Abstract
Whereas human immunodeficiency virus (HIV) infects various cell types by fusion at the plasma membrane, we observed a different entry route in human primary macrophages, in which macropinocytosis is active. Shortly after exposure of macrophages to HIV-1 and irrespective of viral envelope-receptor interactions, particles were visible in intracellular vesicles, which were identified as macropinosomes. Most virions appeared subsequently degraded. However, fusion leading to capsid release in the cytosol and productive infection could take place inside vesicles when particles were properly enveloped. These observations provide new insights into HIV-1 interactions with a cell target relevant to pathogenesis. They may have implications for the design of soluble inhibitors aimed at interfering with the fusion or entry processes.
Enveloped viruses must fuse with cellular membranes to enter into target cells and to deliver their genetic material. This process takes place either at the cell surface or within intracellular vesicles. Viruses requiring low pH for membrane fusion, such as influenza virus, vesicular stomatitis virus (VSV) and Semliki Forest virus, necessitate endocytosis to encounter an acidic environment (for recent reviews, see references 13 and 27). Low pH triggers a series of conformational changes leading to the exposure of a fusion peptide of the viral envelope glycoprotein. By contrast, pH-independent viruses, including most retroviruses, are believed to undergo fusion at the cell surface. Exposure of the highly hydrophobic fusion peptide is secondary to viral envelope glycoproteins binding to a cell surface receptor(s). However, distinctions between entry of pH-dependent and pH-independent viruses may be more subtle than initially thought. Fusion of pH-independent viruses usually occurs at both neutral and acidic pHs, raising the possibility that pH-independent productive infection follows uptake into acidic vesicles (27). Moreover, as studies of virus entry have been mostly performed with established cell lines, such information may not be relevant to the study of virus entry in vivo. Entry may also vary according to the cell line used, as reported for the ecotropic Moloney murine leukemia retrovirus (21). Endocytic pathways leading to viral entry have been characterized by electronic microscopy and immunofluorescence studies or by the use of inhibitors or dominant-negative constructs. For example, pharmaceutical agents removing cholesterol from the plasma membrane indicated that simian virus 40 may enter cells through caveolae (39). On the other hand, a dominant-negative dynamin mutant inhibited infection of Semliki Forest virus, Sindbis virus, and human rhinovirus 14, confirming morphological evidence that receptor-mediated endocytosis through clathrin-coated pits or vesicles is involved in the entry of these viruses (12).
There is controversy about the pathways and mechanisms of the early stages of human immunodeficiency virus type 1 (HIV-1) infection. Seminal studies indicated that HIV-1 infection is pH-independent and does not require endocytosis of the CD4 receptor (25, 29, 51). These conclusions were supported by images showing HIV particles fusing at the cell surface and syncytia formation between Env-expressing and target cells at neutral pH. However, HIV-1 entry through clathrin-coated vesicles and fusion with endosomal membranes were also observed, suggesting that incoming virions gain access to the cytoplasm from endosomes (7, 19, 42). The relevance of these pathways for HIV-1 productive infection is unknown. We previously showed that vesicular uptake is quantitatively the main route of HIV-1 virion internalization but is essentially a dead end with respect to productive infection (26). Furthermore, a recent reported indicated that HIVSF2, but not HIVNL43, infect cells via an endocytic route, following gp41 activation by acidic pH (16). Interestingly, a similar entry pathway depending on a low pH step acting downstream of receptor binding has been demonstrated with another retrovirus, avian leukosis virus (36). However, most, if not all, of the studies regarding HIV-1 entry suffers from the use of continuous cell lines, often HeLa or 293 derivatives, which are not the natural targets of infection. This may not only be responsible for the discrepant results reported in the litterature, but again raises concerns about the in vivo relevance of the observations.
HIV-1 entry routes are poorly documented in lymphocytes, macrophages and dendritic cells, the natural targets of infection. In particular, cells of the macrophage lineage have evolved a variety of strategies for the uptake of exogenous materials and solutes (for reviews, see references 23, 46, and 56) which might be effective for viruses as well. Four morphologically distinct internalization pathways have been identified in mammalian cells. Clathrin-mediated endocytosis is the best-characterized pathway (48). In this pathway, transmembrane receptors bound with their ligand are clustered into clathrin-coated pits. When pits reach a size threshold, pinching results in the formation of vesicles (<150 nm). Vesicles fuse with early endosomes, where receptors are sorted for either recycling or addressing to lysosomes. Non-clathrin-mediated endocytosis includes caveolae, which are small vesicles (50 to 80 nm) enriched with caveolin, cholesterol, and sphingolipids. Caveolae participate in the internalization of macromolecules, glycosyl-phosphatidylinositol-linked proteins, toxins, and in the entry of viruses (i.e., simian virus 40) and bacteria. Macropinocytosis is a cell-type-specific receptor-independent endocytic pathway associated with actin-dependent plasma membrane ruffling (23, 53, 56). It is activated by growth factors or phorbol esters in certain cell types such as macrophages and epithelial cells and operates constitutively in dendritic cells (45). Macropinosomes are large vesicles (0.2 to 3 μm), trapping large amounts of macromolecules and fluids. They play an important immunological role in professional antigen-presenting cells by taking up extracellular antigens for presentation by major histocompatibility complex class I (MHC-I) and MHC-II molecules (23, 56). Of note, macropinocytosis, but not other endocytic pathways, is inhibited by amiloride analogs (38, 45, 55). Phagocytosis consists in the uptake of large particles (>500 nm), microorganisms, cell debris, and apoptotic cells (1). Phagocytosis is initiated by the interaction of cell surface receptors (such as mannose Fc or complement receptors) with ligands on the particles, leading to internalization through an actin-dependent mechanism (1).
Whether HIV-1 uses one or more of these routes for entry into macrophages has not yet been determined. Macrophages play a crucial role in HIV-1 infection. They propagate viral infection, support virus replication in nonlymphoid organs such as lung or brain, and probably represent a viral reservoir established shortly after infection that may persist during highly active antiretroviral therapy (28, 30, 41). Macrophages express CD4 and the coreceptors CCR5 and CXCR4. They can be efficiently infected with R5-tropic strains, whereas the literature is inconsistent about whether CXCR4 can be used for X4-tropic strains. In this study, we examined the mode of HIV-1 entry in primary macrophages by using a comprehensive approach associating morphological, biochemical, and viral replication analyses. We show that HIV-1 internalization in macrophages is a nonspecific process which does not require envelope-receptor interactions. However, after internalization by macropinocytosis, envelope-mediated fusion of HIV-1 virions with the vesicular membrane can occur, leading to pH-independent productive infection of macrophages.
MATERIALS AND METHODS
Preparation of macrophages.
Buffy-coat peripheral blood mononuclear cells (PBMCs) from healthy donnors were isolated by Ficoll centrifugation. PBMCs were resuspended at 106 cells per ml in RPMI 1640 medium (Gibco-BRL, Paisley, Scotland) containing 1% human AB-positive serum and plated. After 1 h of adhesion at 37°C, nonadherent cells were removed by two washes in Ca2+- and Mg2+-free phosphate-buffered saline (PBS). To allow differentiation of macrophages, monocytes were cultured for 7 days before use in RPMI 1640 medium supplemented with 10% human AB-positive serum, 2 mM glutamine, and antibiotics. Cells were >95% CD14+.
Cells, viruses, and reagents.
P4C5 cells are HeLa CD4+ CCR5+ cells in which transactivation by Tat induces expression of the Escherichia coli lacZ gene from the HIV long terminal repeat (LTR) (3). HIVNLAD8, HIVΔenv (from the NL43 strain), and HIV(VSV) pseudotypes were obtained by transfecting pNLAD8 (15), pNL43Δenv (5), pNL43Δenv, and a VSV-G expression vector in HeLa cells (26). The R5-tropic HIVNLAD8 strain, a kind gift of E. Freed, carries a portion of the env gene from the R5-tropic clone AD8 on a HIVNL4–3 backbone (15). Viral stocks were analyzed for their HIV-1 p24 content by enzyme-linked immunosorbent assay (Dupont de Nemours) and frozen. The infectivity of viral supernatants was determined with P4C5 cells (26). Dimethyl amiloride (DMA), bafilomycin A1, and zidovudine (AZT) were from Sigma. Infections throughout the study were performed in the absence of any reagent such as polybrene or DEAE-dextran.
Electronic microscopy analysis.
Macrophages were exposed to HIVNLAD8 (500 ng of p24 for ∼106 cells) for 30 to 45 min at 37°C, washed, fixed in PBS–3% glutaraldehyde for 1 h, and postfixed in PBS–1% osmium tetroxide for 2 h. After being rinsed in PBS, cells were transferred to 0.2 M cacodylate buffer for 30 min. Cells were washed in 30% methanol for 10 min, stained in 2% uranyl acetate–30% methanol for 1 h, and washed in 30% methanol. Cells were then dehydrated in an ethanol series to propylene oxyde and embedded in Epon 812. When stated, cells were fixed in 0.1 M cacodylate buffer containing 0.075% ruthenium red for 1 h at 4°C and processed as described previously (37). Cells were examined with a JEOL 1200EX2 microscope.
Immunofluorescence microscopy and confocal analysis.
Macrophages were cultured on glass coverslips in 24-well plates and exposed to the indicated viruses (150 ng of p24) for 30 min at 37°C. Cells were then fixed and stained with anti-Gag monoclonal antibodies (MAbs) (a kind gift of F. Traincart) as described previously (26). Cells were analyzed with a Leica TCS4D confocal microscope. Representative medial sections were mounted by using Adobe Photoshop software.
Entry assay.
An entry assay was performed using a cell fractionation protocol modified from that described in reference 26. Subconfluent cultures of macrophages (∼2 × 106 cells in 25-cm2 flasks) were exposed to the indicated HIV-1 preparations (450 ng of p24/ml) in culture medium containing 20 mM HEPES for 2 h at 37°C. Cells were then washed three times in ice-cold PBS and removed from the plastic culture flask with a scraper. To remove virus adsorbed at the cell surface, cells were then treated for 2 min with 1 mg of pronase (Boerhinger Mannheim)/ml in ice-cold RPMI and 20 mM HEPES. Cells were washed three times in RPMI supplemented with 10% fetal calf serum to eliminate pronase. Cells were then treated with an ice-cold digitonine buffer (10 mM TRIS [pH 7.5], 10 mM NaCl, 0.15 mM spermine, 0.5 mM spermidine, 1 mM EDTA, and 100 μg/ml digitonine [RBI-Sigma]) for 10 min at 4°C to selectively permeabilize plasma membranes. Cells were then centrifuged (Heraeus Biofuge) at 3,000 rpm for 4 min at 4°C. Supernatants and pellets, corresponding to cytosolic and vesicular fractions, respectively, were adjusted to 0.5% Triton X-100. Samples were then briefly centrifuged (Heraeus Biofuge) at 10,000 rpm to remove debris before measurement of p24 concentrations. When stated, cells were preincubated with bafilomycin A1 (1 h) or DMA (3 h) before viral exposure. Inhibitors were maintained during viral exposure. A similar fractionation protocol was used for P4C5 cells, except that cells were treated with 7 mg of pronase/ml for 10 min.
Single-cycle viral replication assays.
Subconfluent cultures of macrophages (in 12-well plates) were exposed to the indicated HIV-1 preparations (50 ng of p24 per well) for 4 h at 37°C. When indicated, target cells were incubated with inhibitors before viral exposure (15 min and 1 h of preincubation for bafilomycin A1 and dimethyl amiloride, respectively). Inhibitors were maintained during viral exposure. Cells were then washed to remove extracellular virions. Twenty hours later, AZT (5 μM) was added to the culture medium to prevent secondary replication cycles and maintained throughout the study. Viral replication was assessed by measuring p24 production in cell supernatants. In P4C5 cells, single-cycle assays were performed as described previously (26).
RESULTS
Ultrastructural analysis of HIV-1 internalization in macrophages.
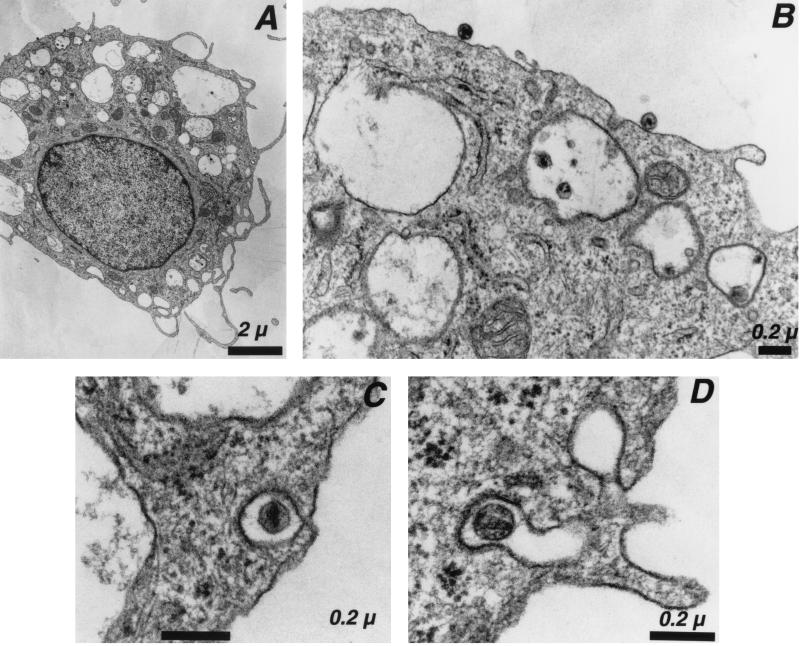
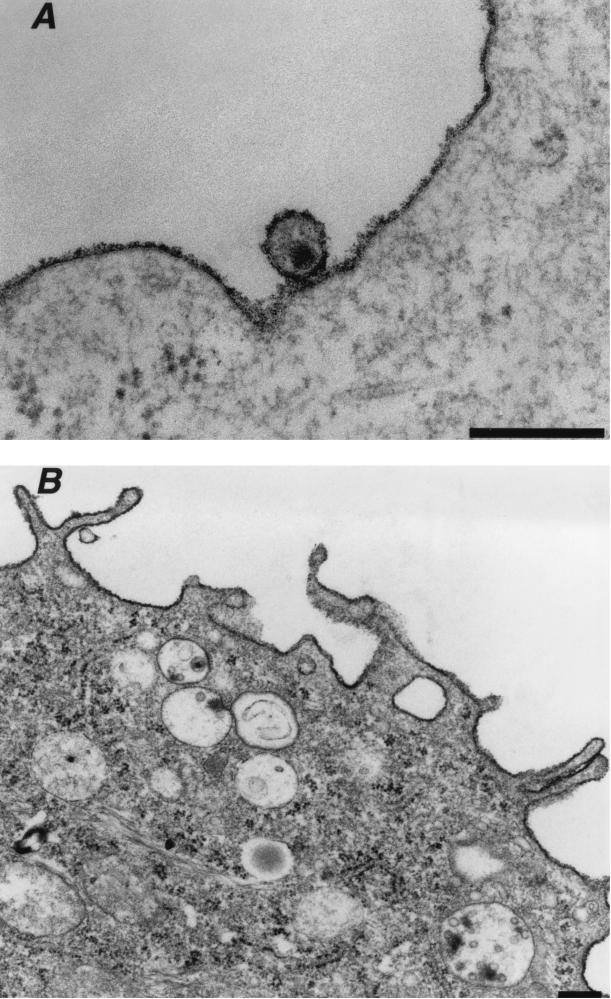
Monocyte-derived macrophages were prepared from PBMCs of healthy donors. Cells expressed CD14 (a marker of the macrophage lineage) and the HIV receptors CD4, CCR5, and CXCR4 and displayed high phagocytic activity (not shown). As expected, R5-tropic HIVNLAD8 and HIVYU-2 strains efficiently replicated in these cells, whereas the X4-tropic laboratory-adapted HIVNL43 strain was unable to grow (not shown). With the aim of following the early steps of viral entry, macrophages were pulsed at 37°C with HIVNLAD8, fixed, and processed for electron microscopy. The virus inoculum contained 500 ng of Gag p24 for ∼106 cells, and the exposure period was 30 to 45 min. We were unable to detect cells harboring virus particles in smaller amounts of inoculum or at shorter incubation periods (not shown). The proportion of cells harboring virus particles was low (<2%). At low magnification, virus particles appeared both in intracellular vesicles and at the cell surface (Fig. 1A). Higher magnification showed virions tightly bound to the plasma membrane (Fig. 1B), whereas others were engulfed in large intracellular vesicles (200 to >500 nm in diameter) (Fig. 1B) or in smaller coated-pits (∼100 nm diameter) (Fig. 1C and D). Virus particles seemed more frequently located in large vesicles than attached at the plasma membrane or captured into small coated-pits (Fig. 1 to 3 and data not shown). Virus particles could be identified as HIV-1 virions, with respect to their typical morphology and by staining with anti-Gag antibodies (not shown). They were not detected in cells that had not been exposed to virus (not shown).
FIG. 1.
Electron microscopy analysis of HIV-1 entry in macrophages. Macrophages were exposed to the R5-tropic HIVNLAD8 for 30 to 45 min and processed for electron microscopy. (A) Low magnification of an HIV-1-infected macrophage. (B) Detail of panel A, showing HIV-1 virions bound at the cell surface and within large intracellular vesicles. (C and D) HIV-1 particles in coated pits. Data are representative of three independent experiments.
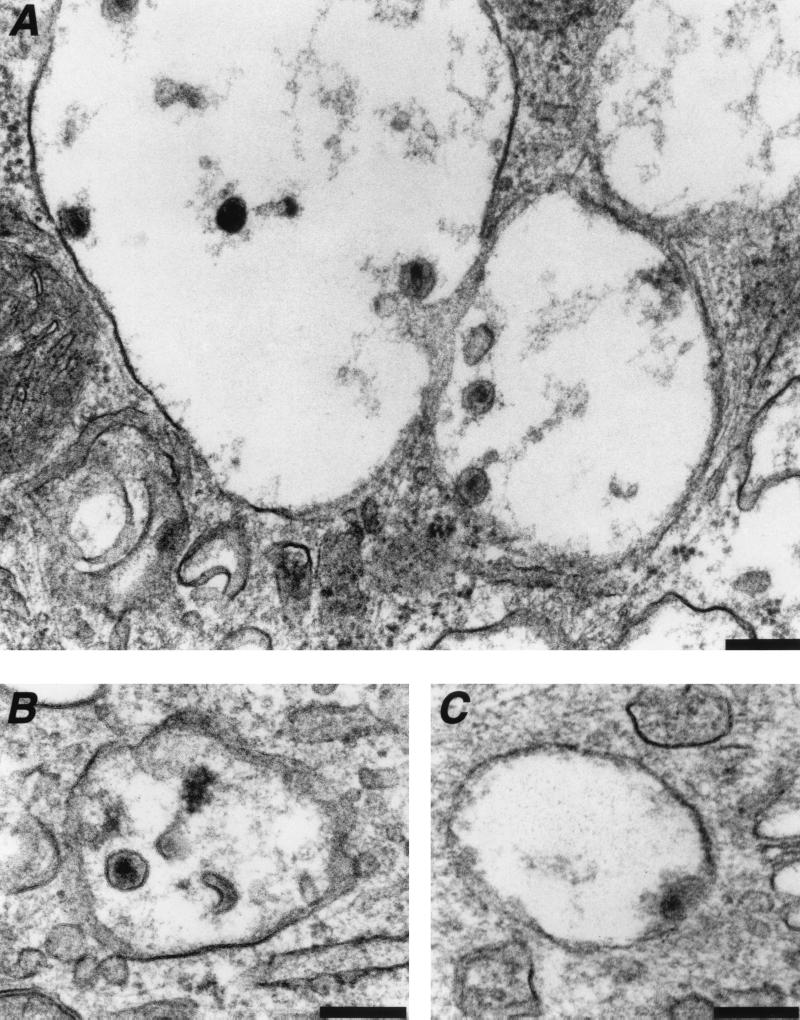
FIG. 3.
Fate of HIV-1 virions internalized in intracellular vesicles. Macrophages were exposed to the R5-tropic HIVNLAD8 for 30 to 45 min and processed for electron microscopy. (A and B) Intact or degraded HIV-1 virions present in the lumen or bound to the membrane of intracellular vesicles. (C) Fusion between viral and vesicular membranes. Data are representative of three independent experiments. Bar = 0.2 μm.
It was important to ensure that virions were truly internalized into intracellular vesicles rather than trapped by plasma membrane invaginations. To this aim, cells were stained with ruthenium red. This electron-dense dye stains the outer leaflet of the plasma membrane and any continuous invaginations at the time of fixation (20). Plasma membranes and extracellular HIV-1 particles were densely stained with the dye (Fig. 2A). In contrast, ruthenium red was absent from the large intracellular vesicles and their viral content (Fig. 2B), indicating that they were not accessible to the extracellular milieu.
FIG. 2.
HIV-1 entry analyzed by ruthenium red staining and electron microscopy. Macrophages were exposed to the R5-tropic HIVNLAD8 for 30 to 45 min and processed for electron microscopy with ruthenium red included in the fixative to stain the surface membranes. (A) Extracellular HIV-1 virion bound at the plasma membrane is densely stained by ruthenium red. (B) HIV-1 virions in large intracelular vacuoles are protected from ruthenium red staining. Data are representative of two independent experiments. Bar = 0.2 μm.
To document HIV-1 vesicular uptake further, we carefully examined the morphology and the location of internalized virions (Fig. 3). Whereas some virions were located in the lumen of the vesicle, others seemed to bind to the vesicular membranes. A high proportion of internalized and unbound particles apparently underwent degradation. This was evidenced by images of disruption of the viral envelope (Fig. 3A and B) and of disorganization or ejection of the viral core from its envelope (Fig. 3A and B). On the other hand, some virus particles tightly interacted with the vesicular membrane (Fig. 3A). In rare cases, images evoking fusion between the viral and the vesicular membrane were observed (Fig. 3C). This would suggest that a fraction of intravesicular virions escapes the endocytic pathway and gains access to the cytoplasm.
These observations indicate that the uptake of HIV-1 particles by primary macrophages could involve a variety of internalization pathways, including entrapment into large intracellular vesicles. However, electronic microscopy did not allow us to draw any functional conclusions concerning these pathways. We thus developed biochemical and virological techniques aimed at addressing whether viral internalization via intracellular vesicles can lead to productive infection.
Cytosolic p24 is associated with productive infection of macrophages.
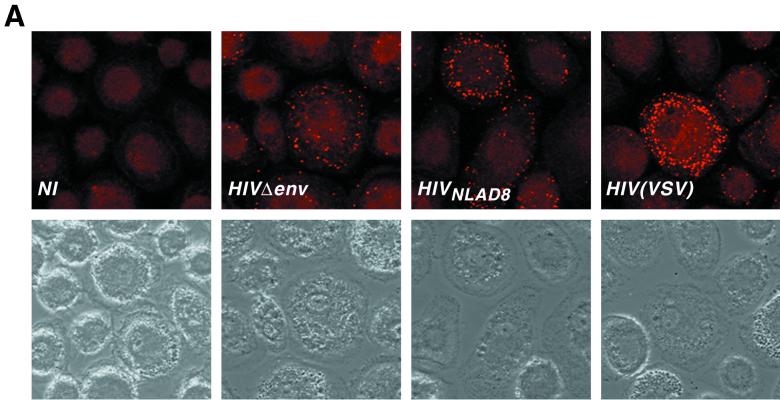
We examined the uptake of viral material by macrophages by immunofluorescence analysis. Macrophages were exposed either to HIVNLAD8 or to an HIV(VSV) pseudotype, which mediates infection through a pH-dependent endocytic pathway (2, 26). Noninfectious HIV-1 particles that lacked a viral envelope (HIVΔenv) were used to assess nonspecific viral uptake. Macrophages were incubated for 30 min at 37°C with equal amounts of p24 for each viral preparation and analyzed by confocal fluorescence microscopy using anti-Gag MAbs. Multiple intracellular dots were observed with the three viral strains (Fig 4A). However, intracellular dots were less abundant with HIVΔenv than with HIVNLAD8 or HIV(VSV). These data strongly suggest that virus material was internalized into macrophages irrespective of adequate envelope-receptor interactions. It is thus presumable that most of the internalized viral material does not participate in the infectious process.
FIG. 4.
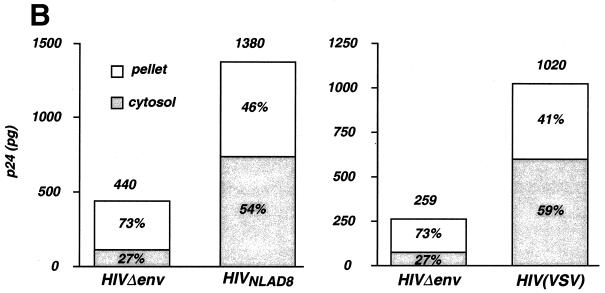
HIV-1 entry analyzed by immunofluorescence and by cellular fractionation. (A) Confocal microscopy analysis of intracellular p24 after macrophage exposure to HIV-1. Macrophages were exposed to the indicated strains for 30 min at 37°C, washed, fixed, and labeled with anti-Gag MAbs. (Top) Immunofluorescence analysis. (Bottom) Phase contrast of the same fields. Noninfected (NI) macrophages were similarly stained as a negative control. (B) p24 levels in subcellular extracts of macrophages exposed to HIV-1. Macrophages were exposed to the R5-tropic HIVNLAD8 strain (left) or to HIV(VSV) pseudotype (right). Noninfectious HIV-1 particles devoid of envelope protein (HIVΔenv) were used as a control. Viral input corresponded to 450 ng of p24 for 2 h at 37°C. After viral exposure, cells were treated by pronase to eliminate virus adsorbed at the cell, and p24 contents were measured in the cytosolic and vesicular (pellet) fractions. Total intracellular p24 levels (in picograms) are indicated over the bars. The percentages of cytosolic and vesicular p24 are shown inside the bars. Data are the means of triplicate measurements (the standard deviation [SD] was below 10%) and are representative of at least three experiments.
We previously described a subcellular fractionation assay that measures cystosolic and vesicular HIV-1 Gag p24 content early after viral exposure (26). In the absence of envelope glycoprotein on virions or of viral receptors, p24 was incorporated in intracellular vesicles but was not detected in the cytosolic fraction. In contrast, when appropriate envelope-receptor interactions could occur, the cytosolic fraction represented 10 to 40% of intracellular p24, indicating that cytosolic p24 is a reliable indicator of virus internalization leading to authentic infection. However, we reported that infectious virus undergoes nonspecific vesicular uptake similarly as nonenveloped virions, and that the majority of internalized particles ends up being degraded in lysosomes. (26). In our previous report, target cells were exposed to virions, treated with pronase with the purpose to remove extracellular virus particles, disrupted, and postnuclear cell extracts were separated into cytosolic and vesicular fractions. Here, we modified this protocol for primary macrophages, which are more fragile than immortalized cell lines (HeLa and lymphoid cell lines) that we initially used, by using lower pronase concentrations and by permeabilizing plasma membranes with digitonin (22) (see Material and Methods).
Macrophages were exposed to similar amounts of HIVNLAD8 and HIVΔenv (450 ng of p24 in 1 ml per ∼2 × 106 cells, for 2 h at 37°C) and fractionated. The lysosomal Lamp1 and Lamp2 proteins were detected by Western blotting in the vesicular fraction only, indicating that the cytosolic fraction was free of detectable vesicular contaminants (not shown). In the representative experiment depicted in Fig. 4B, the total intracellular p24 content reached 1,380 pg for HIVNLAD8 and 440 pg for HIVΔenv. This represented 0.31 and 0.1% of the viral input, respectively. These data show that the uptake of viral material by macrophages was low and did not require specific envelope-receptor interactions. However, uptake was ∼3-fold more efficient when virus particles were coated with envelope glycoproteins. These results confirmed the observations made by immunofluorescence (Fig. 4A). Cytosolic p24 represented about 55% of the internalized material for HIVNLAD8 (corresponding to 759 pg of p24) (Fig. 4B) and only 27% upon exposure to HIVΔenv (corresponding to 110 pg of p24). This was confirmed in six independent experiments where the p24 cytosolic content was 4.2-fold higher with HIVNLAD8 than with HIVΔenv (not shown). Detection of p24 in the cytosolic fraction after exposure to HIVΔenv likely corresponded to background contamination. We also measured cytosolic p24 after exposure to HIV(VSV) pseudotypes. A representative experiment depicted in Fig. 4B shows that the total intracellular uptake was approximatively fourfold higher with HIV(VSV) than with HIVΔenv. Moreover, with HIV(VSV), 59% of the internalized p24 was detected in the cytosolic fraction (Fig. 4B). When compared to noninfectious HIVΔenv virions, the cytosolic content was 6.7-fold higher (in three independent experiments; results not shown). Altogether, these experiments suggest that the majority of internalized virions, which were found in vesicles, do not participate to the infectious process. A significant cytosolic p24 content was detected only when incoming virions were able to infect target cells productively. Therefore, measuring cytosolic p24 content provides a convenient indicator of authentic HIV-1 entry in macrophages.
pH-independent HIV-1 entry in macrophages.
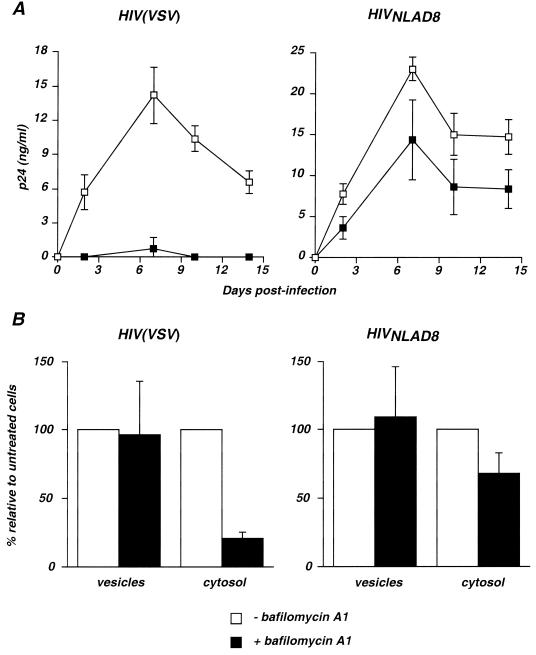
As ultrastructural analysis indicated that a significant part of incoming HIV-1 virions is present in large vesicles, we examined whether infection requires vesicular acidification. Macrophages were treated with bafilomycin A1, an inhibitor of vacuolar proton-ATPases that impairs vesicle acidification, blocks infection by pH-dependent viruses, and inhibits endosomal and lysosomal degradation systems (2, 4, 26). The effect of bafilomycin A1 on HIV(VSV) and HIVNLAD8 infection was first examined in a single-cycle replication assay. In this assay, in order to avoid secondary replication cycles, macrophages were treated with the reverse transcriptase inhibitor AZT at 20 h after virus exposure. Viral production was monitored by measuring p24 production in cell supernatants over a 12- to 15 day-culture period. In the absence of bafilomycin A1, a peak of 15 to 25 ng of p24/ml was measured at day 6 postinfection (Fig. 5A). Of note, p24 was not detected after exposure of macrophages to HIVΔenv virions or when cells were treated with AZT before infection by HIVNLAD8 and HIV(VSV) (not shown). Thus, p24 production in supernatants was not due to nonspecific regurgitation of the viral inoculum. Bafilomycin A1 (at 0.5 μM) fully abrogated p24 production after exposure to HIV(VSV) pseudotypes (Fig. 5A). A subcellular fractionation assay was also performed 2 h after exposure to HIV(VSV). A dramatic decrease in the amount of cytosolic p24 (a fivefold reduction) was observed with bafilomycin A1, whereas levels of vesicular p24 were unchanged in comparison with untreated cells (Fig. 5B). These results indicated that the drug inhibited the release of HIV(VSV) virions from intracellular vesicles, in agreement with previous reports documenting the pH-dependency of HIV(VSV) pseudotypes (2, 26).
FIG. 5.
Effect of bafilomycin A1 on HIV replication and entry into macrophages. (A) Single-cycle replication assay. Macrophages were preincubated with or without bafilomycin A1 (0.5 μM) and exposed to either HIV(VSV) (left) or HIVNLAD8 (right ). The virus inoculum corresponded to 50 ng of p24 for 4 h at 37°C. Macrophages were washed to eliminate drug and virus; 20 h after viral exposure, AZT was added to the culture medium to prevent secondary replication cycles. Virus replication was assessed by measuring p24 production in the cell supernatants. Data are means ± SD of triplicate measurements and are representative of at least three independent experiments. (B) Intracellular p24 levels in cytosolic and vesicular fractions. Macrophages were preincubated with or without bafilomycin A1 (0.5 μM) and exposed to HIV(VSV) (left ) or HIVNLAD8 (right ). Viral input corresponded to 450 ng of p24 for 2 h at 37°C. p24 contents in subcellular fractions were then measured. Values represent relative p24 levels in treated cells, with 100% corresponding to untreated cells. Data are means ± SD of at least three independent experiments.
In contrast, infection of macrophages by HIVNLAD8 was only moderatly affected by bafilomycin A1 (Fig. 5A). Subcellular fractionation showed a proportional decrease in cytosolic p24 content (Fig. 5B). This weak effect of bafilomycin A1 on HIVNLAD8 internalization was likely due to side effects of the compound, which is known to affect various stages of vesicular transport through the endosomal compartment (4). Taken together, these results indicate that in macrophages, HIV(VSV) pseudotype entry requires an acidification step, whereas that of R5-tropic HIV-1 is pH independent.
HIV-1 entry in macrophages mediated by macropinocytosis.
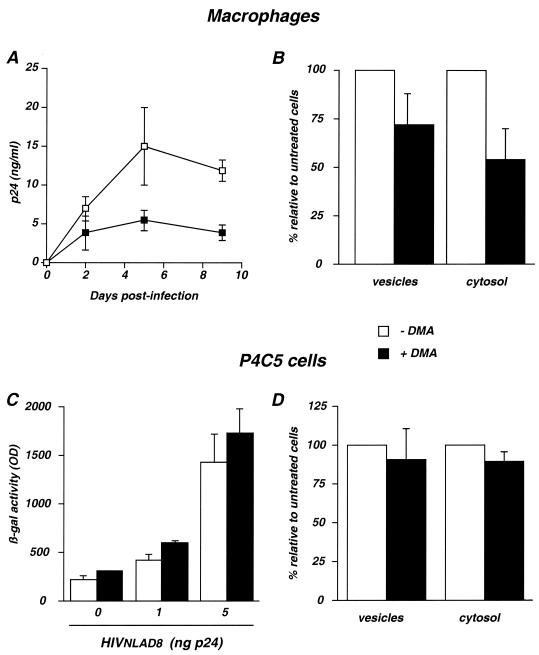
Macrophages actively form large vesicles, or macropinosomes, containing fluid taken up from the surrounding medium. Macropinosomes are formed at the cell margin by membrane ruffles (53). These pinosomes move centripetally and shrink as they approach the nucleus. Membrane ruffling and macropinocytosis can be inhibited by amiloride and more potent analogs, such as DMA. Amiloride analogs are Na+/H+ channel inhibitors which selectively block macropinocytosis without affecting receptor-mediated endocytosis (14, 38, 45, 55). As the size and aspect of vacuoles containing HIV-1 virions (Fig. 1 to 3) were reminiscent of those of macropinosomes (44, 45, 53), we examined whether HIV-1 uses this pathway as an entry route in macrophages. To this aim, we tested the inhibitory effects of DMA on both viral replication and entry. We first verified that DMA (at 100 μM) did not affect cell viability (not shown) (14, 38, 45, 55). Single-cycle assays showed that DMA significantly inhibited HIVNLAD8 replication in macrophages (Fig. 6A). Inhibition was associated with a decrease of internalized p24 in the viral entry assay (Fig. 6B). Both vesicular and cytosolic p24 contents were reduced (Fig. 6B). These results indicated that DMA impaired uptake of virus particles into vesicles and also significantly decreased access of viral material to the cytoplasm. They are consistent with previous reports indicating that DMA inhibits the formation of ruffles and macropinosomes, as well as the uptake of fluid phase markers (11, 14, 45, 53).
FIG. 6.
Effect of DMA on HIV replication and entry. (A and B) Effect of DMA in macrophages. Cells were preincubated with or without DMA (100 μM) and exposed to HIVNLAD8. (A) Single-cycle replication assay. Experimental conditions were similar to those described in the legend to Fig. 5A. Data are means ± SD of triplicate measurements and are representative of three independent experiments. (B) Entry assay. Experimental conditions were similar to those described in the legend to Fig. 5B. Values represent relative p24 levels in treated cells, with 100% corresponding to untreated cells. Data are means ± SD of at least three independent experiments. (C and D) Effect of DMA in P4C5 cells. P4C5 cells are HeLa CD4+ CCR5+ cells carrying an integrated HIV LTR-lacZ cassette. (C) Single-cycle replication assay. Cells were preincubated with or without DMA (100 μM) and exposed to the indicated doses of HIVNLAD8 for 2 h at 37°C. Infection was assessed by measuring β-galactosidase activity in cell extracts 24 h later. Data are means ± SD of triplicates and are representative of two independent experiments. OD, optical density. (D) Entry assay in P4C5 cells. Cells were treated and processed as for macrophages. Data are means ± SD of three independent experiments.
Macropinocytosis has been described for a few cell types only, including macrophages and dendritic cells (44, 45, 53). In contrast, epithelial cells display little or no macropinocytic activity in the absence of stimulation by growth factors or phorbol esters (40, 53, 55). We thus tested the effects of DMA on HIV-1 replication and entry in the P4C5 cell line, an epithelial-derived HeLa CD4+ CCR5+ clone (3). P4C5 cells carry an integrated HIV-LTR lacZ cassette which is activated by Tat upon infection. β-Galactosidase expression levels correlated with infection efficiency in a single-cycle viral replication assay (3, 26). HIVNLAD8 infection of P4C5 cells was not affected by DMA at 100 μM (Fig. 6C). Similar results were obtained when viral growth was monitored by measuring p24 release in the supernatants (not shown). Accordingly, in the entry assay, vesicular and cytosolic p24 levels were not significantly inhibited (Fig. 6D).
These data indicate that DMA significantly inhibited HIV-1 entry and replication in primary macrophages but was unefficient in HeLa cells, which display little or no macropinocytic activity. These results are consistent with a significant participation of macropinocytosis in the entry process of HIV-1 in macrophages.
DISCUSSION
The literature is inconsistent about the mechanism of HIV-1 entry into target cells. Previous studies have mostly been performed using permanent cell lines, and there was controversy about whether virions fuse at the plasma membrane or are internalized via receptor-mediated endocytosis (7, 19, 25, 29, 42, 51). Here, we have studied the entry of a R5-tropic HIV-1 strain in primary macrophages, in a system relevant to the pathophysiology of the infection. Electronic microscopy analysis at an early time point after viral exposure showed HIV-1 virions at multiple cellular locations: at the plasma membrane, inside clathrin-coated pits, and in spacious intracellular vacuoles which were reminiscent of macropinosomes. Immunofluorescence analysis indicated that macrophages internalize noninfectious HIVΔenv particles as well as virions coated with HIV-1 or VSV-G envelope glycoproteins. Thus, there is little selectivity with regard to HIV-1 uptake by macrophages. However, with HIVΔenv, incoming p24 was primarily located in intracellular vesicules and was virtually absent from the cytosol, indicating that in the absence of envelope, incoming virions were not delivered to the cytoplasm and thus were unable to perform subsequent steps of the viral cycle. When adequate envelope-receptor interactions took place and fusion occurred, overall p24 uptake was higher, and 50% of the internalized material was detected in the cytosolic fraction. It is noticeable that during the course of an effective infectious process, the majority of internalized virions ends up being degraded (26), as visualized here by electron microscopy images of virions undergoing destruction inside intracellular vesicles. However, we provide several lines of evidence strongly suggesting that a fraction of the virions that are internalized into intracellular vesicles escapes destruction and leads to productive infection in macrophages.
Images of virus particles undergoing fusion with vesicular membranes were observed by electronic microscopy. This morphological evidence was supported by subcellular fractionation and viral replication assays. Since the size and aspect of the spacious vacuoles containing virus particles were reminiscent of macropinosomes, we examined the activity of DMA, a selective inhibitor of macropinocytosis (14, 38, 45, 55). DMA significantly impaired the uptake of virions as well as the delivery of p24 proteins in the cytoplasm (twofold reduction of cytosolic p24) but was inefficient in P4C5 epithelial cells, which display low levels of macropinocytosis (40, 53, 55). DMA similarly inhibited entry of virions coated with HIV-1 and with VSV-G envelope (not shown). This was not unexpected, since macropinocytosis does not involve specific envelope-receptor interactions (53). Interestingly, the effect of DMA activity on viral entry was associated with a direct inhibitory effect on viral replication. Altogether, these results strongly suggest that incoming HIV-1 virions can be internalized via macropinocytosis in macrophages. A large part of macropinocytosed virions is degraded, likely because macropinocytosis intersects the endosome/lysosome pathway in these cells (44, 53). However, after internalization by macropinocytosis, envelope-mediated fusion of HIV-1 virions with the vesicular membrane can occur, leading to productive infection of macrophages. Entry by macropinocytosis does not necessarily mean that a pH-dependent step is required for fusion. For instance, pH-independent entry via endosomal vesicles has been reported for poliovirus (43). Accordingly, we show here that HIV-1 productive entry in macrophages was insensitive to inhibitors of vesicular acidification such as bafilomycin A1. In contrast, entry and replication of HIV(VSV) pseudotypes, which require a low pH to fuse in endosomes, was inhibited by bafilomycin A1. Our study shows that viral entry is DMA sensitive and bafilomycin A1 resistant in primary macrophages and DMA and bafilomycin A1 resistant in an HeLa-derived cell line, indicating that HIV-1 entry routes vary according to the cell type.
There are multiple possibilities for virions to interact with target cells. Entry via macropinocytosis does not require any binding at the cell surface, since in this process the extracellular fluid is engulfed by cellular ruffles (53). Initial virus-cell interactions may also involve semi- or nonspecific binding of Env with cell surface heparan sulfate proteoglycans (31, 47) or of virion and cellular adhesion factors such as intercellular adhesion molecule 1 and leukocyte function-associated antigen 1 (17). Binding of glycan moieties of Env with cell surface lectins, such as DC-SIGN in dendritic cells, also promotes virus internalization (18). More specific virus-cell interactions also occur. After or during initial binding of virions to target cells, Env gp120 interacts with CD4. This initiates a conformational change which facilitates its binding to a coreceptor molecule, mainly CCR5 or CXCR4. Further conformational modifications of gp120/gp41 complexes will then lead to fusion of viral and cellular membranes (10, 33). Therefore, the multiplicity of virion locations observed by electronic microscopy early after viral exposure likely reflects these various possible virion-cell interactions. Besides macropinocytosis, other internalization pathways, such as phagocytosis, may also be involved in HIV-1 uptake by macrophages. Phagocytosis can be mediated by interaction of HIV-1 with receptors for mannosylated proteins. On the other hand, HIV-1 is opsonized in vivo with antibodies and with complement fragments (32, 52). In vitro, infection is enhanced by sera from certain HIV-infected patients. Infection enhancement by antibody or complement is likely mediated by HIV-1 interaction with Fc or complement receptors and entry by phagocytosis (6, 30, 32, 52).
Evidence for HIV-1 fusion within intracellular vesicles is supported by numerous reports indicating that macrophages are particularly refractory to entry inhibitors. The β-chemokines RANTES, macrophage inflammatory protein 1α (MIP-1α) and MIP-1β, as well as sCD4 and the anti-CCR5 MAb PRO140 are 5- to 100-fold less potent in macrophages than in lymphocytes (30, 50, 54, 57). Levels of CD4 and chemokine receptors or proteoglycans, which vary according to differentiation and activation states of macrophages, are probably involved in these impotencies. However, the composition of the milieu outside the cell is different from that of the lumens of endosomes or macropinosomes (11, 53). Local conditions of pH and concentrations of solutes, proteins, and enzymes will likely interfere with the efficacy of inhibitors in macrophages. Moreover, CCR5 is expressed in multiple conformational states (24), which could vary upon its cellular location and influence both viral fusion and inhibitor potency. Additionally, to be active, inhibitors will have to reach intracellular sites of viral fusion. That HIV-1 productively infects macrophages after macropinocytosis or other internalization pathways should therefore be taken in account when designing new inhibitors aimed at blocking viral entry or fusion (10, 33).
Our results provide an explanation for the puzzling observations that replication of SIVmac239 as well as of some X4-tropic HIV-1 strains is blocked in macrophages after entry (9, 34, 35, 49). These strains synthesize normal or subnormal amounts of proviral DNA, without pursuing the viral cycle (9, 34, 35, 49). It has been proposed that Env-receptor interactions are required for postentry steps such as nuclear translocation of preintegration complexes (9, 49). One can also hypothesize that virions endocytosed in macrophages encounter an environment allowing reverse transcription to proceed, at least partially. The viral cycle will then be aborted without adequate envelope-receptor interactions and viral fusion.
In dendritic cells, macropinocytosis is highly active and should play an important role for HIV-1 entry as well. Macropinocytosis is a key mechanism of antigen capture (23, 45). We recently reported that epitopes derived from incoming HIV-1 virions are presented by MHC-I in dendritic cells and macrophages, leading to cytotoxic T-lymphocyte activation in the absence of viral replication (8). Therefore, HIV-1 macropinocytosis has potentially important immunological implications, by providing an entry route leading to the exogenous presentation of HIV-1 antigens in dendritic cells and macrophages.
In infected individuals, HIV-1 particles have been observed within vacuoles of macrophages (41), suggesting that the entry pathway described here is relevant to the in vivo situation. Deciphering the mechanisms of HIV-1 entry in macrophages and dendritic cells may more clearly define the physiopathology of the disease and strategies for therapeutic intervention aimed at blocking viral entry.
ACKNOWLEDGMENTS
We thank David Ojcius for critical reading of the manuscript and Christine Schmitt for preparing the cells for electron microscopy. We thank Eric Freed, A. Miyanohara, and François Traincart for the kind gift of reagents.
This work was supported by grants from the Agence Nationale de Recherche sur le SIDA, SIDACTION, and the Pasteur Institute.
REFERENCES
- 1.Aderem A, Underhill D. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 2.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara A, Le Gall S, Schwartz O, Salamero J, Montes M, Loetscher P, Baggioloni M, Virelizier J L, Arenzana-Seisdedos F. HIV co-receptor down-regulation as anti-viral principle. SDF-1α dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer N, Schober D, Prchla E, Murphy R F, Blaas D, Fuchs R. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J Virol. 1998;72:9645–9655. doi: 10.1128/jvi.72.12.9645-9655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borman A W, Quillent C, Charneau P, Dauguet C, Clavel F. Human immunodeficiency virus type 1 Vif-mutant particles from restrictive cells: role of Vif in correct particle assembly and infectivity. J Virol. 1995;69:2058–2067. doi: 10.1128/jvi.69.4.2058-2067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouhlal H, Galon J, Kazatchkine M D, Fridman W H, Sautes-Fridman C, Haeffner Cavaillon N. Soluble CD16 inhibits CR3 (CD11b/CD18)-mediated infection of monocytes/macrophages by opsonized primary R5 HIV-1. J Immunol. 2001;166:3377–3383. doi: 10.4049/jimmunol.166.5.3377. [DOI] [PubMed] [Google Scholar]
- 7.Bourinbaiar A S, Phillips D M. Transmission of human immunodeficiency virus from monocytes to epithelia. J Acquir Immune Defic Syndr. 1991;4:56–63. [PubMed] [Google Scholar]
- 8.Buseyne F, Le Gall S, Boccaccio C, Abastado J P, Lifson J D, Arthur L O, Rivière Y, Heard J M, Schwartz O. MHC-I-restricted presentation of HIV-1 virion antigens without viral replication. Nat Med. 2001;7:344–349. doi: 10.1038/85493. [DOI] [PubMed] [Google Scholar]
- 9.Chackerian B, Long E M, Luciw P A, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan D C, Kim P S. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 11.de Baey A, Lanzavecchia A. The role of aquaporins in dendritic cell macropinocytosis. J Exp Med. 2000;191:743–748. doi: 10.1084/jem.191.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeTulleo L, Kirchhausen T. The clathrin endocytic pathway in viral infection. EMBO J. 1998;17:4585–4593. doi: 10.1093/emboj/17.16.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitrov D. Cell biology of virus entry. Cell. 2000;101:697–702. doi: 10.1016/s0092-8674(00)80882-x. [DOI] [PubMed] [Google Scholar]
- 14.Dowrick P, Kenworthy P, McCann B, Warn R. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/scatter factor-treated cells. Eur J Cell Biol. 1993;61:44–53. [PubMed] [Google Scholar]
- 15.Englund G, Theodore T S, Freed E O, Engelman A, Martin M A. Integration is required for productive infection of monocyte-derived macrophages by human immunodeficiency virus type 1. J Virol. 1995;69:3216–3219. doi: 10.1128/jvi.69.5.3216-3219.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fackler O T, Peterlin B M. Endocytic entry of HIV-1. Curr Biol. 2000;10:1005–1008. doi: 10.1016/s0960-9822(00)00654-0. [DOI] [PubMed] [Google Scholar]
- 17.Fortin J F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geijtenbeek T B, Kwon D S, Torensma R, Van Vliet S J, Van Duijnhoven G C, Middel J, Cornelissen I L, Nottet H, KewalRamani V, Littman D, Figdor C G, Van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 19.Grewe C, Beck A, Gelderblom H R. HIV: early virus-cell interactions. J Acquir Immune Defic Syndr. 1990;3:965–974. [PubMed] [Google Scholar]
- 20.Henley J R, Krueger E W, Oswald B J, McNiven M A. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kizhatil K, Albritton L M. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J Virol. 1997;71:7145–7156. doi: 10.1128/jvi.71.10.7145-7156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight D E, Scrutton M C. Gaining access to the cytosol: the technique and some applications of electropermeabilization. Biochem J. 1986;234:497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzavecchia A. Mechanisms of antigen uptake for presentation. Curr Opin Immunol. 1996;8:348–354. doi: 10.1016/s0952-7915(96)80124-5. [DOI] [PubMed] [Google Scholar]
- 24.Lee B, Sharron M, Blanpain C, Doranz B J, Vakili J, Setoh P, Berg E, Liu G, Guy H R, Durell S R, Parmentier M, Chang C N, Price K, Tsang M, Doms R W. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–9626. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 25.Maddon P J, McDougal J S, Clapham P R, Dalgleish A G, Jamal S, Weiss R A, Axel R. HIV infection does not require endocytosis of its receptor, CD4. Cell. 1988;54:865–874. doi: 10.1016/s0092-8674(88)91241-x. [DOI] [PubMed] [Google Scholar]
- 26.Maréchal V, Clavel F, Heard J M, Schwartz O. Cytosolic Gag p24 as an index of productive entry of human imunodeficiency virus type 1. J Virol. 1998;72:2208–2212. doi: 10.1128/jvi.72.3.2208-2212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh M, Pelchen-Matthews A. Endocytosis in viral replication. Traffic. 2000;1:525–532. doi: 10.1034/j.1600-0854.2000.010701.x. [DOI] [PubMed] [Google Scholar]
- 28.Martin J C, Bandres J C. Cells of the monocyte-macrophage lineage and pathogenesis of HIV-1 infection. J Acquir Immune Defic Syndr. 1999;22:413–429. doi: 10.1097/00126334-199912150-00001. [DOI] [PubMed] [Google Scholar]
- 29.McClure M O, Marsh M, Weiss R A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988;7:513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meltzer M S, Skillman D R, Hoover D L, Hanson B D, Turpin J A, Kalter D C, Gendelman H E. Macrophages and the immunodeficiency virus. Immunol Today. 1990;11:217–223. doi: 10.1016/0167-5699(90)90086-o. [DOI] [PubMed] [Google Scholar]
- 31.Mondor I, Ugolini S, Sattentau Q J. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J Virol. 1998;72:3623–3634. doi: 10.1128/jvi.72.5.3623-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montefiori D C. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Springer Semin Immunopathol. 1997;18:371–390. doi: 10.1007/BF00813504. [DOI] [PubMed] [Google Scholar]
- 33.Moore J P, Stevenson M. New targets for inhibitors of HIV-1 replication. Nat Rev Mol Cell Biol. 2000;1:40–49. doi: 10.1038/35036060. [DOI] [PubMed] [Google Scholar]
- 34.Mori K, Ringler D J, Desrosiers R C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993;67:2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori K, Rosenzweig M, Desrosiers R C. Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J Virol. 2000;74:10852–10859. doi: 10.1128/jvi.74.22.10852-10859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mothes W, Boerger A L, Narayan S, Cunningham J M, Young J A. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell. 2000;103:679–689. doi: 10.1016/s0092-8674(00)00170-7. [DOI] [PubMed] [Google Scholar]
- 37.Neyrolles O, Brenner C, Prevost M C, Fontaine T, Montagnier L, Blanchard A. Identification of two glycosylated components of Mycoplasma penetrans: a surface-exposed capsular polysaccharide and a glycolipid fraction. Microbiology. 1998;144:1247–1255. doi: 10.1099/00221287-144-5-1247. [DOI] [PubMed] [Google Scholar]
- 38.Norbury C C, Hewlett L J, Prescott A R, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 39.Norkin L C. Simian virus 40 infection via MHC class I molecules and caveolae. Immunol Rev. 1999;168:13–22. doi: 10.1111/j.1600-065x.1999.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 40.Ojcius D M, Bravo de Alba Y, Kanellopoulos J M, Hawkins R A, Kelly K A, Rank R G, Dautry-Varsat A. Internalization of Chlamydia by dendritic cells and stimulation of Chlamydia-specific T cells. J Immunol. 1998;160:1297–1303. [PubMed] [Google Scholar]
- 41.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 42.Pauza C D, Price T M. Human Immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J Cell Biol. 1988;107:959–968. doi: 10.1083/jcb.107.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez L, Carrasco L. Entry of polivirus into cells does not require a low-pH step. J Virol. 1993;67:4543–4548. doi: 10.1128/jvi.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Racoosin E L, Swanson J A. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011–1020. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandvig K, van Deurs B. Entry of ricin and shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 2000;19:5943–5950. doi: 10.1093/emboj/19.22.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saphire A C, Bobardt M D, Gallay P A. Host cyclophilin A mediates HIV-1 attachment to target cells via heparans. Embo J. 1999;18:6771–6785. doi: 10.1093/emboj/18.23.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmid S L. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 49.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 51.Stein B S, Gowda S D, Lifson J D, Penhallow R C, Bensch K G, Engleman E G. pH-independent HIV entry into CD4-positive cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 52.Stoiber H, Clivio A, Dierich M P. Role of complement in HIV infection. Annu Rev Immunol. 1997;15:649–674. doi: 10.1146/annurev.immunol.15.1.649. [DOI] [PubMed] [Google Scholar]
- 53.Swanson J A, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- 54.Trkola A, Ketas T J, Nagashima K A, Zhao L, Cilliers T, Morris L, Moore J P, Maddon P J, Olson W C. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J Virol. 2001;75:579–588. doi: 10.1128/JVI.75.2.579-588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West M A, Bretscher M S, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yewdell J W, Norbury C C, Bennink J R. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 57.Ylisastigui L, Bakri Y, Amzazi S, Gluckman J C, Benjouad A. Soluble glycosaminoglycans do not potentiate RANTES antiviral activity on the infection of primary macrophages by human immunodeficiency virus type 1. Virology. 2000;278:412–422. doi: 10.1006/viro.2000.0670. [DOI] [PubMed] [Google Scholar]