers.
Fig. 1.
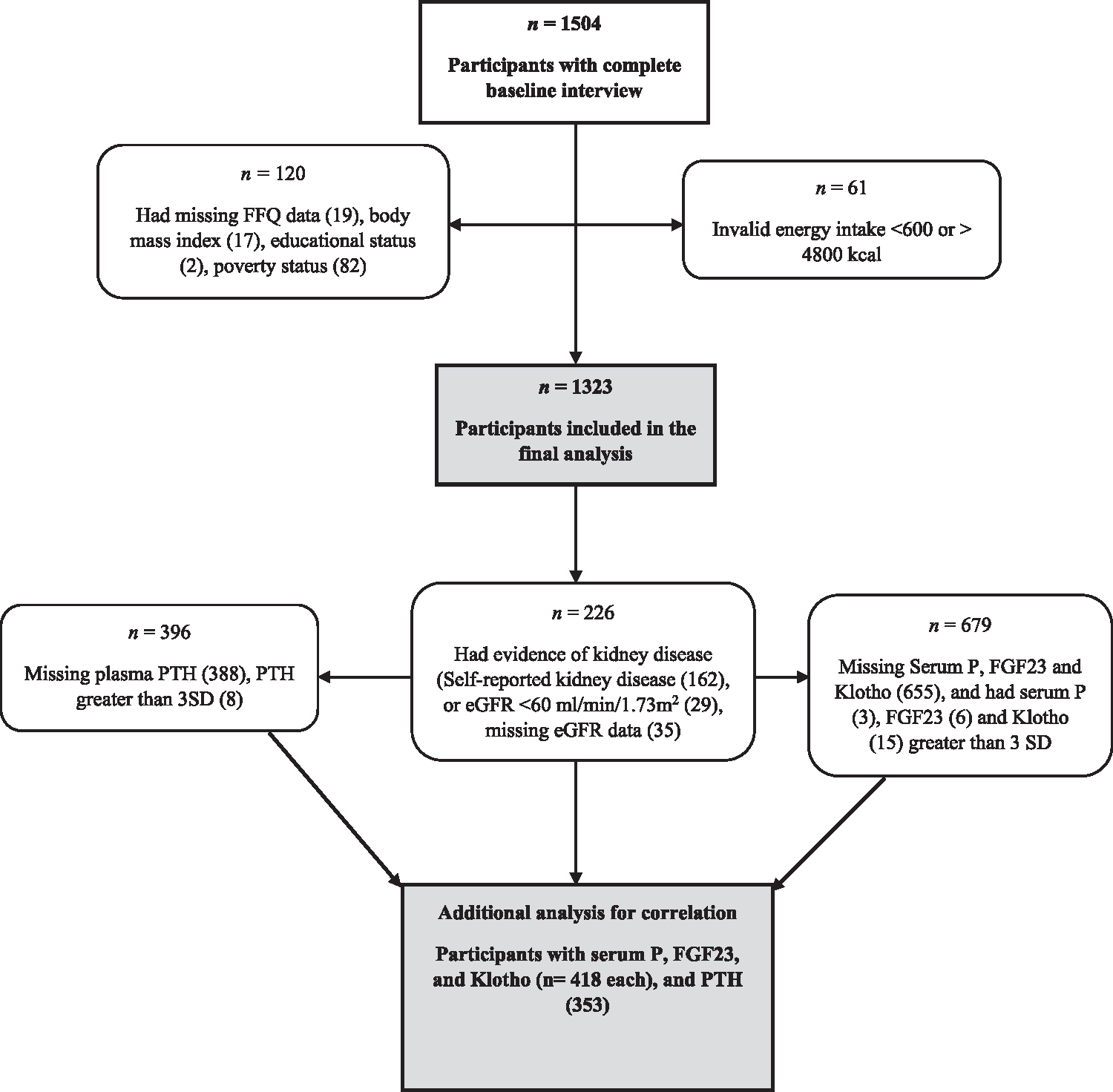
Flow Chart of Participants in the Boston Puerto Rican Health Study.
2.4.2. Dietary data
Interviews were conducted in the home by bilingual interviewers in Spanish or English, based on preference of the participant. Questionnaires were designed based on measures from National Health and Nutrition Examination Survey (NHANES) III (Dreon et al., 1993; McDowell et al., 1990), Hispanic Health and Nutrition Examination Survey (HHANES) (Delgado et al., 1990; McDowell and Loria, 1989), and the National Health Interview Survey Supplement on Aging (Block and Subar, 1992). Dietary intake was assessed at all visits using a food frequency questionnaire (FFQ) adapted for use with this population (Tucker et al., 1998) and validated with plasma measures of vitamin B6 (Ye et al., 2010), B12 (Kwan et al., 2002), vitamin E (Gao et al., 2006), plasma carotenoids (Bermudez et al., 2005), erythrocyte ω-3, and trans-FA (Bigornia et al., 2016). Average daily nutrient intakes were calculated using the Nutritional Data System for Research (NDS-R version 2016, University of Minnesota).
2.4.3. Estimation of bioavailable phosphorus
Estimation of bioavailable P in foods listed on the FFQ was calculated using an algorithm that estimates distinct P metrics for individual foods. A detailed methodology has been described previously (Duong et al., 2022). First, we attributed P content to added or natural sources. As the USDA nutrient database (Food Data Central, FDC) does not discriminate by source of P, a comprehensive list of added vs. natural P and their estimated bio-availabilities were developed, guided by published literature (Calvo et al., 2014; Kalantar-Zadeh et al., 2010; Nouri et al., 2010; Shastak and Rodehutscord, 2015). These were linked to each line item (foods) on the FFQ. Foods containing no P were excluded. Unprocessed or minimally processed foods with no P additives were assigned 0 g for added P. Foods with minimal or zero natural P were assigned 100 % total P as added P. When available, specific proportions from published literature were assigned for added P or by using added P percentages of similar products.
To the extent possible, unknown added P proportions were estimated by subtracting natural from total P and by comparing the P to protein ratio (P:Protein) in processed and unprocessed forms of similar foods in the database. Percent added P in processed mixed dishes were estimated by comparing its total P content to the sum of its corresponding raw ingredients. To determine the level of processing, details were inferred from the literature or by direct analysis of commonly used local products. Lastly, the product of the appropriate added or natural P proportion of the individual food items and their bioavailable weights were estimated. Weighted intakes reflecting bioavailability were derived from the literature or based on expert consensus. Total bioavailable P was calculated with an equation using approximate bioavailability weights for differing sources. All forms of P intake including original total, natural, added, and bioavailable total, natural, and added P (P indicators) were estimated, top coded at three standard deviations and adjusted for total energy intake using the residual method (Willett et al., 1997).
2.4.4. Phosphorus biomarkers
Blood samples were drawn by a certified phlebotomist at each visit after a 12-hour fast and immediately taken to the University of Massachusetts Lowell in coolers with dry ice; cooled to 4 °C and separated within 2 hours in a refrigerated centrifuge. Plasma aliquots were saved in 1 mL cryogenic, screw-cap tubes, and stored at −70 °C. Serum P was determined by Colorimetry with ammonium molybdate (Fiske and Subbarow, 1925) on an EasyRA clinical chemistry analyzer (Medica Corporation, USA). The CV range was 0.68–1.03 %. Plasma fibroblast growth factor 23 C-terminal (FGF23) (Gutiérrez et al., 2015) was estimated using enzyme-labeled immunometric assay (ELISA) assay kit (Eagle Biosciences Inc, USA). Intra- and Inter-assay CVs were ≤ 12 % and ≤ 10 %, respectively. Plasma parathyroid hormone (PTH) was measured with ELISA kit (Abcam, USA). Intra- and Inter-assay CVs were ≤ 1.5 % and 3.8 %, respectively. Plasma Klotho was measured with ELISA kit (Assay Solution Inc, USA). Intra- and Inter-assay CVs were < 10 % and < 8 %, respectively.
2.4.5. Participant characteristics
Data on age, education (≤ 8th grade, 9–12 th grade or GED, some college or college degree, or some graduate school), household income, and household size were obtained from participants during home visits. Poverty status was determined using the poverty threshold published annually by the US Census Bureau. This was estimated by comparing total household income of each participant to the threshold based on the age of household head, household size, and year of interview. A participant was classified as living in poverty if total household income was below this threshold.
2.4.6. Anthropometric measures
Standing height and weight were measured in duplicate in the home during each interview, and body mass index (BMI) was derived by dividing weight (kg) by height (m) squared.
2.4.7. Statistical analysis
Mean intake of the differing forms of P was examined by sex, age, BMI, poverty status and education level. T-tests or one-way analysis of variance (ANOVA) were used to compare differences between groups. The P biomarkers (serum P, FGF23, PTH, and Klotho) were described among participants without evidence of kidney disease (self-reported kidney disease, estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2, or missing eGFR). Adjusting for age and BMI, mean biomarkers were compared between sex using least squares general linear models. Partial Pearson correlations were used to assess relationships between the P indicators, adjusted for age, sex, and total energy intake, and between biomarkers of P status, adjusted for age, sex, and BMI. Statistical analyses were performed using SAS statistical software (SAS version 9.4; SAS Institute Inc.).
3. Results
3.1. P in foods using colorimetric and ICP-MS analysis
Table 1 shows the P content (mean, SD, min, max) from each analyzed food product, using ICP-MS with the total P content of a corresponding food from Food Data Central. Concise descriptions of each food sample are in Table S1. American cheese and cheddar cheese had more P, as analyzed, relative to the values in FDC. P was highest in dairy products (range: 282 ± 227–919 ± 25 mg/100 g), meat (range: 164 ± 23–314 ± 41 mg/100 g), baked sweets (214 ± 45 mg/100 g), legumes (103 ± 14 mg/100 g), and cereal grains (range: 37.2 ± 14.3–320 ± 59 mg/100 g). As changing these values in the database did not result in intakes that differed from those using original USDA values, we did not adjust the database based on our values for the final analysis.
Table 1.
Phosphorus value of food samples using ICP-MS.
| Food | Phosphorus (mg/100 g) | USDAa | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Food group | Product | N | Min | Max | Mean±SD | |
| Cereal grains | Bread | 6 | 85.9 | 102 | 93.6 ± 6.3 | 113 |
| Corn flakes | 7 | 20.7 | 56.7 | 37.2 ± 14.3 | 89 | |
| Oats, whole grain, rolled, old fashioned | 6 | 227 | 380 | 320 ± 59 | 410 | |
| Legumes | Canned beans | 6 | 87.1 | 128 | 103 ± 14 | 153 |
| Dairy | American cheese | 2 | 902 | 937 | 919 ± 25 | 641 |
| Cheddar cheese | 2 | 558 | 709 | 634 ± 107 | 458 | |
| Cream cheese | 4 | 107 | 610 | 282 ± 227 | 107 | |
| Meat | Chicken drumstick | 6 | 132 | 200 | 164 ±23 | 176 |
| Chicken breast | 7 | 187 | 297 | 229 ± 42 | 183 | |
| Codfish | 3 | 52.7 | 541 | 247 ± 259 | 203 | |
| Whiting | 1 | 178 | 178 | 178 | 222 | |
| Pollock | 1 | 238 | 238 | 238 | 221 | |
| Ham | 7 | 174 | 277 | 222 ± 37 | 276 | |
| Shrimp | 6 | 121 | 336 | 214 ± 91 | 214 | |
| Salmon | 4 | 162 | 391 | 239 ± 103 | 200 | |
| Farmed salmon | 2 | 285 | 343 | 314 ± 41 | 240 | |
| Tuna, canned | 6 | 139 | 239 | 184 ± 41 | 237 | |
| Sardines | 7 | 170 | 425 | 287 ± 104 | 490 | |
| Sweets | Doughnuts | 7 | 140 | 284 | 214 ± 45 | 178 |
Corresponding phosphorus value in the United States Department of Agriculture (USDA) Food Data Central
3.2. P intake in the BPRHS
The major food sources of total P intake in this population included milk (~20 %), cheese (~8 %), fish (~7 %), poultry and rice (~6 %), beans and beef (~5 %), processed meat and white bread (~4 %) (Table 2). Major contributors to added P intake included poultry (~14 %), processed meat (~13 %), beef (~11), fish (~10 %), yogurt (~7 %), pork and cheese (~6 %), and white bread (~5 %). Major sources of natural P intake included milk (~23 %), cheese (~8 %), fish, rice (each ~7 %), beans (~6 %), poultry (5 %), beef, white bread (each ~4 %), and eggs, hot breakfast cereal, and processed meat (each ~3 %). Major sources of bioavailable P intake included milk (~19 %), meat turnovers, fish, cheese, and poultry (each ~8 %), beef, processed meat (each ~5 %), rice and white bread (~4 %).
Table 2.
Top contributors to total, added, natural, and bioavailable phosphorus intake in the Boston Puerto Rican Health Studya (n = 1491).
| Food Groups | Total P intake (mg/d) | Contribution to total P (%) | Contribution to added P (%) | Contribution to natural P (%) | Contribution to bioavailable P (%) |
|---|---|---|---|---|---|
|
| |||||
| Milk | 54.5 | 19.5 | 1.5 | 22.6 | 19.0 |
| Cheese | 5.0 | 7.8 | 5.6 | 8.2 | 7.7 |
| Fish | 3.5 | 7.3 | 10.1 | 6.8 | 8.0 |
| Chicken/turkey | 3.6 | 6.4 | 14.4 | 5.0 | 7.5 |
| Rice | 5.9 | 5.8 | 1.1 | 6.6 | 4.3 |
| Beans/legumes | 5.1 | 5.3 | 0.0 | 6.3 | 1.7 |
| Beef | 2.2 | 4.6 | 10.6 | 3.6 | 5.3 |
| Processed meat, sausage, frank | 2.8 | 4.0 | 12.5 | 2.5 | 5.1 |
| White bread | 2.6 | 3.8 | 5.3 | 3.5 | 4.1 |
| Eggs | 3.0 | 3.0 | 1.5 | 3.2 | 2.7 |
| Yogurt | 10.2 | 2.7 | 7.0 | 1.9 | 2.9 |
| Hot breakfast cereal | 5.9 | 2.3 | 0.8 | 2.6 | 1.0 |
| Pork | 3.4 | 2.2 | 5.7 | 1.6 | 2.6 |
| White potatoes | 2.6 | 1.9 | 0.8 | 2.1 | 1.4 |
| Other vegetables | 0.3 | 1.6 | 0.0 | 1.8 | 0.8 |
| Ice cream, sherbet, frozen yogurt | 2.2 | 1.3 | 1.2 | 1.3 | 1.3 |
| Pizza | 4.9 | 1.3 | 1.3 | 1.3 | 1.3 |
| Whole grain breads | 3.2 | 1.2 | 1.3 | 1.2 | 1.1 |
| Cereal (cold, ready-to-eat) | 0.2 | 1.2 | 0.6 | 1.3 | 0.6 |
| Citrus fruit juices | 4.6 | 1.2 | 0 | 1.4 | 1.0 |
| Tortillas/tacos/turnovers | 1.3 | 1.1 | 0.9 | 1.2 | 8.2 |
| Soups | 0.9 | 1.1 | 1.0 | 1.1 | 0.9 |
| Cakes, cookies, pies, doughnuts | 0.6 | 1.1 | 2.7 | 0.8 | 1.2 |
| Liver and organ meats | 4.0 | 1.0 | 2.6 | 0.8 | 1.2 |
| Alcohol beverages | 1.7 | 1.0 | 0.1 | 1.2 | 0.8 |
Ranking using 64 food groups; items provide minimum of 1 % to total phosphorus intake.
After applying the algorithm described above, the mean original total, natural, and added P intakes were 1484 ± 341, 1233 ± 321, and 238 ± 87 mg/d, respectively (Table 3). The mean bioavailable total, natural, and added P intakes were 909 ± 253, 680 ± 219, and 239 ± 83 mg/d, respectively. Original and bioavailable added P were higher among women than men (241 ± 85 vs. 230 ± 91 mg/d, P = 0.035, and 242 ± 81 vs. 231 ± 86 mg/d, P = 0.035) (Table 4). Original and bioavailable added P intakes were higher among younger, vs. older, women (247 ± 89 vs. 232 ± 79 mg/d, P < 0.01, and 248 ± 84 vs. 233 ± 75 mg/d, P < 0.01, respectively), and younger, vs. older, men (242 ± 97.6 vs. 211 ± 75 mg/d, P = 0.001, and 243 ± 93 vs. 213 ± 71 mg/d, P = 0.001, respectively). Older, vs. younger, women had higher original and bioavailable total P (1538 ± 334 vs. 1464 ± 333 mg/d, P < 0.001, and 937 ± 250 vs. 896 ± 242 mg/d, P < 0.0001, respectively) and original and bioavailable natural P intake (1296 ± 308 vs. 1205 ± 319 mg/d, P < 0.0001, and 716 ± 220 vs. 659 ± 210 mg/d, P < 0.0001, respectively) (Table 4). Older, vs. younger, men had higher original natural P (1255 ± 359 vs. 1188 ±307 mg/d, P = 0.05) while younger. vs. older, men had higher original and bioavailable added P (242 ± 98 vs. 211 ± 75 mg/d, P = 0.001, and 243 ± 93 vs. 213 ± 71 mg/d, P = 0.001, respectively).
Table 3.
| Intake | Mean | SD | Q1 | Q2 | Q3 | Range | n >3 SD |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Original P | |||||||
| Total P | 1484 | 341 | 1283 | 1426 | 1634 | 204 – 3498 | 12 |
| Natural P | 1233 | 321 | 1039 | 1172 | 1361 | −26 – 3109 | 11 |
| Added P | 238 | 87 | 187 | 231 | 278 | −62 – 712 | 17 |
| Bioavailable P | |||||||
| Total P | 909 | 253 | 766 | 871 | 1003 | 174 – 2449 | 18 |
| Natural P | 680 | 219 | 556 | 635 | 751 | −30 – 1882 | 18 |
| Added P | 239 | 83 | 190 | 232 | 277 | −46 – 689 | 17 |
Energy adjusted variables, using the residual method; negative values may appear due to this adjustment
Abbreviations: Boston Puerto Rican Health Study (BPRHS), P (phosphorus), SD (standard deviation)
Table 4.
Descriptive characteristics of baseline BPRHS study participants by phosphorus intake (mg/d) (n = 1323).
| Original mean (SD) |
Bioavailable mean (SD) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | n | Totala | Naturala | Addeda | Totala | Naturala | Addeda | |
|
| ||||||||
| Female | 952 | 1493 (335) | 1240 (317) | 241 (85.2) | 912 (246) | 682 (215) | 242 (81.0) | |
| Male | 371 | 1461 (354) | 1214 (329) | 230 (90.6) | 901 (270) | 676 (230) | 231 (86.0) | |
| p b | 0.13 | 0.18 | 0.035 | 0.46 | 0.70 | 0.035 | ||
| Age | 45–59 | 805 | 1460 (333) | 1200 (315) | 246 (91.1) | 900 (248) | 662 (213) | 246 (86.6) |
| 60–75 | 518 | 1521 (349) | 1284 (323) | 226 (78.3) | 923 (260) | 708 (227) | 228 (74.4) | |
| p b | <0.01 | <0.001 | <0.001 | 0.1 | <0.001 | <0.001 | ||
| Female | 45–59 | 579 | 1464 (333) | 1205 (319) | 247 (88.6) | 896 (242) | 659 (210) | 248 (84.1) |
| 60–75 | 373 | 1538 (334) | 1296 (308) | 232 (79.0) | 937 (250) | 716 (220) | 233 (75.1) | |
| p b | <0.001 | <0.001 | <0.01 | 0.01 | <0.001 | <0.01 | ||
| Male | 45–59 | 226 | 1451 (335) | 1188 (307) | 242 (97.6) | 909 (263) | 670 (221) | 243 (92.7) |
| 60–75 | 145 | 1477 (383) | 1255 (359) | 211 (74.9) | 888 (281) | 686 (243) | 213 (71.1) | |
| p b | 0.49 | 0.05 | 0.001 | 0.47 | 0.50 | 0.001 | ||
Energy adjusted variables, using the residual method
P difference in population means (t-test)
Women with obesity consumed higher added P than those with overweight or ideal weight (244 ± 84 vs. 241 ± 85 and 222 ± 94 mg/d, respectively, P = 0.05) (Table 5), and had higher intake of bioavailable added P (245 ± 80 vs. 242 ± 80 and 224 ± 89 mg/d, P = 0.05). Original natural P intake was higher among men with obesity than those with overweight or ideal weight (1259 ± 363 vs. 1180 ± 285 and 1165 ± 310 mg/d, respectively, P = 0.05). No clear associations were observed in comparing the means of P intake measures with educational attainment and poverty status (Table 6).
Table 5.
Baseline body mass index (BMI) category for participants of the BPRHS by phosphorus intake (mg/d) (n = 1323).
| Original mean (SD) |
Bioavailable mean (SD) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Body mass index1 | n | Total2 | Natural2 | Added2 | Total2 | Natural2 | Added2 |
|
| ||||||||
| Female | Normal | 103 | 1447 (333) | 1211 (304) | 222 (94) | 868 (241) | 655 (196) | 224 (89) |
| Overweight | 256 | 1497 (361) | 1244 (347) | 241 (85) | 905 (237) | 676 (215) | 242 (80) | |
| Obesity | 593 | 1499 (323) | 1244 (306) | 244 (84) | 923 (250) | 689 (219) | 245 (80) | |
| P 2 | 0.33 | 0.62 | 0.05 | 0.1 | 0.30 | 0.05 | ||
| Male | Normal | 66 | 1405 (354) | 1165 (310) | 231 (98) | 868 (268) | 650 (221) | 233 (93) |
| Overweight | 134 | 1430 (310) | 1180 (285) | 228 (92) | 875 (256) | 651 (214) | 229 (87) | |
| Obesity | 171 | 1507 (382) | 1259 (363) | 231 (87) | 933 (279) | 706 (242) | 233 (83) | |
| P 2 | 0.06 | 0.05 | 0.93 | 0.1 | 0.06 | 0.93 | ||
Normal BMI is <25 kg/m2; overweight is 25–<30; obesity is>30
Energy adjusted variables, using the residual method.
P difference in population means (ANOVA)
Table 6.
Phosphorus intake (mg/d) by baseline socioeconomic characteristics for participants of the BPRHS (n = 1323).
| n | Original mean (SD) |
Bioavailable mean (SD) |
||||||
|---|---|---|---|---|---|---|---|---|
| Totala | Naturala | Addeda | Totala | Naturala | Addeda | |||
|
| ||||||||
| Educational status | ≤8th grade | 621 | 1481 (336) | 1232 (312) | 235 (88.2) | 913 (261) | 687 (226) | 236 (83.8) |
| 9–12 th grade | 502 | 1479 (337) | 1227 (318) | 239 (84.5) | 910 (254) | 679 (218) | 240 (80.3) | |
| Some college or above | 200 | 1507 (365) | 1250 (355) | 246 (88.5) | 896 (222) | 662 (203) | 246 (84.1) | |
| P b | 0.57 | 0.70 | 0.27 | 0.72 | 0.38 | 0.27 | ||
| Poverty status (Poverty threshold) | Below | 775 | 1484 (343) | 1235 (325) | 234 (86.8) | 914 (256) | 688 (225) | 236 (82.5) |
| Above | 548 | 1484 (338) | 1230 (315) | 243 (86.8) | 902 (249) | 669 (211) | 244 (82.5) | |
| P c | 0.99 | 0.78 | 0.07 | 0.38 | 0.12 | 0.07 | ||
Energy adjusted variables, using the residual method
P difference in population means (ANOVA)
P difference in population means (t-test)
Mean serum P concentration was 3.2 mg/dL, which is within the normal range (2.5–4.5 mg/dl) (Bazydlo et al., 2014), and mean FGF23, PTH, and Klotho concentrations were 1.7 pmol/L, 50.6 pg/mL, and 988 pg/mL, respectively. After excluding participants with evidence of kidney disease (self-reported kidney disease or eGFR < 60 mL/min/1.73 m2), women, vs. men, had higher serum P (3.27 ± 0.05 vs. 3.03 ± 0.07, P < 0.001), FGF23 (1.75 ± 0.10 vs. 1.38 ± 0.14, P = 0.02), and PTH (51.2 ± 1.31 vs. 46.8 ± 1.78, P = 0.03) (Table 7).
Table 7.
Baseline distribution of P status biomarkers stratified by sex among BPRHS study participants.
| Women | Men | ||
|---|---|---|---|
| P biomarkers | Mean (SE)a | Mean (SE)a | P b |
|
| |||
| Serum P, mg/dL (n=418) | 3.27 (0.05) | 3.03 (0.07) | < 0.001 |
| FGF23, pmol/L (n=418) | 1.75 (0.10) | 1.38 (0.14) | 0.02 |
| PTH, pg/mL (n=701) | 51.2 (1.31) | 46.8 (1.78) | 0.03 |
| Klotho, pg/mL (n=418) | 993 (184) | 872 (261) | 0.67 |
Least-squares means and standard errors, using the residuals from the regression models adjusting for age, sex and body mass index
P difference in least-squares means (general linear models)
Serum P tended to be associated with intakes of total and bioavailable total P, while PTH was negatively associated with all P intakes (P = 0.05–0.17). Klotho tended to be positively associated with total and natural P (P = 0.11 and 0.09, respectively, but not with added P (Table 8). As expected, FGF23 was associated positively with PTH (r = 0.09, P = 0.09) and negatively with Klotho (r = −0.10, P = 0.04) (Table 9).
Table 8.
Pearson correlations of baseline P status biomarkers and total, added, natural, and bioavailable P intake (mg/d) among BPRHS participantsa.
| Original phosphorus variables |
Bioavailable phosphorus variables |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P biomarkers | Totalb | Naturalb | Addedb | Totalb | Naturalb | Addedb | ||||||
|
| ||||||||||||
| r | P | r | P | r | P | r | P | r | P | r | P | |
| Serum P, mg/dL (n=418) | 0.08 | 0.10 | 0.05 | 0.23 | 0.02 | 0.63 | 0.07 | 0.15 | 0.05 | 0.33 | 0.02 | 0.63 |
| FGF23, pmol/L (n=418) | −0.07 | 0.18 | −0.07 | 0.16 | 0.02 | 0.68 | −0.07 | 0.16 | −0.08 | 0.12 | 0.02 | 0.68 |
| PTH, pg/mL (n=701) | −0.07 | 0.05 | −0.06 | 0.12 | −0.05 | 0.17 | −0.07 | 0.05 | −0.06 | 0.10 | −0.05 | 0.17 |
| Klotho, pg/mL (n=418) | 0.08 | 0.11 | 0.08 | 0.09 | 0.02 | 0.68 | 0.06 | 0.19 | 0.06 | 0.20 | 0.02 | 0.68 |
BPRHS participants without evidence of kidney disease
Variable adjusted for age, sex, and total energy intake using the residuals from the regression models
Table 9.
Pearson correlations among baseline phosphorus status biomarkers among BPRHS participantsa (n = 418).
| P biomarkersb |
Serum P |
FGF23 |
PTHc |
Klotho |
||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
|
| ||||||||
| Serum P, mg/dL | 1 | 0.03 | 0.58 | − 0.12 | 0.03 | 0.04 | 0.37 | |
| FGF23, pmol/L | 1 | 0.07 | 0.18 | −0.10 | 0.035 | |||
| PTH, pg/mL | 1 | 0.02 | 0.76 | |||||
| Klotho, pg/mL | 1 | |||||||
BPRHS participants without evidence of kidney disease
Variable adjusted for age, sex, and BMI using the residuals from the regression models
Correlation with parathyroid hormone (n = 353)
4. Discussion
Unlike previous reports that food databases may underestimate the P content of foods (Benini et al., 2011; Carrigan et al., 2014; Sherman and Mehta, 2009; Sullivan et al., 2007) the P content in food products reported in FDC were, for the most part, within the range of the chemically analyzed P content. Rather, variation within category was high.
The first aim of this study was to update the P values used in our algorithm, by checking analyzed values of key foods consumed that may be sources of added P. In contrast with most earlier studies, where underestimations were reported in the nutrient database (Benini et al., 2011; Carrigan et al., 2014; Sherman and Mehta, 2009; Sullivan et al., 2007), our food analysis supported the FDC database values, with few exceptions. The leading sources of total P intake in this population were protein rich foods, including milk, cheese, fish, poultry, beef, and processed meats; and grains including rice, beans or legumes, and white bread. Top added P sources were like those of total P, with the addition of yogurt and pork, and exception of milk, rice, and beans. Prior studies similarly found that dairy, meat, and grains were major sources of total P intake (Duong et al., 2022; Fulgoni and Fulgoni, 2021; McClure et al., 2017).
The P intake variables were modeled to adjust for energy intake. This approach has been shown to reduce bias from under or over reporting on the FFQ (Willett et al., 1997). The total dietary P intake in this study (mean ± SD, 1484 ± 341 mg/d) was more than twice the US recommended dietary allowance (RDA) (700 mg/d) and is greater than NHANES 2005–2006 estimates (mean 1359 mg/d) for ages 20 y and older (Fulgoni and Fulgoni III, 2021). The added P intake (238 ± 86.9 mg/d) also exceeds NHANES 2005–2006 derived added P intake (mean 183 mg/d) (Fulgoni and Fulgoni III, 2021).
In this sample of Puerto Rican adults, woman had higher intake of total P than men; and younger (45 - < 60 y) men and women had higher P intake, compared to those ≥ 60 y. Though there remains contradictory evidence on the impact of sex on dietary P intake (Chang et al., 2014), our results support findings that consumption of total P among women has increased over time (Fulgoni and Fulgoni III, 2021). Further, several studies have found that women are more likely than men to have higher serum P attributable to dietary intake (de Boer et al., 2009; Dhingra et al., 2010; Foley et al., 2009). Older women and men had higher natural P intake compared with younger participants. This suggests that older adults in this cohort may have better dietary quality compared to those who are younger. Dietary quality is associated with acculturation. Americans consume large amounts of processed foods, many of which contain added P (León et al., 2013). Among Mexican Americans, acculturation has been shown to have a negative influence on dietary quality (Ayala et al., 2008; Pérez-Escamilla and Putnik, 2007), due to exposure to the “USA mainstream culture” (Pérez-Escamilla, 2009). We previously found that younger adults in this population were more acculturated than older adults, based on language and psychological acculturation (Tucker et al., 2010), consistent with higher natural P intake among older adults and higher added P intake among younger adults, who are more likely to use processed foods.
Further, we found that women with obesity consumed more added P than those who were overweight or normal weight. The prevalence of obesity in this population is greater among women than men (60.5 % vs. 43 %), and among those < 60 y (Tucker et al., 2010). Further, more women than men had abdominal obesity (Tucker et al., 2010).
Added P has greater bioavailability than natural P (> 90 % vs. 20–60 %) (Calvo et al., 2014; Cupisti and Kalantar-Zadeh, 2013; Nouri et al., 2010; Takeda et al., 2017; Williams et al., 2014; Winger et al., 2012), thus, increased intake of added P may raise FGF23, a potent phosphaturic agent. Several studies have described a positive association between BMI and FGF23 (Mirza et al., 2011; Zaheer et al., 2017). Hu et al. (2018) found that BMI and abdominal obesity were independently correlated with FGF23 in 591 postmenopausal women. Though obesity is marked by expression of elevated FGF23, the mechanisms in which FGF23 increases BMI remains to be elucidated. Another possibility is that BMI may be influenced indirectly, as added P tends to be from processed food sources. This is particularly concerning as this population suffers disproportionately from obesity and other comorbid conditions, compared to other Hispanic groups and non-Hispanic Whites.(Andrews and Elixhauser, 2000; Tucker, 2005; Tucker et al., 2000)
Serum P, FGF23, and PTH were higher among women, compared to men, consistent with the findings on dietary intake. However, in the assessment of added P and the P biomarkers, only weak associations were observed. P status is a function of multiple factors, including age, sex, diet, hormones, transporters, effector organs, genetics and others (Lederer, 2014). The mechanism of interaction of these factors remains unknown, and additional studies are needed to understand how these factors leads to blood concentrations of P biomarkers.
Contrary to expectation, total P intake was inversely correlated with PTH, and serum P was inversely correlated with PTH. However, as expected, FGF23 correlated negatively with Klotho. Elevated serum P, PTH and FGF23, with decline in Klotho concentration have been observed with increased P intake among kidney disease patients (Cannata-Andía et al., 2014). However, the P biomarkers analyzed here were for participants without evidence of kidney disease. Circulating P in human plasma is about 72 % organic and 28 % inorganic P, with inorganic P comprising 20 % protein bound (Peacock, 2021). P-bound proteins such as albumin were not accounted for in our analysis and have the potential to affect the observed relationship with circulating P (Webster et al., 2016). Also, phosphate-binding medication use and health conditions such as kidney and bone status, and estrogenic status may influence the observed relationship (Peacock, 2021; Webster et al., 2016).
High P intake (Calvo et al., 2014) suggests use of added P in food processing, hence, increased exposure to added P by consumers. Although adequate P status is required for calcium balance, and ideal intestinal P to calcium absorption improves bone mineralization and turnover (Masuyama et al., 2003), excess P due to the use of P additive in food processing and supplements may lead to hormonal imbalance in P homeostasis and disruption in kidney function (Calvo and Uribarri, 2013; Kemi et al., 2009). Further analysis of these relationships is needed.
Strengths of this study include the use of our novel algorithm to distinguish natural from added P intake in a Puerto Rican cohort and comparing the P intake measures with a panel of P biomarkers. Dietary intake was assessed with a validated FFQ adapted for this population. This enabled assessment of P intake by source. Although our analyzed food values support the existing nutrient database, a limitation of this study is the relatively small number of food products selected for direct chemical analysis. It is possible that variation exists in the estimated dietary P in the nutrient database that was not identified. Also, the type and amount of added P used during food processing appears to vary greatly between brands and products, limiting precision of estimation from FFQs. Future studies may analyze more food samples and obtain more precise descriptions of foods consumed. This may help capture a wider range of low-quality processed foods consumed which are not accounted for when averaging out nutrient estimates in the nutrient database.
5. Conclusion
The P content of the food samples analyzed in this study generally support the values in the existing nutrient database. High total and added P intakes were observed among Puerto Rican adults. Total and added P intake had similar food sources, thus, more studies are needed to understand how this relates to health outcomes. Interventions that promote increased consumption of quality diets are needed in this population, especially among individuals at a high risk of chronic kidney disease.
Supplementary Material
Acknowledgments
The authors thank Kushal Biswas for assistance with food sample analysis.
Funding
This research was funded by the National Institutes of Health (P50 HL105185, P01 AG023394 and R01 AG055948).
Abbreviations:
- BPRHS
Boston Puerto Rican Health Study
- ANOVA
one-way analysis of variance
- CVD
Cardiovascular disease
- SRM
certified reference material
- EGFR
estimated glomerular filtration rate
- FDA
Food and Drug Administration
- ELISA
enzyme-labeled immunometric assay
- FFQ
food frequency questionnaire
- FGF23
fibroblast growth factor 23
- GRAS
generally recognized as safe
- HHANES
Hispanic Health and Nutrition Examination Survey
- ICP-MS
Inductively coupled plasma mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantitation
- MMSE
Mini-Mental State Exam
- NIST
National Institute of Standards and Technology
- NHANES
National Health and Nutrition Examination Survey
- NDS-R
Nutrition Data System for Research
- PTH
parathyroid hormone
- P
Phosphorus
- PSS
Phosphorus standard solution
- PTFE
polytetrafluoroethylene
- RDA
recommended dietary allowance
Footnotes
Declaration of Competing Interest
None
CRediT authorship contribution statement
Sabrina E Noel: Writing – review & editing. Dhimiter Bello: Writing – review & editing, Validation, Supervision, Resources, Formal analysis. Wenjun Li: Writing – review & editing, Supervision. Mahdi Garelnabi: Writing – review & editing. Chi N Duong: Validation. Oladimeji J Akinlawon: Writing – original draft, Visualization, Software, Project administration, Methodology, Investigation, Formal analysis. Xiyuan Zhang: Software, Formal analysis, Data curation. Katherine L Tucker: Writing – review & editing, Validation, Supervision, Funding acquisition, Conceptualization.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jfca.2024.106681.
Data Availability
Data will be made available on request.
References
- Andrews RM, Elixhauser A, 2000. Use of major therapeutic procedures: are Hispanics treated differently than non-Hispanic whites? Ethn. Dis. 10 (3), 384–394. [PubMed] [Google Scholar]
- Assembly Standing Committee on Mental Health Mental Retardation and Developmental Disabilities, (2007). Notice of Public Hearing: Food additives and behavioral disorders. Purpose: To examine the potential relationship between food additives and hyperactivity in children. Tuesday, October 30, 2007, New York City. [Google Scholar]
- Ayala GX, Baquero B, Klinger S, 2008. A systematic review of the relationship between acculturation and diet among Latinos in the United States: implications for future research. J. Am. Diet. Assoc. 108 (8), 1330–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazydlo LA, Needham M, Harris NS, 2014. Calcium, magnesium, and phosphate. J. Lab. Med. 45 (1), e44–e50. [Google Scholar]
- Benini O, D’Alessandro C, Gianfaldoni D, Cupisti A, 2011. Extra-phosphate load from food additives in commonly eaten foods: a real and insidious danger for renal patients. J. Ren. Nutr. 21 (4), 303–308. [DOI] [PubMed] [Google Scholar]
- Bermudez OI, Ribaya-Mercado JD, Talegawkar SA, Tucker KL, 2005. Hispanic and non-Hispanic white elders from Massachusetts have different patterns of carotenoid intake and plasma concentrations. J. Nutr. 135 (6), 1496–1502. [DOI] [PubMed] [Google Scholar]
- Bigornia SJ, Lichtenstein AH, Harris WS, Tucker KL, 2016. Associations of erythrocyte fatty acid patterns with insulin resistance. Am. J. Clin. Nutr. 103 (3), 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G, Subar A, 1992. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J. Am. Diet. Assoc. 92 (8), 969–977. [PubMed] [Google Scholar]
- de Boer IH, Rue TC, Kestenbaum B, 2009. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am. J. Kidney Dis. 53 (3), 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MS, Moshfegh AJ, Tucker KL, 2014. Assessing the health impact of phosphorus in the food supply: issues and considerations. J. Adv. Nutr. 5 (1), 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MS, Uribarri J, 2013. Contributions to Total Phosphorus Intake: All Sources Considered, Seminars in Dialysis. Wiley Online Library, pp. 54–61. [DOI] [PubMed] [Google Scholar]
- Cannata-Andía JB, Carrillo-López N, Rodriguez-García M, Torregrosa J-V, 2014. Mineral and Bone Disorders in Chronic Kidney Disease, Management of Chronic Kidney Disease. Springer, pp. 223–239. [Google Scholar]
- Carrigan A, Klinger A, Choquette SS, Luzuriaga-McPherson A, Bell EK, Darnell B, Gutierrez OM, 2014. Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J. Ren. Nutr. 24 (1), 13–19, 19e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AR, Lazo M, Appel LJ, Gutierrez OM, Grams ME, 2014. High dietary phosphorus intake is associated with all-cause mortality: results from NHANES III. Am. J. Clin. Nutr. 99 (2), 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke A, 2017. Dietary food-additive phosphate and human health outcomes. Compr. Rev. Food Sci. Food Saf. 16 (5), 906–1021. [DOI] [PubMed] [Google Scholar]
- Cupisti A, Kalantar-Zadeh K, 2013. Management of Natural and Added Dietary Phosphorus Burden in Kidney Disease. Seminars in Nephrology. Elsevier, pp. 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado JL, Johnson CL, Roy I, Trevino FM, 1990. Hispanic health and nutrition examination survey: methodological considerations. Am. J. Public Health 80 (Suppl), 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R, Gona P, Benjamin EJ, Wang TJ, Aragam J, D’Agostino RB Sr, Kannel WB, Vasan RS, 2010. Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur. J. Heart Fail. 12 (8), 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreon DM, John EM, DiCiccio Y, Whittemore AS, 1993. Use of NHANES data to assign nutrient densities to food groups in a multiethnic diet history questionnaire. Nutr. Cancer 20 (3), 223–230. [DOI] [PubMed] [Google Scholar]
- Duong CN, Akinlawon OJ, Gung J, Noel SE, Bigornia S, Flanagan K, Pourafshar S, Lin P-H, Davenport CA, Pendergast J, 2022. Bioavailability of phosphorus and kidney function in the Jackson Heart study. Am. J. Clin. Nutr. 116 (2), 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske CH, Subbarow Y, 1925. The colorimetric determination of phosphorus. J. Biol. Chem. 66 (2), 375–400. [Google Scholar]
- Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA, 2009. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J. Am. Soc. Nephrol. 20 (2), 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgoni K, Fulgoni III, 2021. Trends in total, added, and natural phosphorus intake in adult Americans, NHANES 1988–1994 to NHANES 2015–2016. Nutrients 13 (7), 2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wilde PE, Lichtenstein AH, Bermudez OI, Tucker KL, 2006. The maximal amount of dietary α-tocopherol intake in US adults (NHANES 2001–2002). J. Nutr. 136 (4), 1021–1026. [DOI] [PubMed] [Google Scholar]
- Gliszczyńska-Świgło A, Rybicka I, 2021. Fast and sensitive method for phosphorus determination in dairy products. J. Consum. Prot. Food Saf. 16 (3), 213–218. [Google Scholar]
- Gutiérrez OM, 2013. Sodium-and phosphorus-based food additives: persistent but surmountable hurdles in the management of nutrition in chronic kidney disease. Adv. Chronic Kidney Dis. 20 (2), 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez OM, Luzuriaga-McPherson A, Lin Y, Gilbert LC, Ha S-W, Beck GR Jr, 2015. Impact of phosphorus-based food additives on bone and mineral metabolism. J. Clin. Endocrinol. Metab. 100 (11), 4264–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Ma X, Luo Y, Xu Y, Xiong Q, Pan X, Xiao Y, Bao Y, Jia W, 2018. Associations of serum fibroblast growth factor 23 levels with obesity and visceral fat accumulation. Clin. Nutr. 37 (1), 223–228. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, Noori N, Hirschberg R, Benner D, Nissenson AR, 2010. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 5 (3), 519–530. [DOI] [PubMed] [Google Scholar]
- Kemi VE, Rita HJ, Kärkkäinen MU, Viljakainen HT, Laaksonen MM, Outila TA, Lamberg-Allardt CJ, 2009. Habitual high phosphorus intakes and foods with phosphate additives negatively affect serum parathyroid hormone concentration: a cross-sectional study on healthy premenopausal women. Public Health Nutr. 12 (10), 1885–1892. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, 2019. The Klotho proteins in health and disease. Nat. Rev. Nephrol. 15 (1), 27–44. [DOI] [PubMed] [Google Scholar]
- Kwan LL, Bermudez OI, Tucker KL, 2002. Low vitamin B-12 intake and status are more prevalent in Hispanic older adults of Caribbean origin than in neighborhood-matched non-Hispanic whites. J. Nutr. 132 (7), 2059–2064. [DOI] [PubMed] [Google Scholar]
- Lederer E, 2014. Regulation of serum phosphate. J. Physiol. 592 (18), 3985–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León JB, Sullivan CM, Sehgal AR, 2013. The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J. Ren. Nutr. 23 (4), 265–270 e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama R, Nakaya Y, Katsumata S, Kajita Y, Uehara M, Tanaka S, Sakai A, Kato S, Nakamura T, Suzuki K, 2003. Dietary calcium and phosphorus ratio regulates bone mineralization and turnover in vitamin D receptor knockout mice by affecting intestinal calcium and phosphorus absorption. J. Bone Miner. Res. 18 (7), 1217–1226. [DOI] [PubMed] [Google Scholar]
- McClure ST, Chang AR, Selvin E, Rebholz CM, Appel LJ, 2017. Dietary sources of phosphorus among adults in the United States: results from NHANES 2001–2014. Nutrients 9 (2), 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell M, Briefel R, Warren R, Buzzard I, Feskanich D, Gardner S, 1990. the Dietary Data Collection System. An automated interview and coding system for NHANES III. Proceedings of the 14th National Nutrient Databank Conference. CBORD Group, Inc, Ithaca, New York, pp. 125–131. [Google Scholar]
- McDowell M, Loria C, 1989. Cultural considerations in analyzing dietary data from the Hispanic Health and Nutrition Examination Survey. Natl. Nutr. Database Conf. 1989. 43–46. [Google Scholar]
- Mirza MA, Alsiö J, Hammarstedt A, Erben RG, Michaëlsson K, Tivesten Å, Marsell R, Orwoll E, Karlsson MK, Ljunggren Ö, 2011. Circulating Fibroblast Growth factor-23 is Associated with Fat Mass and Dyslipidemia in Two Independent Cohorts of Elderly Individuals. Arterioscler. Thromb. Vasc. Biol. 31 (1), 219–227. [DOI] [PubMed] [Google Scholar]
- Molins RA, 1990. Phosphates in Food. CRC Press. [Google Scholar]
- Moore LW, Nolte JV, Gaber AO, Suki WN, 2015. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. Am. J. Clin. Nutr. 102 (2), 444–453. [DOI] [PubMed] [Google Scholar]
- Nouri N, Sims JJ, Kopple JD, Shah A, Colman S, Shinaberger CS, Bross R, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K, 2010. Organic and inorganic dietary phosphorus and its management in chronic kidney disease. Iran. J. Kidney Dis. 4 (2), 89–100. [PubMed] [Google Scholar]
- Onufrak SJ, Bellasi A, Shaw LJ, Herzog CA, Cardarelli F, Wilson PW, Vaccarino V, Raggi P, 2008. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis 199 (2), 424–431. [DOI] [PubMed] [Google Scholar]
- Peacock M, 2021. Phosphate metabolism in health and disease. Calcif. Tissue Int. 108, 3–15. [DOI] [PubMed] [Google Scholar]
- Pehrsson P, Haytowitz D, Holden J, Perry C, Beckler D, 2000. USDA’s national food and nutrient analysis program: food sampling. J. Food Compos. Anal. 13 (4), 379–389. [Google Scholar]
- Pérez-Escamilla R, 2009. Dietary quality among Latinos: is acculturation making us sick? J. Am. Diet. Assoc. 109 (6), 988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Escamilla R, Putnik P, 2007. The role of acculturation in nutrition, lifestyle, and incidence of type 2 diabetes among Latinos. J. Nutr. 137 (4), 860–870. [DOI] [PubMed] [Google Scholar]
- Shastak Y, Rodehutscord M, 2015. Recent developments in determination of available phosphorus in poultry. J. Appl. Poult. Res. 24 (2), 283–292. [Google Scholar]
- Sherman RA, Mehta O, 2009. Dietary phosphorus restriction in dialysis patients: potential impact of processed meat, poultry, and fish products as protein sources. Am. J. Kidney Dis. 54 (1), 18–23. [DOI] [PubMed] [Google Scholar]
- Suki WN, Moore LW, 2016. Phosphorus regulation in chronic kidney disease. Methodist Debakey Cardiovasc. J 12 (4 Suppl), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CM, Leon JB, Sehgal AR, 2007. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J. Ren. Nutr. 17 (5), 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda E, Yamamoto H, Taketani Y, 2017. Effects of natural and added phosphorus compounds in foods in health and disease. Clin. Asp. Nat. Added Phosphorus Foods 111–121. [Google Scholar]
- Tucker KL, 2005. Stress and nutrition in relation to excess development of chronic disease in Puerto Rican adults living in the Northeastern USA. J. Med. Investig. 52 (Supplement), 252–258. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Bianchi LA, Maras J, Bermudez OI, 1998. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am. J. Epidemiol. 148 (5), 507–518. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Falcon LM, Bianchi LA, Cacho E, Bermudez OI, 2000. Self-reported prevalence and health correlates of functional limitation among Massachusetts elderly. J. Gerontol. A Biol. Sci. Med. Sci. 55. M90–M97. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM, 2010. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health 10 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, Calvo MS, 2003. Hidden Sources of Phosphorus in the Typical American Diet: Does It Matter in Nephrology? Seminars in Dialysis. Wiley Online Library, pp. 186–188. [DOI] [PubMed] [Google Scholar]
- Webster R, Sheriff S, Faroqui R, Siddiqui F, Hawse JR, Amlal H, 2016. Klotho/fibroblast growth factor 23-and PTH-independent estrogen receptor-α-mediated direct downregulation of NaPi-IIa by estrogen in the mouse kidney. Am. J. Physiol. Ren. Physiol. 311 (2), F249–F259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Howe GR, Kushi LH, 1997. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 65 (4), S1220–S1228. [DOI] [PubMed] [Google Scholar]
- Williams C, Ronco C, Kotanko P, 2014. Whole grains in the renal diet-is it time to reevaluate their role? Blood Purif. 36 (3–4), 210–214. [DOI] [PubMed] [Google Scholar]
- Winger RJ, Uribarri J, Lloyd L, 2012. Phosphorus-containing food additives: an insidious danger for people with chronic kidney disease. Trends Food Sci. Technol. 24 (2), 92–102. [Google Scholar]
- Ye X, Maras JE, Bakun PJ, Tucker KL, 2010. Dietary intake of vitamin B-6, plasma pyridoxal 5′-phosphate, and homocysteine in Puerto Rican adults. J. Am. Diet. Assoc. 110 (11), 1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C-Y, Park JT, Jhee JH, Noh J, Kee YK, Seo C, Lee M, Cha M-U, Kim H, Park S, Yun H, Jung S, SH H, Yoo T, Kang S, 2017. High dietary phosphorus density is a risk factor for incident chronic kidney disease development in diabetic subjects: a community-based prospective cohort study. Am. J. Clin. Nutr. 106 (1), 311–321. [DOI] [PubMed] [Google Scholar]
- Zaheer S, De Boer IH, Allison M, Brown JM, Psaty BM, Robinson-Cohen C, Michos ED, Ix JH, Kestenbaum B, Siscovick D, 2017. Fibroblast growth factor 23, mineral metabolism, and adiposity in normal kidney function. J. Clin. Endocrinol. Metab. 102 (4), 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


