Abstract
OBJECTIVES:
Continuous electroencephalogram (cEEG) monitoring is recommended for status epilepticus (SE) management in ICU but is still underused due to resource limitations and inconclusive evidence regarding its impact on outcome. Furthermore, the term “continuous monitoring” often implies continuous recording with variable intermittent review. The establishment of a dedicated ICU-electroencephalogram unit may fill this gap, allowing cEEG with nearly real-time review and multidisciplinary management collaboration. This study aimed to evaluate the effect of ICU-electroencephalogram unit establishing on SE outcome and management.
DESIGN:
Single-center retrospective before-after study.
SETTING:
Neuro-ICU of a Swiss academic tertiary medical care center.
PATIENTS:
Adult patients treated for nonhypoxic SE between November 1, 2015, and December 31, 2023.
INTERVENTIONS:
None.
MEASUREMENT AND MAIN RESULTS:
Data from all SE patients were assessed, comparing those treated before and after ICU-electroencephalogram unit introduction. Primary outcomes were return to premorbid neurologic function, ICU mortality, SE duration, and ICU SE management. Secondary outcomes were SE type and etiology. Two hundred seven SE patients were included, 149 (72%) before and 58 (38%) after ICU-electroencephalogram unit establishment. ICU-electroencephalogram unit introduction was associated with increased detection of nonconvulsive SE (p = 0.003) and SE due to acute symptomatic etiology (p = 0.019). Regression analysis considering age, comorbidities, SE etiology, and SE semeiology revealed a higher chance of returning to premorbid neurologic function (p = 0.002), reduced SE duration (p = 0.024), and a shift in SE management with increased use of antiseizure medications (p = 0.007) after ICU-electroencephalogram unit introduction.
CONCLUSIONS:
Integrating neurology expertise in the ICU setting through the establishment of an ICU-electroencephalogram unit with nearly real-time cEEG review, shortened SE duration, and increased likelihood of returning to premorbid neurologic function, with an increased number of antiseizure medications used. Further studies are warranted to validate these findings and assess long-term prognosis.
Keywords: continuous electroencephalogram, electroencephalogram monitoring, intensive care unit, seizure, status epilepticus
KEY POINTS.
Question: What is the impact of establishing an ICU-electroencephalogram unit on the management and outcome of status epilepticus (SE) patients?
Findings: Our before-after study found that the ICU-electroencephalogram unit introduction was associated with reduced SE duration and higher rate of return to premorbid neurologic function, with increased nonconvulsive and acute symptomatic SE detection.
Meaning: Integrating neurology expertise within the critical care setting through the establishment of an ICU-electroencephalogram unit may reduce SE duration and improve patient outcome, underscoring the importance of real-time electroencephalogram monitoring in guiding tailored management strategies for SE patients.
Continuous electroencephalogram (cEEG) monitoring, a noninvasive and portable tool (1, 2), is strongly recommended by guidelines (3–5) for diagnosing and managing status epilepticus (SE), although the supporting evidence is of low quality. This holds true especially in ICU, considering high prevalence of nonconvulsive seizures (NCSs) and nonconvulsive SE (NCSE) (6, 7). However, despite its established impact in clinical decision-making (8, 9). cEEG monitoring is still underused due to resource limitations and inconclusive data regarding its impact on outcome (10–14). While three cross-sectional studies relied on administrative data suggest that cEEG use may be associated with better outcome (10, 14, 15), the multicenter randomized clinical Continuous EEG Randomized Trial in Adults trial demonstrated that cEEG monitoring, compared with repetitive electroencephalogram (rEEG) monitoring, has advantages in detecting seizures, but no impact in terms of outcome, in patients without recent seizures or SE (13).
Electroencephalogram is primarily a diagnostic tool and does not directly influence outcome, but rather treatment decisions. A noteworthy omission in this context is the lack of attention to the crucial aspect of timely access to electroencephalogram information. The term “continuous monitoring” implies continuous real-time acquisition and analysis but, in practice, cEEG recording with intermittent check/review (usually once bid) is far more common (16–18). Thus, electroencephalogram real-time review is pivotal for SE patients, where early treatment plays a crucial role in mitigating both morbidity and mortality (19, 20).
From November 2020, a dedicated ICU-electroencephalogram unit has been established at the University Hospital of Geneva (HUG) (Switzerland), with neurologists/neurophysiologists and electroencephalogram qualified technicians performing cEEG monitoring fully integrated in the critical care setting. The aim of this study was to evaluate the impact of ICU-electroencephalogram unit creation on SE management, with a focus on variations in SE characteristics, treatment, and prognosis.
MATERIALS AND METHODS
Data Collection
This is a monocentric retrospective before-after study performed at the ICU of the HUG, a Swiss academic tertiary medical care center. The Strengthening the Reporting of Observational Studies in Epidemiology guidelines were followed to improve the quality of our study (21).
Data from all consecutive SE adult patients (i.e., ≥ 18 yr old) admitted to the ICU between November 1, 2015, and December 31, 2023, were retrospectively assessed.
Until October 2020, patients with SE were monitored using rEEG (30 min) or underwent on-demand cEEG monitoring (at the discretion of the neurologist, typically for patients experiencing super-refractory SE and usually 2–4/yr) reviewed once daily during daytime shifts. Weekend shifts were managed by on-call technicians and consultant neurologists only in the mornings (8:00–12:00), with no coverage during night shifts.
Following the establishment of the ICU-electroencephalogram unit in November 2020, cEEG monitoring indications included management of refractory and super-refractory SE, suspicion of NCSE after a convulsive SE or seizure (if there was no return to neurologic baseline), unexplained altered mental status, detection of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage, neuroprognostication in post-cardiac arrest comatose patients, and calibration of sedation. cEEG monitoring was conducted 24/7, with continuous and real-time interpretation provided by a dedicated epileptologist during daytime shifts and an electroencephalogram-trained technician and on-call neurologist during weekend shifts. Interpretation during night shifts was not systematically covered. Written cEEG reports were typically provided bid, supplemented by more frequent oral reports and discussions as dictated by clinical needs.
All electroencephalogram recordings were conducted using a 10–20 system (with 19–25 scalp electrodes plus electrocardiogram) on a Deltamed and Micromed system (Natus System, Middleton, WI) with a sampling rate of 1000 Hz.
Data were collected and managed with the password encrypted online browser-based, metadata-driven database organizer Research Electronic Data Capture (22). SE Patients with SE following cardiorespiratory arrest were excluded, as this etiology is associated with a high mortality independent of treatment.
The following patient demographic, management data and SE features, were assessed: age, sex, SE etiology, SE semeiology, SE duration, number of antiseizure medications (ASMs), intubation and IV anesthetics, and days of ICU stay.
SE types were defined as recommended by the current guidelines of the International League Against Epilepsy (19) (i.e., focal NCSE without coma [with or without altered consciousness and absence SE], SE with motor symptoms [myoclonic and convulsive], and NCSE with coma). SE etiology was defined as acute symptomatic, remote symptomatic, progressive symptomatic, and unknown (19) and was categorized as potential nonfatal and fatal, following previous reports (23). Illness severity was quantified by the Status Epilepticus Severity Score (STESS; range, 0–6) (24), the Charlson Comorbidity Index (CCI) (range, 0–37) (25), the Acute Physiology and Chronic Health Evaluation II (range, 0–299) (26), and the Simplified Acute Physiology Score II (SAPS II; range, 0–163) (27).
The duration of SE was defined as the period between SE diagnosis and the clinical and/or electroencephalogram evidence of seizure termination, as previously described (28). The time of SE onset was clinically defined for SE with prominent motor symptoms and electroencephalogram-defined (according to the Salzburg Criteria and the 2021 American Clinical Neurophysiology Society terminology [29–31]) for patients with NCSE. For patients treated with anesthesia (32), the duration of SE was determined as the period from seizure onset until the establishment of an electroencephalogram seizure-free pattern (with either simple seizure cessation or achievement of a burst-suppression pattern), provided that the patient did not experience a relapse into SE after weaning off anesthetics.
Patients were categorized into those enrolled before (November 1, 2015, to October 31, 2020) or after (November 1, 2020, to December 31, 2023) ICU-electroencephalogram unit introduction. Primary analysis was conducted including the entire pool of SE ICU patients, while secondary analyses considering: 1) patients suffering NCSE and 2) patients with SE detected at least 24 hours after ICU admission.
Outcomes
Primary outcomes were return to premorbid neurologic function, ICU death, SE duration, and ICU SE management (number of ASMs, number of intubated patients, number of patients receiving anesthetics during SE, and days of ICU stay). Return to premorbid neurologic function was defined as the full recovery of all patients’ neurologic abilities or restoration to the neurologic functioning present before SE, based on physicians’ notes and examination, and assessed at ICU discharge (33, 34).
Secondary outcomes were SE type and etiology.
Statistics
Univariable comparisons were performed by the chi-square test or the Fisher exact test for categorical variables. For continuous variables, the Shapiro-Wilk test was used to distinguish between normally and not normally distributed variables. Normally distributed variables were analyzed with the Student t test, whereas variables violating the normal distribution were analyzed with the Mann-Whitney U test.
Subsequently, a regression model was performed to identify independent associations between ICU-electroencephalogram unit introduction and primary outcomes, considering unbalanced baseline variables and other well-established outcome-related features (age, comorbidity burden expressed as CCI, and potentially fatal etiology).
Patients’ subgroup univariable analyses were performed to assess the impact of the ICU-electroencephalogram unit introduction in: 1) patients suffering NCSE and 2) patients with SE detected greater than 24 hours after ICU admission. Two-sided p values of less than or equal to 0.05 were considered significant. Statistical analysis was performed with iamovi, 2022 Version (Sydney, NSW, Australia).
Ethic
The study protocol was reviewed and approved by the local ethic committee (“STEP-UP GENEVA,” protocol number CCER 2019-00836, date approved June 2019), and patients’ consent was waived in compliance with the Declaration of Helsinki first published in 1964 and its following amendments.
RESULTS
Univariable Comparisons
Two hundred seven nonhypoxic SE patients were included, 149 (72%) before and 58 (38%) after ICU-electroencephalogram unit introduction. ICU-electroencephalogram unit establishment led to a slight annual increase in the number of SE diagnosis, with a trend toward a more balanced gender distribution (p = 0.08). Univariable comparisons of demographics, clinical, management, and outcome variables are summarized in Table 1. After ICU-electroencephalogram unit introduction the proportions of SE with prominent motor features and SE with remote symptomatic etiology decreased (p = 0.003 and p < 0.001, respectively), while those of NCSE and of SE with an acute symptomatic etiology increased (p = 0.003 and p = 0.019, respectively). Individual etiologies, the proportion of SE due to potentially fatal etiologies and patient severity scores did not differ between the two groups (except for SAPS II, significantly higher after ICU-electroencephalogram unit establishment [p = 0.004]).
TABLE 1.
Univariable Comparisons Assessing Relationship Between ICU-Electroencephalogram Unit Introduction and Baseline Demographic Data, Status Epilepticus Features, Illness Severity Scores, Management Data, and Outcome
| Cohort Features | Without ICU-Electroencephalogram Unit (149) | With ICU-Electroencephalogram Unit (58) | p |
|---|---|---|---|
| Demographics data | |||
| SE patients/yr, mean ± sd | 24.0 (± 4.2) | 28.7 (± 6.1) | 0.2 |
| Male: female ratio | 99: 50 | 31: 27 | 0.08 |
| Age, mean ± sd | 57.6 (± 18.2) | 58.1 (± 17.1) | 0.8 |
| SE features | |||
| SE duration, d, mean ± sd | 1.7 (± 2.8) | 1.0 (± 0.8) | 0.7 |
| SE type: | |||
| SE with prominent motor symptoms, n (%) | 120 (81) | 35 (60) | 0.003 |
| Convulsive SE | 105 | 30 | |
| Myoclonic SE | 15 | 5 | |
| NCSE, n (%) | 29 (19) | 23 (40) | 0.003 |
| Focal NCSE without coma, n (%) | 13 (8) | 10 (17) | 0.08 |
| With altered consciousness | 11 | 8 | |
| Without altered consciousness | 2 | 2 | |
| NCSE with coma, n (%) | 16 (11) | 13 (22) | 0.030 |
| SE etiology, n (%) | |||
| Acute symptomatic | 58 (39) | 33 (57) | 0.019 |
| Remote symptomatic | 57 (39) | 7 (12) | < 0.001 |
| Progressive symptomatic | 20 (13) | 10 (17) | 0.5 |
| Unknown | 14 (9) | 8 (14) | 0.4 |
| SE etiology (nonmutually exclusive): | |||
| Potentially fatal, n (%) | 29 (19) | 15 (26) | 0.3 |
| Known epilepsy | 49 | 19 | 0.9 |
| Fast-growing brain tumors | 16 | 6 | 0.9 |
| Old stroke | 19 | 4 | 0.2 |
| Acute intracranial hemorrhage | 24 | 12 | 0.6 |
| Acute subarachnoid hemorrhage | 11 | 6 | 0.5 |
| Alcohol/ drug withdrawal | 19 | 8 | 0.8 |
| Metabolic | 12 | 3 | 0.5 |
| Neurodegenerative | 3 | 2 | 0.5 |
| Infectious (meningo-) encephalitis | 7 | 4 | 0.5 |
| Acute ischemic stroke | 3 | 4 | 0.08 |
| Acute severe traumatic brain injury | 8 | 6 | 0.2 |
| Acute autoimmune encephalitis | 2 | 5 | 0.009 |
| Unknown | 13 | 4 | 0.7 |
| Illness severity scores, mean ± sd | |||
| Status Epilepticus Severity Score (range, 0–6) | 3.1 (± 1.5) | 3.2 (± 1.5) | 0.5 |
| Charlson Comorbidity Index score (range, 0–37) | 3.6 (± 3.0) | 2.6 (± 1.9) | 0.07 |
| Acute Physiology and Chronic Health Evaluation II (range, 0–71) | 22.2 (± 5.7) | 24.0 (± 5.4) | 0.08 |
| Simplified Acute Physiology Score II (range, 0–163) | 45.2 (± 14.3) | 52.8 (± 12.8) | 0.004 |
| Management data | |||
| ICU stay, d, mean ± sd | 6.1 (± 8.8) | 7.8 (± 6.8) | 0.02 |
| Number of nonanesthetic antiseizure medications, mean ± sd | 2.1 (± 1.2) | 2.6 (± 1.1) | < 0.001 |
| SE patients intubated during SE, n (%) | 143 (96) | 50 (86) | 0.012 |
| SE patients with IV anesthetics during SE, n (%) | 140 (94) | 46 (79) | 0.002 |
| IV anesthetics (nonmutually exclusive): | |||
| Propofol | 136/140 | 46/46 | |
| Midazolam | 24/140 | 3/46 | |
| Thiopental | 0/140 | 1/46 | |
| Outcome, n (%) | |||
| Death | 9 (6) | 6 (10) | 0.3 |
| Return to premorbid neurologic function | 71 (48) | 37 (64) | 0.037 |
NCSE = nonconvulsive status epilepticus, SE = status epilepticus.
Boldface values indicate statistical significance.
Univariable analysis revealed a significant association between ICU-electroencephalogram unit introduction and longer ICU stay (mean, 7.8 d [± 6.8 d] vs. 6.1 d [± 8.8 d]; p = 0.02), as well as a significant increase in the number of ASMs prescribed (mean, 2.6 [± 1.1] vs. 2.1 [± 1.2]; p < 0.001). The number of intubated patients (50 [86%] vs. 143 [96%]; p = 0.012) and the number of patients treated with anesthetics (46 [79%] vs. 150 [94%]; p = 0.002) decreased significantly. Univariable analysis did not reveal differences in terms of ICU death, while ICU-electroencephalogram unit presence was associated with a higher percentage of patients returning to premorbid neurologic condition after SE resolution (37 [64%] vs. 71 [48%]; p = 0.037).
Regression Model
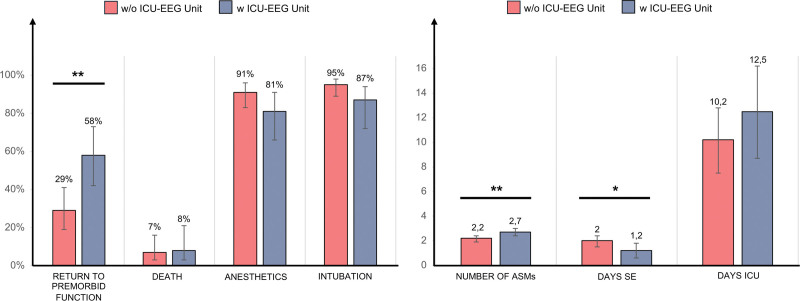
Figure 1 displays regression model results, showing primary outcomes assessment in relation to the establishment of the ICU-electroencephalogram unit.
Figure 1.
Regression analysis results assessing the association between ICU-electroencephalogram (EEG) unit introduction and primary outcomes, after correction for age, status epilepticus (SE) etiology, SE semeiology, potentially fatal etiology, and Charlson Comorbidity Index. *p < 0.05, **p < 0.01, ***p < 0.001. ASMs = antiseizure medications.
After adjustments for potential confounders (age, CCI, SE etiology, and SE semeiology), the only statistically significant associations with ICU-electroencephalogram unit establishment were augmented chance to return to premorbid neurologic function (58.0% [95% CI, 42–73%] vs. 29% [95% CI, 19–41%]; p = 0.002), reduced SE duration (1.2 [95% CI, 0.6–1.8] vs. 2.0 [95% CI, 1.5–2.4]; p = 0.024), and augmented number of ASMs (2.7 [95% CI, 2.4–3.0] vs. 2.2 [95% CI, 1.9–2.4]; p = 0.007). A trend (although not statistically significant) was observed toward a relationship between ICU-electroencephalogram unit introduction and fewer intubated patients (88% [95% CI, 72–94%] vs. 95% [95% CI, 89–98%]; p = 0.078). The presence of the ICU-electroencephalogram unit was not significantly associated to ICU death (8% [95% CI, 3–21%] vs. 7% [95% CI, 3–16%]; p = 0.078), the number of patients requiring anesthetics (81% [95% CI, 66–91%] vs. 91% [95% CI, 83–96%]; p = 0.101), and to days of ICU stay (12.5 [95% CI, 8.7–16.2] vs. 10.2 [95% CI, 7.5–12.8]; p = 0.254).
Subgroups Analyses
NCSE was detected in 29 patients (4.7/yr) before ICU-electroencephalogram unit introduction and in 23 (8.5/yr) after its establishment, with a trend toward a more frequent detection after the unit establishment. Univariable analysis revealed a significant association with the ICU-electroencephalogram unit presence and reduced SE duration (1.3 d [± 1.2 d] vs. 3.7 d [± 4.6 d]; p = 0.017), fewer SE with remote etiology (45 vs. 28%; p = 0.028), and an augmented chance to return to premorbid neurologic function (47% vs. 21%; p = 0.038). Results are highlighted in Table 2 (upper part).
TABLE 2.
Univariable Comparisons Considering Only Patients Suffering Nonconvulsive Status Epilepticus (Upper Part) and Patients With Status Epilepticus Detected Greater Than 24 Hours After ICU Admission (Lower Part)
| NCSE | Without ICU-Electroencephalogram Unit (29) | With ICU-Electroencephalogram Unit (23) | p |
|---|---|---|---|
| Demographics data | |||
| SE patients/yr, mean ± sd | 4.7 (± 1.1) | 8.5 (± 0.7) | 0.08 |
| Male: female ratio | 14: 15 | 14: 9 | 0.5 |
| Age, mean ± sd | 63.0 (± 17.8) | 59.0 (± 16.8) | 0.6 |
| SE features | |||
| SE duration, d, mean ± sd | 3.7 (± 4.6) | 1.3 (± 1.2) | 0.017 |
| NCSE type: | |||
| Focal NCSE without coma, n (%) | 13 (45) | 10 (55) | 0.9 |
| With altered consciousness | 11 | 8 | |
| Without altered consciousness | 2 | 2 | |
| NCSE with coma, n (%) | 16 (55) | 13 (57) | 0.9 |
| SE etiology, n (%): | |||
| Acute symptomatic | 14 (48) | 17 (74) | 0.06 |
| Remote symptomatic | 8 (28) | 1 (4) | 0.028 |
| Progressive symptomatic | 5 (17) | 4 (17) | 0.9 |
| Unknown | 2 (7) | 1 (4) | 0.7 |
| Potentially fatal etiology, n (%) | 13 (45) | 10 (44) | 0.9 |
| Management data | |||
| ICU stay, d, mean ± sd | 14 (± 15.6) | 12 (± 7.7) | 0.6 |
| Number of ASMs, mean ± sd | 2.7 (± 1.6) | 2.9 (± 1.4) | 0.3 |
| SE patients intubated during SE, n (%) | 26 (90) | 19 (83) | 0.5 |
| SE patients with IV anesthetics during SE, n (%) | 24 (83) | 14 (61) | 0.08 |
| Outcome, n (%) | |||
| Death | 5 (17) | 5 (22) | 0.7 |
| Return to premorbid neurologic function | 6 (21) | 11 (47) | 0.038 |
| SE Detected > 24 hr After ICU Admission | Without ICU-Electroencephalogram Unit (14) | With ICU-Electroencephalogram Unit (13) | p |
|---|---|---|---|
| Demographics data | |||
| SE patients/yr, mean ± sd | 2.3 (± 1.37) | 5.5 (± 0.71) | 0.07 |
| Male: female ratio | 6:8 | 8:5 | 0.3 |
| Age, mean ± sd | 58.5 (± 18.5) | 58.5 (± 19.8) | 0.9 |
| SE features | |||
| SE duration, d, mean ± sd | 4.8 (± 5.4) | 1.0 (± 0.5) | 0.003 |
| SE type, n (%) | 0.9 | ||
| SE with prominent motor symptoms patients | 12 (86) | 11 (85) | |
| NCSE | 2 (14) | 2 (15) | |
| SE etiology, n (%): | |||
| Acute symptomatic | 11 (79) | 12 (92) | 0.3 |
| Remote symptomatic | 1 (7) | 0 (0) | 0.3 |
| Progressive symptomatic | 1 (7) | 1 (7) | 0.9 |
| Potentially fatal etiology, n (%) | 7 (50) | 9 (69) | 0.3 |
| Management data | |||
| ICU stay, d, mean ± sd | 20 (± 20) | 24 (± 38) | 0.9 |
| Number of antiseizure medications, mean ± sd | 2.42 (± 1.16) | 2.54 (± 1.39) | 0.9 |
| SE patients intubated during SE, n (%) | 12 (86) | 9 (69) | 0.3 |
| SE patients with IV anesthetics during SE, n (%) | 11 (79) | 4 (31) | 0.013 |
| Outcome, n (%) | |||
| Death | 3 (21) | 2 (15) | 0.7 |
| Return to premorbid neurologic function | 1 (7) | 6 (46) | 0.021 |
NCSE = nonconvulsive status epilepticus, SE = status epilepticus.
Boldface values indicate statistical significance.
SE was detected after at least 24 hours from ICU admission in 14 patients from November 1, 2015, to October 31, 2020 (2.3/yr), and in 13 patients from November 1, 2020, to December 31, 2023 (5.5/yr), suggesting a tendency toward a more frequent detection with the ICU-electroencephalogram unit. The unit introduction was associated with reduced SE duration (1.0 d [± 0.5 d] vs. 4.8 d [± 5.4 d]; p = 0.003), fewer patients treated with anesthetics (31% vs. 79%; p = 0.013), and increased chance of returning to premorbid neurologic function (46% vs. 7%; p = 0.021). Univariable results are highlighted in Table 2 (lower part).
DISCUSSION
This study assessed the impact of the establishment of an ICU-integrated electroencephalogram neuromonitoring unit in terms of SE features, management, and outcome. The introduction of the ICU-electroencephalogram unit was associated with shorter SE duration and increased likelihood of patients returning to premorbid neurologic function. It prompted changes in SE management, with more frequent use of ASMs and a tendency toward a reduced reliance on invasive therapies. Furthermore, ICU-electroencephalogram unit establishment led to a shift in the SE population, with more frequent detection of NCSE and SE due to acute symptomatic etiology, aligning with evidence from the literature (13, 35).
Increased cEEG in critical care settings allowed the detection of NCSE in acute patients, which might otherwise have gone undiagnosed, and, in accordance, we assume that a not negligible number of patients with NCSE with coma or NCSE with altered consciousness had been overlooked before ICU-electroencephalogram unit creation. Robust evidence highlights how NCSE and acute etiology concern to a more severely ill category of patients, due primarily to the underlying brain or systemic pathology (36, 37). Accordingly, in our cohort, nonconvulsive semeiology and acute etiology were both related to prolonged ICU stay, higher STESS values, and increased reliance of invasive therapies. This might explain the apparent relationship between ICU-electroencephalogram unit and longer ICU stay (10, 14), which was not confirmed after adjusting for SE features.
The univariable associations between ICU-electroencephalogram unit and increased number of ASMs, reduced intubation, and decreased anesthetic therapy indicate a change in the SE management framework. It has been demonstrated that systematic cEEG monitoring impacts clinical decision-making with more frequent adjustments in ASMs (8, 9, 12, 13). Additionally, the ICU-electroencephalogram unit establishment has led to more frequent SE management through multiple nonsedating ASMs, decreasing reliance on invasive therapies. However, caution is warranted in interpreting these results, as they have only been partially confirmed after regression analysis, potentially due to an insufficient sample size.
Regression model confirmed a higher likelihood of returning to premorbid neurologic function after ICU-electroencephalogram unit introduction, coupled with reduced SE duration. Probably, the presence of an ICU-electroencephalogram unit enabled more timely SE management, reducing SE duration and consequentially secondary brain injury, ultimately allowing a more ICU death was not significantly associated with ICU-electroencephalogram unit even after adjusting for SE features and this is probably because mortality in the ICU setting is heavily influenced by the determination of goals of care and advance directives, as well as by the severity of the underlying acute pathology (38). In line with these observations, since SE sample studied after the ICU-electroencephalogram unit establishment was more severely ill, a similar ICU death rate should be interpreted in our opinion as a reassuring if not a good result of ICU-electroencephalogram unit SE management.
Patients with NCSE and those with SE detected greater than 24 hours after ICU admission represents two subgroups where the impact of ICU-electroencephalogram unit may be more pronounced. In the first scenario, cEEG is necessary for both NCSE diagnosis and treatment, with diagnostic delay associated with worse outcome (39). In the second one, SE is diagnosed and managed by the ICU-electroencephalogram unit from the early phase rather than solely in later stages. Importantly, the increasing prevalence of patients with NCSE and SE detected greater than 24 hours of ICU stays highlights the historical underdiagnosis of these SE categories before the introduction of the ICU-electroencephalogram unit (40). In both subgroups, the ICU-electroencephalogram unit was associated with more patients returning to premorbid neurologic function and reduced SE duration. However, the small sample size suggests caution in interpreting these results.
Several reports have examined the relationship between cEEG and prognosis, albeit with significant variability (10–15). Furthermore, many studies did not specifically address SE patients, and some even excluded them due to ethical concerns (13). While guidelines prioritize cEEG for monitoring patients at high risk of seizures or SE (3–5), the large cross-sectional study by Hill et al (15) demonstrated better outcomes for several subgroups undergoing cEEG except for those with seizures. Similarly, a population of NCSE patients showed no significant benefit from cEEG compared with spot rEEGs (11). Consequently, despite strong recommendations, the scarcity of unequivocal supporting evidence hampers efforts to implement this technique effectively (17), especially outside the Unites States.
The unclear link between outcome and cEEG monitoring contradicts the almost linear relationship between seizure burden and patient prognosis (41, 42). Seizure burden is strongly associated with neurologic decline (43), and cEEG monitoring allows for greater seizure detection, especially in cases of NCS/NCSE (2). However, the increased cEEG detection do not necessarily translate into better outcomes (13). Furthermore, the electroencephalogram reader and the consultant neurologist for the ICU may not be the same person or may change day-by-day, lacking in global vision and management continuity. This is particularly important in the ICU, a setting that, to provide a 24/7 days service, needs frequent changes in medical team. Although cEEG offers advantages in terms of better understanding seizure burden and patient trajectories, what matters most in severely ill SE patients is a prompt response, achievable only with as close to real-time analysis as possible, and deep integration within the ICU treating team.
It might be more appropriate to assess the impact not of cEEG compared with rEEGs, but rather the difference between neurologists with competence in electroencephalogram reading integrated within the ICU team, providing complementary expertise in monitoring and therapeutic strategies, vs. consultants outside the ICU. Only in the former scenario cEEG could become a true real-time extension of clinical assessment and not merely a tool to intercept seizures. The establishment of the ICU-electroencephalogram unit and our study align with this direction, aiming for a more accurate follow-up of epileptic activity to guide better, tailored management, reducing the risk of under- or over-treatment, and ultimately leading to better outcomes. In this context, our study was not specifically designed to differentiate solely between rEEG and cEEG, nor between systematic cEEG recording and an integrated ICU-electroencephalogram unit, but primarily aimed to compare rEEG with few cases of cEEG (typically 2–4/yr) with the establishment of an ICU-integrated electroencephalogram unit providing systemized cEEG monitoring with nearly real-time interpretation and feedback.
Our study has several limitations. First, it is a single-center before-after study and residual confounding, such as variations in prehospital and ICU patients’ management, which could potentially affect the relationship with endpoints, might not have been fully considered. However, while the study spanned almost a decade, during the reference years, only minor changes were made to treatment protocols without significant prognostic impact.
The latency between admission and electroencephalogram recording onset was not assessed. ICU patients could develop clinical suspicion of SE even long after admission, and in patients admitted for therapeutic SE escalation, electroencephalograms were often initiated before admission. Additionally, the latency between clinical suspicion of SE and electroencephalogram recording onset was not assessed due to lack of data.
We solely focused on short-term outcomes, without assessing long-term prognosis. In this context, an outcome less reliant on ICU-related variables could be the development of long-term epilepsy, an analysis we could not undertake due to insufficient follow-up.
We did not find significant changes in the ASMs used in the cohort, but we did not specific investigate variations related to ASMs dosages before and after ICU-electroencephalogram unit introduction.
Finally, one limitation of our organizational model is the lack of medical expertise during weekend and night shifts. However, we have assessed a hybrid approach at least for weekends, where electroencephalogram-ICU trained technical staff remain on duty to communicate with consultant neurologists, aiming to minimize the impact on patient care management.
CONCLUSIONS
In our before-after study, the ICU-electroencephalogram unit introduction shortened SE duration and improved patient outcome, with a concomitant increase in the number of ASM prescribed. While requiring more human, technical, and economic resources, ICU-electroencephalogram unit might refine cEEG monitoring selection, and increasing NCSE and acute symptomatic SE detection, while reducing instances of patients being mistakenly treated for SE based solely on clinical criteria.
Footnotes
Dr. Misirocchi is supported by the 2023 International Federation of Clinical Neurophysiology Research Fellowship Grant. Dr. Kleinschmidt has received honoraria for consulting from Abbvie, Eli Lilly, Lundbeck, Mitsubishi Tanabe, Novartis, and TEVA that were paid to a teaching and research fund at the University Hospital Geneva. Dr. Seeck is a shareholder of Epilog NV (Ghent, Belgium); she received grants from the Swiss National Science Foundation (163398, CRS115-180365). Dr. De Stefano was supported by the Swiss National Science Foundation (163398, CRS115-180365) and is supported by the 2022 Swiss League Against Epilepsy Research Support Prize; she is supported by the Swiss National Science Foundation (163398, CRS115-180365) and is supported by the 2022 Swiss League Against Epilepsy Research Support Prize. The remaining authors have disclosed that they do not have any potential conflicts of interest.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Contributor Information
Hervé Quintard, Email: herve.quintard@hug.ch.
Andreas Kleinschmidt, Email: andreas.kleinschmidt@hcuge.ch.
Karl Schaller, Email: karl.schaller@hug.ch.
Jérôme Pugin, Email: jerome.pugin@hug.ch.
Margitta Seeck, Email: margitta.seeck@hcuge.ch.
REFERENCES
- 1.Caricato A, Melchionda I, Antonelli M: Continuous electroencephalography monitoring in adults in the intensive care unit. Crit Care 2018; 22:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenthal ES: Seizures, status epilepticus, and continuous eeg in the intensive care unit. Continuum (Minneap Minn) 2021; 27:1321–1343 [DOI] [PubMed] [Google Scholar]
- 3.Brophy GM, Bell R, Claassen J, et al. ; Neurocritical Care Society Status Epilepticus Guideline Writing Committee: Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17:3–23 [DOI] [PubMed] [Google Scholar]
- 4.Claassen J, Taccone FS, Horn P, et al. ; Neurointensive Care Section of the European Society of Intensive Care Medicine: Recommendations on the use of EEG monitoring in critically ill patients: Consensus statement from the neurointensive care section of the ESICM. Intensive Care Med 2013; 39:1337–1351 [DOI] [PubMed] [Google Scholar]
- 5.Herman ST, Abend NS, Bleck TP, et al. : Consensus statement on continuous EEG in critically ill adults and children, part I: Indications. J Clin Neurophysiol 2015; 32:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laccheo I, Sonmezturk H, Bhatt AB, et al. : Non-convulsive status epilepticus and non-convulsive seizures in neurological ICU patients. Neurocrit Care 2015; 22:202–211 [DOI] [PubMed] [Google Scholar]
- 7.Florea B, Beniczky SA, Demény H, et al. : Semiology of subtle motor phenomena in critically ill patients. Seizure 2017; 48:33–35 [DOI] [PubMed] [Google Scholar]
- 8.Kilbride RD, Costello DJ, Chiappa KH: How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol 2009; 66:723–728 [DOI] [PubMed] [Google Scholar]
- 9.Holm-Yildiz S, Richter Hansen J, Thonon V, et al. : Does continuous electroencephalography influence therapeutic decisions in neurocritical care? Acta Neurol Scand 2021; 143:290–297 [DOI] [PubMed] [Google Scholar]
- 10.Ney JP, Van Der Goes DN, Nuwer MR, et al. : Continuous and routine EEG in intensive care: Utilization and outcomes, United States 2005-2009. Neurology 2013; 81:2002–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskioglou E, Stähli C, Rossetti AO, et al. : Extended EEG and non-convulsive status epilepticus: Benefit over routine EEG? Acta Neurol Scand 2017; 136:272–276 [DOI] [PubMed] [Google Scholar]
- 12.Khawaja AM, Wang G, Cutter GR, et al. : Continuous electroencephalography (cEEG) monitoring and outcomes of critically ill patients. Med Sci Monit 2017; 23:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossetti AO, Schindler K, Sutter R, et al. : Continuous vs routine electroencephalogram in critically ill adults with altered consciousness and no recent seizure: A multicenter randomized clinical trial. JAMA Neurol 2020; 77:1225–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amerineni R, Sun H, Lee H, et al. : Using electronic health data to explore effectiveness of ICU EEG and anti-seizure treatment. Ann Clin Transl Neurol 2021; 8:2270–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill CE, Blank LJ, Thibault D, et al. : Continuous EEG is associated with favorable hospitalization outcomes for critically ill patients. Neurology 2019; 92:e9–e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavvala J, Abend N, LaRoche S, et al. ; Critical Care EEG Monitoring Research Consortium (CCEMRC): Continuous EEG monitoring: A survey of neurophysiologists and neurointensivists. Epilepsia 2014; 55:1864–1871 [DOI] [PubMed] [Google Scholar]
- 17.Rossetti AO, Lee JW: What’s new on EEG monitoring in the ICU. Minerva Anestesiol 2021; 87:1139–1145 [DOI] [PubMed] [Google Scholar]
- 18.Kowoll CM, Klein M, Salih F, et al. ; On Behalf Of The Ignite Group: IGNITE status epilepticus survey: A nationwide interrogation about the current management of status epilepticus in Germany. J Clin Med 2022; 11:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinka E, Cock H, Hesdorffer D, et al. : A definition and classification of status epilepticus—report of the ILAE task force on classification of status epilepticus. Epilepsia 2015; 56:1515–1523 [DOI] [PubMed] [Google Scholar]
- 20.Susman E: Is continuous EEG needed in the intensive care unit? Neurol Today 2019; 19:36–37 [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007; 370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumann SM, Semmlack S, Rybitschka A, et al. : Prolonged mechanical ventilation in patients with terminated status epilepticus and outcome: An observational cohort study. Epilepsia 2021; 62:3042–3057 [DOI] [PubMed] [Google Scholar]
- 24.Rossetti AO, Logroscino G, Bromfield EB: A clinical score for prognosis of status epilepticus in adults. Neurology 2006; 66:1736–1738 [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373–383 [DOI] [PubMed] [Google Scholar]
- 26.Knaus WA, Draper EA, Wagner DP, et al. : APACHE II: A severity of disease classification system. Crit Care Med 1985; 13:818–829 [PubMed] [Google Scholar]
- 27.Gall JR, Lemeshow S, Saulnier F: A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270:2957–2963 [DOI] [PubMed] [Google Scholar]
- 28.Baumann SM, De Stefano P, Kliem PSC, et al. : Sex-related differences in adult patients with status epilepticus: A seven-year two-center observation. Crit Care 2023; 27:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leitinger M, Beniczky S, Rohracher A, et al. : Salzburg consensus criteria for non-convulsive status epilepticus—approach to clinical application. Epilepsy Behav 2015; 49:158–163 [DOI] [PubMed] [Google Scholar]
- 30.Leitinger M, Gaspard N, Hirsch LJ, et al. : Diagnosing nonconvulsive status epilepticus: Defining electroencephalographic and clinical response to diagnostic intravenous antiseizure medication trials. Epilepsia 2023; 64:2351–2360 [DOI] [PubMed] [Google Scholar]
- 31.Hirsch LJ, Fong MWK, Leitinger M, et al. : American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol 2021; 38:1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisch U, Jünger AL, Baumann SM, et al. : Association between induced burst suppression and clinical outcomes in patients with refractory status epilepticus. Neurology 2023; 100:e1955–e1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumann SM, Semmlack S, De Marchis GM, et al. : Frequency and implications of complications in the ICU after status epilepticus: No calm after the storm. Crit Care Med 2020; 48:1779–1789 [DOI] [PubMed] [Google Scholar]
- 34.Marchi NA, Novy J, Faouzi M, et al. : Status epilepticus: Impact of therapeutic coma on outcome. Crit Care Med 2015; 43:1003–1009 [DOI] [PubMed] [Google Scholar]
- 35.Limotai C, Ingsathit A, Thadanipon K, et al. : How and whom to monitor for seizures in an ICU. Crit Care Med 2019; 47:e366–e373 [DOI] [PubMed] [Google Scholar]
- 36.Leitinger M, Trinka E, Giovannini G, et al. : Epidemiology of status epilepticus in adults: A population-based study on incidence, causes, and outcomes. Epilepsia 2019; 60:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misirocchi F, Zilioli A, Mannini E, et al. : Prognostic value of Salzburg nonconvulsive status epilepticus criteria: The SACE score. Epilepsia 2024; 65:138–147 [DOI] [PubMed] [Google Scholar]
- 38.Yuan F, Damien C, Gaspard N: Severity scores for status epilepticus in the ICU: Systemic illness also matters. Crit Care 2023; 27:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young BG, Jordan KG, Doig GS: An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring. Neurology 1996; 47:83–89 [DOI] [PubMed] [Google Scholar]
- 40.Dedeoglu O, Akça H, Emeksiz S, et al. : Management of status epilepticus by different pediatric departments: Neurology, intensive care, and emergency medicine. Eur Neurol 2023; 86:315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vespa PM, Nuwer MR, Nenov V, et al. : Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg 1999; 91:750–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Marchis GM, Pugin D, Meyers E, et al. : Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology 2016; 86:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Payne ET, Zhao XY, Frndova H, et al. : Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014; 137:1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]