Maintaining a healthy weight is associated with less severe menopausal symptoms in middle-aged women. The association between physical activity and the severity of menopausal symptoms varied based on the differences in total and lean body mass.
Key Words: Exercise, Hot flashes, Menopause, Night sweats, Obesity, Women's health
Abstract
Objective
The aim of the study was to conduct exploratory analyses on the role of cardiorespiratory fitness (CRF) and body composition in the association between physical activity and menopausal symptoms.
Methods
This was a cross-sectional (N = 298) study of women aged 51–59 years including a subsample of 82 women followed for 4 years. The severity of menopausal symptoms was assessed with the Menopause Rating Scale in total symptoms as well as using the somato-vegetative, psychological, and urogenital subscales. Physical activity was assessed with accelerometers and self-reports, body composition with dual-energy x-ray absorptiometry, and CRF with a custom-made prediction model based on the six-minute walking distance and spiroergometry. The associations of interest were studied using unstandardized regression coefficients derived from multiple linear regression models with the severity of menopausal symptoms as the outcome.
Results
Higher total body and fat mass (kg) were associated with more severe total symptoms (B = 0.06 [95% CI, 0.01 to 0.12] and 0.07 [0.01 to 0.14], respectively) as well as somato-vegetative (0.03 [0.01 to 0.05]; 0.04 [0.01 to 0.06]) and psychological symptoms (0.03 [0.00 to 0.05]; 0.03 [0.00 to 0.06]) in cross-sectional design. Total and lean body mass interacted with physical activity in total and psychological symptoms with stronger indirect associations being observed in participants with lower total and lean body mass. CRF was not associated with menopausal symptoms and did not interact with physical activity.
Conclusions
Maintaining a healthy weight is associated with less severe menopausal symptoms in middle-aged women. The association between physical activity and the severity of menopausal symptoms varied based on the differences in total and lean body mass.
Menopausal symptoms are caused by hormonal changes that arise from the menopause-related gradual cessation of ovarian function. They include but are not limited to vasomotor symptoms (VMS; eg, hot flashes and night sweats), sleep disturbances, mood instability, and sexual dysfunction.1,2 The majority of middle-aged women experience these symptoms that significantly impair the quality of life during the time when women have important roles in society and within the family.2
The role of physical activity in the alleviation of menopausal symptoms has been studied in several observational studies as well as in randomized controlled trials, but the results of these studies have been contradictory, and the overall evidence is still inconclusive.3-5 Physical activity is known to affect cardiorespiratory fitness (CRF) and body composition,6-8 which both have also been associated with menopausal symptoms.9-11 Furthermore, previous studies have shown that the aging related decline in CRF and accumulation of adipose tissue accelerates in midlife women.12,13 However, the role of CRF and body composition has rarely been investigated in the studies focusing on the associations of physical activity and menopausal symptoms. Notably, exercise interventions that have also included CRF measurement have reported intervention to alleviate menopausal symptoms more in women with better CRF at baseline14 and in women who improved their CRF more during the intervention.15 Nonetheless, it has also been observed that the changes in CRF and body composition do not modify the exercise intervention effect on menopausal symptoms.16
Therefore, the role of CRF and body composition in the association between physical activity and menopausal symptoms is still unclear, and further insight on this topic could provide tools for promotion of health and quality of life in middle-aged and elderly women. Thus, this exploratory study aimed to investigate the associations of CRF, body composition, and physical activity with menopausal symptoms.
METHODS
Study design and population
This study was conducted using Estrogenic Regulation of Muscle Apoptosis (ERMA) cohort data (dataset: doi.10.17011/jyx/dataset/83491).17 The used data sets includes data from the ERMA and the Estrogen, MicroRNAs and the risk of Metabolic Dysfunction (EsmiRs) studies. The ERMA measurements were conducted in 2015–2016 and the EsmiRs measurements in 2019–2020. The EsmiRs measurements were discontinued due to coronavirus disease 2019 (COVID-19) restrictions on March 16, 2020.
The primary cross-sectional analyses of this study were carried out using the data from the EsmiRs study, which is a 4-year follow-up study for the ERMA study.18 The EsmiRs study included questionnaires for severity of the menopausal symptoms, range of physiological measurements, and EsmiRs metabolism substudy19 including detailed CRF assessment. For the auxiliary longitudinal analyses for participants with no symptoms at ERMA baseline, we utilized the data from the ERMA measurements and the data from the EsmiRs measurements conducted 4 years after ERMA baseline. The studies were performed in accordance with the Declaration of Helsinki, and they were approved by the ethical committee of the Central Finland Health Care District. All participants provided written informed consent.
The recruitment and participant selection procedures for the ERMA,17 EsmiRs,18 and EsmiRs metabolism studies19 have been described elsewhere. Briefly, a random sample of 6,878 women aged 47–55 years living in the Central Finland drawn from the Population Information System were invited to participate in the ERMA baseline measurements. Of those women, 1,393 consented and were not excluded. Exclusion criteria of the study were conditions affecting ovarian function, systemic hormone levels, or inflammatory status, such as bilateral oophorectomy, pregnancy, lactation, and severe obesity (self-reported body mass index [BMI] ≥ 35 kg/m2).17 Of the 1,393 participants in the ERMA measurements in 2015‐2016, 298 participated in the EsmiRs measurements after the 4-year follow-up in 2019–2020.18 The loss of participants from ERMA baseline to EsmiRs is illustrated in Figure 1.
FIG. 1.
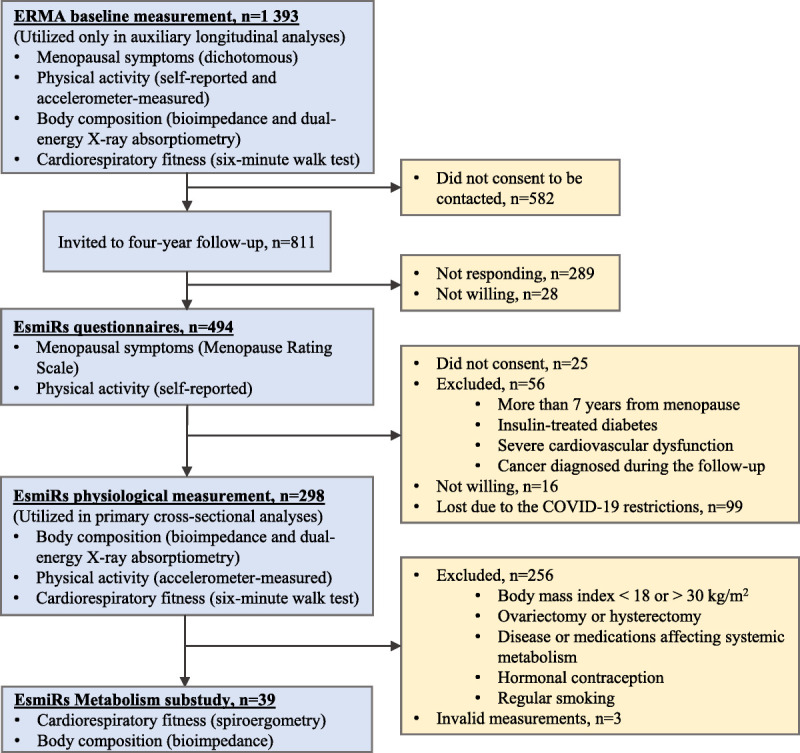
Flow chart of the study.
For the EsmiRs metabolism substudy, participants who were either pre- or perimenopausal, postmenopausal, or postmenopausal hormone therapy users were recruited. Furthermore, the aim was to recruit an even number of participants with normal weight and overweight (BMI threshold 25 kg/m2) with similar physical activity levels based on the single question for leisure time physical activity.19,20 The other exclusion criteria of the EsmiRs metabolism substudy are illustrated in Figure 1, and more detailed participant characteristics are shown in Table 1. EsmiRs metabolism substudy measurements were conducted after the EsmiRs measurements, the median (interquartile range) time between the measurements being 9 (7‐19) weeks.
TABLE 1.
Characteristics of the study population
| Cross-sectional study | Longitudinal studya | |||
|---|---|---|---|---|
| EsmiRsb (n = 298) | EsmiRs metabolismb (n = 39) | Baseline, ERMAc (n = 82) | Follow-up, EsmiRsb (n = 82) | |
| Age (year) | 55.1 ± 1.8 | 55.4 ± 1.6d | 50.9 ± 1.7 | 54.7 ± 1.7 |
| Body height (m) | 1.66 ± 0.06 | 1.66 ± 0.05 | 1.66 ± 0.05 | 1.66 ± 0.05 |
| Total body mass (kg) | 70.9 ± 11.5 | 68.3 ± 8.2 | 67.0 ± 11.1 | 70.4 ± 11.7 |
| Body mass index (kg/m2) | 25.8 ± 4.1 | 24.8 ± 2.7 | 24.9 ± 3.7 | 25.4 ± 3.9 |
| Menopausal statuse | ||||
| Pre | 5 (15) | 3 (1) | 59 (48) | 7 (6) |
| Peri | 14 (42) | 15 (6) | 34 (28) | 22 (18) |
| Post | 81 (241) | 82 (32) | 7 (6) | 71 (58) |
| Menopause rating scale score (n) | 298 | 39 | 82 | 82 |
| Total | 8.60 ± 5.00 | 7.87 ± 4.74 | 7.19 ± 4.26 | |
| Somato-vegetative | 3.90 ± 2.18 | 3.72 ± 2.03 | No | 3.45 ± 2.06 |
| Psychological | 2.53 ± 2.40 | 2.18 ± 2.15 | Symptoms | 1.85 ± 1.83 |
| Urogenital | 2.15 ± 1.89 | 1.97 ± 1.71 | 1.88 ± 1.70 | |
| Cardiorespiratory fitness (n) | – | 39 | – | – |
| VO2max (l/min) | – | 2.15 ± 0.34d | – | – |
| VO2max (ml/kg/min) | – | 31.7 ± 5.1d | – | – |
| Dual-energy x-ray absorptiometry (n) | 293 | 39 | 72 | 81 |
| Body fat mass (kg) | 25.9 ± 9.0 | 24.3 ± 6.2 | 23.4 ± 9.0 | 25.0 ± 9.3 |
| Lean body mass (kg) | 42.2 ± 4.2 | 41.3 ± 3.8 | 42.9 ± 3.9 | 42.6 ± 4.0 |
| Fat percentage (%) | 37.1 ± 7.7 | 36.6 ± 5.7 | 34.2 ± 8.3 | 35.9 ± 7.9 |
| Bioelectrical impedance analysis (n) | 292 | 39 | 75 | 79 |
| Fat mass (kg) | 23.6 ± 8.9 | 21.7 ± 5.4d | 20.3 ± 8.5 | 22.7 ± 9.1 |
| Fat free mass (kg) | 47.5 ± 4.9 | 46.5 ± 4.6d | 48.6 ± 4.7 | 47.8 ± 4.7 |
| Skeletal muscle mass (kg) | 26.2 ± 2.9 | 25.6 ± 2.8d | 26.9 ± 2.8 | 26.4 ± 2.8 |
| Accelerometer-measured PA (n) | 283 | 36 | 69 | 77 |
| ACC-PA (mg) | 28.3 ± 8.6 | 29.8 ± 9.2 | 30.3 ± 11.3 | 29.0 ± 8.9 |
| Self-reported leisure time PA (n) | 298 | 39 | 82 | 82 |
| SR-PA (MET-h/d) | 4.91 ± 3.93 | 5.20 ± 3.63 | 5.26 ± 5.41 | 5.14 ± 3.93 |
| 6-minute walk test (n) | 259 | 38 | 69 | 76 |
| Sitting heart rate (bpm) | 71.1 ± 9.5 | 69.8 ± 9.7 | 78.5 ± 12.4 | 71.1 ± 9.0 |
| 6-minute heart rate (bpm) | 152 ± 17 | 156 ± 17 | 156 ± 15 | 154 ± 18 |
| 6-minute RPE | 14.4 ± 2.0 | 14.4 ± 2.1 | 14.4 ± 1.8 | 14.5 ± 2.2 |
| Distance walked (m) | 672 ± 61 | 683 ± 49 | 686 ± 60 | 678 ± 68 |
| Lifestyle habits (n) | 298 | 39 | 82 | 82 |
| Alcohol consumption (portions/wk) | 3.24 ± 3.43 | 2.79 ± 2.86 | 3.50 ± 2.80 | 3.01 ± 2.65 |
| Smokinge | ||||
| Nonsmoker | 94 (280) | 95 (37) | 96 (79) | 94 (77) |
| Smoker | 6 (18) | 5 (2) | 4 (3) | 6 (5) |
| Educatione | ||||
| Primary or secondary | 55 (165) | 51 (20) | 63 (52) | 63 (52) |
| Tertiary | 45 (133) | 49 (19) | 37 (30) | 37 (30) |
| Use of hormonal preparationse | ||||
| Nonuser | 60 (180) | 82 (32) | 59 (48) | 57 (47) |
| Progestogen | 19 (56) | 0 (0) | 42 (34) | 31 (25) |
| Estrogen | 3 (10) | 0 (0) | 0 (0) | 2 (2) |
| Progestogen + estrogen | 18 (52) | 18 (7) | 0 (0) | 10 (8) |
ACC-PA, accelerometer-measured physical activity mean amplitude deviation; CRF, cardiorespiratory fitness; ERMA, Estrogenic Regulation of Muscle Apoptosis; Estrogen, MicroRNAs and the risk of Metabolic Dysfunction; mg, milligravity (0.00981 m/s2); PA, physical activity; RPE, rating of perceived exertion; SR-PA, self-reported physical activity; VO2max, maximal oxygen consumption.
Data are mean ± standard deviation unless otherwise specified.
aParticipants who did not report any menopausal symptoms at baseline ERMA measurement were included in the longitudinal study.
bMeasurements were conducted in 2019‐2020.
cMeasurements were conducted in 2015‐2016.
dMeasured on the EsmiRs metabolism day.
eData are % (n).
Menopausal symptoms
Menopausal symptoms were assessed using structured questionnaires in ERMA and EsmiRs measurements. The questionnaire used in the EsmiRs was the Menopause Rating Scale (MRS), a standardized and validated measure of health-related quality of life and severity of menopausal symptoms.21,22 The MRS includes 11 symptoms for which the respondent reports one of the five categories of the perceived symptom severity (none, mild, moderate, severe, very severe). The individual score of each symptom varies from 0 (none) to 4 (very severe), and the total symptom score (ranging from 0 to 44) is the sum of all symptom scores. The three subscales of the MRS (with their respective symptoms) are somato-vegetative (hot flashes/sweating, heart discomfort, sleep problems, and joint/muscular discomfort), psychological (depressive mood, irritability, anxiety, and physical/mental exhaustion), and urogenital (sexual problems, bladder problems, vaginal dryness).21
In the ERMA questionnaire, participants were asked to report dichotomously if they had menopausal symptoms from the list of 10 common symptoms.17,23 The questionnaire also included the option to describe a maximum of three additional symptoms. There were 82 participants who did not report any symptoms at baseline ERMA measurements.
Physical activity
Accelerometer-measured physical activity (ACC-PA) was assessed with mean amplitude deviations of hip-worn accelerometer data. Participants were instructed to wear accelerometers (ActiGraph GT3X and wGT3X, Actigraph, Pensacola, FL) for seven consecutive days during waking hours, except for water-based activities. The data were collected at 60 Hz, and the Euclidian norm of the resultant acceleration was computed for each timepoint. Mean amplitude deviation values were computed for nonoverlapping 5-second epochs, and ACC-PA was computed as their mean value.18
Additionally, self-reported physical activity (SR-PA) was assessed with a structured questionnaire that included questions about the average frequency, intensity, and duration of leisure time physical activity as well as the average duration of commuting activity. Finally, leisure time physical activity in metabolic equivalent (MET) hours per day was computed based on the responses to these four questions.24
Anthropometrics and body composition
Body composition was measured with dual-energy x-ray absorptiometry (DXA; LUNAR Prodigy, GE Healthcare, Chicago, IL) and bioelectrical impedance analysis (BIA; InBody720, Biospace, Seoul, Korea) after overnight fasting. BMI was calculated using total body mass and height measured with standard procedures. DXA, BIA, and BMI measurements were conducted in both timepoints. Furthermore, BIA was carried out in the EsmiRs metabolism substudy.
Cardiorespiratory fitness
In the EsmiRs metabolism substudy (n = 39), CRF was determined as maximal oxygen consumption (VO2max) measured with metabolic cart (Vmax Encore 92, Sensormedics, Yorba Linda, CA) during maximal incremental bicycle ergometer (Ergoselect 200, Ergoline, Bitz, Germany) test. A more detailed description of the protocol has been reported previously.19 All participants (n = 298) performed a 6-minute walk test on a 20-meter indoor track at baseline ERMA measurements and after the 4-year follow-up in EsmiRs measurements. Participants were instructed to complete as many laps as possible, and distance walked during the test (6-minute walking distance, 6MWD) was measured.25 Sitting heart rate was assessed before the test and heart rate as well as the rating of perceived exertion (RPE) were assessed after the test.17
A custom-made prediction model for VO2max based on the 6MWD and body composition measurements was constructed to estimate VO2max for participants without spiroergometry. The VO2max estimate derived from the constructed model was used to assess CRF at ERMA baseline measurements and after the 4-year follow-up in EsmiRs. The constructed model is described in more detail in the statistical analyses and results section.
Confounding factors
The serum concentrations of estradiol and follicle-stimulating hormone (FSH) were determined (IMMULITE 2000 XPi, Siemens Healthineers, Erlangen, Germany) from the fasting blood samples. For participants with predictable menstrual cycles, the blood samples were taken during the first 5 days of the menstrual cycle. Furthermore, the participants provided a diary that included information about the menstrual bleedings during the 6-month period prior to the ERMA measurements. Participants were categorized as pre-, peri-, or postmenopausal based on their serum FSH concentrations and menstrual bleeding diaries using the adapted Stages of Reproductive Aging Workshop (STRAW +10) guidelines.1
Education level (primary/secondary or tertiary), smoking status (nonsmoker or smoker), and alcohol consumption in portions per week were assessed using structured questionnaires. Furthermore, participants reported their current use of hormonal preparations for contraception or menopausal hormone therapy, and they were classified as nonuser, only progestogen, only estrogen, and combined estrogen and progestogen users. All exogenous sex hormone preparations for contraceptive and hormone therapy use were included, except for the intravaginal local estrogen therapy.
Missing data
The number of missing data values was 989 out of 13,112 (7.5%), where 13,112 is the number of potential data values (44 variables in the full wide format data set multiplied by 298 participants). The percentage of missing values varied from 0 to 23% across the variables. Missing data occurred because of invalid or missing measurements and unclear and incomplete questionnaire responses. Missing data were assumed to occur at random.26 Multiple imputation with 50 imputed data sets and 50 iterations for chained equations was used to deal with the missing data. Variables measured at the same timepoint and the target variable measurement from the other timepoint were used for the imputation of each variable. Derived variables, which were imputed with passive imputation, were not used for imputation of their originals. Model parameters were estimated separately for each data set. Multiple imputation and pooling of the model estimates were carried out in R using the standard settings of the “mice” package.27,28 We also performed the complete case analysis, and the results were not notably different from the ones acquired using multiple imputation.
Statistical analyses
The selection of the model for the prediction of absolute VO2max was carried out using the lasso regression in the EsmiRs metabolism subsample using the “glmnet” package in R.29 The 14 candidate predictors for the model included age, height, weight, BMI, DXA fat mass, DXA lean mass, DXA appendicular lean mass, BIA fat mass, BIA skeletal muscle mass, BIA fat-free mass, heart rate before and after the 6MWT, RPE after the 6MWT, and 6MWD. Both DXA and BIA measures were included as candidate predictors because BIA was conducted on the same day with spiroergometry in EsmiRs metabolism substudy, while DXA was conducted earlier during the EsmiRs measurements. To avoid overfitting to our subsample of 39 participants, the number of predictors was limited to three.30 We studied the predictive performance of our model against measured VO2max with Pearson correlation, model errors, and Bland-Altman plot using leave-one-out cross-validation. Furthermore, we calculated intraclass correlation (ICC) estimates in R using the “irr” package based on the single measurement, absolute agreement, and two-way mixed-effect model.31 For sensitivity analyses, we also compared the performance of our model with another model with 6MWD as a predictor published by Mänttäri et al.32
The primary cross-sectional analyses were conducted with multiple linear regression models using the follow-up measurements from the EsmiRs study. MRS total symptoms, and its subscales were used separately as the outcome variable, while physical activity (ACC-PA and SR-PA), CRF (VO2max), and body composition (total body mass, DXA body fat mass, and DXA lean body mass) were included in the model as predictors one at a time. CRF and body composition were used as the predictors with physical activity measures, and their interaction was also included in the model. Additionally, simple linear regression models with physical activity as predictor were fitted for participants in each tertile based on the total body mass, body fat mass, and lean body mass separately. The regression lines of each tertile were plotted on the same figure to further illustrate the interactions between physical activity and body composition measures. In the auxiliary analyses for participants that did not report any symptoms at baseline, multiple linear regression models with baseline physical activity, CRF, body composition measures as predictors, and the follow-up MRS scores as outcome were used. For sensitivity analyses, we ran the same analyses after excluding the premenopausal women. The models were adjusted with menopausal status, age, smoking, alcohol consumption, education, and the use of hormonal preparations. Additionally, VO2max models were adjusted with lean body mass and body composition models were adjusted with body height. Residual plots, Q–Q plots, and correlation analysis between the covariates were used for assessing the model assumptions.
RESULTS
Characteristics of the study population
In the full EsmiRs sample (n = 298), participants reported a total mean MRS score of 8.6 ± SD 5.0 with only two participants being fully asymptomatic (total MRS score = 0). Most severe symptoms were reported in the somato-vegetative subscale (Table 1). On average, participants tended to be slightly overweight with mean BMI being 25.8 ± 4.1. Participants who did not report any symptoms at baseline ERMA measurements and in the EsmiRs metabolism subsample tended to have lower fat mass and BMI, as well as higher physical activity, CRF levels, and less severe symptoms compared to full EsmiRs sample. The mean time between the ERMA and EsmiRs measurements was 3.8 ± 0.1 years.
Model for predicting VO2max
The estimated prediction model for VO2max was:
where VO2max is absolute maximal oxygen consumption (l/min), height is body height (cm), SMM is BIA skeletal muscle mass (kg), and 6MWD is the 6-minute walking distance (m).
With leave-one-out cross-validation, the mean absolute error between the measured and predicted values in the EsmiRs metabolism subsample was 0.19 ± 0.15 l/min for absolute VO2max and 2.75 ± 2.38 ml/min/kg for VO2max relative to body weight. Pearson correlations and ICCs with 95% confidence intervals were 0.71 (0.50 to 0.84) and 0.69 (0.48 to 0.83), respectively (Supplemental Fig. 1A, http://links.lww.com/MENO/B275). Applying the previously published prediction model for VO2max relative to body weight32 to our study population resulted in a mean absolute error of 3.88 ± 2.67 ml/kg/min as well as Pearson correlations and ICCs of 0.69 (0.48 to 0.83) and 0.46 (−0.09 to 0.76), respectively (Supplemental Fig. 1B, http://links.lww.com/MENO/B275).
Associations between physical activity, CRF, body composition, and menopausal symptoms
In the primary analyses, higher total body and fat mass were associated with more severe total symptoms, somato-vegetative symptoms, and psychological symptoms with 1 kg higher mass predicting 0.03 to 0.07 higher symptoms score (Table 2). Physical activity and CRF were not associated with the severity of menopausal symptoms, except for the higher SR-PA being associated with less severe somato-vegetative symptoms. In the auxiliary analyses of the 82 participants that did not report any symptoms at baseline, ERMA baseline physical activity, VO2max, and body composition were not associated with the severity of menopausal symptoms assessed 4 years later (Table 3). The results did not change significantly after excluding the premenopausal women from the analyses (data not shown).
TABLE 2.
Associations of physical activity, cardiorespiratory fitness, and body composition with menopausal symptoms in the cross-sectional study (n = 298)
| Total symptoms | Somato-vegetative symptoms | Psychological symptoms | Urogenital symptoms | |||||
|---|---|---|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | |
| ACC-PA (10 mg) | −0.16 | −0.86 to 0.55 | −0.23 | −0.53 to 0.08 | −0.05 | −0.39 to 0.28 | 0.12 | −0.15 to 0.39 |
| SR-PA (MET-h/d) | −0.13 | −0.27 to 0.02 | −0.07c | −0.13 to −0.00 | −0.03 | −0.10 to 0.04 | −0.02 | −0.08 to 0.04 |
| Predicted VO2max (l/min)a | 1.08 | −1.80 to 3.95 | 0.35 | −0.90 to 1.60 | 0.27 | −1.08 to 1.63 | 0.45 | −0.64 to 1.54 |
| Total body mass (kg)b | 0.06c | 0.01 to 0.12 | 0.03d | 0.01 to 0.05 | 0.03c | 0.00 to 0.05 | 0.01 | −0.01 to 0.03 |
| Body fat mass (kg)b | 0.07c | 0.01 to 0.14 | 0.04c | 0.01 to 0.06 | 0.03c | 0.00 to 0.06 | 0.01 | −0.02 to 0.03 |
| Lean body mass (kg)b | 0.13 | −0.03 to 0.30 | 0.05 | −0.02 to 0.12 | 0.07 | −0.01 to 0.15 | 0.02 | −0.05 to 0.08 |
Multiple imputation was applied in the analyses. All models are adjusted with menopausal status, age, use of hormonal preparations, smoking, education, and alcohol consumption.
ACC-PA, accelerometer-measured physical activity mean amplitude deviation; mg, milligravity (0.00981 m/s2); SR-PA, self-reported physical activity; VO2max, maximal oxygen consumption.
aModel is additionally adjusted with lean body mass.
bModel is additionally adjusted with body height.
cP < 0.05
dP < 0.01
TABLE 3.
Associations of baseline physical activity, cardiorespiratory fitness, and body composition with follow-up menopausal symptoms in the longitudinal study (n = 82)
| Total symptoms | Somato-vegetative symptoms | Psychological symptoms | Urogenital symptoms | |||||
|---|---|---|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | |
| ACC-PA (10 mg) | −0.18 | −1.08 to 0.72 | −0.13 | −0.55 to 0.29 | 0.03 | −0.38 to 0.44 | −0.08 | −0.43 to 0.27 |
| SR-PA (MET-h/d) | −0.01 | −0.19 to 0.18 | 0.01 | −0.08 to 0.10 | 0.00 | −0.08 to 0.08 | −0.01 | −0.09 to 0.06 |
| Predicted VO2max (l/min)a | −2.06 | −7.23 to 3.12 | −1.12 | −3.63 to 1.39 | −0.56 | −2.84 to 1.71 | −0.37 | −2.40 to 1.67 |
| Total body mass (kg)b | −0.03 | −0.13 to 0.06 | −0.03 | −0.07 to 0.02 | 0.01 | −0.03 to 0.05 | −0.02 | −0.05 to 0.02 |
| Body fat mass (kg)b | −0.04 | −0.39 to 0.31 | 0.01 | −0.16 to 0.17 | −0.05 | −0.20 to 0.10 | 0.01 | −0.13 to 0.14 |
| Lean body mass (kg)b | 0.04 | −0.29 to 0.37 | 0.00 | −0.16 to 0.16 | 0.06 | −0.09 to 0.20 | −0.02 | −0.15 to 0.11 |
Multiple imputation was applied in the analyses. All models are adjusted with baseline menopausal status, age, use of hormonal preparations, smoking, education, and alcohol consumption.
ACC-PA, accelerometer-measured physical activity mean amplitude deviation; mg, milligravity (0.00981 m/s2); SR-PA, self-reported physical activity; VO2max, maximal oxygen consumption.
aModel is additionally adjusted with baseline lean body mass.
bModel is additionally adjusted with baseline body height.
In separate models with interaction between VO2max and physical activity as well as body composition measures and physical activity, all body composition measures showed positive interactions with ACC-PA or SR-PA (Supplemental Tables 1–2, http://links.lww.com/MENO/B275). Notably, interactions of total body and fat mass with physical activity measures were strongest with urogenital symptoms. Total body mass and especially lean body mass interacted with physical activity measures in total and psychological symptoms. VO2max showed no interaction with physical activity measures.
Figure 2 and Supplemental Tables 3–4 (http://links.lww.com/MENO/B275) illustrate that ACC-PA and SR-PA were inversely associated with total, somato-vegetative, and psychological symptoms in participants with the lowest lean body mass. In the lowest lean body mass tertile, 10 mg higher ACC-PA predicted decrease of 1.43 (0.26 to 2.60) in total symptom score. With participants in lowest total body and fat mass tertiles, inverse associations of physical activity and menopausal symptoms were not as distinct as with participants in lowest lean body mass tertile. However, in participants with highest body fat mass ACC-PA associated directly with psychological and urogenital symptoms.
FIG. 2.
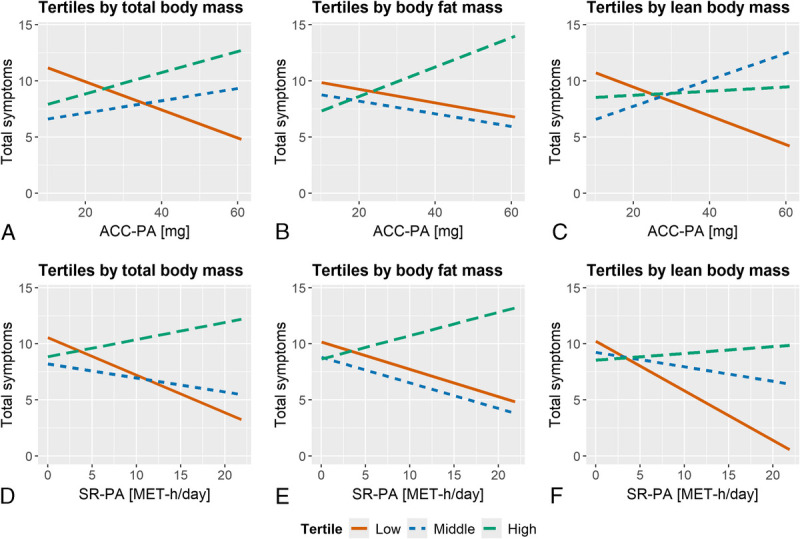
Simple linear regression lines for total menopausal symptoms with ACC-PA and SR-PA as predictors by total body mass, body fat mass, and lean body mass tertiles. (A) ACC-PA and tertiles by body mass (n = 298), (B) ACC-PA tertiles by body fat mass (n = 293), (C) ACC-PA and tertiles by lean body mass (n = 293), (D) SR-PA and tertiles by body mass (n = 298), (E) SR-PA tertiles by body fat mass (n = 293), (F) SR-PA and tertiles by lean body mass (n = 293). Tertile mean values in kg from lowest to highest are 59.0, 69.6, and 84.3 for total body mass, 16.8, 24.7, and 36.3 for body fat mass, and 37.6, 42.3, and 46.8 for lean body mass. Multiple imputation was applied in the analyses. ACC-PA, accelerometer-measured physical activity; SR-PA, self-reported physical activity.
DISCUSSION
We observed that the association between physical activity and menopausal symptoms varied based on the differences in body composition. The association of physical activity with total symptoms and psychological symptoms varied based on the differences in total and lean body mass with stronger indirect associations being observed in participants with lower total and lean body mass. The association between physical activity and urogenital symptoms was affected by the differences in total body and fat mass with direct associations being observed in participants with increased total body and fat mass. Furthermore, higher total body and fat mass were associated with more severe menopausal symptoms in the cross-sectional study, but CRF was not observed to associate with menopausal symptoms or to interact with physical activity.
In the cross-sectional analyses, physical activity and CRF were not associated with menopausal symptoms. Our results are in line with the previous literature proposing very weak or negligible indirect associations between physical activity and menopausal symptoms.3–5 Contradictory to our results, the few previous observational studies focusing on the role of CRF in this context have reported inverse associations between CRF and menopausal symptoms.9,33 Similarly, an inverse association between CRF and menopause-related quality of life has also been reported in one randomized controlled trial with exercise intervention in middle-aged women.15 However, another exercise intervention study did not observe an association between CRF and menopausal symptoms in postmenopausal women.16
Our results from the cross-sectional analyses with interactions included in the models suggested that the association between physical activity and the severity of menopausal symptoms fluctuates based on body composition. Notably, the association between physical activity and urogenital symptoms varied based on the differences in total body mass and especially body fat mass with the strongest direct associations between physical activity and urogenital symptoms being observed in participants with highest total body and fat mass. This may be accounted for by the fact that more active women are more likely to engage in activities with higher intensity; however, women with higher total body and fat mass may be less accustomed to high-intensity exercise, predisposing them to pelvic floor disorders such as urinary incontinence.34
Furthermore, body weight and especially lean body mass explained the differences in association of physical activity with menopausal symptoms. Participants with the lowest lean body mass had strong direct associations between physical activity and the severity of somato-vegetative, psychological, and total symptoms. This observation may be due to the differences in physical activity type among participants with different lean body mass. However, with the physical activity assessment methods used in this study, we were not able to identify the type of physical activity participants are engaging. Nonetheless, the exercise that does not significantly increase muscle mass (eg, aerobic training) may be more beneficial for alleviating symptoms compared to exercise that builds muscle mass (eg, resistance training). This hypothesis is supported by the results of the study with the aerobic and resistance training interventions in which the severity of menopausal symptoms decreased in both intervention groups, but the decrease was more significant in the group with aerobic training intervention.35 Moreover, the exercise intervention studies for menopausal symptoms have mainly focused on aerobic training and yoga rather than resistance training,3–5 and therefore the difference between aerobic and resistance training for menopausal symptoms is not well known.
Overall, the association between physical activity and menopausal symptoms varied significantly between the different body composition tertiles as well as between the different body composition measures. Consequently, because of this significant variation and relatively small sample size in this study, the results of this study should be considered as preliminary findings on this topic that should be verified in future studies.
When studying the associations between body composition and menopausal symptoms, we observed that participants with higher total body and fat mass had more severe total symptoms, somato-vegetative symptoms, and psychological symptoms in the cross-sectional analyses. However, in the auxiliary analyses with a smaller sample size, baseline body composition measures were not associated with menopausal symptoms assessed after the four-year follow-up. The direct association between BMI and body adiposity with menopausal symptoms has also been reported previously in cross-sectional and longitudinal studies, especially with vasomotor symptoms.10,36 Direct association between obesity and the severity of urogenital symptoms has also been reported.37,38 Therefore, in agreement with previous studies, our results indicate that obesity may be a risk factors for a variety of menopausal symptoms.
The strengths of the study are the assessment of menopausal symptoms with a validated questionnaire that accounts for the severity of the symptoms and the assessment of CRF with spiroergometry. Furthermore, body composition measured accurately with DXA, the use of accelerometer-measured physical activity, a comprehensive set of measured confounders including the use of hormonal preparations. The limitation of the study is the homogeneous sample of White women with exclusion of women with severe obesity and severe medical disorders, thus limiting the generalizability of the results. Furthermore, the statistically significant findings should be interpreted with caution due to the multiple statistical comparisons conducted in the study.
CONCLUSIONS
Maintaining healthy weight is associated with less severe menopausal symptoms in middle-aged women. Furthermore, the results of this study provide preliminary evidence that the association between physical activity and menopausal symptoms varies based on the differences in total and lean body mass highlighting the need for further research on this topic.
Acknowledgments
We thank Anja Ahlgren, Eeva-Maija Palonen, Mervi Matero, Hanne Tähti, and the other laboratory staff in Faculty of Sport and Health Sciences for their invaluable help with the data collection, as well as the participants in ERMA and EsmiRs studies who volunteered their time and effort. Gerontology Research Center is a joint effort between the University of Jyväskylä and the University of Tampere.
Footnotes
Funding/support: This work was supported by the Academy of Finland (grant number 275323 to V.K.; grant numbers 309504, 314181, and 335249 to E.K.L.; grant numbers 321336 and 328818 to T.R.; grant numbers 339391 and 346462 to L.K.).
Financial disclosures/conflicts of interest: None reported.
Data availability: The datasets generated and/or analyzed during the current study are not publicly available due to EU and Finnish legislation and the consent provided by the participants, which do not permit open access to individual level personal data. However, they are available from the corresponding author on reasonable request. More information about the datasets: doi.10.17011/jyx/dataset/83491.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Website (www.menopause.org).
ORCID ID: Matti Hyvärinen, https://orcid.org/0000-0002-5086-3635
Contributor Information
Juha Karvanen, Email: juha.t.karvanen@jyu.fi.
Jari E. Karppinen, Email: jari.karppinen@helsinki.fi.
Laura Karavirta, Email: laura.i.karavirta@jyu.fi.
Hanna-Kaarina Juppi, Email: hanna.k.juppi@jyu.fi.
Tuija H. Tammelin, Email: tuija.tammelin@jamk.fi.
Vuokko Kovanen, Email: vukekovanen@gmail.com.
Jari Laukkanen, Email: jari.laukkanen@ksshp.fi.
Pauliina Aukee, Email: pauliina.aukee@hyvaks.fi.
Sarianna Sipilä, Email: sarianna.sipila@jyu.fi.
Timo Rantalainen, Email: timo.rantalainen@jyu.fi.
Eija K. Laakkonen, Email: eija.k.laakkonen@jyu.fi.
REFERENCES
- 1.Harlow SD Gass M Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Menopause 2012;19:387–395. doi: 10.1097/gme.0b013e31824d8f40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteleone P, Mascagni G, Giannini A, Genazzani AR, Simoncini T. Symptoms of menopause — global prevalence, physiology and implications. Nat Rev Endocrinol 2018;14:199–215. doi: 10.1038/nrendo.2017.180 [DOI] [PubMed] [Google Scholar]
- 3.Daley A, Stokes-Lampard H, Thomas A, MacArthur C. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev 2014;11:CD006108. doi: 10.1002/14651858.CD006108.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettee Gabriel K, Mason JM, Sternfeld B. Recent evidence exploring the associations between physical activity and menopausal symptoms in midlife women: perceived risks and possible health benefits. Womens Midlife Health 2015;1:1. doi: 10.1186/s40695-015-0004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen TM, Do TTT, Tran TN, Kim JH. Exercise and quality of life in women with menopausal symptoms: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health 2020;17:7049. doi: 10.3390/ijerph17197049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Donoghue G, Blake C, Cunningham C, Lennon O, Perrotta C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes Rev 2021;22:e13137. doi: 10.1111/obr.13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muñoz-Martínez FA, Rubio-Arias JÁ, Ramos-Campo DJ, Alcaraz PE. Effectiveness of resistance circuit-based training for maximum oxygen uptake and upper-body one-repetition maximum improvements: a systematic review and meta-analysis. Sports Med 2017;47:2553–2568. doi: 10.1007/s40279-017-0773-4 [DOI] [PubMed] [Google Scholar]
- 8.Irwin ML Yasui Y Ulrich CM, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA 2003;289:323–330. doi: 10.1001/jama.289.3.323 [DOI] [PubMed] [Google Scholar]
- 9.Moradpour F, Koushkie Jahromi M, Fooladchang M, Rezaei R, Sayar Khorasani MR. Association between physical activity, cardiorespiratory fitness, and body composition with menopausal symptoms in early postmenopausal women. Menopause 2020;27:230–237. doi: 10.1097/GME.0000000000001441 [DOI] [PubMed] [Google Scholar]
- 10.Thurston RC Sowers MR Sternfeld B, et al. Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women's health across the nation. Am J Epidemiol 2009;170:766–774. doi: 10.1093/aje/kwp203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harlow SD Karvonen-Gutierrez C Elliott MR, et al. It is not just menopause: symptom clustering in the Study of Women's Health Across the Nation. Women's Midlife Health 2017;3:2. doi: 10.1186/s40695-017-0021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson AS, Sui X, Hébert JR, Church TS, Blair SN. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med 2009;169:1781–1787. doi: 10.1001/archinternmed.2009.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambikairajah A, Walsh E, Tabatabaei-Jafari H, Cherbuin N. Fat mass changes during menopause: a metaanalysis. Am J Obstet Gynecol 2019;221:393–409.e50. doi: 10.1016/j.ajog.2019.04.023 [DOI] [PubMed] [Google Scholar]
- 14.Sternfeld B Guthrie KA Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause 2014;21:330–338. doi: 10.1097/GME.0b013e31829e4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elavsky S, McAuley E. Physical activity and mental health outcomes during menopause: a randomized controlled trial. Ann Behav Med 2007;33:132–142. doi: 10.1007/BF02879894 [DOI] [PubMed] [Google Scholar]
- 16.Aiello EJ Yasui Y Tworoger SS, et al. Effect of a yearlong, moderate-intensity exercise intervention on the occurrence and severity of menopause symptoms in postmenopausal women. Menopause 2004;11:382–388. doi: 10.1097/01.GME.0000113932.56832.27 [DOI] [PubMed] [Google Scholar]
- 17.Kovanen V Aukee P Kokko K, et al. Design and protocol of Estrogenic Regulation of Muscle Apoptosis (ERMA) study with 47 to 55-year-old women's cohort: novel results show menopause-related differences in blood count. Menopause 2018;25:1020–1032. doi: 10.1097/GME.0000000000001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyvärinen M Juppi HK Taskinen S, et al. Metabolic health, menopause, and physical activity—a 4-year follow-up study. Int J Obes (Lond) 2022;46:544–554. doi: 10.1038/s41366-021-01022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karppinen JE Juppi HK Hintikka J, et al. Associations of resting and peak fat oxidation with sex hormone profile and blood glucose control in middle-aged women. Nutr Metab Cardiovasc Dis 2022;32:2157–2167. doi: 10.1016/j.numecd.2022.06.001 [DOI] [PubMed] [Google Scholar]
- 20.Hyvärinen M Sipilä S Kulmala J, et al. Validity and reliability of a single question for leisure-time physical activity assessment in middle-aged women. J Aging Phys Act 2020;28:231–241. doi: 10.1123/japa.2019-0093 [DOI] [PubMed] [Google Scholar]
- 21.Potthoff P, Heinemann LA, Schneider HP, Rosemeier HP, Hauser GA. The Menopause Rating Scale (MRS II): methodological standardization in the German population [in German]. Zentralbl Gynakol 2000;122:280–286. [PubMed] [Google Scholar]
- 22.Schneider HP, Heinemann LA, Rosemeier HP, Potthoff P, Behre HM. The Menopause Rating Scale (MRS): comparison with Kupperman index and quality-of-life scale SF-36. Climacteric 2000;3:50–58. doi: 10.3109/13697130009167599 [DOI] [PubMed] [Google Scholar]
- 23.Hyvärinen M Karvanen J Juppi HK, et al. Menopausal symptoms and cardiometabolic risk factors in middle-aged women: a cross-sectional and longitudinal study with 4-year follow-up. Maturitas 2023;174:39–47. doi: 10.1016/j.maturitas.2023.05.004 [DOI] [PubMed] [Google Scholar]
- 24.Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: The Finnish Twin Cohort. JAMA 1998;279:440–444. doi: 10.1001/jama.279.6.440 [DOI] [PubMed] [Google Scholar]
- 25.Enright PL. The six-minute walk test. Respir Care 2003;48:783–785. [PubMed] [Google Scholar]
- 26.Seaman S, Galati J, Jackson D, Carlin J. What is meant by “missing at random”? Statistical Science 2013;28:257–268. doi: 10.1214/13-STS415 [DOI] [Google Scholar]
- 27.R Core Team . R: A language and environment for statistical computing. Version 4.3.1. R Foundation for Statistical Computing. Published online 2023. Available at: https://www.R-project.org/
- 28.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 29.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 30.Harrell FE. Regression Modeling Strategies. 2nd ed,. New York: Springer; 2015. [Google Scholar]
- 31.Gamer M, Lemon J, Fellows I, Singh P. irr: various coefficients of interrater reliability and agreement. Published online 2019. Available at: https://CRAN.R-project.org/package=irr
- 32.Mänttäri A Suni J Sievänen H, et al. Six-minute walk test: a tool for predicting maximal aerobic power (VO2 max) in healthy adults. Clin Physiol Funct Imaging 2018;38:1038–1045. doi: 10.1111/cpf.12525 [DOI] [PubMed] [Google Scholar]
- 33.Aparicio VA Borges-Cosic M Ruiz-Cabello P, et al. Association of objectively measured physical activity and physical fitness with menopause symptoms. The Flamenco Project. Climacteric 2017;20:456–461. doi: 10.1080/13697137.2017.1329289 [DOI] [PubMed] [Google Scholar]
- 34.Bø K. Urinary incontinence, pelvic floor dysfunction, exercise and sport. Sports Med 2004;34:451–464. doi: 10.2165/00007256-200434070-00004 [DOI] [PubMed] [Google Scholar]
- 35.Ağıl A, Abıke F, Daşkapan A, Alaca R, Tüzün H. Short-term exercise approaches on menopausal symptoms, psychological health, and quality of life in postmenopausal women. Obstet Gynecol Int 2010;2010:e274261. doi: 10.1155/2010/274261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gold EB Crawford SL Shelton JF, et al. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms: the Study of Women's Health Across the Nation (SWAN). Menopause 2017;24:9–26. doi: 10.1097/GME.0000000000000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pastore LM, Carter RA, Hulka BS, Wells E. Self-reported urogenital symptoms in postmenopausal women: Women's Health Initiative. Maturitas 2004;49:292–303. doi: 10.1016/j.maturitas.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 38.Pomian A, Lisik W, Kosieradzki M, Barcz E. Obesity and pelvic floor disorders: a review of the literature. Med Sci Monit 2016;22:1880–1886. doi: 10.12659/MSM.896331 [DOI] [PMC free article] [PubMed] [Google Scholar]


