Abstract
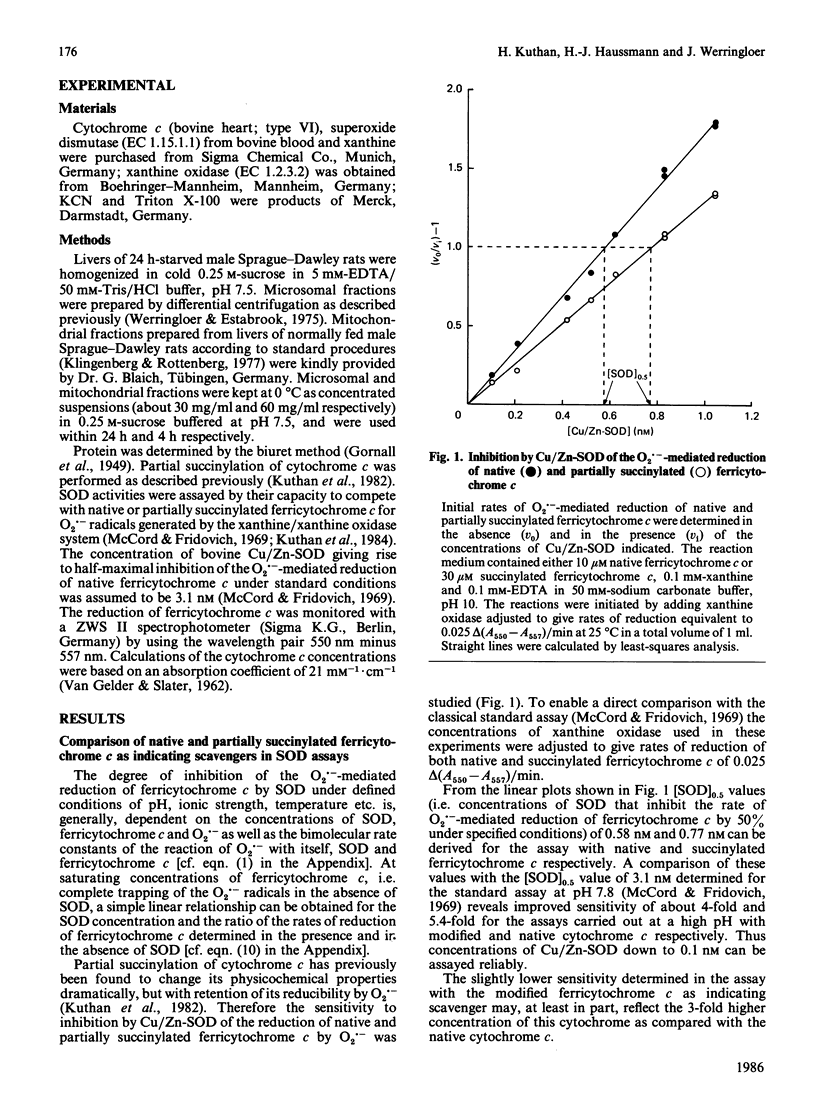
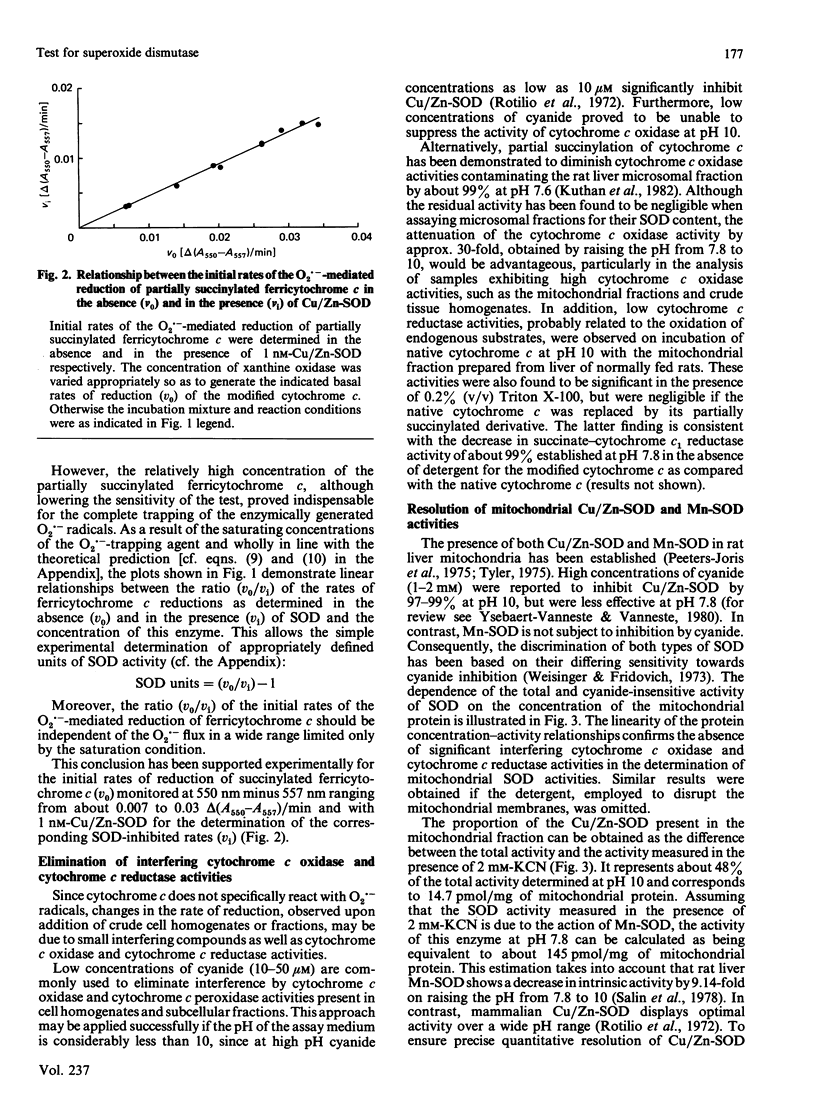
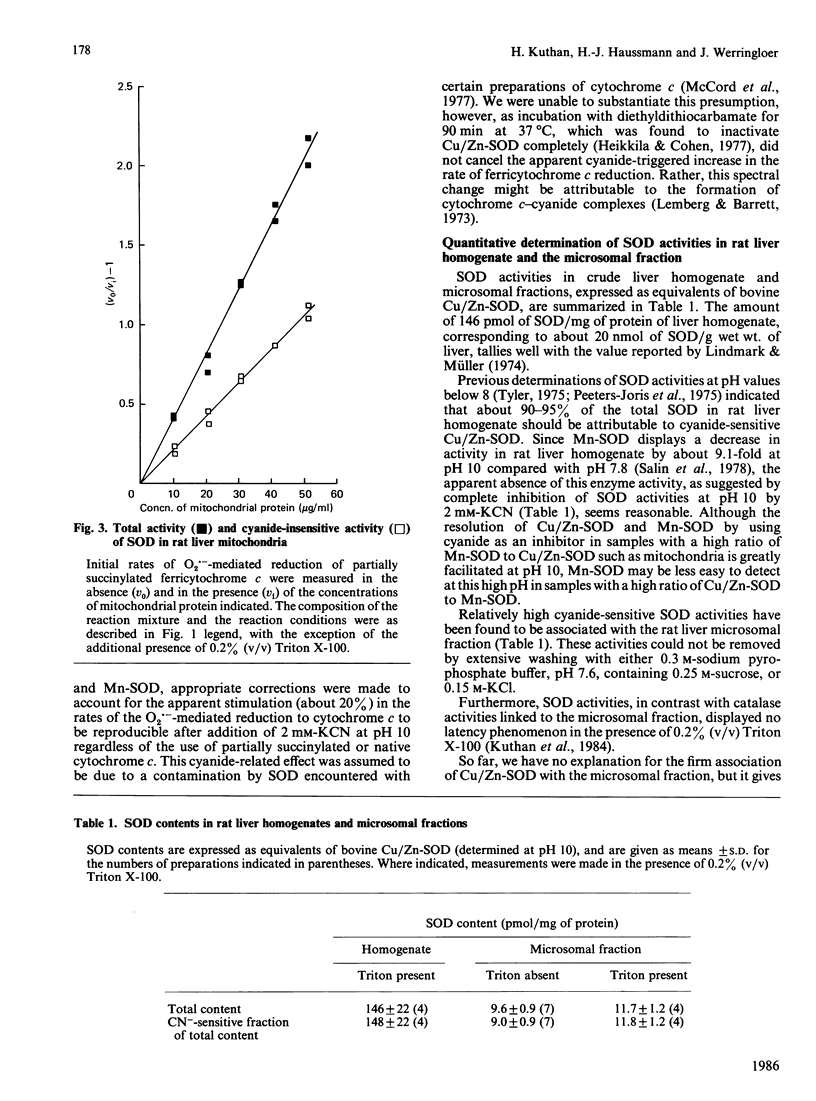
A sensitive and reliable assay method was developed to characterize crude cell homogenates and subcellular fractions with regard to their superoxide dismutase (SOD) activities. The determination of SOD activities was based on the well-known spectrophotometric assay introduced by McCord & Fridovich [(1969) J. Biol. Chem. 244, 6049-6055], with partially succinylated (3-carboxypropionylated) rather than native ferricytochrome c as indicating scavenger. Partial succinylation of cytochrome c resulted in minimization of interference associated with the interaction of cytochrome c with mitochondrial cytochrome c oxidase or cytochrome c reductases. The further increase in specificity, with regard to exclusion of cytochrome c oxidase interference, gained as a consequence of the high pH of 10 enabled the analysis of samples as rich in cytochrome c oxidase activity as the mitochondrial fraction in the presence or absence of membrane-disrupting detergents. Linear relationships for the dependence of the SOD activities with protein concentration were obtained with rat liver homogenate, mitochondrial and microsomal fractions, indicating negligible interference. Furthermore, by choosing a high pH for the assay medium, a 4-fold increase in sensitivity compared with the classical SOD assay, carried out at pH 7.8, was gained as well as a more precise resolution of Cu/Zn-SOD and Mn-SOD by 2 mM-KCN in samples with a high ratio of Mn-SOD to Cu/Zn-SOD, such as mitochondria. The complete trapping of the O2.- radicals, which was more feasible at pH 10 than at pH 7.8, enabled the application of a simple equation derived for the calculation of appropriately defined units of SOD activity from a single experiment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A., Montecucco C., Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun. 1975 Jul 22;65(2):597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Flohé L., Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- Geller B. L., Winge D. R. A method for distinguishing Cu,Zn- and Mn-containing superoxide dismutases. Anal Biochem. 1983 Jan;128(1):86–92. doi: 10.1016/0003-2697(83)90348-2. [DOI] [PubMed] [Google Scholar]
- Kirby T. W., Fridovich I. A picomolar spectrophotometric assay for superoxide dismutase. Anal Biochem. 1982 Dec;127(2):435–440. doi: 10.1016/0003-2697(82)90200-7. [DOI] [PubMed] [Google Scholar]
- Klingenberg M., Rottenberg H. Relation between the gradient of the ATP/ADP ratio and the membrane potential across the mitochondrial membrane. Eur J Biochem. 1977 Feb 15;73(1):125–130. doi: 10.1111/j.1432-1033.1977.tb11298.x. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H., van Buuren K. J., Butler J., Braams R. The kinetics of the reduction of cytochrome c by the superoxide anion radical. Biochim Biophys Acta. 1976 Nov 9;449(2):157–168. doi: 10.1016/0005-2728(76)90130-4. [DOI] [PubMed] [Google Scholar]
- Kuthan H., Ullrich V., Estabrook R. W. A quantitative test for superoxide radicals produced in biological systems. Biochem J. 1982 Jun 1;203(3):551–558. doi: 10.1042/bj2030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuthan H., Ullrich V., Estabrook R. W. A quantitative test for superoxide radicals produced in biological systems. Biochem J. 1982 Jun 1;203(3):551–558. doi: 10.1042/bj2030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Superoxide dismutase in the anaerobic flagellates, Tritrichomonas foetus and Monocercomonas sp. J Biol Chem. 1974 Jul 25;249(14):4634–4637. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. Superoxide dismutase: "positive" spectrophotometric assays. Anal Biochem. 1977 May 1;79(1-2):553–560. doi: 10.1016/0003-2697(77)90429-8. [DOI] [PubMed] [Google Scholar]
- Peeters-Joris C., Vandevoorde A. M., Baudhuin P. Subcellular localization of superoxide dismutase in rat liver. Biochem J. 1975 Jul;150(1):31–39. doi: 10.1042/bj1500031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotilio G., Bray R. C., Fielden E. M. A pulse radiolysis study of superoxide dismutase. Biochim Biophys Acta. 1972 May 12;268(2):605–609. doi: 10.1016/0005-2744(72)90359-2. [DOI] [PubMed] [Google Scholar]
- Salin M. L., Day E. D., Jr, Crapo J. D. Isolation and characterization of a manganese-containing superoxide dismutase from rat liver. Arch Biochem Biophys. 1978 Apr 15;187(1):223–228. doi: 10.1016/0003-9861(78)90027-9. [DOI] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMORI S., WADA K., ANDO K., HOSOKAWA M., SEKUZU I., OKUNUKI K. Studies on cytochrome a. VIII. Reaction of cytochrome a with chemically modified cytochrome c and basic proteins. J Biochem. 1962 Jul;52:28–37. doi: 10.1093/oxfordjournals.jbchem.a127568. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. Polarographic assay and intracellular distribution of superoxide dismutase in rat liver. Biochem J. 1975 Jun;147(3):493–504. doi: 10.1042/bj1470493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- Werringloer J., Estabrook R. W. Heterogeneity of liver microsomal cytochrome P-450: the spectral characterization of reactants with reduced cytochrome P-450. Arch Biochem Biophys. 1975 Mar;167(1):270–286. doi: 10.1016/0003-9861(75)90463-4. [DOI] [PubMed] [Google Scholar]
- Ysebaert-Vanneste M., Vanneste W. H. Quantitative resolution of Cu,Zn- and Mn-superoxide dismutase activities. Anal Biochem. 1980 Sep 1;107(1):86–95. doi: 10.1016/0003-2697(80)90496-0. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


