Abstract
Background
Ventricular assist device (VAD) implantation has become an alternative treatment for patients with end-stage heart failure. In Germany, valid and reliable instruments to assess health-related quality of life in patients with VAD are lacking.
Objective
The aim of this study was to present the psychometric validation of the German version of the Quality of Life with a Ventricular Assist Device questionnaire.
Methods
In a multicenter, cross-sectional study, 393 participants (mean age, 58.3 years; 85.8% male, 60.3% bridge to transplant, and 72.8% living with VAD for ≤2 years) completed the German Quality of Life with a Ventricular Assist Device questionnaire of physical, emotional, social, cognitive, and meaning/spiritual domains. Item and confirmatory factor analyses were conducted to test item difficulty and discrimination and the underlying structure, respectively. To examine internal consistency, Cronbach α was assessed. Convergent construct validity was tested using the Kansas City Cardiomyopathy Questionnaire and the Patient Health Questionnaire-9. Readability was examined using Flesch Reading Ease index and Vienna Factual Text Formula.
Results
The Quality of Life with a Ventricular Assist Device showed reasonable item difficulty (Ptotal = .67) and mostly moderate to high discriminatory power (rit > 0.30). In confirmatory factor analysis, root-mean-square error of approximation (0.07) was acceptable for model fit, but no other indices. Acceptable internal consistency was found (α ≥ 0.79), with the exception of the cognitive domain (α = 0.58). The overall questionnaire and single domains demonstrated convergent validity (r ≥ 0.45, P < .001). The questionnaire showed adequate readability (Flesch Reading Ease, 64.11; Vienna Factual Text Formula, 6.91).
Conclusion
Findings indicate a promising standardized clinical instrument to assess health-related quality of life in patients with VAD.
KEY WORDS: health-related quality of life, instrument development, psychometric evaluation, ventricular assist device
The implantation of ventricular assist devices (VADs) has emerged as an alternative treatment strategy for the growing number of patients with end-stage heart failure.1–4 A VAD is an electromechanical cardiovascular assist device that either partially or completely replaces the function of a failing heart.5 The technological advancement of VADs has resulted in a prolonged life and increased use of the devices in long-term therapy.1,2,6 Thus, many patients will spend longer or even the rest of their lives on VAD support. As a consequence, not only the pure survival but also the health-related quality of life (HRQoL) of those patients plays a significant role.7,8
In addition, recent studies indicate that patients may benefit considerably from VAD implantation in terms of improvements in their HRQoL.7,9–12 However, despite VADs being a lifesaving therapy, patients face the risk of serious complications, dependence on a caregiver, and the need to adjust their lifestyles.1–3,6,12
A number of validated instruments are currently available for assessing generic as well as disease-specific HRQoL in patients with heart failure, such as the SF-36 Health Status Questionnaire13 or the Kansas City Cardiomyopathy Questionnaire.14 However, these instruments cannot comprehensively capture the unique demands on patients with durable VAD support (eg, dressing changes, no bathing or swimming, ensuring adequate access to electrical power, device-specific complications or fears),7,15,16 which can impact HRQoL outcomes. To assess HRQoL in this population adequately, the use of a reliable, valid, and VAD-specific HRQoL instrument is needed. Therefore, it is important to develop and psychometrically test instruments targeting the VAD-specific needs of this growing patient population.
Recently, the patients' perspective has been gaining more attention,16,17 with the impact of VAD therapy on patient-reported outcomes becoming increasingly prominent.7,8,18 Quality of life, as well as HRQoL, have been evaluated as patient-reported outcomes.15 The terms quality of life and HRQoL are often used interchangeably.19 Yet, HRQoL focuses on the health-related aspects of quality of life and generally reflects the impact of illness and treatment on disability and daily functioning of an individual.20,21
Within this context, the Quality of Life with a Ventricular Assist Device (QoLVAD) questionnaire, a disease-specific self-report instrument to assess HRQoL in patients on ongoing continuous-flow VAD support, was developed by Sandau and colleagues.15 The questionnaire was developed based on a comprehensive qualitative study that established a conceptual definition of HRQoL for patients on VAD support.15,16 It assesses the experience of HRQoL in 5 disease-specific domains: physical, emotional, social, cognitive, and meaning/spiritual. Initial results of the psychometric validation of the original US version showed acceptable psychometric properties.15,16 Furthermore, in the Ex-VAD study,22 the first nonvalidated German version of the QoLVAD questionnaire was translated.
The overall aim of this study was to evaluate the quality of the German QoLVAD questionnaire using data from 393 patients on VAD support from a multicenter study. Specific aims were (1) to test item function, (2) to assess the instrument's internal consistency, and (3) to estimate its convergent construct validity and underlying structure.
Methods
Study Design
This study was part of phase 1 of the 3-phase national multicenter Self-management for Patients on VAD Support study, which focused on self-management for patients on VAD support (ClinicalTrials.gov ID: NCT04234230).3 Therefore, a cross-sectional observational study was performed in the context of patient-reported outcome research. For more details, please see the study protocol.3 The study was approved by the institutional review board (EK-No. 304/19) and confirmed by the participating centers' review boards before initiation of the data collection process. All study procedures were performed in concordance with the Declaration of Helsinki23 and the European Data Protection Regulation.24 The complete translation process of the QoLVAD questionnaire was performed as part of the Ex-VAD study22 and thus was not part of this project. In this study, the psychometric validation was based on the standardized criteria for the international cultural adaptation of QoL questionnaires.25,26 Findings are reported based on the Strengthening the Reporting of Observational Studies in Epidemiology statement for cross-sectional studies.27
Setting and Participants
The sample was recruited as part of the SELMA study at 4 established heart centers in Germany. Stable patients with ongoing VAD support in the outpatient setting were considered for this study. Other eligibility criteria were cognitive ability and sufficient language skills to consent and participate in a survey-based study, follow-up at the respective site, and in the window between 3 and 36 months after VAD implantation. Patients living in long-term care or rehabilitation centers were intentionally excluded from this study.3
Instruments and Measures
A paper-based questionnaire booklet was administered during outpatient clinical visits. Besides the German QoLVAD questionnaire, the following patient-reported outcome instruments were used to determine convergent construct validity: Kansas City Cardiomyopathy Questionnaire28 and Patient Health Questionnaire-9.29
The Quality of Life with a Ventricular Assist Device Questionnaire
The QoLVAD questionnaire was designed as a quantitative, disease-specific self-report instrument to assess HRQoL in people with VAD support. It was based on a qualitative study designed to develop a conceptual definition of quality of life.15,16
The original QoLVAD was translated from English into German by the working group within the Ex-VAD trial based on International Society for Pharmacoeconomics and Outcomes Research guidelines in 2018.22,30 Two researchers fluent in English, but with German as their first language, made individual first translations. After resolving disagreements, including, where needed, discussion of semantic ambiguities with the author of the original English version, the resulting German version was shared for backward translation with a third bilingual researcher, whose first language was English. The backward-translated questionnaire was checked for discrepancies with the original version, which were mostly minor. All discrepancies were discussed between one of the initial translators and the person who had performed the backward translation, and then resolved.
In the Ex-VAD trial, the standard instruments used (ie, European Quality of Life 5 Dimensions, Kansas City Cardiomyopathy Questionnaire, SF-36 Physical Functioning scale) were supplemented with this German translation of the QoLVAD to cover device-specific aspects of quality of life as a secondary research question.
The US version of the QoLVAD was psychometrically validated15; at the same time, it was being translated and used in the Ex-VAD and SELMA studies. As part of the psychometric validation, items 7, 8a to 8g, 9, 12, and 13 were removed from the US (English) version consistent with the results of content validation by experts, another review by the author team, and item discrimination analysis. For this reason, the US and German versions currently consist of a different number of items. After the validation process, the latest US version consists of 43 items within the 5 subscales as well as additional summary items including 1 open-ended question.15 The German version of the QoLVAD used in the Ex-VAD and SELMA studies includes 54 items in 5 key HRQoL domains for patients with ongoing VAD support: physical (26 items), emotional (10 items), social (10 items), cognitive (3 items), and meaning/spiritual (5 items). Beyond the 5 domains, there are 4 additional items: 2 items on perceived adjustment and improvement, 1 item on global HRQoL, and an open-ended question. Following the developer's instructions,15 the open-ended question serves as an additional method to assess the validity of the newly developed instrument. This open-ended question allows patients to provide comments on their HRQoL with a VAD and can be used to compare the answers given in the questionnaire.
All items of the 5 domains are answered on a 5-point Likert scale (0–4) with an option of “not applicable” for a few select items. To calculate domain scores, at least 80% of items within a respective domain must have been answered.31 The scores obtained are standardized for each of the 5 domains and range from 0 to 100, with higher scores indicating better HRQoL. A total score for the questionnaire assessing overall HRQoL was calculated using the mean of the 5 domains. Therefore, the total score also ranges from 0 to 100, with higher scores indicating better HRQoL.15
Kansas City Cardiomyopathy Questionnaire
The Kansas City Cardiomyopathy Questionnaire comprises 23 items measuring 7 domains of disease-specific health status in patients with heart failure: physical limitations, symptom stability, symptom frequency, symptom burden, self-efficacy, quality of life, and social limitations. For each domain, the scores obtained are transformed to a scale from 0 to 100, with 0 representing the worst and 100 representing the best possible status. In addition, an overall summary score (0–100) was calculated according to the developers' recommendations using the average of the following domains: physical limitations, symptom domains, quality of life, and social limitation.28
Patient Health Questionnaire-9
The patient-health questionnaire measures depressive symptoms on a 4-point Likert scale. Depressive symptoms were assessed using a sum score with possible values between 0 and 27. Higher scores indicate more severe depressive symptoms.29
Demographic and Clinical Data
Demographic data including age, gender, and marital status were collected in the demographic section of the questionnaire. Clinical characteristics including implant strategy and days since VAD implantation were collected from the patient chart with permission from the patient.
Statistical Methods
Statistical analyses were conducted in IBM SPSS Statistics version 26 and R version 3.6.3 using the packages psych, lavaan, semPlot, and GPArotation. Significance level was set at P < .05.
Missing and Nonapplicable Values
Missing values were examined using a missing values analysis. Little's32 test of missing completely at random was used to test for randomness of missing values. All analyses except confirmatory factor analysis were performed using a list-wise exclusion approach. Because of loss of data, a full-information maximum likelihood method was conducted for confirmatory factor analysis.33,34 Following the patient reported outcome research approach, nonapplicable values were not included in further inferential statistical analyses because participants explicitly withheld themselves from this particular item.35
Item Difficulty and Discrimination
To examine item difficulty, a difficulty index (Pi) was calculated from the item mean value divided by its maximum value.36,37 In addition, the average difficulty per domain was assessed. Higher values of Pi indicate low item difficulty. For good differentiation, item difficulties should be evenly distributed between 0.05 ≤ Pi ≤ 0.95.37
To evaluate item discrimination, the discriminatory power coefficient rit was computed. The coefficient rit was defined as the product-moment correlation between the item values and the corrected total domain value. For good discriminatory power, rit should be within a range of 0.40 to 0.70.36,37 In our analysis, values of 0.30 were considered medium, and values of 0.50 were considered high.38
Confirmatory Factor Analysis
Because there was an a priori theoretical structure of the original US version, confirmatory factor analysis with robust maximum likelihood estimation method was conducted to test the structural validity of the German QoLVAD questionnaire.15 Fit of the underlying model was tested using root-mean-square error of approximation, comparative fit index, Tucker-Lewis index, and standardized root-mean-square residual.39 Root-mean-square error of approximation values ≤ 0.05 were considered as a good fit, and values between 0.05 and 0.08 were considered as an adequate fit. For comparative fit index and Tucker-Lewis index, values ≥ 0.97 were interpreted as a good, and values ≥ 0.95 were interpreted as an acceptable fit. Values of standardized root-mean-square residual ≤ 0.05 were defined as a good fit, and values ≤ 0.10 were defined as an acceptable fit.39,40 Regarding model-based factor loadings, values > 0.30 were interpreted as good.41
Internal Consistency
On the basis of the cross-sectional design of the study, reliability estimation was performed using internal consistency. For this purpose, Cronbach α was assessed for each domain as well as for the total score. For α, coefficient values > 0.70 were considered as acceptable, values > 0.80 were considered as good, and values > 0.90 were considered as excellent.42
Convergent Construct Validity
Convergent construct validity was tested using the Kansas City Cardiomyopathy Questionnaire and the Patient Health Questionnaire. Pearson correlations were calculated for the following comparisons: QoLVAD total score with Kansas City Cardiomyopathy Questionnaire quality of life, QoLVAD physical domain with Kansas City Cardiomyopathy Questionnaire physical limitation, QoLVAD emotional domain with Patient Health Questionnaire (sum score), QoLVAD social domain with Kansas City Cardiomyopathy Questionnaire social limitation, and QoLVAD cognitive domain with Kansas City Cardiomyopathy Questionnaire overall summary score. No comparison instrument was available for the meaning/spiritual domain. As noted, no disease-specific standardized instrument for the assessment of spiritual well-being in people with VAD or even chronic heart failure could be identified in German. Results of correlation coefficient r were interpreted according to Cohen: values of r < 0.3 were considered as small, 0.3 ≤ r < 0.5 were considered as medium, and r ≥ 0.5 were considered as large.38
Readability
All items were examined for their readability. For this purpose, the German version of the established Flesch Reading Ease index43 and the Vienna Factual Text Formula44 were used. The comprehensibility of the text increases with the Flesch Reading Ease index. Index values between 60 and 70 indicate that the texts are easy to understand.45 The Vienna Factual Text Formula evaluates the readability on a scale from 4 to 15 points. The scale corresponds to the years of schooling that a reader must have completed to understand a text.44,45
Results
Participants
A total of 1003 patients were screened for study participation. Of those, 434 were included in the study and 393 participated in the analysis, indicating a dropout rate of 9.4%. Loss of contact, death, external aftercare, VAD explant, and heart transplantation were stated reasons for dropout.
Sample Characteristics
Mean age of the sample was 58.3 ± 11.3 years (Table 1). The majority was male (85.8%) and married or in a stable relationship (68.9%). The subjects were predominantly on a bridge-to-transplant therapy (60.3%) and had been living with the device for up to 2 years (72.8%).
TABLE 1.
Sample Characteristics (N = 393)
| Variables | Mean ± SD (Range)/n (%)a |
|---|---|
| Age, y | 58.3 ± 11.3 (18–85) |
| Gender | |
| Female | 56 (14.2) |
| Male | 337 (85.8) |
| Marital status | |
| Married/stable relationship | 268 (68.9) |
| Single/divorced/separated | 121 (31.1) |
| Implant strategy | |
| Bridge to transplant | 237 (60.3) |
| Destination therapy | 89 (22.6) |
| Bridge to recovery | 58 (14.8) |
| Years since initial implant | |
| <1 | 154 (39.2) |
| 1–2 | 132 (33.6) |
| >2 | 107 (27.2) |
aDiscrepancies to total in variables are due to missing values.
Missing Values
Low rates of missing values were found on the item level (minimum, 0.3%; maximum, 6.6%). On the domain level, Little's test of missing completely at random revealed a missing completely random (P > .05) for 4 of the 5 domains. In the physical domain, lack of random absence (χ2[1177] = 1338.40, P = .001) was identified. However, because of the predominantly fulfilled “missing completely at random” condition, the low missing rates within the physical domain, and the preceding analysis of missing values, no bias due to missing values was expected. Therefore, a missing-at-random condition was assumed for missing values within the physical domain.
Item Difficulty and Discrimination
The QoLVAD showed reasonable item difficulty (Ptotal = .67) and mostly moderate to high discriminatory power (rit > 0.30). The individual item difficulty indices ranged from 0.34 to 0.97. The cognitive domain showed the lowest level of difficulty (Pcognitive = .79). The remaining domains possessed comparable levels of difficulty (Pdomain = .61–.65). For individual items, discriminatory power ranged from −0.06 to 0.72. Details are given in Table 2. Overall, 7 items (highlighted in gray in Table 2) showed both extremely high difficulty indices (reflecting low difficulty) (Pi ≥ 0.80) and low discriminatory power (rit ≤ 0.30):
TABLE 2.
Item Difficulty (Pi) and Discrimination (rit)
| Items and HRQoL Domains | N Valid | Mean (SD) | Difficulty (Pi) | Discrimination (rit) |
|---|---|---|---|---|
| Physical | 0.65 | |||
| 1. I have been satisfied with my ability to: | ||||
| a. bathe (ie, showering or sponging) | 380 | 1.89 (1.23) | 0.47 | 0.58 |
| b. get dressed by myself | 388 | 2.93 (1.10) | 0.73 | 0.55 |
| c. change my VAD dressing | 247 | 1.81 (1.70) | 0.45 | 0.44 |
| d. go shopping | 368 | 2.42 (1.25) | 0.61 | 0.65 |
| e. do housekeeping/minor household repairs | 377 | 2.00 (1.28) | 0.50 | 0.72 |
| f. drive a car | 331 | 2.50 (1.52) | 0.63 | 0.56 |
| 2. I feel satisfied with the quality of my sleep. | 387 | 2.14 (1.19) | 0.54 | 0.51 |
| 3. I feel like my appetite is at a healthy level for me. | 391 | 2.87 (1.05) | 0.72 | 0.44 |
| 4. I feel like I can do activities that I enjoy. | 388 | 1.99 (1.17) | 0.50 | 0.63 |
| 5. I feel satisfied with my physical strength. | 391 | 1.51 (1.08) | 0.38 | 0.71 |
| 6. I feel satisfied with how long my energy lasts. | 390 | 1.37 (1.07) | 0.34 | 0.69 |
| 7. It frustrates me not being able to drink alcohol. | 387 | 3.59 (0.81) | 0.90 | −0.06 |
| 8. In the past 2 weeks, I was bothered by: | ||||
| a. swelling of the legs or ankles | 386 | 3.37 (1.06) | 0.84 | 0.37 |
| b. difficulty breathing | 388 | 2.96 (1.07) | 0.74 | 0.49 |
| c. nosebleed | 383 | 3.37 (0.98) | 0.84 | 0.19 |
| d. infections related to my VAD | 383 | 3.45 (1.07) | 0.86 | 0.20 |
| e. intestinal bleeding | 382 | 3.89 (0.49) | 0.97 | 0.23 |
| f. malfunctions of the device (the VAD did not work properly) | 382 | 3.82 (0.71) | 0.96 | 0.08 |
| g. hospitalization due to my heart or the VAD | 383 | 3.50 (1.10) | 0.88 | 0.18 |
| 9. It frustrates me that I can't fully submerge myself in water (eg, in the bathtub or while swimming). | 390 | 1.62 (1.52) | 0.41 | 0.13 |
| 10. I am comfortable working with my VAD equipment. | 390 | 2.43 (1.19) | 0.61 | 0.52 |
| 11. I have figured out some tricks for daily living with a VAD. | 383 | 2.17 (1.17) | 0.54 | 0.30 |
| 12. It bothers me how the VAD affects my sitting position on chairs or in the car. | 391 | 2.21 (1.19) | 0.55 | 0.55 |
| 13. Because of the VAD, it is difficult to maintain balance. | 390 | 3.12 (1.07) | 0.78 | 0.35 |
| 14. I feel like I cannot move freely…like I'm “tied down” by my VAD. | 389 | 2.07 (1.18) | 0.52 | 0.65 |
| 15. I have physical discomfort related to wearing equipment. | 391 | 2.54 (1.26) | 0.64 | 0.57 |
| Emotional | 0.65 | |||
| 16. I feel hopeful. | 381 | 2.29 (1.22) | 0.57 | 0.56 |
| 17. I feel sad. | 386 | 2.99 (1.04) | 0.75 | 0.64 |
| 18. I worry that my VAD might stop working properly. | 390 | 2.53 (1.32) | 0.63 | 0.53 |
| 19. I can laugh. | 387 | 2.99 (1.08) | 0.75 | 0.48 |
| 20. I am angry about my heart problems. | 390 | 1.80 (1.28) | 0.45 | 0.50 |
| 21. I have found ways to help cope with life's challenges. | 392 | 2.51 (1.05) | 0.63 | 0.52 |
| 22. I am confident my healthcare providers can help me if I have questions about living with my VAD. | 391 | 2.97 (0.99) | 0.74 | 0.45 |
| 23. I worry when I travel a distance from my VAD center. | 380 | 2.98 (1.13) | 0.75 | 0.54 |
| 24. I feel stressed by health issues not related to my VAD. | 389 | 2.73 (1.26) | 0.68 | 0.40 |
| 25. I am anxious about the uncertainty of my future. | 392 | 2.35 (1.25) | 0.59 | 0.69 |
| Social | 0.61 | |||
| 26. I am less likely to go places because of my supplies. | 389 | 2.27 (1.38) | 0.57 | 0.55 |
| 27. I am burdened by extra costs related to my VAD. | 391 | 2.87 (1.21) | 0.72 | 0.27 |
| 28. I can contribute to the well-being of others. | 384 | 1.92 (1.23) | 0.48 | 0.51 |
| 29. I have someone I can talk to about my condition. | 392 | 3.18 (1.23) | 0.80 | 0.31 |
| 30. It is difficult to talk with healthy people about the challenges I have living with my VAD. | 391 | 2.73 (1.23) | 0.68 | 0.39 |
| 31. I am bothered by how my VAD makes me look. | 392 | 2.79 (1.19) | 0.70 | 0.42 |
| 32. My VAD keeps me from working outside the home. | 322 | 2.40 (1.44) | 0.60 | 0.48 |
| 33. I can do the things I need to fulfill my role in my family. | 371 | 2.29 (1.27) | 0.57 | 0.61 |
| 34. I am satisfied with my ability to cuddle/hold loved ones. | 367 | 2.55 (1.31) | 0.64 | 0.62 |
| 35. I am satisfied with my ability to be intimate with my partner. | 272 | 1.34 (1.38) | 0.34 | 0.48 |
| Cognitive | 0.79 | |||
| 36. It is hard to remember things. | 382 | 3.02 (1.07) | 0.76 | 0.46 |
| 37. My mind is clear enough to do my everyday activities. | 389 | 3.20 (1.11) | 0.80 | 0.22 |
| 38. It is hard for me to concentrate for longer than 30 min. | 389 | 3.21 (1.08) | 0.80 | 0.50 |
| Meaning/spiritual | 0.63 | |||
| 39. I am a valuable human being. | 376 | 3.02 (1.19) | 0.76 | 0.55 |
| 40. My life has meaning and purpose. | 379 | 3.04 (1.17) | 0.76 | 0.58 |
| 41. I have peace no matter what happen. | 378 | 2.60 (1.29) | 0.65 | 0.58 |
| 42. I believe God or a higher power cares for me. | 289 | 1.79 (1.63) | 0.45 | 0.58 |
| 43. I feel support from others who share my faith. | 230 | 2.18 (1.56) | 0.55 | 0.64 |
“N valid” indicates number of valid values per item. Those shaded in gray have extremely high difficulty indices (Pi ≥ 0.80) and low discriminatory power (rit ≤ 0.30). Values in bold indicate mean domain difficulty.
Abbreviations: HRQoL, health-related quality of life; SD, standard deviation; VAD, ventricular assist device.
7. It frustrates me not being able to drink alcohol.
8c. In the past 2 weeks I was bothered by: nosebleed.
8d. In the past 2 weeks I was bothered by: infections related to my VAD.
8e. In the past 2 weeks I was bothered by: intestinal bleeding.
8f. In the past 2 weeks I was bothered by: malfunctions of the device.
8g. In the past 2 weeks I was bothered by: hospitalization due to my heart or the VAD.
37. My mind is clear enough to do my everyday activities.
Confirmatory Factor Analysis
The results of the CFA are presented in the Figure. Most items demonstrated significant associations with the overall HRQoL and the 5 specific domains. Whereas the fit index root-mean-square error of approximation demonstrated a good fit (0.07; 90% confidence interval, 0.06–0.07), other indices of model fit did not reach the respective cutoff value (comparative factor index/ Tucker-Lewis index, 0.66/0.65; standardized root-mean-square residual, 0.11). Factor loadings varied widely across items and ranged from −0.07 to 0.89 when standardized. Eight items with low factor loadings (<0.30) were identified including all 7 previously mentioned items with high difficulty indices and low discriminatory power, supplemented by item 9, “It frustrates me that I can't fully submerge myself in water (eg, in the bathtub or while swimming).”
FIGURE.
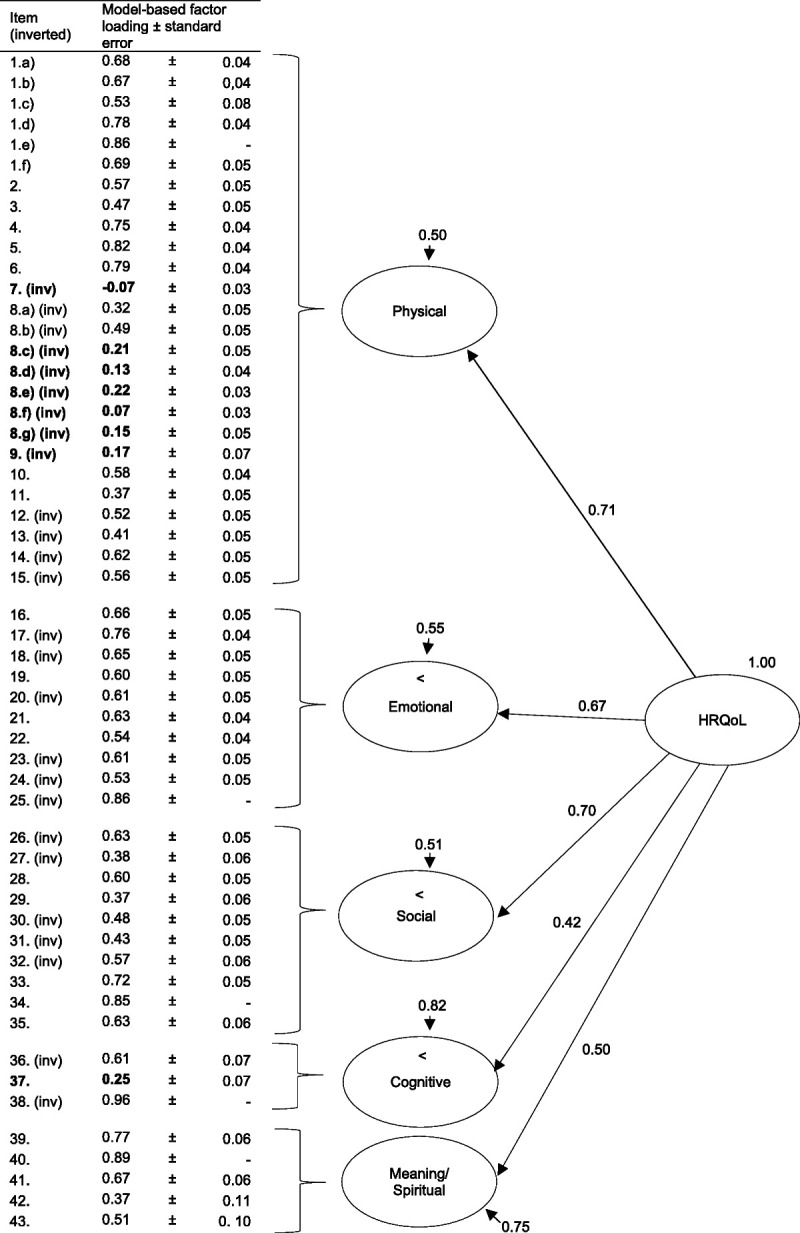
Confirmatory factor analysis of the 5 Quality of Life with a Ventricular Assist Device questionnaire domains (completely standardized solution). Presented are: model-based factor loadings ± standard errors, residual variances of the first-order factors and loadings of the domains on the general factor. Bold letters highlight low factor loadings. Abbreviations: HRQoL, health-related quality of life; inv, inverted item.
Overall, mean factor loadings were found to be in the medium range (total, 0.55; physical domain, 0.48; emotional domain, 0.65; social domain, 0.57; cognitive domain, 0.61; spiritual domain, 0.64). Whereas the physical, emotional, and social domains were explained to a large extent (≥45.0%) by the general factor of HRQoL, low proportions of explained variance (≤25%) for the cognitive and meaning/spiritual domains were identified.
Internal Consistency
Except for the cognitive domain, all domains and the total score showed acceptable to very good internal consistencies with α ranging from 0.79 to 0.93 (Table 3).
TABLE 3.
Internal Consistency of the Quality of Life with a Ventricular Assist Device Questionnaire Total Score and the Individual Domains
| QoLVAD Domains | Cronbach α | n (%)a |
|---|---|---|
| Total | 0.93 | 69 (17.6) |
| Physical | 0.88 | 168 (42.7) |
| Emotional | 0.84 | 358 (91.1) |
| Social | 0.79 | 224 (57.0) |
| Cognitive | 0.58b | 379 (96.4) |
| Meaning/spiritual | 0.80 | 222 (56.5) |
Abbreviation: QoLVAD, Quality of Life with a Ventricular Assist Device questionnaire.
aValue of 100% = 393.
bα = 0.71 if item 37 is deleted.
Convergent Construct Validity
The considered domains as well as the total score of the QoLVAD showed significant convergent construct validity with known validated measures for the patient population under investigation (Kansas City Cardiomyopathy Questionnaire, Patient Health Questionnaire). Moderate to strong correlations were observed (P ≤ .01). When compared with the Kansas City Cardiomyopathy Questionnaire, positive correlations were identified, which is in line with the solving direction of both questionnaires (higher values indicating better outcomes). Following the direction of items and sum score of the Patient Health Questionnaire (higher values indicate more severe depressive symptoms), a negative correlation was found. With better scores on the emotional scale correlating with lower depressive symptoms, good construct validity for this scale can be further supported. No comparison instrument was available for the meaning/spiritual domain; thus, construct validity could not be calculated for this domain (Table 4).
TABLE 4.
Convergent Construct Validity of the Quality of Life with a Ventricular Assist Device Questionnaire Total Score and the Individual Domains
| QoLVAD Domains | Comparator Assessment | Correlation (r) Between Tests |
|---|---|---|
| Total | KCCQ QoL | 0.70a |
| Physical | KCCQ Physical Limitations | 0.63a |
| Emotional | PHQ-9 | −0.68a |
| Social | KCCQ Social Limitations | 0.54a |
| Cognitive | KCCQ Summary | 0.45a |
| Meaning/spiritual | No comparator available | — |
Abbreviations: KCCQ, Kansas City Cardiomyopathy Questionnaire; PHQ-9, Patient Health Questionnaire-9; QoL, quality of life; QoLVAD, Quality of Life with a Ventricular Assist Device questionnaire; r, Pearson correlation coefficient r.
aCorrelation is significant at the .01 level (2-tailed).
Readability
The readability of the questionnaire was classified as “medium difficult” (Flesch Reading Ease, 64.11; Vienna Factual Text Formula, 6.91). Readers need approximately 7 school years of education to fully understand the text.
Discussion
The objective of this study was to assess the psychometric properties of a newly developed self-report instrument in its German version to capture disease-specific HRQoL of patients on durable VAD support. The German QoLVAD questionnaire presented a moderate readability and low proportions of missing values at the item level. Item analysis identified a rather low item difficulty with high values for the overall index (Ptotal = .67) and mostly medium to high discriminatory power (rit ≥ 0.30), which was adequate for this population. However, in its present form, not all model fit indices could reach the respective cutoff value. Except for the cognitive domain, total score and individual domains demonstrated acceptable to good internal consistencies. In addition, domains and total score showed significant correlations with established measures, demonstrating convergent validity for this new measure.
A rather low item difficulty was found within the item analysis. This might be a reflection of the sample's fairly high subjective health status (mean Kansas City Cardiomyopathy Questionnaire total score of 65.35; 95% confidence interval, 60.12–65.01). However, lower item difficulties can be classified as adequate for this specific patient population due to potential limitations in cognitive functioning.46,47
Despite the good results in the analysis of missingness and item analysis, as well as readability, our confirmatory factor analysis revealed a need for a more optimal model fit. The inadequate results for factor analysis may have several explanations.
First, our item and factor analysis identified 8 items with low item difficulty, discriminatory power, and/or factor loading. Those items may have contributed to the suboptimal model fit. However, we did test convergent construct validity for the following item combinations: (a) items of the German version, (b) items with poor results in German analysis eliminated, and (c) same item count as the US version. Although the values for comparative factor index and Tucker-Lewis index have improved slightly, they still do not reach the corresponding limits (see Supplemental Data 1, http://links.lww.com/JCN/A257). These results, together with our good values for Cronbach α, acceptable root-mean-square error of approximation fit index as well as the acceptable medium mean factor loadings for the total score and each domain might suggest that the dimensionality of the questionnaire needs to be reassessed. This assumption is further supported by an additional exploratory factor analysis, which we additionally performed, that yielded in a single-factor solution with a reduction of 8 items (see Supplemental Data 2, http://links.lww.com/JCN/A258). This could be due to cultural differences between the US population, where the QoLVAD was originally developed, and the German sample of this study. The observed sample was from well-established cardiac centers with high-quality care programs, including psychological care. Low proportions of explained variance (≤25%) were identified for the cognitive and meaning/spiritual domains. These may be due to the cognitive abilities of the sample under consideration, as well as attitudes toward spirituality and faith within German-speaking countries. Inclusion criterion for the sample was sufficient cognitive ability to participate in the study. It cannot be excluded that the sample therefore possessed a basic standard of cognitive ability in order not to feel restricted in everyday life. Regarding attitudes toward spirituality and faith, whereas the “belief in god” is very widespread in the United States with approval rates of 95.0%, it was found to be much less prevalent in German-speaking countries with approval rates of 13.0% in former Eastern Germany, 47.0% in Austria, 59.0% in former Western Germany, and 59.0% in Switzerland.48,49 Moreover, a high proportion of nondenominational people and a further decrease in religious commitment can be observed in German-speaking countries.49
Second, our sample size may have contributed to poor values within the confirmatory factor analysis. Although our sample of 393 patients represents a large sample for the field of VAD and is seen as a strength of our study, it is too small for a confirmatory factor analysis with 54 items in a higher-order model. For example, goodness-of-fit indices are sensitive to sample size in confirmatory factor analysis, with larger sample sizes being required with increased item count and model complexity.50,51 However, because people with VAD still represent a small population, the difficulty of finding an appropriate sample size for confirmatory factor analysis will remain in future studies.
Third, we interpreted items on a continuous scale and used the robust maximum likelihood estimation method within confirmatory factor analysis. One might argue that an ordinal scale and a weighted least square mean and variance estimator might be more appropriate. We considered using weighted least square mean and variance estimator and calculated it separately showing comparable results (see Supplemental Data, http://links.lww.com/JCN/A259). Because of high loss of data, we aimed to use the full-information maximum likelihood method, which is not available for the weighted least square mean and variance estimator. Because of the comparable results together with the possibility to use the full-information maximum likelihood method, we decided on the robust maximum likelihood estimator for our final analysis. This decision is supported by a simulation study by Rhemtulla et al52 (2012), where the maximum likelihood estimator achieved good results for categorical variables with at least 5 categories.
Finally, HRQoL is a complex and dynamic construct that might not work well in strict mathematical equations of confirmatory factor analysis. Within multidimensional models, it is assumed that items are indicators for only one of the dimensions each, so they can be assigned to exactly 1 factor.53 The single domains of the German QoLVAD might be too interlaced to fit this assumption (see also Supplemental Data 2, http://links.lww.com/JCN/A258). Further studies are needed to clarify the underlying factors of the German QoLVAD questionnaire.
Acceptable to high internal consistency was shown for the total score and domains, except for the cognitive domain. This may be due to the fact that the cognitive domain only contained 3 items contributing to a lower α. Furthermore, as an inclusion criterion, a sufficient cognitive ability to participate in the study was defined. Our analysis revealed an increase of α to 0.71 if item 37 (“My mind is clear enough to do my everyday activities”) would be deleted. This has to be discussed for future adaptations. We are aware of the limitations of Cronbach α and that McDonald's ω coefficient is increasingly recommended.54,55 However, the model fit is crucial for the interpretability of ω which was not given at this state of the analysis. Therefore, α was used but has to be interpreted with caution because it tends to overestimate reliability with given item and sample count.
Convergent construct validity of the German QoLVAD with known validated measures for the population of patients on VAD support (Kansas City Cardiomyopathy Questionnaire, Patient Health Questionnaire) was confirmed showing moderate to strong effect sizes. It is noteworthy that no comparison instrument was available for the evaluation of the meaning/spiritual scale. In addition, to assess the construct validity of the cognitive scale, the Kansas City Cardiomyopathy Questionnaire total score was used in the present work. However, the Kansas City Cardiomyopathy Questionnaire total score is not a specific measure for cognition but instead represents a measure of overall status. Because of the strong correlation of the QoLVAD total score with the Kansas City Cardiomyopathy Questionnaire quality of life score, a good convergent construct validity for the questionnaire can be inferred (r = 0.70, P < .001).
The findings of this study support the initial psychometric validation for the original US instrument regarding the readability, internal consistency, and convergent construct validity of the questionnaire.15 However, in contrast to the German QoLVAD, the original US questionnaire showed an acceptable model fit.15 It has to be mentioned that items 7, 8a to 8g, 9, 12, and 13 were already removed from the US version before confirmatory factor analysis.15 These items remained in the initial German translation to provide an opportunity to assess whether the questionable items were also low scorers in the German sample. Our findings underlined the recommendations for item elimination from the developers15 showing poor values for almost the same set of items within item and factor analysis: alcohol use (item 7), reporting of medical side effects (items 8c–8g), and submersion under water (item 9). However, final recommendations for item deletion can only been given after an improved model fit for the German QoLVAD.
In our work, we have shown that the questionnaire in its current form has some limitations reflected in the confirmatory factor analysis as well as the internal reliability in 1 domain. However, acceptable results for readability, internal construct validity, and convergent construct validity were identified. Furthermore, despite some low-scoring items, item analysis further revealed adequate overall item difficulty and mostly medium to high discriminatory power. Therefore, the German version of the QoLVAD instrument can be considered a promising instrument to assess HRQoL in patients with VAD support. We anticipate that our findings may support future research.
Strengths and Limitations
Our study presents the psychometric validation of the QoLVAD's German translation, the first disease-specific instrument in German to assess HRQoL in patients on ongoing VAD support. Conclusions have been drawn from a sample of 393 patients on durable VAD support based on a multicenter survey from across the country. The observed sample is reflective of the overall patient population in terms of age, gender, and goal of VAD therapy as described in the third report of the European Registry for Patients with Mechanical Circulatory Support Registry.2 Despite the cross-sectional design, data from 4 different centers located in northern, northeastern, eastern, and southern Germany are readily generalizable to different geographic areas and clinical practices in Germany. The high response rate and the low dropout rate represent a strength of this study.
To further interpret the results, some limitations must be considered. One important limitation is the use of cross-sectional data, which limits information on the questionnaire's sensitivity to measure changes over time. Because of the cross-sectional design, the results of this study can only be generalized to the limits of this design in general. Furthermore, no comparable instrument was available for the evaluation of the spiritual/meaningful scale in German; thus, this scale could not be tested individually for convergent validity. In addition, there is a risk that participants who felt too ill or too weak could not take part in the survey. This could have led to a selection bias and to a more positive bias in the results. The high mean quality of life value of the sample in Kansas City Cardiomyopathy Questionnaire (65.35; 95% confidence interval, 60.12–65.01) supports this statement, because they indicate a restricted distribution of quality of life values. Self-reported data are limited by recall bias and by the option that respondents might give socially desirable answers. Within the study, we aimed to counteract those biases by a 2-week recall period of the instrument as well as pseudonymized data.
Conclusion
Our results provide a promising standardized German instrument to assess HRQoL in patients on durable continuous-flow VAD support. The individual domains support a patient-oriented and multiprofessional care approach necessary to meet the needs of these patients. However, in its current form, the questionnaire represents a lengthy instrument. Adjusting the scales and reducing the items according to the exploratory factor analysis indications could increase the model fit and further improve its applicability and use in routine clinical practice. The resulting validated QoLVAD questionnaire can be used, with limitations, as the first German instrument for the specific assessment of HRQoL in patients on VAD support in further research and clinical practice. In the long term, improved care and understanding of the patient's HRQoL may help reduce rehospitalization rates and associated costs.
What’s New and Important
The QoLVAD questionnaire is the first German-language instrument to assess HRQoL in patients on ongoing continuous-flow VAD support.
The questionnaire showed acceptable results for convergent construct validity, internal consistency for the total score and most domains, and readability.
The questionnaire aims to support a patient-oriented and multiprofessional care approach.
Acknowledgments
The authors thank Johannes Beller, Maja Kammerlander, and Veronika Soetedjo for their statistical and methodological support. They would also like to thank Maiken Seemann for her support in the data collection and administration.
Footnotes
Hannah Spielmann, ORCID ID: 0000-0001-8352-5424
Paulina Staus, ORCID ID: 0000-0003-0792-9229
Christiane Kugler, ORCID ID: 0000-0002-1422-2718
This analysis was part of the SELMA study (ClinicalTrials.gov identifier: NCT04234230), which is supported by the Federal Joint Committee (G-BA) (grant number 01VSF18012). Translation of the German Quality of Life with a Ventricular Assist Device (QoLVAD) questionnaire was funded by the German Center for Cardiovascular Research (DZHK). Dr K.E.S. is coauthor of the QoLVAD questionnaire and holds it as intellectual property and copyright. All other authors have no competing interests to report.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jcnjournal.com).
Contributor Information
Hannah Spielmann, Email: hannah.spielmann@uniklinik-freiburg.de.
Katharina Tigges-Limmer, Email: KTigges-Limmer@hdz-nrw.de.
Wolfgang Albert, Email: albert@DHZB.de.
Christine Spitz-Köberich, Email: christine.spitz@uniklinik-freiburg.de.
Sandra Semmig-Könze, Email: Sandra.Semmig-Koenze@helios-gesundheit.de.
Paulina Staus, Email: paulina.staus@uniklinik-freiburg.de.
Christoph Herrmann-Lingen, Email: cherrma@gwdg.de.
Kristin E. Sandau, Email: Ksandau@umn.edu.
Brynn Okeson, Email: brynn.okeson@gmail.com.
Siegfried Geyer, Email: Geyer.Siegfried@mh-hannover.de.
REFERENCES
- 1.de By TMMH Mohacsi P Gahl B, et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS) of the European Association for Cardio-Thoracic Surgery (EACTS): second report. Eur J Cardiothorac Surg. 2018;53(2):309–316. doi: 10.1093/ejcts/ezx320. [DOI] [PubMed] [Google Scholar]
- 2.de By TMMH Schoenrath F Veen KM, et al. The European Registry for Patients with Mechanical Circulatory Support of the European Association for Cardio-Thoracic Surgery: third report. Eur J Cardiothorac Surg. 2022;62(1):ezac032. doi: 10.1093/ejcts/ezac032. [DOI] [PubMed] [Google Scholar]
- 3.Kugler C Spielmann H Seemann M, et al. Self-management for patients on ventricular assist device support: a national, multicentre study: protocol for a 3-phase study. BMJ Open. 2021;11(5):e044374. doi: 10.1136/bmjopen-2020-044374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spielmann H Seemann M Friedrich N, et al. Self-management with the therapeutic regimen in patients with ventricular assist device (VAD) support—a scoping review. Heart Lung. 2021;50(3):388–396. doi: 10.1016/j.hrtlng.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Chmielinski A, Koons B. Nursing care for the patient with a left ventricular assist device. Nursing. 2017;47(5):34–40. doi: 10.1097/01.NURSE.0000515503.80037.07. [DOI] [PubMed] [Google Scholar]
- 6.Kirklin JK Pagani FD Kormos RL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36(10):1080–1086. doi: 10.1016/j.healun.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Adams EE, Wrightson ML. Quality of life with an LVAD: a misunderstood concept. Heart Lung. 2018;47(3):177–183. doi: 10.1016/j.hrtlng.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Bidwell JT Lyons KS Mudd JO, et al. Quality of life, depression, and anxiety in ventricular assist device therapy: longitudinal outcomes for patients and family caregivers. J Cardiovasc Nurs. 2017;32(5):455–463. doi: 10.1097/JCN.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stehlik J Mountis M Haas D, et al. Quality of life and treatment preference for ventricular assist device therapy in ambulatory advanced heart failure: a report from the REVIVAL study. J Heart Lung Transplant. 2020;39(1):27–36. doi: 10.1016/j.healun.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kugler C, Meng M, Rehn E, Morshuis M, Gummert JF, Tigges-Limmer K. Sexual activity in patients with left ventricular assist devices and their partners: impact of the device on quality of life, anxiety and depression. Eur J Cardiothorac Surg. 2018;53(4):799–806. doi: 10.1093/ejcts/ezx426. [DOI] [PubMed] [Google Scholar]
- 11.Grady KL Andrei AC Elenbaas C, et al. Health-related quality of life in older patients with advanced heart failure: findings from the SUSTAIN-IT study. J Am Heart Assoc. 2022;11(4):e024385. doi: 10.1161/JAHA.121.024385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grady KL Wissman S Naftel DC, et al. Age and gender differences and factors related to change in health-related quality of life from before to 6 months after left ventricular assist device implantation: findings from interagency registry for mechanically assisted circulatory support. J Heart Lung Transplant. 2016;35(6):777–788. doi: 10.1016/j.healun.2016.01.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ware JE, Jr. SF-36 health survey update. Spine (Phila Pa 1976). 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/S0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 15.Sandau KE Lee CS Faulkner KM, et al. Health-related quality of life in patients with a left ventricular assist device (QOLVAD) questionnaire: initial psychometrics of a new instrument. J Cardiovasc Nurs. 2021;36(2):172–184. doi: 10.1097/JCN.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 16.Sandau KE, Hoglund BA, Weaver CE, Boisjolie C, Feldman D. A conceptual definition of quality of life with a left ventricular assist device: results from a qualitative study. Heart Lung. 2014;43(1):32–40. doi: 10.1016/j.hrtlng.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Schlander M. PRO (“patient-reported outcomes”) und lebensqualität in der onkologie. Forum. 2020;35(5):382–390. doi: 10.1007/s12312-020-00841-9. [DOI] [Google Scholar]
- 18.Brouwers C, Denollet J, de Jonge N, Caliskan K, Kealy J, Pedersen SS. Patient-reported outcomes in left ventricular assist device therapy: a systematic review and recommendations for clinical research and practice. Circ Heart Fail. 2011;4(6):714–723. doi: 10.1161/CIRCHEARTFAILURE.111.962472. [DOI] [PubMed] [Google Scholar]
- 19.Karimi M, Brazier J. Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics. 2016;34(7):645–649. doi: 10.1007/s40273-016-0389-9. [DOI] [PubMed] [Google Scholar]
- 20.Haraldstad K Wahl A Andenæs R, et al. A systematic review of quality of life research in medicine and health sciences. Qual Life Res. 2019;28(10):2641–2650. doi: 10.1007/s11136-019-02214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa DSJ, Mercieca-Bebber R, Rutherford C, Tait MA, King MT. How is quality of life defined and assessed in published research? Qual Life Res. 2021;30(8):2109–2121. doi: 10.1007/s11136-021-02826-0. [DOI] [PubMed] [Google Scholar]
- 22.Bobenko A Schoenrath F Knierim JH, et al. Exercise training in patients with a left ventricular assist device (Ex-VAD): rationale and design of a multicentre, prospective, assessor-blinded, randomized, controlled trial. Eur J Heart Fail. 2019;21(9):1152–1159. doi: 10.1002/ejhf.1431. [DOI] [PubMed] [Google Scholar]
- 23.The World Medical Association . Declaration of Helsinki. https://www.wma.net/what-we-do/medical-ethics/declaration-of-helsinki/doh-oct2004/. Published 2004. Accessed July 14, 2023.
- 24.Amtsblatt der Europäischen Union . Verordnung (EU) 2016/679 des Europäischen Parlaments und des Rates vom 27. April 2016 zum schutz natürlicher personen bei der verarbeitung personenbezogener daten, zum freien datenverkehr und zur aufhebung der richtlinie 95/46/EG (Datenschutz-Grundverordnung). 2016. https://www.bmj.de/DE/Themen/FokusThemen/DSGVO/_documents/Amtsblatt_EU_DSGVO.pdf;jsessionid=17985984EAAA5A18359CA636290D56D3.2_cid297?__blob=publicationFile&v=1. Accessed July 14, 2023.
- 25.Bullinger M, Anderson R, Cella D, Aaronson N. Developing and evaluating cross-cultural instruments from minimum requirements to optimal models. Qual Life Res. 1993;2(6):451–459. doi: 10.1007/BF00422219. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt S, Bullinger M. Current issues in cross-cultural quality of life instrument development. Arch Phys Med Rehabil. 2003;84:S29–S34. doi: 10.1053/apmr.2003.50244. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. [DOI] [PubMed] [Google Scholar]
- 28.Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8(5):469–476. doi: 10.1161/CIRCOUTCOMES.115.001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wild D Grove A Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8(2):94–104. doi: 10.1111/j.1524-4733.2005.04054.x. [DOI] [PubMed] [Google Scholar]
- 31.Tourangeau R, Rips LJ, Rasinski KA. The Psychology of Survey Response. Cambridge, England: Cambridge University Press; 2000. [Google Scholar]
- 32.Little RJ. A test of missing completely at random for multivariate data with missing values. JASA. 1988;83(404):1198–1202. [Google Scholar]
- 33.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model Multidiscip J. 2001;8(3):430–457. doi: 10.1207/S15328007SEM0803_5. [DOI] [Google Scholar]
- 34.Zhang X, Savalei V. Examining the effect of missing data on RMSEA and CFI under normal theory full-information maximum likelihood. Struct Equ Model Multidiscip J. 2020;27(2):219–239. doi: 10.1080/10705511.2019.1642111. [DOI] [Google Scholar]
- 35.Food and Drug Administration, Department of Health and Human Services . Guidance for industry, patient-reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims. Published 2009. Accessed May 3, 2021. [DOI] [PMC free article] [PubMed]
- 36.Döring N, Bortz J. Forschungsmethoden und Evaluation in den Sozial- und Humanwissenschaften. 5. Vollständig Überarbeitete, Aktualisierte und Erweiterte Auflage. Berlin, Germany: Springer; 2016. [Google Scholar]
- 37.Moosbrugger H, Kelava A. Deskriptivstatistische evaluation von items (itemanalyse) und testwertverteilungen. In: Moosbrugger H, Kelava A, eds. Testtheorie und Fragebogenkonstruktion. 2nd ed. Berlin, Germany: Springer; 2020:75–102. doi: 10.1007/978-3-662-61532-4 [DOI] [Google Scholar]
- 38.Cohen J. Statistical Power Analysis for the Behavioral Sciences (reprint). 2nd ed. New York, NY: Psychology Press; 2009. [Google Scholar]
- 39.Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Meth Psychol Res. 2003;8(2):23–74. [Google Scholar]
- 40.Schreiber JB, Nora A, Stage FK, Barlow EA, King J. Reporting structural equation modeling and confirmatory factor analysis results: a review. J Educ Res. 2006;99(6):323–337. [Google Scholar]
- 41.Brown TA. Confirmatory Factor Analysis for Applied Research. 2nd ed. New York, NY: The Guilford Press; 2015. [Google Scholar]
- 42.George D, Mallery P. SPSS for Windows Step by Step: A Simple Guide and Reference, 11.0 Update. 4th ed. Boston, MA: Allyn & Bacon; 2003. [Google Scholar]
- 43.Amstad T. Wie verstaendlich sind unsere zeitungen? 1978. https://katalog.ub.uni-freiburg.de/link?kid=1077145446. Accessed July 14, 2023.
- 44.Bamberger R, Vanacek E. Lesen-Verstehen-Lernen-Schreiben. Austria: Diesterweg; 1984. [Google Scholar]
- 45.Luers JC, Gostian AO, Roth KS, Beutner D. Lesbarkeit von medizinischen texten im Internetangebot deutscher HNO-universitätskliniken. HNO. 2013;61(8):648–654. doi: 10.1007/s00106-013-2674-7. [DOI] [PubMed] [Google Scholar]
- 46.Cowger J, Romano MA, Stulak J, Pagani FD, Aaronson KD. Left ventricular assist device management in patients chronically supported for advanced heart failure. Curr Opin Cardiol. 2011;26(2):149–154. doi: 10.1097/HCO.0b013e3283438258. [DOI] [PubMed] [Google Scholar]
- 47.Faulkner KM Chien CV Denfeld QE, et al. Longitudinal effects of left ventricular assist device implantation on global and domain-specific cognitive function. J Cardiovasc Nurs. 2022;37(1):31–40. doi: 10.1097/JCN.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 48.Huber S. Religiosität in Deutschland, Österreich und der Schweiz. In: Balck F, ed. Gesundheit-Religion-Spiritualität. Konzepte, Befunde Und Erklärungsansätze. Weinheim, Germany: Juventa; 2011:163–187. [Google Scholar]
- 49.Zwingmann C, Hodapp B. Religiosität/spiritualität und psychische gesundheit: zentrale ergebnisse einer metaanalyse über studien aus dem deutschsprachigen raum. Spiritual Care. 2017;7(1):69–80. doi: 10.1515/spircare-2017-0019. [DOI] [Google Scholar]
- 50.Kyriazos TA. Applied psychometrics: sample size and sample power considerations in factor analysis (EFA, CFA) and SEM in general. PSYCH. 2018;9(8):2207–2230. doi: 10.4236/psych.2018.98126. [DOI] [Google Scholar]
- 51.Kenny DA, McCoach DB. Effect of the number of variables on measures of fit in structural equation modeling. Struct Equ Model Multidiscip J. 2003;10(3):333–351. doi: 10.1207/S15328007SEM1003_1. [DOI] [Google Scholar]
- 52.Rhemtulla M, Brosseau-Liard PÉ, Savalei V. When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychol Methods. 2012;17(3):354–373. doi: 10.1037/a0029315. [DOI] [PubMed] [Google Scholar]
- 53.Gäde JC, Schermelleh-Engel K, Brandt H. Konfirmatorische faktorenanalyse (CFA). In: Moosbrugger H, Kelava A, eds. Testtheorie und Fragebogenkonstruktion. Berlin, Germany: Springer; 2020:615–659. doi: 10.1007/978-3-662-61532-4_24 [DOI] [Google Scholar]
- 54.Goodboy AK, Martin MM. Omega over alpha for reliability estimation of unidimensional communication measures. Ann Int Commun Assoc. 2020;44(4):422–439. doi: 10.1080/23808985.2020.1846135. [DOI] [Google Scholar]
- 55.Hayes AF, Coutts JJ. Use omega rather than Cronbach's alpha for estimating reliability. But. Commun Methods Meas. 2020;14(1):1–24. doi: 10.1080/19312458.2020.1718629. [DOI] [Google Scholar]
- 56.Blumer V, Mendirichaga R, Hernandez GA, Zablah G, Chaparro SV. Sex-specific outcome disparities in patients receiving continuous-flow left ventricular assist devices: a systematic review and meta-analysis. ASAIO J. 2018;64(4):440–449. doi: 10.1097/MAT.0000000000000695. [DOI] [PubMed] [Google Scholar]


