Abstract
PURPOSE
Phase III studies of intravenous amivantamab demonstrated efficacy across epidermal growth factor receptor (EGFR)–mutated advanced non–small cell lung cancer (NSCLC). A subcutaneous formulation could improve tolerability and reduce administration time while maintaining efficacy.
PATIENTS AND METHODS
Patients with EGFR-mutated advanced NSCLC who progressed after osimertinib and platinum-based chemotherapy were randomly assigned 1:1 to receive subcutaneous or intravenous amivantamab, both combined with lazertinib. Coprimary pharmacokinetic noninferiority end points were trough concentrations (Ctrough; on cycle-2-day-1 or cycle-4-day-1) and cycle-2 area under the curve (AUCD1-D15). Key secondary end points were objective response rate (ORR) and progression-free survival (PFS). Overall survival (OS) was a predefined exploratory end point.
RESULTS
Overall, 418 patients underwent random assignment (subcutaneous group, n = 206; intravenous group, n = 212). Geometric mean ratios of Ctrough for subcutaneous to intravenous amivantamab were 1.15 (90% CI, 1.04 to 1.26) at cycle-2-day-1 and 1.42 (90% CI, 1.27 to 1.61) at cycle-4-day-1; the cycle-2 AUCD1-D15 was 1.03 (90% CI, 0.98 to 1.09). ORR was 30% in the subcutaneous and 33% in the intravenous group; median PFS was 6.1 and 4.3 months, respectively. OS was significantly longer in the subcutaneous versus intravenous group (hazard ratio for death, 0.62; 95% CI, 0.42 to 0.92; nominal P = .02). Fewer patients in the subcutaneous group experienced infusion-related reactions (IRRs; 13% v 66%) and venous thromboembolism (9% v 14%) versus the intravenous group. Median administration time for the first infusion was reduced to 4.8 minutes (range, 0-18) for subcutaneous amivantamab and to 5 hours (range, 0.2-9.9) for intravenous amivantamab. During cycle-1-day-1, 85% and 52% of patients in the subcutaneous and intravenous groups, respectively, considered treatment convenient; the end-of-treatment rates were 85% and 35%, respectively.
CONCLUSION
Subcutaneous amivantamab-lazertinib demonstrated noninferiority to intravenous amivantamab-lazertinib, offering a consistent safety profile with reduced IRRs, increased convenience, and prolonged survival.
PALOMA-3 shows noninferior PK, efficacy, better safety, and faster administration subcutaneous versus IV amivantamab.
INTRODUCTION
Amivantamab is an epidermal growth factor receptor (EGFR)-MET bispecific antibody with immune cell–directing activity.1-5 The intravenous formulation of amivantamab is approved in combination with chemotherapy in the first-line setting (phase III PAPILLON trial) and as a monotherapy after disease progression on platinum-based chemotherapy (phase I CHRYSALIS trial) in patients with locally advanced or metastatic non–small cell lung cancer (NSCLC) with EGFR exon 20 insertion mutations.6
CONTEXT
Key Objective
Is subcutaneous amivantamab combined with lazertinib noninferior (for pharmacokinetics and efficacy) versus intravenous amivantamab-lazertinib, and does it have a similar safety profile?
Knowledge Generated
Subcutaneous amivantamab-lazertinib demonstrated noninferior pharmacokinetics and objective response rates, with potentially longer response duration, progression-free survival, and overall survival compared with intravenous amivantamab-lazertinib. The subcutaneous formulation also exhibited reduced infusion-related reactions and venous thromboembolic events, with shorter treatment administration times and enhanced patient convenience compared with the intravenous formulation.
Relevance (T.E. Stinchcombe)
The subcutaneous formulation of amivantamab in combination with lazertinib or with carboplatin and pemetrexed may become a treatment option in the future.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
Amivantamab has been combined with lazertinib, a central nervous system–penetrant, third-generation EGFR tyrosine kinase inhibitor (EGFR-TKI). Amivantamab-lazertinib demonstrated superior progression-free survival (PFS) versus osimertinib in patients with treatment-naïve, EGFR-mutated advanced NSCLC on the basis of the phase III MARIPOSA trial.7
Infusion-related reactions (IRRs) are observed in two thirds of patients receiving intravenous amivantamab, with most occurring on cycle-1-day-1 and being grade 1 to 2.8 The initial dose of intravenous amivantamab is split over 2 days to reduce IRRs, resulting in a minimum total infusion time of 2-4 hours.6 The subcutaneous formulation of amivantamab was first evaluated in the phase I PALOMA trial, revealing low rates of IRRs and associated symptoms, with an administration time ≤7 minutes for the once-every-2-week and once-every-3-week regimens, and up to 10 minutes for the once-every-4-week regimen.9,10
The goal of the subcutaneous amivantamab development program is to reduce administration time and improve patient convenience. PALOMA-3 (ClinicalTrials.gov identifier: NCT05388669) is a phase III, international, randomized trial assessing the noninferiority of pharmacokinetics, efficacy, and safety of subcutaneous versus intravenous amivantamab, both combined with lazertinib, in patients with EGFR-mutated, advanced NSCLC after disease progression on osimertinib and platinum-based chemotherapy.
PATIENTS AND METHODS
Patients
Eligible patients were age 18 years and older, had confirmed advanced or metastatic NSCLC harboring classical EGFR exon 19 deletions (Ex19del) or exon 21 L858R mutations with disease progression on or after osimertinib (or another approved third-generation EGFR-TKI) and platinum-based chemotherapy, irrespective of sequence. For additional criteria, see the protocol (online only).
Study Design and Treatment
Patients were randomly assigned (1:1) to receive subcutaneous amivantamab-lazertinib or intravenous amivantamab-lazertinib in 28-day cycles (Appendix Fig A1, online only). Subcutaneous amivantamab (concentration, 160 mg/mL), coformulated with hyaluronidase (rHuPH20), was administered by manual injection at a dose of 1,600 mg (2,240 mg for ≥80 kg weight) once-weekly for the first 4 weeks and every 2 weeks thereafter.9 Intravenous amivantamab (concentration, 50 mg/mL) was administered at the approved dose of 1,050 mg (1,400 mg for ≥80 kg weight) on the same interval, with the first infusion split over 2 days (350 mg on cycle-1-day-1, the remainder on cycle-1-day-2). Lazertinib was administered orally at a dose of 240 mg once daily.
Random assignment was stratified by history of brain metastases (yes or no), EGFR mutation type (Ex19del v L858R), race (Asian v non-Asian), and type of last therapy (osimertinib v chemotherapy).
An increased risk of venous thromboembolism (VTE) associated with intravenous amivantamab-lazertinib was initially observed in the MARIPOSA trial.7 This increase appears specific to amivantamab-lazertinib, as amivantamab monotherapy, lazertinib monotherapy, and amivantamab-chemotherapy did not show notable rises in VTE incidence.11-14 Consequently, the study protocol was amended to recommend prophylactic anticoagulation for the first 4 months of amivantamab-lazertinib treatment as per local guidelines.
End Points and Assessments
The coprimary pharmacokinetic outcomes for noninferiority were trough concentrations (Ctrough; either predose on cycle-2-day-1 or at steady state [cycle-4-day-1], per regional health authority guidance) and area under the curve from cycle-2-day-1 to day-15 (AUCD1-D15). Key secondary outcomes were objective response rate (ORR) and PFS. Overall survival (OS) was a predefined exploratory end point. A complete list of outcomes and definitions is available in the protocol.
Disease assessments (computed tomography, magnetic resonance imaging, or other imaging) were performed within 28 days before random assignment, then at 6 weeks (maximum, 7 weeks) after random assignment and subsequently every 6 weeks (within a 1-week window) for the first 18 months and every 12 weeks (within a 1-week window) thereafter until disease progression. All response assessments were performed by the investigator according to RECIST, v1.1. All patients underwent brain imaging at baseline; subsequent imaging was performed every 6 weeks in patients with baseline brain metastases or as clinically indicated.
Adverse events (AEs), vital signs, and laboratory tests were assessed at each visit and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v5.0. Pharmacokinetic and immunogenicity assessments for amivantamab were conducted using validated assays on blood serum and plasma samples collected throughout the trial until the end of treatment. Patient-reported cancer therapy satisfaction was assessed using a modified version of the Therapy Administration Satisfaction Questionnaire (TASQ), completed by patients after treatment administration in cycle-1, cycle-3, and at the end of treatment.
Trial Oversight
The trial was conducted in accordance with the provisions of the Declaration of Helsinki, Good Clinical Practice guidelines (as defined by the International Council for Harmonisation), and applicable regulatory and country-/territory-specific requirements. The protocol was approved by the local institutional review board and independent ethics committees of the participating centers. Patients provided written informed consent at screening. Protocol amendments made after the study started are described in the protocol.
Statistical Analysis
The pharmacokinetic analysis included patients who received all doses without modification and provided the required pharmacokinetic samples through the final required sample relevant to the end point. Efficacy analysis included all randomly assigned patients. Safety analysis included all patients who received ≥1 dose of any treatment. For calculating primary and key secondary outcomes, we estimated that a sample size of 400 patients would provide >95% power for a one-sided alpha of .05 allocated to each of the coprimary pharmacokinetic end points and 80% power with a one-sided alpha of .025 allocated to ORR. For the coprimary end points, the power analysis assumes true geometric mean ratios of Ctrough and AUCD1-D15 to be 1 between the two treatment groups, and a coefficient of variation (CV) of 56% for both end points. Additional details can be found in Section 9 of the protocol.
The primary hypotheses were that the lower bounds of the 90% CI for the geometric mean ratios for subcutaneous versus intravenous amivantamab would be ≥80% (noninferiority margin of 20%) for both coprimary pharmacokinetic end points. The noninferiority criterion for the pharmacokinetic primary end point is based on the US Food and Drug Administration–recommended lower limit for the bioequivalence.15,16 ORR was analyzed using logistic regression, with noninferiority established if the lower bound of the relative risk's 95% CI was ≥60% (on the basis of regulatory precedence from other subcutaneous formulations17; additional details can be found on the statistical analysis plan, Data Supplement, online only). PFS was evaluated using the P value generated from the stratified log-rank test, with EGFR mutation type, Asian race, history of brain metastasis, and last therapy as stratification factors. The hazard ratio (HR) and 95% CI were estimated using a stratified Cox regression model, with treatment as the sole explanatory variable. Medians and corresponding 95% CIs were estimated using the Kaplan-Meier method. A hierarchical testing approach was used for the coprimary pharmacokinetic end points (noninferiority, at a two-sided alpha of .05), followed by the key secondary end points of ORR (noninferiority) and then PFS (superiority). The key secondary end points were tested using a combined two-sided alpha of .05.
Analyses of additional secondary or other outcomes, including subgroup analyses, were not part of hypothesis testing in the trial, and these results are reported as descriptive statistics without adjustment for multiplicity. All data reported here are based on the primary analysis and were reported before the January 3, 2024, data cutoff date.
RESULTS
Patients
From August 2022 to October 2023, 635 patients were screened and 418 were randomly assigned (206 to subcutaneous amivantamab-lazertinib and 212 to intravenous amivantamab-lazertinib; Fig 1). Overall, 416 patients received ≥1 dose of trial treatment. Pharmacokinetic samples were available from 414 patients. Demographic and baseline characteristics were well balanced (Table 1); median number of previous therapy lines was 2 (range, 1-5 [subcutaneous], 1-4 [intravenous]). Most patients were female, were Asian or White, and had never smoked.
FIG 1.
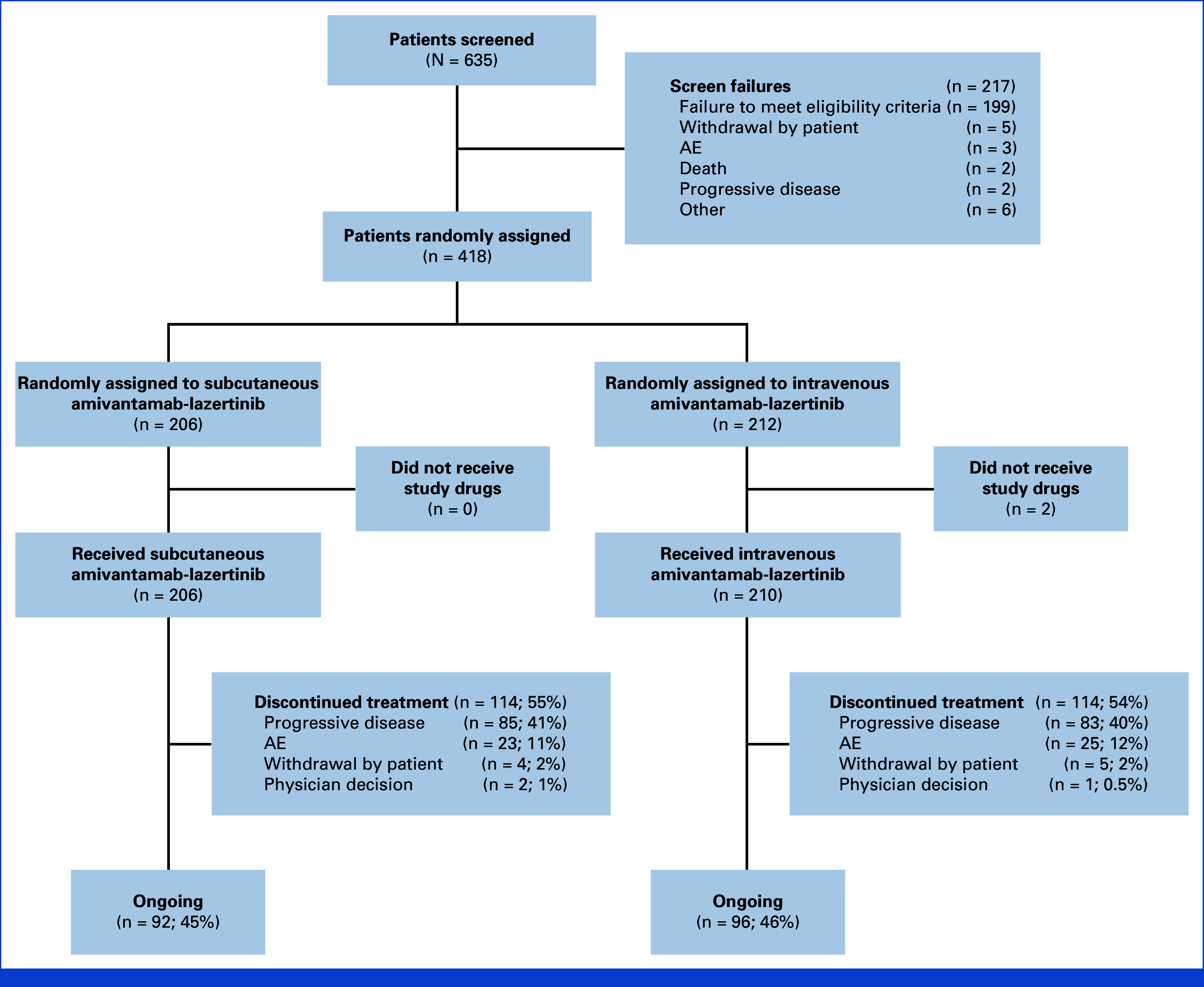
CONSORT diagram of patient disposition. AE, adverse event.
TABLE 1.
Demographic and Clinical Characteristics of Patients at Baseline
| Characteristic | Subcutaneous Group (n = 206) | Intravenous Group (n = 212) |
|---|---|---|
| Age, years | ||
| Median (range) | 61 (35-82) | 62 (29-81) |
| Distribution, No. (%) | ||
| <65 years | 133 (65) | 120 (57) |
| ≥65 to <75 years | 55 (27) | 70 (33) |
| ≥75 years | 18 (9) | 22 (10) |
| Sex, No. (%) | ||
| Female | 138 (67) | 141 (67) |
| Male | 68 (33) | 71 (33) |
| Race or ethnic group, No. (%) | ||
| Asian | 126 (61) | 129 (61) |
| White | 78 (38) | 77 (36) |
| Black or African American | 1 (0.5) | 3 (1) |
| Multiple | 0 | 1 (0.5) |
| Not reported | 1 (0.5) | 2 (0.9) |
| Body weight | ||
| Median, kg (range) | 61.8 (35-130) | 60.1 (33-150) |
| Distribution, No. (%) | ||
| <80 kg | 184 (89) | 184 (87) |
| ≥80 kg | 22 (11) | 28 (13) |
| Region of enrollment, No. (%)a | ||
| North America | 19 (9) | 30 (14) |
| South America | 11 (5) | 17 (8) |
| Europe | 38 (18) | 40 (19) |
| Asia | 126 (61) | 120 (57) |
| Oceania | 12 (6) | 5 (2) |
| ECOG PS, No. (%) | ||
| 0 | 58 (28) | 61 (29) |
| 1 | 148 (72) | 151 (71) |
| History of smoking, No. (%) | ||
| No | 141 (68) | 145 (68) |
| Yes | 65 (32) | 67 (32) |
| Median time from initial diagnosis, months (range) | 34.5 (2.8-191.3) | 33.7 (6.1-156.9) |
| Median time from metastatic diagnosis, months (range) | 32.7 (0.9-169.0) | 29.7 (0.6-142.6) |
| Histologic type, No. (%) | ||
| Adenocarcinoma | 204 (99) | 207 (98) |
| Large cell carcinoma | 1 (0.5) | 1 (0.5) |
| Squamous cell carcinoma | 1 (0.5) | 3 (1) |
| Other | 0 | 1 (0.5) |
| EGFR mutation type at random assignment, No. (%) | ||
| Exon 19 deletion | 135 (66) | 138 (65) |
| L858R | 71 (34) | 74 (35) |
| History of brain metastasis, No. (%) | ||
| Yes | 70 (34) | 72 (34) |
| No | 136 (66) | 140 (66) |
| Last therapy before random assignment, No. (%) | ||
| Osimertinib | 91 (44) | 96 (45) |
| Chemotherapy | 115 (56) | 116 (55) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; NSCLC, non–small cell lung cancer; TKI, tyrosine kinase inhibitor.
Russia was counted as part of Europe; Turkey and Israel were counted as part of Asia.
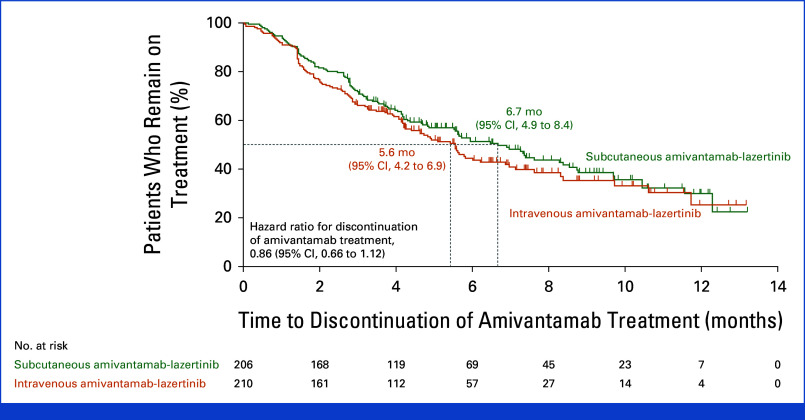
At a median follow-up of 7.0 months (range, 0.1-14.4), median treatment duration was 4.7 months (range, 0.1-13.2) in the subcutaneous group and 4.1 (range, 0.0-13.2) in the intravenous group. Median duration of amivantamab administration on cycle-1-day-1 was 4.8 minutes (range, 0-18) in the subcutaneous and 5.0 hours (range, 0.2-9.9) for the first infusion on cycle-1-day-1 in the intravenous group; corresponding values on cycle-3-day-1 were 4.8 minutes (range, 0-12) and 2.3 hours (range, 0.5-4.4). At data cutoff, 92 (45%) and 96 (46%) patients were undergoing treatment in the subcutaneous and intravenous groups, respectively. Time to amivantamab discontinuation is shown in Appendix Figure A2.
Pharmacokinetics
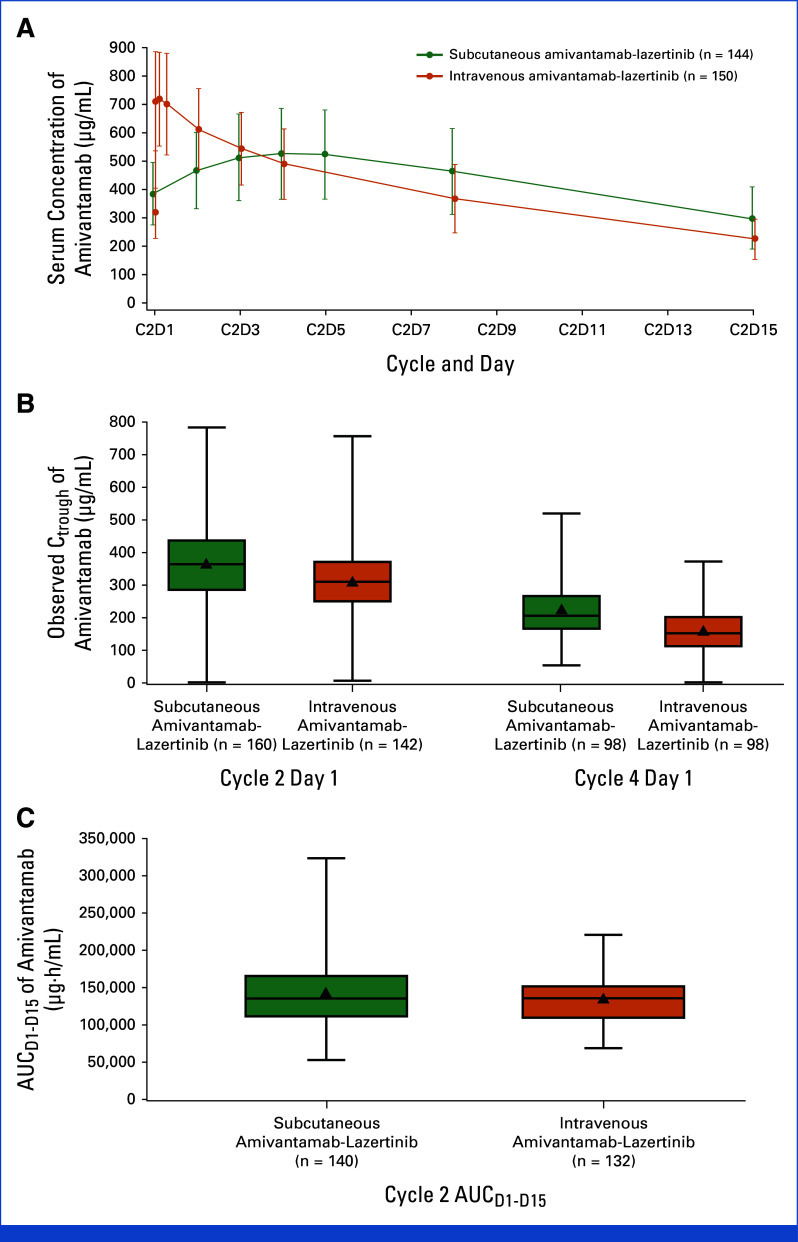
Mean (%CV) Ctrough at cycle-2-day-1 was 365 (33) µg/mL and 314 (32) µg/mL in the subcutaneous and intravenous groups, respectively; corresponding values at cycle-4-day-1 were 224 (39) µg/mL and 162 (42) µg/mL (Table 2). The geometric mean ratio for Ctrough for subcutaneous to intravenous group was 1.15 (90% CI, 1.04 to 1.26) at cycle-2-day-1 and 1.43 (90% CI, 1.27 to 1.61) at cycle-4-day-1. Cycle-2 AUCD1-D15 mean (%CV) was 142,236 (31) µg·h/mL and 135,552 (24) µg·h/mL in the subcutaneous and intravenous groups, respectively. The geometric mean ratio for cycle-2 AUCD1-D15 was 1.03 (90% CI, 0.98 to 1.09). These results indicate that the noninferiority criteria were met. Observed amivantamab concentration-time profiles and boxplots of Ctrough and AUCD1-D15 are shown in Appendix Figure A3.
TABLE 2.
Coprimary Pharmacokinetic and Key Efficacy End Points
| End Point | Subcutaneous Group (n = 206) | Intravenous Group (n = 212) | Treatment Effect (95% CI) | P |
|---|---|---|---|---|
| Coprimary pharmacokinetic end pointsa | ||||
| Ctrough, µg/mL (%CV) | Geometric mean ratio (90% CI) | |||
| Cycle-2-day-1 | 365 (33) | 314 (32) | 1.15 (1.04 to 1.26) | |
| Cycle-4-day-1 (steady state) | 224 (39) | 162 (42) | 1.43 (1.27 to 1.61) | |
| AUCD1-D15, µg·h/mL (%CV) | Geometric mean ratio (90% CI) | |||
| Cycle 2 | 142,236 (31) | 135,552 (24) | 1.03 (0.98 to 1.09) | |
| Secondary end points | ||||
| Objective responseb | ||||
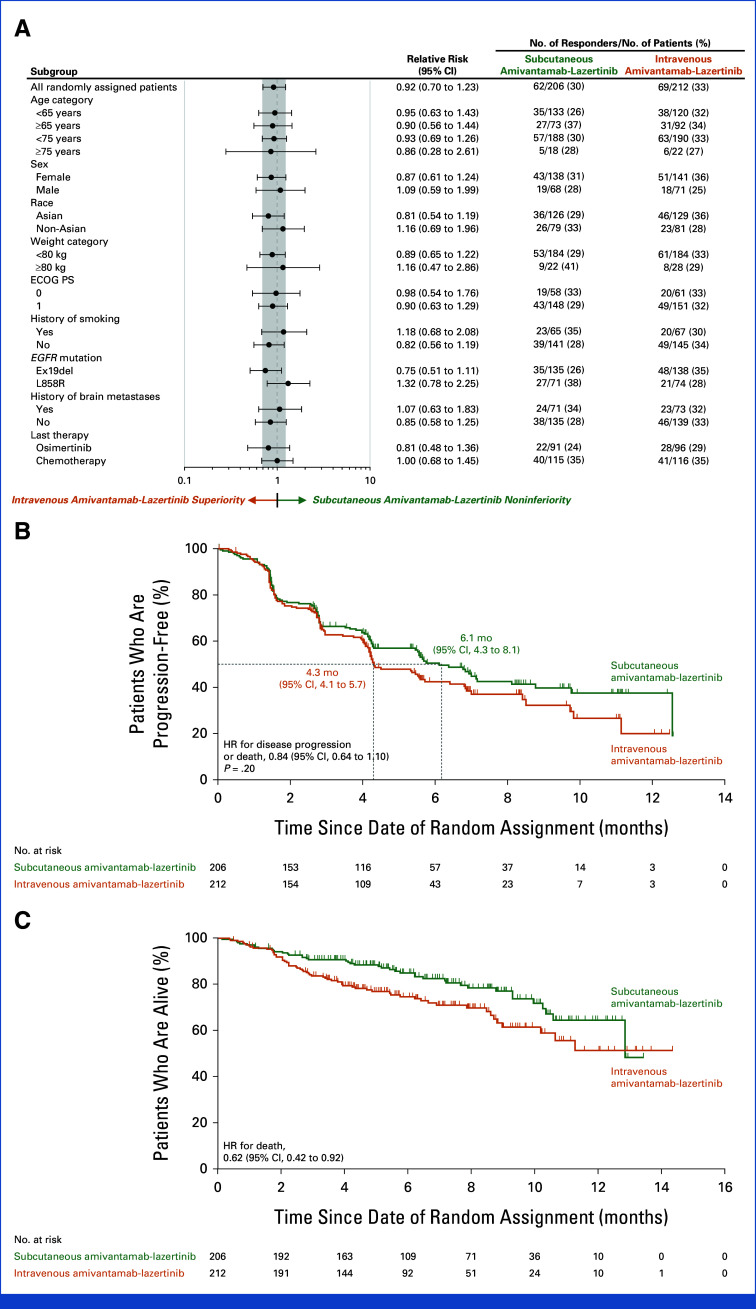
| Patients, % (95% CI) | 30 (24 to 37) | 33 (26 to 39) | Relative risk for noninferiority, 0.92 (0.70 to 1.23)c | .001 |
| Progression-free survival | ||||
| Median, mo (95% CI) | 6.1 (4.3 to 8.1) | 4.3 (4.1 to 5.7) | HR, 0.84 (0.64 to 1.10) | .20 |
| Patients, % (95% CI) | ||||
| At 6 months | 50 (43 to 58) | 42 (35 to 50) | ||
| At 12 months | 37 (28 to 46) | 20 (8 to 35) | ||
| Overall survival | ||||
| Median, mo (95% CI) | 12.9 (12.9 to NE) | NE (10.2 to NE) | HR, 0.62 (0.42 to 0.92)d | .02d |
| Patients, % (95% CI) | ||||
| At 6 months | 85 (79 to 89) | 75 (68 to 80) | ||
| At 12 months | 65 (52 to 74) | 51 (37 to 64) | ||
Abbreviations: %CV, % coefficient of variation; AUCD1-D15, area under the curve from cycle-2 day-1 to day-15; Ctrough, observed serum concentration of amivantamab at steady state; EGFR, epidermal growth factor receptor; Ex19del, exon 19 deletion; HR, hazard ratio; NE, not estimable; TKI, tyrosine kinase inhibitor.
The pharmacokinetic population for evaluating the coprimary pharmacokinetic end points included all patients who received all doses without dose modifications before the respective end point and who provided the pharmacokinetic samples necessary to derive each parameter. The efficacy population included all the patients who had undergone random assignment.
The objective response (complete or partial response as best response) was assessed by the investigator among all responders.
Odds ratio (95% CI), 0.87 (0.58 to 1.32); P = .52. P value is calculated via a logistic regression model stratified by brain metastases at baseline (yes v no), EGFR mutation (L858R v Ex19del), race (Asian v non-Asian), and last therapy (osimertinib [or another third-generation EGFR-TKI] v chemotherapy).
For overall survival, 95% CIs were not adjusted for multiplicity and should not be used in place of hypothesis testing; P value is nominal.
Treatment-emergent antiamivantamab antibodies were detected in one (0.6%) patient in the subcutaneous and none in the intravenous group. Treatment-emergent anti-rHuPH20 antibodies occurred in 15 (8%) patients in the subcutaneous group without impact on amivantamab pharmacokinetics.
Efficacy
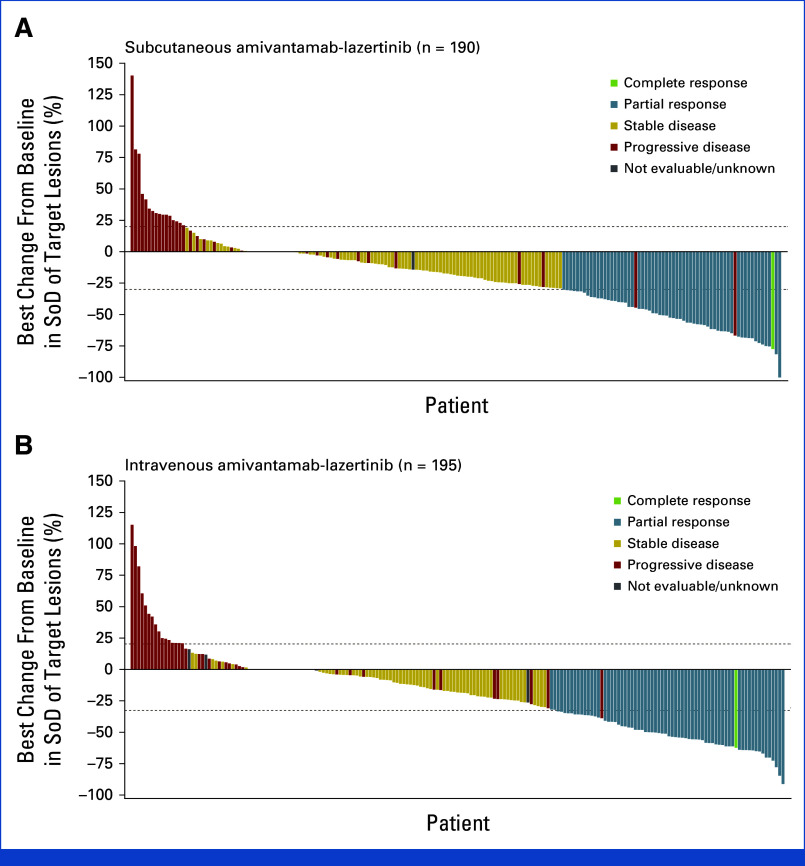
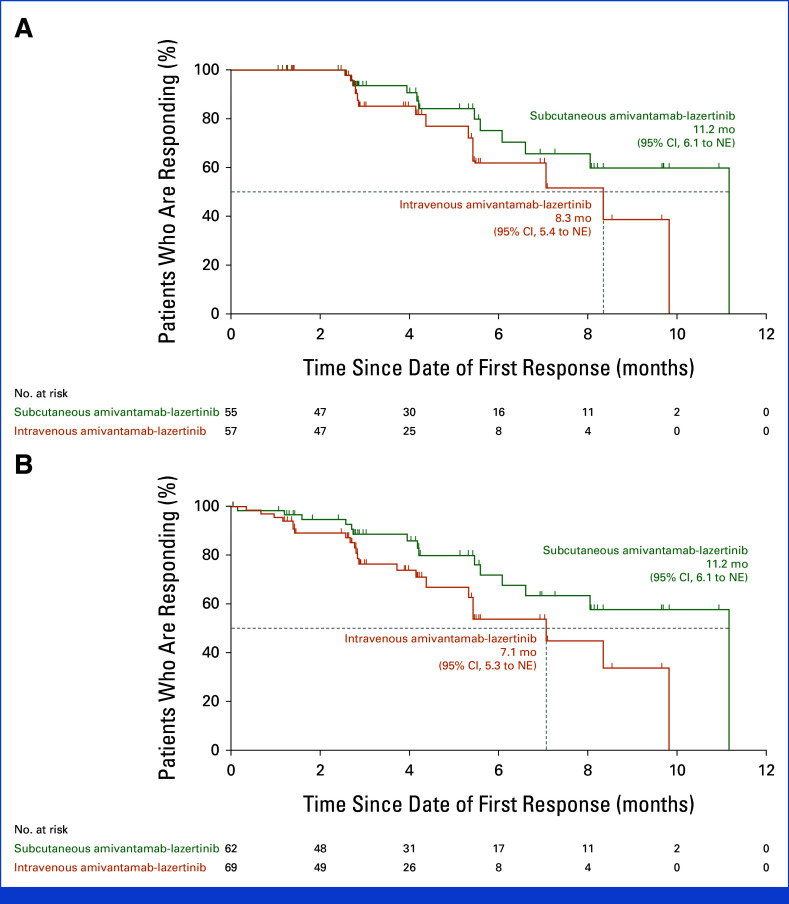
An objective response (complete or partial) was reported in 30% of patients (95% CI, 24 to 37) in the subcutaneous group and 33% (95% CI, 26 to 39) in the intravenous group (relative risk, 0.92; 95% CI, 0.70 to 1.23; Table 2 and Appendix Fig A4). The ORR in the subcutaneous group met the noninferiority criterion (lower 95% CI bound equals 70%) by retaining ≥60% of the ORR in the intravenous group. Objective response for predefined subgroups is shown in Figure 2A. Median time to response was 1.5 months (range, 1.2-6.9) in the subcutaneous group and 1.5 months (range, 1.2-9.9) in the intravenous group. Among confirmed responders, median response duration (DoR) was 11.2 months (95% CI, 6.1 to not estimable [NE]) in the subcutaneous group and 8.3 months (95% CI, 5.4 to NE) in the intravenous group; 29% and 14% of patients, respectively, had a DoR ≥6 months (Appendix Fig A5 and Table A1).
FIG 2.
(A) Objective response forest plot, (B) PFS, and (C) OS. The efficacy population included all patients who had undergone random assignment. (A) The shaded area indicates 95% CIs for the relative risk in all patients; the subgroup analyses were not part of the hypothesis testing and results are reported without adjustment for multiplicity. (B, C) The dashed lines indicate the median PFS and OS, respectively, in the two groups, and the tick marks indicate censoring of data. ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
The percentage of patients exhibiting stable disease was 45% in the subcutaneous group and 38% in the intravenous group. Disease control rate was 75% (95% CI, 69 to 81) in the subcutaneous group and 71% (95% CI, 64 to 77) in the intravenous group (Appendix Table A1). PFS was tested for superiority of subcutaneous versus intravenous amivantamab, with a median PFS of 6.1 months (95% CI, 4.3 to 8.1) and 4.3 months (95% CI, 4.1 to 5.7), respectively, but did not reach statistical significance (HR for disease progression or death, 0.84; 95% CI, 0.64 to 1.10; P = .20; Fig 2B).
Death occurred in 43 patients in the subcutaneous and 62 patients in the intravenous group, with 35/43 (81%) and 50/62 (81%) deaths caused by progressive disease, respectively. The percentage of patients who were alive at 6 and 12 months, respectively, was 85% (95% CI, 79 to 89) and 65% (95% CI, 52 to 74) in the subcutaneous group, and 75% (95% CI, 68 to 80) and 51% (95% CI, 37 to 64) in the intravenous group. OS was significantly longer for the subcutaneous compared with the intravenous group (HR for death, 0.62; 95% CI, 0.42 to 0.92; nominal P = .02; Fig 2C).
Safety
Most patients had ≥1 AE (Table 3). The most common grade ≥3 AEs (≥5% in either group) were dermatitis acneiform (9% and 6% in the subcutaneous and intravenous groups, respectively) and lymphopenia (<1% and 8%). Serious AEs were reported in 29% and 30% of patients in the subcutaneous and intravenous groups, respectively (Appendix Table A2).
TABLE 3.
Overview of AEs
| AEa | Subcutaneous Group (n = 206), No. (%) | Intravenous Group (n = 210), No. (%) | ||
|---|---|---|---|---|
| Any event | 204 (99) | 209 (99) | ||
| Grade ≥3 | 107 (52) | 118 (56) | ||
| Any serious event | 59 (29) | 64 (30) | ||
| Any event resulting in death | 7 (3) | 10 (5) | ||
| Any event leading to: | ||||
| Interruption of any study agentb | 127 (62) | 127 (60) | ||
| Reduction of any study agent | 63 (31) | 52 (25) | ||
| Discontinuation of any study agent | 26 (13) | 29 (14) | ||
| AEs reported in ≥15% of patients in either groupc | All | Grade ≥3 | All | Grade ≥3 |
| Paronychia | 111 (54) | 8 (4) | 108 (51) | 3 (1) |
| Hypoalbuminemia | 96 (47) | 9 (4) | 77 (37) | 8 (4) |
| Rash | 95 (46) | 8 (4) | 91 (43) | 8 (4) |
| Dermatitis acneiform | 64 (31) | 18 (9) | 69 (33) | 12 (6) |
| Nausea | 60 (29) | 1 (0.5) | 52 (25) | 3 (1) |
| Stomatitis | 57 (28) | 1 (0.5) | 69 (33) | 5 (2) |
| Peripheral edema | 52 (25) | 6 (3) | 58 (28) | 1 (0.5) |
| Increased alanine aminotransferase | 46 (22) | 6 (3) | 56 (27) | 8 (4) |
| Decreased appetite | 45 (22) | 1 (0.5) | 52 (25) | 3 (1) |
| Fatigue | 44 (21) | 3 (1) | 43 (20) | 5 (2) |
| Vomiting | 44 (21) | 2 (1) | 41 (20) | 1 (0.5) |
| Diarrhea | 43 (21) | 3 (1) | 39 (19) | 2 (1) |
| Constipation | 42 (20) | 0 | 42 (20) | 1 (0.5) |
| Headache | 42 (20) | 1 (0.5) | 36 (17) | 1 (0.5) |
| Increased aspartate aminotransferase | 42 (20) | 2 (1) | 45 (21) | 3 (1) |
| Anemia | 39 (19) | 4 (2) | 40 (19) | 5 (2) |
| Pruritus | 33 (16) | 0 | 25 (12) | 0 |
| Hypocalcemia | 33 (16) | 0 | 27 (13) | 0 |
| Myalgia | 32 (16) | 0 | 13 (6) | 0 |
| Asthenia | 31 (15) | 4 (2) | 23 (11) | 2 (1) |
| Thrombocytopenia | 29 (14) | 4 (2) | 33 (16) | 2 (1) |
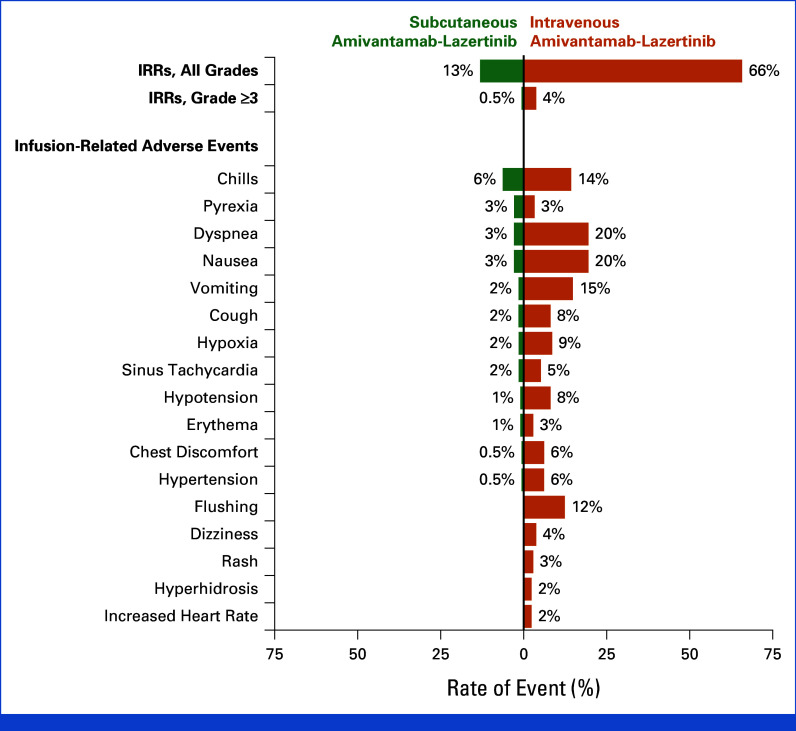
| IRR | 27 (13) | 1 (0.5) | 138 (66) | 8 (4) |
Abbreviations: AE, adverse event; IRR, infusion-related reaction.
The safety population included all patients who had undergone random assignment and received at least one dose of any trial treatment.
Excluding infusion-/administration-related reactions.
Events in this category are listed according to decreasing incidence in the subcutaneous group.
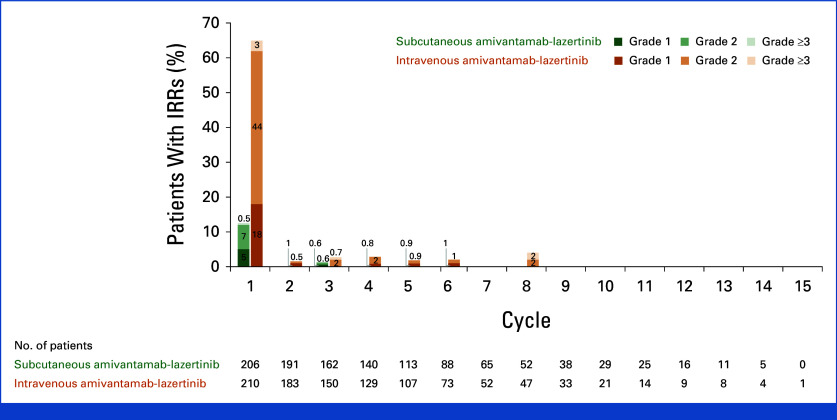
The proportion of patients reporting an IRR was 13% in the subcutaneous group and 66% in intravenous group (Fig 3), with one (0.5%) and eight (4%) patients experiencing a grade 3 event, respectively (no grade 4 or 5 events were reported). All infusion-related AEs ranged between 0% and 6% in the subcutaneous group and 2% and 20% in the intravenous group. Most IRRs occurred during cycle-1 (Appendix Fig A6). There were no discontinuations because of IRRs in the subcutaneous group versus four (2%) in the intravenous group.
FIG 3.
IRRs and infusion-related AEs. The safety population included all patients who had undergone random assignment and received at least one dose of any trial treatment. AE, adverse event; IRR, infusion-related reaction.
VTE was reported in 9% of patients in the subcutaneous group and 14% in the intravenous group, with pulmonary embolism and deep-vein thrombosis being the most common (Appendix Table A3). Among all VTE, most occurred in the first 4 months (74% and 67% in the subcutaneous and intravenous groups, respectively). Overall, 80% and 81% of patients in the subcutaneous and intravenous groups, respectively, received prophylactic anticoagulation (Appendix Table A4). Among those receiving prophylactic anticoagulation, VTE occurred in 7% and 12% of patients, respectively; the rates of VTE among patients who did not receive anticoagulation were 17% and 26%, respectively (Appendix Tables A5 and A6). Grade ≥3 bleeding events occurred in 2% and 0.6% of patients receiving anticoagulation in the subcutaneous and intravenous groups, respectively; one patient in the subcutaneous group receiving anticoagulation discontinued treatment because of bleeding.
AEs leading to dose interruptions, reductions, and discontinuations of any trial agent are shown in Table 3. The dose reduction rate was 31% in the subcutaneous group and 25% in the intravenous group; corresponding rates because of grade ≥3 AEs were 3% and 4%, respectively. Rash was the leading cause for dose reductions in the subcutaneous and intravenous groups (8% v 4%) with similar incidence of all-grade rash (46% v 43%) and grade ≥3 rash (3% v 4%) between both groups (Appendix Table A7). The median duration of rash was 31 days in the subcutaneous group and 44 days in the intravenous group. Most common reasons for discontinuation are presented in Appendix Table A7. Discontinuation of all agents because of treatment-related AEs was 9% and 12% in the subcutaneous and intravenous groups, respectively. Treatment-related AEs are shown in Appendix Table A8.
Death due to AEs occurred in seven (3%) and 10 (5%) patients in the subcutaneous and intravenous groups, respectively. All grade 5 AEs are listed in Appendix Table A9.
Patient Convenience
The subcutaneous injection during cycle-1-day-1 was reported as very convenient or convenient by 85% of patients, versus 52% of patients for the intravenous infusion (nominal P < .001; Fig 4). Data at cycle-3-day-1 were consistent with cycle-1-day-1. At the end of treatment, the subcutaneous injection was reported as very convenient or convenient by 85% of patients versus 35% for the intravenous infusion (nominal P < .001).
FIG 4.
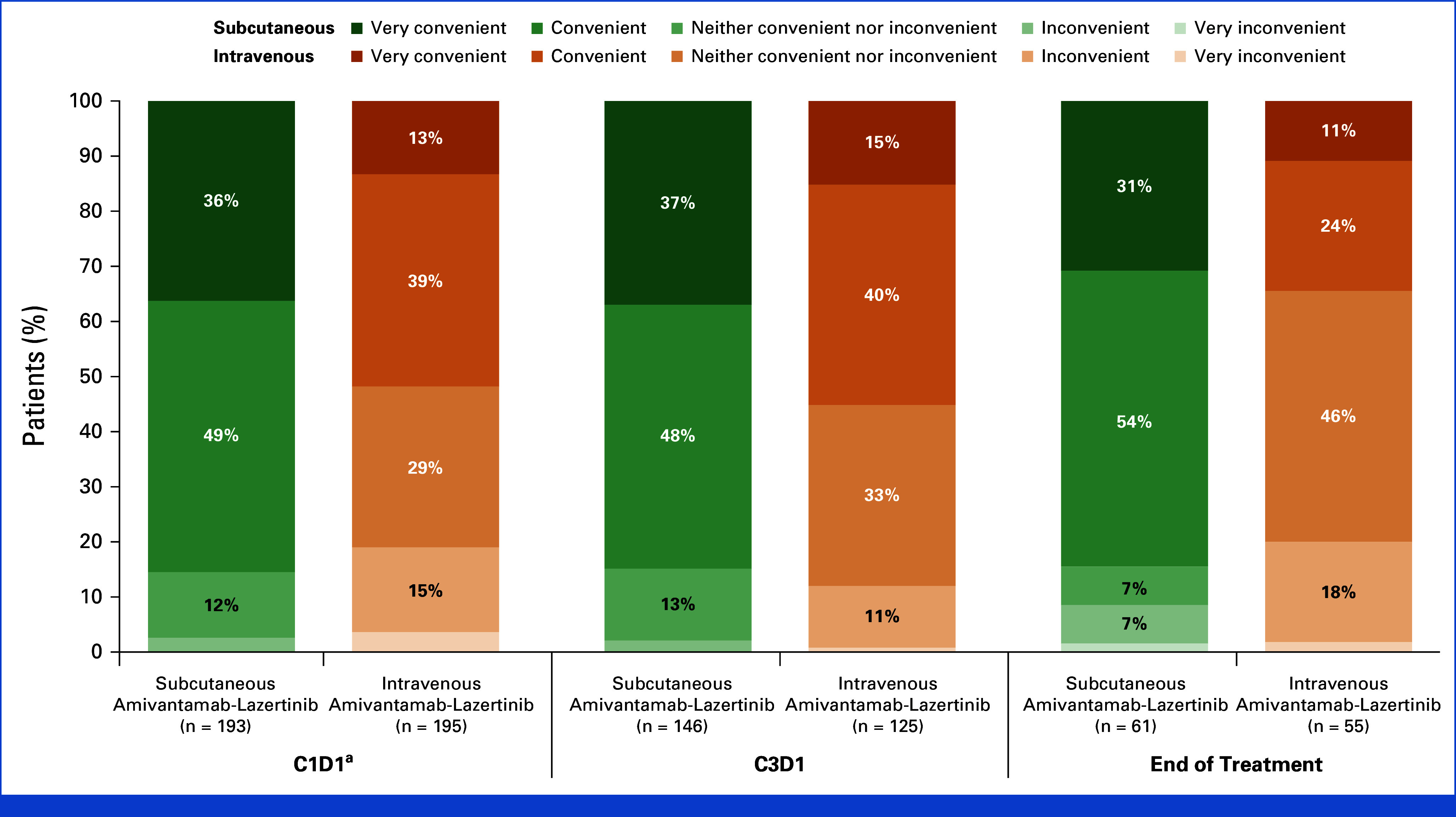
Patient-reported convenience of the subcutaneous injection and intravenous infusion. Item 6 of the modified TASQ asked, “How convenient is it for you to have your [IV infusion/SC injection]?” The modified TASQ was completed by patients after treatment administration in cycle-1 (baseline), cycle-3, and at EOT. EOT data could have been collected after administration of the last dose. aC1D2 for patients who received IV amivantamab because of split dosing. C, cycle; D, day; EOT, end of treatment; IV, intravenous; SC, subcutaneous; TASQ, Therapy Administration Satisfaction Questionnaire.
DISCUSSION
Subcutaneous amivantamab-lazertinib demonstrated noninferior pharmacokinetics and antitumor activity (objective response) compared with intravenous amivantamab-lazertinib. The geometric mean ratio for Ctrough was 1.15 at cycle-2-day-1 and 1.43 at cycle-4-day-1, indicating that noninferior trough concentrations were maintained with subcutaneous versus intravenous administration, although total systemic exposure (cycle-2 AUCD1-D15) remained similar between groups. Consistent with the established flat exposure-safety relationships previously reported,18 the higher Ctrough observed with subcutaneous amivantamab-lazertinib did not negatively affect its safety profile, as AE incidence was comparable between groups.
Although ORR was noninferior for the subcutaneous group versus the intravenous group, DoR was numerically longer and there was a higher proportion of patients with stable disease in the subcutaneous group. These results indicate that there may be a potential clinical benefit for subcutaneous amivantamab-lazertinib in disease control. Furthermore, subcutaneous amivantamab resulted in a similar time to response as intravenous amivantamab. Although statistical significance for superiority was not achieved, PFS was also numerically longer in the subcutaneous group versus the intravenous group. The predefined exploratory OS analysis showed a significantly improved survival with subcutaneous versus intravenous amivantamab-lazertinib (HR for death, 0.62, nominal P = .02). The observed benefit in the subcutaneous group was consistent across all efficacy end points and may be driven by better tolerability, as indicated by the longer time to treatment discontinuation. Furthermore, the impact of subcutaneous administration on lymphatic absorption and immune stimulation is unknown but may also play a role.19-21 Although study follow-up is 7.0 months and further investigation is needed, our trial shows consistent evidence of clinically relevant improvement with subcutaneous amivantamab.
There were no unexpected toxicities from subcutaneous and intravenous amivantamab-lazertinib, consistent with previous reports.7,22 Although dose reduction rates were marginally higher in the subcutaneous group, dose reductions because of grade ≥3 AEs were comparable (3% v 4%). Subcutaneous amivantamab-lazertinib demonstrated a safety profile consistent with historical intravenous data, with a 5-fold reduced rate and lower severity of IRRs and a reduced administration time of <5 minutes versus up to 5 hours for intravenous amivantamab-lazertinib.9 Patient-reported convenience was also significantly higher with subcutaneous versus intravenous administration of amivantamab.
To our knowledge, our trial is the first to prospectively evaluate the impact of prophylactic anticoagulation on the risk of VTE with amivantamab-lazertinib. The prevalence of VTE in lung cancer is 14%-30%, with higher values in patients with molecular driver alterations.23,24 Moreover, an elevated risk of VTE in the first 4 months of treatment was specifically identified for amivantamab-lazertinib combinations in the MARIPOSA (ClinicalTrials.gov identifier: NCT04487080) and MARIPOSA-2 (ClinicalTrials.gov identifier: NCT04988295) trials.11 For this reason, prophylactic anticoagulation was recommended, with uptake occurring in approximately 80% of all patients, leading to an observed incidence of 9% (subcutaneous) to 14% (intravenous). We also found that reduced VTE rates were observed with prophylactic anticoagulation, with low risk of clinically important bleeding across both administration routes, demonstrating that anticoagulation can be safely implemented. The safety and efficacy of prophylactic anticoagulation seen here was comparable with previous studies of patients with similar risk profiles.25 Regardless of prophylactic anticoagulation, VTE rates were lower for the subcutaneous group than for the intravenous group.
Importantly, the increased tolerability and convenience of the subcutaneous formulation seen in PALOMA-3 may improve patient and provider experiences while maintaining efficacy. Intravenous amivantamab-based combinations are efficacious in the first- and second-line treatment of patients with advanced NSCLC harboring EGFR mutations.7,12,13 The findings from our trial are expected to positively affect clinical practice and may enhance outcomes for patients with advanced NSCLC. The PALOMA-2 trial (ClinicalTrials.gov identifier: NCT05498428) is evaluating the efficacy and safety of subcutaneous amivantamab-based combinations in various patient populations across advanced NSCLC.7,12,13 In addition, the PALOMA trial has established extended dosing intervals for subcutaneous amivantamab administered once every 3 weeks and once every 4 weeks,9,26 which may further increase convenience for the patient and the provider.
In summary, subcutaneous amivantamab-lazertinib demonstrated noninferior pharmacokinetics and ORR versus intravenous amivantamab-lazertinib, with numerically longer DoR and PFS. Surprisingly, significantly longer OS was observed with the subcutaneous formulation. Incidence of VTE was lower in both groups with the use of prophylactic anticoagulation. Compared with the intravenous formulation, subcutaneous amivantamab maintains efficacy, improves patient and health care provider experience, and substantially reduces the rate of IRRs.
ACKNOWLEDGMENT
The authors thank the patients who participated in the study and their families and caregivers, the physicians and nurses who cared for patients, and the staff members who supported this clinical trial. Medical writing and editorial support were provided by Suparna Abraham, PharmD, of Lumanity Communications Inc and were funded by Janssen Global Services, LLC. A complete list of investigators in the PALOMA-3 trial is provided in the Appendix Table A10.
APPENDIX
FIG A1.
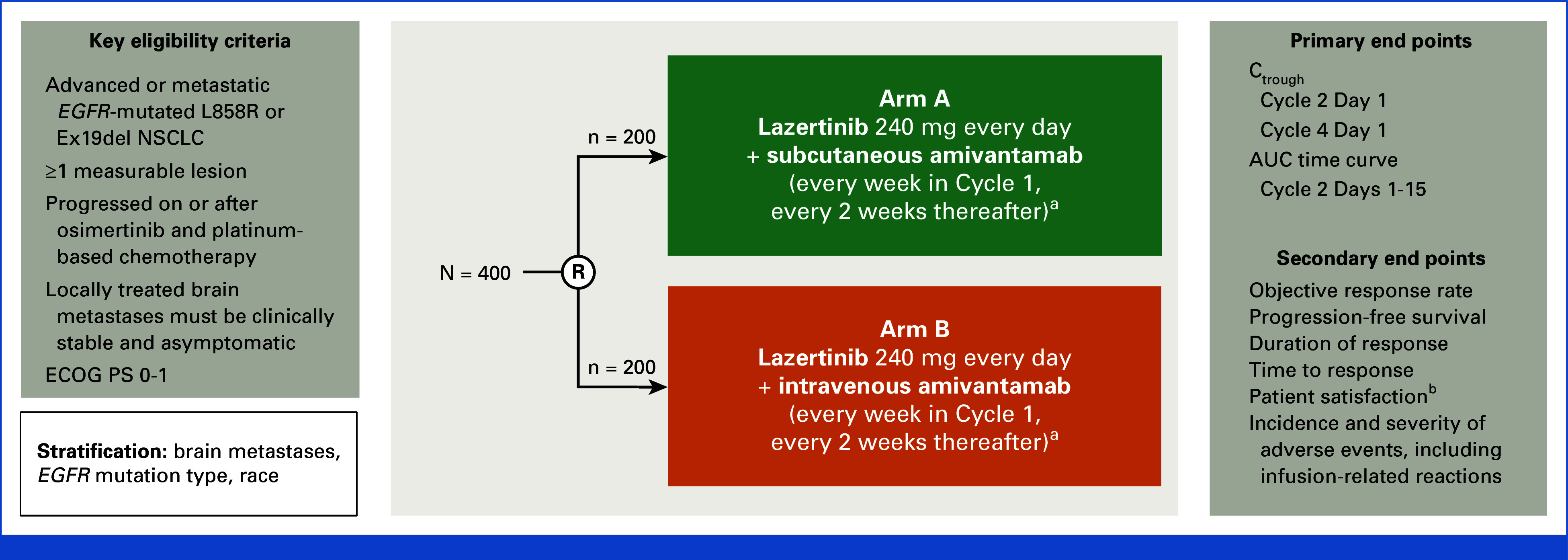
PALOMA-3 study design. aCycle 1 for intravenous amivantamab-lazertinib: Days 1, 2 (Day 2 applies to intravenous split dose only), 8, 15, and 22; Cycle 1 for subcutaneous amivantamab-lazertinib: Days 1, 8, 15, and 22; after Cycle 1 for all: Days 1, 15 (28-day cycles). Subcutaneous amivantamab is coformulated with recombinant human hyaluronidase. bAssessed by using modified TASQ. AUC, area under the concentration-time curve; Ctrough, observed serum concentration of amivantamab at steady state; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; Ex19del, exon 19 deletion mutation; NSCLC, non–small cell lung cancer; R, random assignment; TASQ, Therapy Administration Satisfaction Questionnaire.
FIG A2.
Time to amivantamab discontinuation. The dashed lines indicate the median time to amivantamab discontinuation in the two groups, and the tick marks indicate censoring of data.
FIG A3.
(A) Observed concentration-time profiles, (B) Ctrough, and (C) AUCD1-D15 of amivantamab. To capture the peak concentration of intravenous amivantamab, two samples were analyzed soon after the end of infusion (at 10 minutes and 2 hours after intravenous infusion). The upper and lower end of the boxes indicate the 25th and 75th quartiles, respectively, the triangles indicate the means, the horizontal lines within the boxes indicate the medians, and the error bars indicate 95% CIs. AUC, area under the concentration-time curve; AUCD1-D15, AUC between Cycle 2 Day 1 and Day 15; C, Cycle; Ctrough, observed serum concentration of amivantamab at steady state; D, Day.
FIG A4.
Best percentage change from baseline in target lesions: (A) subcutaneous group and (B) intravenous group. Target lesions were measured as the sum of the longest diameters. The number of patients with measurable disease at baseline was 206 in the subcutaneous group and 212 in the intravenous group; 190 and 195 patients, respectively, had postbaseline tumor assessments. SoD, sum of diameters.
FIG A5.
DoR: (A) among confirmed responders and (B) among all responders. The efficacy population included all patients who had undergone random assignment. Included in this analysis were the 55 (confirmed) and 62 (including unconfirmed) responders (of the 206 patients with measurable disease at baseline by RECIST, v1.1) in the subcutaneous group and the 57 (confirmed) and 69 (including unconfirmed) responders (of 212 patients) in the intravenous group, respectively. Tick marks indicate censoring of data. DoR, response duration; NE, not estimable.
FIG A6.
Incidence of IRRs by treatment cycle. IRR was counted only once per time frame per patient, and the event experienced by the patient with the worst toxicity was used. AE, adverse event; IRR, infusion-related reaction.
TABLE A1.
Response End Points
| End Point | Subcutaneous Group (n = 206) | Intravenous Group (n = 212) |
|---|---|---|
| Objective responsea | ||
| Patients including all responders, % (95% CI) | 30 (24 to 37) | 33 (26 to 39) |
| Patients including only confirmed responders, % (95% CI) | 27 (21 to 33) | 27 (21 to 33) |
| Best overall response, No. (%)a | ||
| Complete responseb | 1 (0.5) | 1 (0.5) |
| Partial responseb | 61 (30) | 68 (32) |
| Stable disease | 93 (45) | 81 (38) |
| Progressive disease | 37 (18) | 42 (20) |
| Not evaluable | 14 (7) | 20 (9) |
| Disease control rate, % (95% CI)c | 75 (69 to 81) | 71 (64 to 77) |
| DoR | ||
| Median among all responders, months (95% CI) | 11.2 (6.1 to NE) | 7.1 (5.3 to NE) |
| Median among confirmed responders, months (95% CI) | 11.2 (6.1 to NE) | 8.3 (5.4 to NE) |
| Time to response | ||
| Median, months (range) | 1.5 (1.2-6.9) | 1.5 (1.2-9.9) |
NOTE. The efficacy population included all the patients who had undergone random assignment.
Abbreviations: DoR, response duration; NE, not estimable.
The objective response (complete or partial response as best response) was assessed using RECIST, v1.1 and analyzed using logistic regression.
Among all responders.
Not protocol-specified; calculated as the sum of complete response, partial response, and stable disease; all responders were included.
TABLE A2.
Treatment-Emergent Serious AEs Occurring in at Least Two Patients
| Eventa | Subcutaneous Group (n = 206), No. (%) | Intravenous Group (n = 210), No. (%) |
|---|---|---|
| Pneumonitis | 9 (4) | 6 (3) |
| COVID-19 | 4 (2) | 4 (2) |
| Alanine aminotransferase increased | 4 (2) | 3 (1) |
| Pneumonia | 3 (1) | 7 (3) |
| Interstitial lung disease | 3 (1) | 1 (0.5) |
| Fatigue | 3 (1) | 1 (0.5) |
| Deep vein thrombosis | 2 (1) | 4 (2) |
| Asthenia | 2 (1) | 2 (1) |
| Respiratory failure | 2 (1) | 1 (0.5) |
| Vomiting | 2 (1) | 0 |
| Femur fracture | 2 (1) | 0 |
| Dyspnea | 1 (0.5) | 2 (1) |
| Pulmonary embolism | 1 (0.5) | 2 (1) |
| Skin infection | 1 (0.5) | 2 (1) |
| Aspartate aminotransferase increased | 1 (0.5) | 2 (1) |
| Back pain | 1 (0.5) | 2 (1) |
| Cerebral infarction | 0 | 3 (1) |
| Nausea | 0 | 3 (1) |
| Infusion-related reaction | 0 | 2 (1) |
| Hypoalbuminemia | 0 | 2 (1) |
| Rash | 0 | 2 (1) |
NOTE. The safety population included all patients who were randomly assigned and received at least one dose of any trial treatment.
Events in this category are listed according to decreasing incidence in the subcutaneous group.
TABLE A3.
Venous Thromboembolic Events
| Event | Subcutaneous Group (n = 206), No. (%) | Intravenous Group (n = 210), No. (%) |
|---|---|---|
| Any venous thromboembolic event | 19 (9) | 30 (14) |
| Grade 1 | 1 (0.5) | 7 (3) |
| Grade 2 | 16 (8) | 16 (8) |
| Grade 3 | 2 (1) | 6 (3) |
| Grade 4 | 0 | 1 (0.5) |
| Grade 5 | 0 | 0 |
| Any venous thromboembolic event leading to death | 0 | 0 |
| Any venous thromboembolic event leading to discontinuation of any agent | 0 | 2 (1) |
| Venous thromboembolic eventsa | ||
| Pulmonary embolism | 6 (3) | 9 (4) |
| Deep vein thrombosis | 5 (2) | 11 (5) |
| Embolism venous | 3 (1) | 3 (1) |
| Venous thrombosis limb | 3 (1) | 3 (1) |
| Embolism | 2 (1) | 3 (1) |
| Thrombosis | 2 (1) | 1 (0.5) |
| Subclavian vein thrombosis | 1 (0.5) | 0 |
| Superficial vein thrombosis | 1 (0.5) | 0 |
| Pulmonary infarction | 0 | 1 (0.5) |
| Venous thrombosis | 0 | 3 (1) |
NOTE. The safety population included all patients who were randomly assigned and received at least one dose of any trial treatment.
Events in this category are listed according to decreasing incidence in the subcutaneous group.
TABLE A4.
Concomitant Anticoagulants
| Anticoagulant Use | Subcutaneous Group (n = 206), No. (%) | Intravenous Group (n = 210), No. (%) |
|---|---|---|
| Patients with one or more concomitant anticoagulants | 164 (80) | 171 (81) |
| Antithrombotic agents | ||
| Direct factor Xa inhibitors | 132 (64) | 143 (68) |
| Rivaroxaban | 89 (43) | 76 (36) |
| Apixaban | 38 (18) | 54 (26) |
| Edoxaban | 7 (3) | 17 (8) |
| Heparin group | 48 (23) | 45 (21) |
| Enoxaparin | 39 (19) | 35 (17) |
| Heparin | 4 (2) | 2 (1) |
| Tinzaparin | 3 (2) | 2 (1) |
| Low molecular weight heparin | 3 (2) | 1 (0.5) |
| Bemiparin | 2 (1) | 3 (1) |
| Nadroparin | 1 (0.5) | 2 (1) |
| Dalteparin | 0 | 1 (0.5) |
| Other antithrombotic agents | 1 (0.5) | 3 (1) |
| Fondaparinux | 1 (0.5) | 3 (1) |
| Direct thrombin inhibitors | 0 | 1 (0.5) |
| Dabigatran | 0 | 1 (0.5) |
| Vitamin K antagonists | 0 | 1 (0.5) |
| Warfarin | 0 | 1 (0.5) |
TABLE A5.
Venous Thromboembolic and Bleeding Events by Anticoagulation Use and by Treatment Group
| Event | Subcutaneous Group (n = 206), No. (%) | Intravenous Group (n = 210), No. (%) | ||
|---|---|---|---|---|
| Any Prophylactic Anticoagulation (n = 164) | No Prophylactic Anticoagulation (n = 42) | Any Prophylactic Anticoagulation (n = 171) | No Prophylactic Anticoagulation (n = 39) | |
| Any venous thromboembolic event | 12 (7) | 7 (17) | 20 (12) | 10 (26) |
| Grade 1 | 0 | 1 (2) | 5 (3) | 2 (5) |
| Grade 2 | 10 (6) | 6 (14) | 13 (8) | 3 (8) |
| Grade 3 to 4 | 2 (1) | 0 | 2 (1) | 5 (13) |
| Grade 5 | 0 | 0 | 0 | 0 |
| Any venous thromboembolic event leading to death | 0 | 0 | 0 | 0 |
| Any venous thromboembolic event leading to discontinuation of any agent | 0 | 0 | 0 | 2 (5) |
| Venous thromboembolic eventsa | ||||
| Pulmonary embolism | 4 (2) | 2 (5) | 6 (4) | 3 (8) |
| Deep vein thrombosis | 3 (2) | 2 (5) | 8 (5) | 3 (8) |
| Venous embolism | 2 (1) | 1 (2) | 2 (1) | 1 (3) |
| Venous thrombosis limb | 3 (2) | 0 | 1 (0.6) | 2 (5) |
| Embolism | 1 (0.6) | 1 (2) | 2 (1) | 1 (3) |
| Thrombosis | 1 (0.6) | 1 (2) | 1 (0.6) | 0 |
| Subclavian vein thrombosis | 1 (0.6) | 0 | 0 | 0 |
| Superficial vein thrombosis | 0 | 1 (2) | 0 | 0 |
| Venous thrombosis | 0 | 0 | 2 (1) | 1 (3) |
| Pulmonary infarction | 0 | 0 | 0 | 1 (3) |
| Any bleeding event | 44 (27) | 5 (12) | 48 (28) | 5 (13) |
| Grade 3 to 4b | 3 (2) | 1 (2) | 1 (0.6) | 0 |
| Grade 5 | 0 | 0 | 0 | 0 |
| Any bleeding event leading to death | 0 | 0 | 0 | 0 |
| Any bleeding event leading to discontinuation of any agent | 1 (0.6) | 0 | 0 | 0 |
NOTE. The safety population included all patients who had undergone random assignment and received at least one dose of any trial treatment. The group with any prophylactic anticoagulation included patients who had anticoagulation before or at Cycle 1 Day 1 plus a 3-day window and continued until disease progression, death, withdrawal from the study, occurrence of venous thromboembolism, or Cycle 5 Day 1.
Events in this category are listed according to decreasing incidence in the subcutaneous group.
Grade 3 to 4 events include contusion, gingival bleeding, hemoptysis, hematemesis, and nail bed bleeding.
TABLE A6.
Venous Thromboembolic and Bleeding Events by Anticoagulation Use Across All Study Patients
| Event | Any Prophylactic Anticoagulation (n = 335), No. (%) | No Prophylactic Anticoagulation (n = 81), No. (%) |
|---|---|---|
| Any venous thromboembolic event | 32 (10) | 17 (21) |
| Grade 1 | 5 (1) | 3 (4) |
| Grade 2 | 23 (7) | 9 (11) |
| Grade 3 to 4 | 4 (1) | 5 (6) |
| Grade 5 | 0 | 0 |
| Any venous thromboembolic event leading to death | 0 | 0 |
| Any venous thromboembolic event leading to discontinuation of any agent | 0 | 2 (2) |
| Venous thromboembolic eventsa | ||
| Deep vein thrombosis | 11 (3) | 5 (6) |
| Pulmonary embolism | 10 (3) | 5 (6) |
| Venous thrombosis limb | 4 (1) | 2 (2) |
| Venous embolism | 4 (1) | 2 (2) |
| Embolism | 3 (0.9) | 2 (2) |
| Venous thrombosis | 2 (0.6) | 1 (1) |
| Thrombosis | 2 (0.6) | 1 (1) |
| Subclavian vein thrombosis | 1 (0.3) | 0 |
| Pulmonary infarction | 0 | 1 (1) |
| Superficial vein thrombosis | 0 | 1 (1) |
| Any bleeding event | 92 (27) | 10 (12) |
| Grade 3 to 4b | 4 (1) | 1 (1) |
| Grade 5 | 0 | 0 |
| Any bleeding event leading to death | 0 | 0 |
| Any bleeding event leading to discontinuation of any agent | 1 (0.3) | 0 |
NOTE. The safety population included all the patients who were randomly assigned and received at least one dose of any trial treatment. The group with any prophylactic anticoagulation included patients who had anticoagulation before at Cycle 1 Day 1 plus a 3-day window and continued until disease progression, death, withdrawal from the study, occurrence of venous thromboembolism, or Cycle 5 Day 1. The group with no prophylactic anticoagulation included patients who never took prophylactic anticoagulation during the first 4 months of amivantamab and lazertinib combination treatment.
Events in this category are listed according to decreasing incidence in the prophylactic anticoagulation group.
Grade 3 to 4 events include contusion, gingival bleeding, hemoptysis, hematemesis, and nail bed bleeding.
TABLE A7.
AEs Leading to Treatment Interruptions, Reductions, and Discontinuations
| Event | Subcutaneous Group (n = 206), No. (%) | Intravenous Group (n = 210), No. (%) |
|---|---|---|
| Any event leading to interruptions of any study agent | 127 (62) | 127 (61) |
| Grade ≥3 events leading to interruptions of any study agent | 73 (35) | 76 (36) |
| Most common events leading to interruptions of any study agenta | ||
| Paronychia | 27 (13) | 10 (5) |
| Dermatitis acneiform | 26 (13) | 15 (7) |
| Rash | 25 (12) | 17 (8) |
| Increased alanine aminotransferase | 10 (5) | 8 (4) |
| COVID-19 | 9 (4) | 12 (6) |
| Peripheral edema | 8 (4) | 7 (3) |
| Hypoalbuminemia | 7 (3) | 6 (3) |
| Pyrexia | 7 (3) | 4 (2) |
| Increased aspartate aminotransferase | 6 (3) | 6 (3) |
| Vomiting | 5 (2) | 6 (3) |
| Nausea | 4 (2) | 10 (5) |
| Stomatitis | 4 (2) | 10 (5) |
| Fatigue | 4 (2) | 9 (4) |
| Asthenia | 4 (2) | 6 (3) |
| Pneumonia | 3 (2) | 7 (3) |
| Hypotension | 0 | 6 (3) |
| Any event leading to dose reductions of any study agent | 63 (31) | 52 (25) |
| Grade ≥3 events leading to dose reductions of any study agent | 6 (3) | 8 (4) |
| Most common events leading to dose reductions of any study agentb | ||
| Rash | 16 (8) | 8 (4) |
| Paronychia | 14 (7) | 8 (4) |
| Dermatitis acneiform | 12 (6) | 9 (4) |
| Increased alanine aminotransferase | 5 (2) | 4 (2) |
| Stomatitis | 4 (2) | 2 (1) |
| Diarrhea | 4 (2) | 0 |
| Fatigue | 3 (1) | 5 (2) |
| Hypoalbuminemia | 2 (1) | 4 (2) |
| Any event leading to discontinuations of any study agent | 26 (13) | 29 (14) |
| Grade ≥3 events leading to discontinuations of any study agent | 20 (10) | 21 (10) |
| Most common events leading to discontinuations of any study agentb | ||
| Pneumonitis | 7 (3) | 6 (3) |
| Dermatitis acneiform | 4 (2) | 1 (0.5) |
| Infusion-related reaction | 0 | 4 (2) |
NOTE. The safety population included all patients who were randomly assigned and received at least one dose of any trial treatment. Events are listed according to decreasing incidence in the subcutaneous group.
Abbreviation: AE, adverse event.
Listed are AEs that were reported in at least 3% of patients in either group.
Listed are AEs that were reported in at least 2% of patients in either group.
TABLE A8.
Treatment-Related AEs
| Event | Subcutaneous Group (n = 206), No. (%) | Intravenous Group (n = 210), No. (%) | ||
|---|---|---|---|---|
| Any event | 196 (95) | 206 (98) | ||
| Grade ≥3 | 79 (38) | 82 (39) | ||
| Any serious event | 33 (16) | 34 (16) | ||
| Any event resulting in death | 3 (1) | 4 (2) | ||
| AEs reported in ≥15% of patients in either groupa | All | Grade ≥3 | All | Grade ≥3 |
| Paronychia | 110 (53) | 8 (4) | 108 (51) | 3 (1) |
| Rash | 90 (44) | 8 (4) | 91 (43) | 8 (4) |
| Hypoalbuminemia | 79 (38) | 5 (2) | 66 (31) | 7 (3) |
| Dermatitis acneiform | 64 (31) | 18 (9) | 69 (33) | 12 (6) |
| Stomatitis | 54 (26) | 1 (0.5) | 67 (32) | 5 (2) |
| Peripheral edema | 46 (22) | 4 (2) | 43 (20) | 1 (0.5) |
| Nausea | 43 (21) | 1 (0.5) | 40 (19) | 3 (1) |
| Increased alanine aminotransferase | 40 (19) | 6 (3) | 49 (23) | 6 (3) |
| Decreased appetite | 37 (18) | 1 (0.5) | 44 (21) | 2 (1) |
| Diarrhea | 36 (17) | 3 (1) | 31 (15) | 2 (1) |
| Increased aspartate aminotransferase | 35 (17) | 2 (1) | 37 (18) | 2 (1) |
| Vomiting | 33 (16) | 2 (1) | 29 (14) | 1 (0.5) |
| Fatigue | 30 (15) | 2 (1) | 30 (14) | 4 (2) |
| Infusion-related reaction | 27 (13) | 1 (0.5) | 136 (65) | 8 (4) |
NOTE. The safety population included all patients who were randomly assigned and received at least one dose of any trial treatment.
Abbreviation: AE, adverse event.
Events in this category are listed according to decreasing incidence in the subcutaneous group.
TABLE A9.
All Grade 5 AEs
| Eventa | Subcutaneous Group (n = 206), No. (%) | Intravenous Group (n = 210), No. (%) |
|---|---|---|
| Pneumonitis | 1 (0.5)b | 3 (1)b |
| Respiratory failure | 1 (0.5)b | 1 (0.5) |
| Sudden death | 1 (0.5)b | 1 (0.5) |
| Respiratory disorder | 1 (0.5) | 0 |
| Pneumonia | 1 (0.5) | 0 |
| Viral pneumonia | 1 (0.5) | 0 |
| Cardiac arrest | 1 (0.5) | 0 |
| Urosepsis | 0 | 1 (0.5) |
| Asthenia | 0 | 1 (0.5) |
| Cerebral infarction | 0 | 2 (1)c |
| Acute myocardial infarction | 0 | 1 (0.5) |
NOTE. The safety population included all patients who had undergone random assignment and received at least one dose of any trial treatment.
Abbreviation: AE, adverse event.
Events are listed according to decreasing incidence in the subcutaneous group.
All events deemed related to any study treatment.
One event deemed related to any study treatment.
TABLE A10.
List of PALOMA-3 Investigators
| Principal Investigator | Clinical Site |
|---|---|
| Hiroaki Akamatsu | Wakayama Medical University Hospital |
| Mariam Alexander | Medical University of South Carolina |
| Annalen Bleckmann | University Hospital Münster |
| Federico Cappuzzo | Istituto Nazionale Tumori Regina Elena |
| Ying Cheng | Jilin Cancer Hospital |
| Byoung Chul Cho | Yonsei Cancer Center |
| Timucin Cil | Adana City Hospital |
| Alexis Cortot | Institute Coeur Poumon |
| Pongwut Danchaivijitr | Siriraj Hospital |
| Till-Oliver Emde | Oncologianova GmbH |
| Dilek Erdem | Medical Park Samsun Hastanesi |
| Enriqueta Felip | Vall d’Hebron Institute of Oncology (VIHO) |
| Fernanda Estevinho | Hospital Pedro Hispano |
| Maria Lurdes Ferreira | Hospital de Braga |
| Flavio Ferreira da Silva | Fundacao Pio XII |
| Maria del Rosario Garcia Campelo | Hospital Universitario A Coruña |
| Laurent Greillier | Aix Marseille University, APHM, INSERM, CNRS, CRCM, Hôpital Nord |
| Alastair Greystoke | Newcastle Freeman Hospital |
| Ji-Youn Han | National Cancer Center |
| Ping-Chih Hsu | Chang Gung University College of Medicine |
| Jen-Yu Hung | Kaohsiung Medical University Chung-Ho Memorial Hospital |
| Mei Ji | The First People's Hospital of Changzhou |
| Thomas John | Peter MacCallum Cancer Centre |
| Rohit Joshi | Cancer Research SA |
| Young-Chul Kim | Chonnam National University Hwasun Hospital |
| Masashi Kondo | Fujita Health University Hospital |
| Ernesto Korbenfeld | British Hospital of Buenos Aires—Central British Hospital |
| Dariusz Kowalski | Maria Sklodowska-Curie National Research Institute of Oncology |
| Se-Hoon Lee | Samsung Medical Center |
| Natasha Leighl | Princess Margaret Cancer Centre |
| Juan Li | Sichuan Cancer Hospital |
| Sheng-Hao Lin | Changhua Christian Hospital |
| Baogang Liu | Harbin Medical University Cancer Hospital |
| Caigang Liu | Shengjing Hospital of China Medical University |
| John Seng-Hooi Low | Pantai Hospital Kuala Lumpur |
| Melina E. Marmarelis | Perelman School of Medicine, University of Pennsylvania |
| Bartomeu Massutí | Alicante University Dr. Balmis Hospital |
| Anna R. Minchom | The Royal Marsden Hospital and The Institute of Cancer Research |
| Sara Moore | The Ottawa Hospital Cancer Centre |
| Mor Moskovitz | Davidoff Cancer Center, Rabin Medical Center |
| Adnan Nagrial | Westmead Hospital |
| Danny Nguyen | City of Hope National Medical Center |
| Silvia Novello | University of Turin, S. Luigi Gonzaga Hospital |
| Yuichiro Ohe | National Cancer Center Hospital |
| Mustafa Özgüroğlu | Istanbul University Cerrahpaşa Medical Faculty |
| Ozgur Ozyilkan | Adana Baskent University Hospital |
| Antonio Passaro | European Institute of Oncology, IRCCS |
| Nir Peled | Shaare Zedek Medical Center |
| Naiyarat Prasongsook | Phramongkutklao Hospital and Medical College |
| Angel Qin | University of Michigan Rogel Cancer Center |
| Elisa F. Ramos | Cetus Oncologia |
| Joshua K. Sabari | Perlmutter Cancer Center, NYU Langone Health |
| Jorge Salinas | Cemaic—Centro Privado de Especialidades Medicas Ambulatorias e Investigacion Clinica |
| Rachel E. Sanborn | Earle A. Chiles Research Institute, Providence Cancer Institute |
| Mehmet Ali Nahit Sendur | Ankara Yıldırım Beyazıt University, Ankara City Hospital |
| Felipe José Silva Melo Cruz | Núcleo de Ensino e Pequisa, Instituto Brasileiro de Controle do Câncer |
| Alexander I. Spira | Virginia Cancer Specialists |
| Thatthan Suksombooncharoen | Chiang Mai University |
| Motohiro Tamiya | Osaka International Cancer Institute |
| Jiunn Liang Tan | University Malaya Medical Centre |
| Encarnacao Teixeira | Hospital CUF Descobertas |
| Rajanikar Tota | St John of God Hospital Murdoch |
| Damien Urban | Chaim Sheba Medical Center |
| Alain Vergnenègre | CHU de Limoges, Hopital Dupuytren |
| Pei Jye Voon | Sarawak General Hospital |
| Vanina Wainsztein | CEMIC (Centro de Educación Médica e Investigaciones Clínicas) |
| Jialei Wang | Fudan University Shanghai Cancer Center |
| Thomas Wehler | University Hospital of Giessen and Marburg |
| James Chih-Hsin Yang | National Taiwan University Cancer Center |
| Hiroshige Yoshioka | Kansai Medical University Hospital |
| Alona Zer | Rambam Medical Center |
| Yanqiu Zhao | The Affiliated Cancer Hospital of Zhengzhou University and Henan Cancer Hospital |
| Bogdan Zurawski | Centrum Onkologii im. Prof. F. Lukaszczyka |
Natasha B. Leighl
Honoraria: BeiGene, Bristol Myers Squibb, Janssen, MSD Oncology, Takeda
Research Funding: MSD (Inst), Lilly (Inst), AstraZeneca Canada (Inst), Inivata/NeoGenomics (Inst), Janssen Oncology (Inst), Novartis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Hiroaki Akamatsu
Honoraria: AstraZeneca, Lilly, Pfizer, MSD, Bristol Myers Squibb Japan, Taiho Pharmaceutical, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Amgen, Nippon Kayaku, Novartis, Takeda
Consulting or Advisory Role: Amgen, Janssen, Sandoz
Research Funding: Amgen (Inst), Chugai Pharma (Inst)
Sun Min Lim
Honoraria: Takeda, Boehringer Ingelheim, Yuhan
Research Funding: Janssen Research & Development
Anna R. Minchom
Honoraria: Janssen, Merck, GSK, Seagen
Consulting or Advisory Role: Janssen Oncology, Faron Pharmaceuticals, GSK, MSD, Immutep, Genmab, Merck
Research Funding: Merck (Inst), MSD Oncology (Inst), Astex Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Janssen Oncology, Amgen
Melina E. Marmarelis
Stock and Other Ownership Interests: Merck, Johnson & Johnson
Honoraria: Novocure, AstraZeneca, Janssen Oncology, Takeda, Blueprint Medicines, Thermo Fisher Scientific
Consulting or Advisory Role: AstraZeneca, Ikena Oncology, Bristol Myers Squibb/Celgene, AstraZeneca, Janssen Oncology, Regeneron
Research Funding: Lilly (Inst), AstraZeneca (Inst), Janssen Oncology (Inst), Ikena Oncology (Inst), Genentech (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Regeneron
Open Payments Link: https://openpaymentsdata.cms.gov/physician/856967
Rachel E. Sanborn
Honoraria: MJH Life Sciences, Targeted Oncology, Curio Science, Illumina
Consulting or Advisory Role: AstraZeneca, EMD Serono, Janssen Oncology, Macrogenics, Sanofi/Aventis, Regeneron, GSK, Illumina, G1 Therapeutics, Daiichi Sankyo, Lilly, Amgen, Gilead Sciences, GE Healthcare
Research Funding: Bristol Myers Squibb (Inst), Merck, AstraZeneca
James Chih-Hsin Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, Takeda, Lilly, Pfizer, Amgen (Inst), AstraZeneca/MedImmune (Inst), Boehringer Ingelheim (Inst), Dizal Pharma (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), Takeda (Inst), Roche/Genentech (Inst), Daiichi Sankyo/AstraZeneca (Inst), MSD Oncology (Inst), BeiGene (Inst), Gilead Sciences (Inst), Sanofi/Regeneron (Inst)
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Clovis Oncology, Lilly (Inst), MSD Oncology, Celgene, Bayer, Pfizer, Ono Pharmaceutical, Bristol Myers Squibb, Boehringer Ingelheim (Inst), Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Takeda, Amgen, Incyte, GSK (Inst), Amgen (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), MSD Oncology (Inst), Janssen Oncology (Inst), Merck KGaA (Inst), Daiichi Sankyo/AstraZeneca (Inst), Puma Biotechnology (Inst), Gilead Sciences (Inst), Pfizer (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), Roche/Genentech (Inst), Sanofi (Inst)
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer
Thomas John
Honoraria: AstraZeneca/MedImmune, Roche/Genentech, Bristol Myers Squibb, MSD Oncology
Consulting or Advisory Role: AstraZeneca, Pfizer, AstraZeneca/MedImmune, Roche/Genentech, Ignyta, Boehringer Ingelheim, Novartis, MSD Oncology, Merck KGaA, Bristol Myers Squibb, Amgen (Inst), PharmaMar (Inst), Specialised Therapeutics, Gilead Sciences, Seagen (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Roche, AstraZeneca, Bristol Myers Squibb, Roche, MSD
Bartomeu Massutí
Consulting or Advisory Role: Roche, AstraZeneca, Janssen, Amgen
Speakers' Bureau: Roche, AstraZeneca, Bristol Myers Squibb, Janssen Oncology, Pfizer, MSD Oncology
Travel, Accommodations, Expenses: Roche, MSD Oncology, AstraZeneca, Pfizer
Alexander I. Spira
Leadership: Next Oncology (Inst)
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer, Prelude Therapeutics, AbbVie, Astellas Pharma
Consulting or Advisory Role: Incyte, Amgen, Novartis, AstraZeneca/MedImmune (Inst), Mirati Therapeutics, Gritstone Bio, Jazz Pharmaceuticals, Merck (Inst), Bristol Myers Squibb (Inst), Takeda, Janssen Research & Development, Mersana, Blueprint Medicines (Inst), Gritstone Bio, Daiichi Sankyo/AstraZeneca, Regeneron, Lilly, Black Diamond Therapeutics, Sanofi, ArriVent Biopharma
Research Funding: Roche (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), MedImmune (Inst), Novartis (Inst), Incyte (Inst), AbbVie (Inst), Ignyta (Inst), Takeda (Inst), MacroGenics (Inst), CytomX Therapeutics (Inst), LAM Therapeutics, Astex Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Loxo (Inst), Gritstone Bio (Inst), Plexxikon (Inst), Amgen (Inst), Loxo (Inst), Daiichi Sankyo (Inst), ADC Therapeutics (Inst), Janssen Oncology (Inst), ADC Therapeutics (Inst), Rubius Therapeutics (Inst), Synthekine (Inst), Mersana (Inst), Blueprint Medicines (Inst), Regeneron (Inst), Alkermes (Inst), Revolution Medicines (Inst), Medikine (Inst), Black Diamond Therapeutics (Inst), BluPrint Oncology (Inst), Nalo Therapeutics (Inst), Scorpion Therapeutics (Inst), ArriVent Biopharma (Inst), Revolution Medicines (Inst), Prelude Therapeutics (Inst)
Se-Hoon Lee
Honoraria: AstraZeneca/MedImmune, Roche, Lilly, Amgen, Yuhan, MSD
Consulting or Advisory Role: AstraZeneca, Roche, Pfizer, Lilly, Bristol Myers Squibb/Ono, Takeda, Janssen, IMBdx, Abion, BeiGene, Daiichi Sankyo, ImmuneOncia, Merck (German), MSD, Novartis
Speakers' Bureau: Abion
Research Funding: AstraZeneca (Inst), Lunit (Inst), MSD (Inst)
Silvia Novello
Consulting or Advisory Role: Sanofi
Speakers' Bureau: AstraZeneca, MSD, Bristol Myers Squibb, Roche, Pfizer, Lilly, Takeda, AbbVie, Boehringer Ingelheim, Bayer, Amgen, BeiGene, Novartis, Janssen
Travel, Accommodations, Expenses: Sanofi
Masashi Kondo
Honoraria: Chugai Pharma, Lilly, Pfizer, AstraZeneca, Ono Pharmaceutical, Bristol Myers Squibb, MSD K.K, Takeda, Pfizer
Speakers' Bureau: Chugai Pharma, Lilly, AstraZeneca, Ono Pharmaceutical, Bristol Myers Squibb, MSD, Takeda, Daiichi Sankyo Healthcare
Research Funding: Chugai Pharma (Inst), Ono Pharmaceutical (Inst), AstraZeneca (Inst), MSD (Inst), Takeda (Inst)
Motohiro Tamiya
Honoraria: Boehringer Ingelheim, Chugai Pharma, AstraZeneca, Taiho Pharmaceutical, Lilly Japan, Asahi Kasei, MSD, Ono Pharmaceutical, Bristol Myers Squibb Japan, Amgen, Takeda, Pfizer, Novartis, Nihonkayaku, Kyowa Kirin International, Bayer
Consulting or Advisory Role: Pfizer
Research Funding: Boehringer Ingelheim, Ono Pharmaceutical, MSD
Ernesto Korbenfeld
Honoraria: AstraZeneca
Consulting or Advisory Role: Pfizer
Mor Moskovitz
Honoraria: MSD, Bristol Myers Squibb, Roche, Takeda, AbbVie, AstraZeneca, Johnson & Johnson/Janssen
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Takeda, Roche, Pfizer, Johnson & Johnson, Novartis, Amgen
Travel, Accommodations, Expenses: Pfizer, MSD, Roche
Ji-Youn Han
Honoraria: AstraZeneca, Takeda, Novartis, Merck, Janssen, Pfizer, Yuhan
Consulting or Advisory Role: Novartis, Merck, Takeda, Janssen, Lantern Pharma, Amgen, AstraZeneca, Daiichi Sankyo/UCB Japan, Oncovix, AbbVie
Research Funding: Roche, Pfizer
Mariam Alexander
Consulting or Advisory Role: Amgen
Rohit Joshi
Honoraria: Bristol Myers Squibb, MSD, Roche, Pfizer, Pfizer/EMD Serono, Ipsen, Gilead Sciences
Consulting or Advisory Role: MSD, AstraZeneca
Speakers' Bureau: Gilead Sciences
Travel, Accommodations, Expenses: Novartis
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GSK, Janssen, Merck Serono, MSD, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Genmab
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, MSD, PeerVoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: Grifols
Uncompensated Relationships: Member of the Scientific Advisory Committee -Hospital Universitari Parc Taulí, SEOM (Sociedad Española de Oncología Médica), President from 2021-2023, “ETOP IBCSG Partners” Member of the Scientific Committee
Pei Jye Voon
Consulting or Advisory Role: AstraZeneca, Ipsen, MSD, Novartis, Pfizer, BeiGene, Amgen
Research Funding: Novartis (Inst), Boehringer Ingelheim (Inst), Viracta Therapeutics (Inst), Roche (Inst), Merck KGaA (Inst), MSD (Inst), BeiGene (Inst), AstraZeneca (Inst), Janssen-Cilag (Inst), Johnson & Johnson (Inst)
Pongwut Danchaivijitr
Consulting or Advisory Role: Merck, AstraZeneca, Pfizer, Roche, Janssen Oncology
Speakers' Bureau: Merck, AstraZeneca, Pfizer, Roche, Janssen Oncology
Research Funding: Merck (Inst), Roche (Inst), AstraZeneca (Inst), Daiichi Sankyo/AstraZeneca (Inst), Boehringer Ingelheim (Inst)
Felipe José Silva Melo Cruz
Consulting or Advisory Role: Pfizer, Bayer, Astellas Pharma, Janssen
Travel, Accommodations, Expenses: Janssen Oncology, Novartis
Thomas Wehler
Honoraria: Roche/Genentech, Boehringer Ingelheim, MSD, Bristol Myers Squibb, Pfizer, AstraZeneca, Janssen, Novocure
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Pfizer, MSD, Bristol Myers Squibb, AbbVie, Merck Serono, Boehringer Ingelheim
Research Funding: AstraZeneca, Roche/Genentech, Boehringer Ingelheim
Travel, Accommodations, Expenses: Pfizer, Celgene, Roche/Genentech, Boehringer Ingelheim, AstraZeneca, Janssen
Laurent Greillier
Honoraria: AstraZeneca, Roche, Bristol Myers Squibb, MSD, Takeda, Novartis, Pfizer, Sanofi, Amgen, Janssen
Consulting or Advisory Role: Roche, Bristol Myers Squibb, Takeda, MSD, AstraZeneca, Novartis, Janssen
Travel, Accommodations, Expenses: Roche, MSD, Bristol Myers Squibb, AstraZeneca, Pfizer, Amgen
Encarnação Teixeira
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Lilly, Takeda, Roche, MSD, Merck Serono
Speakers' Bureau: MSD Oncology, Sanofi/Aventis, Merck Serono, Janssen
Danny Nguyen
Stock and Other Ownership Interests: Intuitive Surgical (I), Teladoc (I)
Consulting or Advisory Role: Janssen Oncology
Other Relationship: Takeda, Novartis, Seagen, Pfizer
Uncompensated Relationships: Takeda, Novartis
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Pfizer, Regeneron, Medscape, Takeda, Janssen, Genentech/Roche, Mirati Therapeutics, AbbVie, Loxo/Lilly, Sanofi, Janssen (Inst), Loxo/Lilly (Inst), Mirati Therapeutics (Inst), Regeneron (Inst)
Angel Qin
Consulting or Advisory Role: Summit Therapeutics, Regeneron, Strata Oncology, Janssen
Research Funding: AstraZeneca (Inst), Roche (Inst), Janssen Oncology (Inst)
Dariusz Kowalski
Consulting or Advisory Role: Bristol Myers Squibb, Boehringer Ingelheim, Merck Serono, Roche/Genentech, AstraZeneca, MSD, Pfizer, Amgen, Johnson & Johnson/Janssen, Takeda, Sanofi/Aventis
Mehmet Ali Nahit Şendur
Honoraria: Amgen, Bristol Myers Squibb, MSD, Pfizer, Roche, Bayer, Novartis, Janssen, Astellas Pharma, Takeda
Speakers' Bureau: Bristol Myers Squibb, Pfizer, Amgen, Novartis, Roche, Janssen, Astellas Pharma, Takeda, Abdi Ibrahim
John Xie
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Debopriya Ghosh
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Johnson & Johnson/Janssen
Ali Alhadab
Employment: Pfizer, Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Pfizer, Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Johnson & Johnson/Janssen
Nahor Haddish-Berhane
Employment: Jonhson and Johnson
Stock and Other Ownership Interests: Johnson and Johnson
Pamela L. Clemens
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Patricia Lorenzini
Employment: Johnson & Johnson/Janssen
Remy B. Verheijen
Employment: AstraZeneca, Johnson & Johnson
Stock and Other Ownership Interests: AstraZeneca, Johnson & Johnson, Aduro Biotech, Chinook Tx, Galapagos NV
Mohamed Gamil
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Johnson & Johnson/Janssen
Joshua M. Bauml
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Mahadi Baig
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Antonio Passaro
Consulting or Advisory Role: Roche/Genentech, Bristol Myers Squibb, AstraZeneca, MSD Oncology, Pfizer, Boehringer Ingelheim, Johnson & Johnson/Janssen, Novartis, Daiichi Sankyo Europe GmbH, Bayer
Speakers' Bureau: AstraZeneca, Johnson & Johnson/Janssen, Daiichi Sankyo Europe GmbH, MSD Oncology
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2024.
SUPPORT
Supported by Janssen Research & Development, LLC.
CLINICAL TRIAL INFORMATION
NCT05388669 (PALOMA-3)
Contributor Information
Collaborators: Hiroaki Akamatsu, Mariam Alexander, Annalen Bleckmann, Federico Cappuzzo, Ying Cheng, Byoung Chul Cho, Timucin Cil, Alexis Cortot, Pongwut Danchaivijitr, Till-Oliver Emde, Dilek Erdem, Enriqueta Felip, Fernanda Estevinho, Maria Lurdes Ferreira, Flavio Ferreira da Silva, Maria del Rosario Garcia Campelo, Laurent Greillier, Alastair Greystoke, Ji-Youn Han, Ping-Chih Hsu, Jen-Yu Hung, Mei Ji, Thomas John, Rohit Joshi, Young-Chul Kim, Masashi Kondo, Ernesto Korbenfeld, Dariusz Kowalski, Se-Hoon Lee, Natasha Leighl, Juan Li, Sheng-Hao Lin, Baogang Liu, Caigang Liu, John Seng-Hooi Low, Melina E. Marmarelis, Bartomeu Massutí, Anna R. Minchom, Sara Moore, Mor Moskovitz, Adnan Nagrial, Danny Nguyen, Silvia Novello, Yuichiro Ohe, Mustafa Özgüroğlu, Ozgur Ozyilkan, Antonio Passaro, Nir Peled, Naiyarat Prasongsook, Angel Qin, Elisa F. Ramos, Joshua K. Sabari, Jorge Salinas, Rachel E. Sanborn, Mehmet Ali Nahit Sendur, Felipe José Silva Melo Cruz, Alexander I. Spira, Thatthan Suksombooncharoen, Motohiro Tamiya, Jiunn Liang Tan, Encarnacao Teixeira, Rajanikar Tota, Damien Urban, Alain Vergnenègre, Pei Jye Voon, Vanina Wainsztein, Jialei Wang, Thomas Wehler, James Chih-Hsin Yang, Hiroshige Yoshioka, Alona Zer, Yanqiu Zhao, and Bogdan Zurawski
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.24.01001.
AUTHOR CONTRIBUTIONS
Conception and design: Natasha B. Leighl, Bartomeu Massutí, Alexander I. Spira, Dariusz Kowalski, John Xie, Debopriya Ghosh, Nahor Haddish-Berhane, Remy B. Verheijen, Mohamed Gamil, Joshua M. Bauml
Financial support: Alexander I. Spira, Ali Alhadab
Administrative support: Alexander I. Spira, Jialei Wang, Ali Alhadab
Provision of study materials or patients: Hiroaki Akamatsu, Sun Min Lim, Ying Cheng, Anna R. Minchom, Rachel E. Sanborn, James Chih-Hsin Yang, Thomas John, Bartomeu Massutí, Alexander I. Spira, Jialei Wang, Silvia Novello, Masashi Kondo, Ernesto Korbenfeld, Mor Moskovitz, Ji-Youn Han, Enriqueta Felip, Pei Jye Voon, Ping-Chih Hsu, Felipe José Silva Melo Cruz, Thomas Wehler, Laurent Greillier, Danny Nguyen, Joshua K. Sabari, Ali Alhadab, Mohamed Gamil
Collection and assembly of data: Natasha B. Leighl, Hiroaki Akamatsu, Sun Min Lim, Ying Cheng, Anna R. Minchom, Rachel E. Sanborn, James Chih-Hsin Yang, Baogang Liu, Thomas John, Bartomeu Massutí, Alexander I. Spira, Jialei Wang, Juan Li, Caigang Liu, Masashi Kondo, Motohiro Tamiya, Ernesto Korbenfeld, Mor Moskovitz, Ji-Youn Han, Mariam Alexander, Rohit Joshi, Enriqueta Felip, Pei Jye Voon, Pongwut Danchaivijitr, Ping-Chih Hsu, Felipe José Silva Melo Cruz, Thomas Wehler, Laurent Greillier, Danny Nguyen, Joshua K. Sabari, Angel Qin, Dariusz Kowalski, Mehmet Ali Nahit Şendur, John Xie, Debopriya Ghosh, Ali Alhadab, Remy B. Verheijen, Mohamed Gamil, Joshua M. Bauml, Mahadi Baig, Antonio Passaro
Data analysis and interpretation: Natasha B. Leighl, Hiroaki Akamatsu, Ying Cheng, Anna R. Minchom, Melina E. Marmarelis, Rachel E. Sanborn, James Chih-Hsin Yang, Thomas John, Bartomeu Massutí, Alexander I. Spira, Se-Hoon Lee, Jialei Wang, Silvia Novello, Ernesto Korbenfeld, Mariam Alexander, Rohit Joshi, Enriqueta Felip, Pei Jye Voon, Pongwut Danchaivijitr, Felipe José Silva Melo Cruz, Thomas Wehler, Laurent Greillier, Encarnação Teixeira, Danny Nguyen, Joshua K. Sabari, Angel Qin, Dariusz Kowalski, John Xie, Debopriya Ghosh, Ali Alhadab, Nahor Haddish-Berhane, Pamela L. Clemens, Patricia Lorenzini, Remy B. Verheijen, Mohamed Gamil, Joshua M. Bauml, Mahadi Baig, Antonio Passaro
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Subcutaneous Versus Intravenous Amivantamab, Both in Combination With Lazertinib, in Refractory Epidermal Growth Factor Receptor–Mutated Non–Small Cell Lung Cancer: Primary Results From the Phase III PALOMA-3 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Natasha B. Leighl
Honoraria: BeiGene, Bristol Myers Squibb, Janssen, MSD Oncology, Takeda
Research Funding: MSD (Inst), Lilly (Inst), AstraZeneca Canada (Inst), Inivata/NeoGenomics (Inst), Janssen Oncology (Inst), Novartis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Hiroaki Akamatsu
Honoraria: AstraZeneca, Lilly, Pfizer, MSD, Bristol Myers Squibb Japan, Taiho Pharmaceutical, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Amgen, Nippon Kayaku, Novartis, Takeda
Consulting or Advisory Role: Amgen, Janssen, Sandoz
Research Funding: Amgen (Inst), Chugai Pharma (Inst)
Sun Min Lim
Honoraria: Takeda, Boehringer Ingelheim, Yuhan
Research Funding: Janssen Research & Development
Anna R. Minchom
Honoraria: Janssen, Merck, GSK, Seagen
Consulting or Advisory Role: Janssen Oncology, Faron Pharmaceuticals, GSK, MSD, Immutep, Genmab, Merck
Research Funding: Merck (Inst), MSD Oncology (Inst), Astex Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Janssen Oncology, Amgen
Melina E. Marmarelis
Stock and Other Ownership Interests: Merck, Johnson & Johnson
Honoraria: Novocure, AstraZeneca, Janssen Oncology, Takeda, Blueprint Medicines, Thermo Fisher Scientific
Consulting or Advisory Role: AstraZeneca, Ikena Oncology, Bristol Myers Squibb/Celgene, AstraZeneca, Janssen Oncology, Regeneron
Research Funding: Lilly (Inst), AstraZeneca (Inst), Janssen Oncology (Inst), Ikena Oncology (Inst), Genentech (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Regeneron
Open Payments Link: https://openpaymentsdata.cms.gov/physician/856967
Rachel E. Sanborn
Honoraria: MJH Life Sciences, Targeted Oncology, Curio Science, Illumina
Consulting or Advisory Role: AstraZeneca, EMD Serono, Janssen Oncology, Macrogenics, Sanofi/Aventis, Regeneron, GSK, Illumina, G1 Therapeutics, Daiichi Sankyo, Lilly, Amgen, Gilead Sciences, GE Healthcare
Research Funding: Bristol Myers Squibb (Inst), Merck, AstraZeneca
James Chih-Hsin Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, Takeda, Lilly, Pfizer, Amgen (Inst), AstraZeneca/MedImmune (Inst), Boehringer Ingelheim (Inst), Dizal Pharma (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), Takeda (Inst), Roche/Genentech (Inst), Daiichi Sankyo/AstraZeneca (Inst), MSD Oncology (Inst), BeiGene (Inst), Gilead Sciences (Inst), Sanofi/Regeneron (Inst)
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Clovis Oncology, Lilly (Inst), MSD Oncology, Celgene, Bayer, Pfizer, Ono Pharmaceutical, Bristol Myers Squibb, Boehringer Ingelheim (Inst), Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Takeda, Amgen, Incyte, GSK (Inst), Amgen (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), MSD Oncology (Inst), Janssen Oncology (Inst), Merck KGaA (Inst), Daiichi Sankyo/AstraZeneca (Inst), Puma Biotechnology (Inst), Gilead Sciences (Inst), Pfizer (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), Roche/Genentech (Inst), Sanofi (Inst)
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer
Thomas John
Honoraria: AstraZeneca/MedImmune, Roche/Genentech, Bristol Myers Squibb, MSD Oncology
Consulting or Advisory Role: AstraZeneca, Pfizer, AstraZeneca/MedImmune, Roche/Genentech, Ignyta, Boehringer Ingelheim, Novartis, MSD Oncology, Merck KGaA, Bristol Myers Squibb, Amgen (Inst), PharmaMar (Inst), Specialised Therapeutics, Gilead Sciences, Seagen (Inst)
Travel, Accommodations, Expenses: Boehringer Ingelheim, Roche, AstraZeneca, Bristol Myers Squibb, Roche, MSD
Bartomeu Massutí
Consulting or Advisory Role: Roche, AstraZeneca, Janssen, Amgen
Speakers' Bureau: Roche, AstraZeneca, Bristol Myers Squibb, Janssen Oncology, Pfizer, MSD Oncology
Travel, Accommodations, Expenses: Roche, MSD Oncology, AstraZeneca, Pfizer
Alexander I. Spira
Leadership: Next Oncology (Inst)
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer, Prelude Therapeutics, AbbVie, Astellas Pharma
Consulting or Advisory Role: Incyte, Amgen, Novartis, AstraZeneca/MedImmune (Inst), Mirati Therapeutics, Gritstone Bio, Jazz Pharmaceuticals, Merck (Inst), Bristol Myers Squibb (Inst), Takeda, Janssen Research & Development, Mersana, Blueprint Medicines (Inst), Gritstone Bio, Daiichi Sankyo/AstraZeneca, Regeneron, Lilly, Black Diamond Therapeutics, Sanofi, ArriVent Biopharma
Research Funding: Roche (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), MedImmune (Inst), Novartis (Inst), Incyte (Inst), AbbVie (Inst), Ignyta (Inst), Takeda (Inst), MacroGenics (Inst), CytomX Therapeutics (Inst), LAM Therapeutics, Astex Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Loxo (Inst), Gritstone Bio (Inst), Plexxikon (Inst), Amgen (Inst), Loxo (Inst), Daiichi Sankyo (Inst), ADC Therapeutics (Inst), Janssen Oncology (Inst), ADC Therapeutics (Inst), Rubius Therapeutics (Inst), Synthekine (Inst), Mersana (Inst), Blueprint Medicines (Inst), Regeneron (Inst), Alkermes (Inst), Revolution Medicines (Inst), Medikine (Inst), Black Diamond Therapeutics (Inst), BluPrint Oncology (Inst), Nalo Therapeutics (Inst), Scorpion Therapeutics (Inst), ArriVent Biopharma (Inst), Revolution Medicines (Inst), Prelude Therapeutics (Inst)
Se-Hoon Lee
Honoraria: AstraZeneca/MedImmune, Roche, Lilly, Amgen, Yuhan, MSD
Consulting or Advisory Role: AstraZeneca, Roche, Pfizer, Lilly, Bristol Myers Squibb/Ono, Takeda, Janssen, IMBdx, Abion, BeiGene, Daiichi Sankyo, ImmuneOncia, Merck (German), MSD, Novartis
Speakers' Bureau: Abion
Research Funding: AstraZeneca (Inst), Lunit (Inst), MSD (Inst)
Silvia Novello
Consulting or Advisory Role: Sanofi
Speakers' Bureau: AstraZeneca, MSD, Bristol Myers Squibb, Roche, Pfizer, Lilly, Takeda, AbbVie, Boehringer Ingelheim, Bayer, Amgen, BeiGene, Novartis, Janssen
Travel, Accommodations, Expenses: Sanofi
Masashi Kondo
Honoraria: Chugai Pharma, Lilly, Pfizer, AstraZeneca, Ono Pharmaceutical, Bristol Myers Squibb, MSD K.K, Takeda, Pfizer
Speakers' Bureau: Chugai Pharma, Lilly, AstraZeneca, Ono Pharmaceutical, Bristol Myers Squibb, MSD, Takeda, Daiichi Sankyo Healthcare
Research Funding: Chugai Pharma (Inst), Ono Pharmaceutical (Inst), AstraZeneca (Inst), MSD (Inst), Takeda (Inst)
Motohiro Tamiya
Honoraria: Boehringer Ingelheim, Chugai Pharma, AstraZeneca, Taiho Pharmaceutical, Lilly Japan, Asahi Kasei, MSD, Ono Pharmaceutical, Bristol Myers Squibb Japan, Amgen, Takeda, Pfizer, Novartis, Nihonkayaku, Kyowa Kirin International, Bayer
Consulting or Advisory Role: Pfizer
Research Funding: Boehringer Ingelheim, Ono Pharmaceutical, MSD
Ernesto Korbenfeld
Honoraria: AstraZeneca
Consulting or Advisory Role: Pfizer
Mor Moskovitz
Honoraria: MSD, Bristol Myers Squibb, Roche, Takeda, AbbVie, AstraZeneca, Johnson & Johnson/Janssen
Consulting or Advisory Role: AstraZeneca, MSD Oncology, Takeda, Roche, Pfizer, Johnson & Johnson, Novartis, Amgen
Travel, Accommodations, Expenses: Pfizer, MSD, Roche
Ji-Youn Han
Honoraria: AstraZeneca, Takeda, Novartis, Merck, Janssen, Pfizer, Yuhan
Consulting or Advisory Role: Novartis, Merck, Takeda, Janssen, Lantern Pharma, Amgen, AstraZeneca, Daiichi Sankyo/UCB Japan, Oncovix, AbbVie
Research Funding: Roche, Pfizer
Mariam Alexander
Consulting or Advisory Role: Amgen
Rohit Joshi
Honoraria: Bristol Myers Squibb, MSD, Roche, Pfizer, Pfizer/EMD Serono, Ipsen, Gilead Sciences
Consulting or Advisory Role: MSD, AstraZeneca
Speakers' Bureau: Gilead Sciences
Travel, Accommodations, Expenses: Novartis
Enriqueta Felip
Consulting or Advisory Role: AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Roche, Gilead Sciences, GSK, Janssen, Merck Serono, MSD, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, Genmab
Speakers' Bureau: Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Lilly, Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, MSD, PeerVoice, Pfizer, Sanofi, Takeda, Touch Oncology
Travel, Accommodations, Expenses: AstraZeneca, Janssen, Roche
Other Relationship: Grifols
Uncompensated Relationships: Member of the Scientific Advisory Committee -Hospital Universitari Parc Taulí, SEOM (Sociedad Española de Oncología Médica), President from 2021-2023, “ETOP IBCSG Partners” Member of the Scientific Committee
Pei Jye Voon
Consulting or Advisory Role: AstraZeneca, Ipsen, MSD, Novartis, Pfizer, BeiGene, Amgen
Research Funding: Novartis (Inst), Boehringer Ingelheim (Inst), Viracta Therapeutics (Inst), Roche (Inst), Merck KGaA (Inst), MSD (Inst), BeiGene (Inst), AstraZeneca (Inst), Janssen-Cilag (Inst), Johnson & Johnson (Inst)
Pongwut Danchaivijitr
Consulting or Advisory Role: Merck, AstraZeneca, Pfizer, Roche, Janssen Oncology
Speakers' Bureau: Merck, AstraZeneca, Pfizer, Roche, Janssen Oncology
Research Funding: Merck (Inst), Roche (Inst), AstraZeneca (Inst), Daiichi Sankyo/AstraZeneca (Inst), Boehringer Ingelheim (Inst)
Felipe José Silva Melo Cruz
Consulting or Advisory Role: Pfizer, Bayer, Astellas Pharma, Janssen
Travel, Accommodations, Expenses: Janssen Oncology, Novartis
Thomas Wehler
Honoraria: Roche/Genentech, Boehringer Ingelheim, MSD, Bristol Myers Squibb, Pfizer, AstraZeneca, Janssen, Novocure
Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Pfizer, MSD, Bristol Myers Squibb, AbbVie, Merck Serono, Boehringer Ingelheim
Research Funding: AstraZeneca, Roche/Genentech, Boehringer Ingelheim
Travel, Accommodations, Expenses: Pfizer, Celgene, Roche/Genentech, Boehringer Ingelheim, AstraZeneca, Janssen
Laurent Greillier
Honoraria: AstraZeneca, Roche, Bristol Myers Squibb, MSD, Takeda, Novartis, Pfizer, Sanofi, Amgen, Janssen
Consulting or Advisory Role: Roche, Bristol Myers Squibb, Takeda, MSD, AstraZeneca, Novartis, Janssen
Travel, Accommodations, Expenses: Roche, MSD, Bristol Myers Squibb, AstraZeneca, Pfizer, Amgen
Encarnação Teixeira
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Lilly, Takeda, Roche, MSD, Merck Serono
Speakers' Bureau: MSD Oncology, Sanofi/Aventis, Merck Serono, Janssen
Danny Nguyen
Stock and Other Ownership Interests: Intuitive Surgical (I), Teladoc (I)
Consulting or Advisory Role: Janssen Oncology
Other Relationship: Takeda, Novartis, Seagen, Pfizer
Uncompensated Relationships: Takeda, Novartis
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Pfizer, Regeneron, Medscape, Takeda, Janssen, Genentech/Roche, Mirati Therapeutics, AbbVie, Loxo/Lilly, Sanofi, Janssen (Inst), Loxo/Lilly (Inst), Mirati Therapeutics (Inst), Regeneron (Inst)
Angel Qin
Consulting or Advisory Role: Summit Therapeutics, Regeneron, Strata Oncology, Janssen
Research Funding: AstraZeneca (Inst), Roche (Inst), Janssen Oncology (Inst)
Dariusz Kowalski
Consulting or Advisory Role: Bristol Myers Squibb, Boehringer Ingelheim, Merck Serono, Roche/Genentech, AstraZeneca, MSD, Pfizer, Amgen, Johnson & Johnson/Janssen, Takeda, Sanofi/Aventis
Mehmet Ali Nahit Şendur
Honoraria: Amgen, Bristol Myers Squibb, MSD, Pfizer, Roche, Bayer, Novartis, Janssen, Astellas Pharma, Takeda
Speakers' Bureau: Bristol Myers Squibb, Pfizer, Amgen, Novartis, Roche, Janssen, Astellas Pharma, Takeda, Abdi Ibrahim
John Xie
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Debopriya Ghosh
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Johnson & Johnson/Janssen
Ali Alhadab
Employment: Pfizer, Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Pfizer, Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Johnson & Johnson/Janssen
Nahor Haddish-Berhane
Employment: Jonhson and Johnson
Stock and Other Ownership Interests: Johnson and Johnson
Pamela L. Clemens
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Patricia Lorenzini
Employment: Johnson & Johnson/Janssen
Remy B. Verheijen
Employment: AstraZeneca, Johnson & Johnson
Stock and Other Ownership Interests: AstraZeneca, Johnson & Johnson, Aduro Biotech, Chinook Tx, Galapagos NV
Mohamed Gamil
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Travel, Accommodations, Expenses: Johnson & Johnson/Janssen
Joshua M. Bauml
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Mahadi Baig
Employment: Johnson & Johnson/Janssen
Stock and Other Ownership Interests: Johnson & Johnson/Janssen
Antonio Passaro
Consulting or Advisory Role: Roche/Genentech, Bristol Myers Squibb, AstraZeneca, MSD Oncology, Pfizer, Boehringer Ingelheim, Johnson & Johnson/Janssen, Novartis, Daiichi Sankyo Europe GmbH, Bayer
Speakers' Bureau: AstraZeneca, Johnson & Johnson/Janssen, Daiichi Sankyo Europe GmbH, MSD Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1. Moores SL, Chiu ML, Bushey BS, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76:3942–3953. doi: 10.1158/0008-5472.CAN-15-2833. [DOI] [PubMed] [Google Scholar]
- 2. Vijayaraghavan S, Lipfert L, Chevalier K, et al. Amivantamab (JNJ-61186372), an Fc enhanced EGFR/cMet bispecific antibody, induces receptor downmodulation and antitumor activity by monocyte/macrophage trogocytosis. Mol Cancer Ther. 2020;19:2044–2056. doi: 10.1158/1535-7163.MCT-20-0071. [DOI] [PubMed] [Google Scholar]
- 3. Yun J, Lee SH, Kim SY, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR exon 20 insertion-driven NSCLC. Cancer Discov. 2020;10:1194–1209. doi: 10.1158/2159-8290.CD-20-0116. [DOI] [PubMed] [Google Scholar]
- 4. Cho BC, Simi A, Sabari J, et al. Amivantamab, an epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor (MET) bispecific antibody, designed to enable multiple mechanisms of action and broad clinical applications. Clin Lung Cancer. 2023;24:89–97. doi: 10.1016/j.cllc.2022.11.004. [DOI] [PubMed] [Google Scholar]
- 5. Besse B, Baik CS, Marmarelis ME, et al. Predictive biomarkers for treatment with amivantamab plus lazertinib among EGFR-mutated NSCLC in the post-osimertinib setting: Analysis of tissue IHC and ctDNA NGS. J Clin Oncol. 2023;41:9013. [Google Scholar]
- 6.RYBREVANT (Amivantamab-vmjw) Injection, for Intravenous Use [prescribing Information] Horsham, PA: Janssen Biotech,; 2024. [Google Scholar]
- 7. Cho BC, Lu S, Felip E, et al. Amivantamab plus lazertinib in previously untreated EGFR-mutated advanced NSCLC. N Engl J Med. doi: 10.1056/NEJMoa2403614. 10.1056/NEJMoa2403614 [epub ahead of print June 26, 2024] [DOI] [PubMed] [Google Scholar]
- 8. Park K, Sabari JK, Haura EB, et al. Management of infusion-related reactions (IRRs) in patients receiving amivantamab in the CHRYSALIS study. Lung Cancer. 2023;178:166–171. doi: 10.1016/j.lungcan.2023.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Minchom A, Krebs MG, Cho BC, et al. Subcutaneous amivantamab in patients with advanced solid malignancies: The PALOMA study—updated safety and confirmation of the recommended phase 2 dose. Presented at American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, June 2-6, 2023.
- 10.Leighl N, Cho BC, Hiret S, et al. Amivantamab in patients with advanced NSCLC and MET exon14 skipping mutation: Results from the CHRYSALIS study. Presented at IASLC 2023 World Conference on Lung Cancer, Singapore, September 9-12, 2023.
- 11. Passaro A, Wang J, Shah S, et al. Letter to the editor regarding 'Correspondence to: Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study' by Moik F, Riedl JM, and Ay C. Ann Oncol. 2024;35:328–329. doi: 10.1016/j.annonc.2023.11.012. [DOI] [PubMed] [Google Scholar]
- 12. Passaro A, Wang J, Wang Y, et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study. Ann Oncol. 2024;35:77–90. doi: 10.1016/j.annonc.2023.10.117. [DOI] [PubMed] [Google Scholar]
- 13. Zhou C, Tang KJ, Cho BC, et al. Amivantamab plus chemotherapy in NSCLC with EGFR exon 20 insertions. N Engl J Med. 2023;389:2039–2051. doi: 10.1056/NEJMoa2306441. [DOI] [PubMed] [Google Scholar]
- 14.Girard N, Cho BC, Spira AI, et al. Risk factors for venous thromboembolism (VTE) among patients with EGFR-mutated advanced non-small cell lung cancer (NSCLC) receiving amivantamab plus lazertinib versus either agent alone. Presented at American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, June 2-6, 2023.
- 15.European Medicines Agency Concept paper for the development of a guideline on non-inferiority and equivalence comparisons in clinical trials. https://www.ema.europa.eu/en/documents/scientific-guideline/concept-paper-development-guideline-non-inferiority-equivalence-comparisons-clinical-trials_en.pdf .
- 16.U.S. Food and Drug Administration Non-inferiority clinical trials to establish effectiveness. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/non-inferiority-clinical-trials
- 17. Mateos MV, Nahi H, Legiec W, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): A multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020;7:e370–e380. doi: 10.1016/S2352-3026(20)30070-3. [DOI] [PubMed] [Google Scholar]
- 18. Haddish-Berhane N, Su Y, Russu A, et al. Determination and confirmation of recommended Ph2 dose of amivantamab in epidermal growth factor receptor exon 20 insertion non-small cell lung cancer. Clin Pharmacol Ther. 2024;115:468–477. doi: 10.1002/cpt.3064. [DOI] [PubMed] [Google Scholar]
- 19. Feeney OM, Gracia G, Brundel DHS, et al. Lymph-directed immunotherapy—Harnessing endogenous lymphatic distribution pathways for enhanced therapeutic outcomes in cancer. Adv Drug Deliv Rev. 2020;160:115–135. doi: 10.1016/j.addr.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 20. Trevaskis NL, Kaminskas LM, Porter CJ. From sewer to saviour—Targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 21. Styles IK, Feeney OM, Nguyen TH, et al. Removal of interstitial hyaluronan with recombinant human hyaluronidase improves the systemic and lymphatic uptake of cetuximab in rats. J Control Release. 2019;315:85–96. doi: 10.1016/j.jconrel.2019.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Cho BC, Felip E, Spira A, et al. Amivantamab plus lazertinib versus osimertinib as first-line treatment in EGFR-mutated advanced NSCLC. Presented at European Society for Medical Oncology (ESMO) Congress, Madrid, Spain, October 20-24, 2023.
- 23. Khorana AA, Palaia J, Rosenblatt L, et al. Venous thromboembolism incidence and risk factors associated with immune checkpoint inhibitors among patients with advanced non-small cell lung cancer. J Immunother Cancer. 2023;11:e006072. doi: 10.1136/jitc-2022-006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mulder FI, Horváth-Puhó E, van Es N, et al. Venous thromboembolism in cancer patients: A population-based cohort study. Blood. 2021;137:1959–1969. doi: 10.1182/blood.2020007338. [DOI] [PubMed] [Google Scholar]
- 25. Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 26.Leighl NB, Minchom AR, Lee KH, et al. Subcutaneous amivantamab administered every 4 weeks (Q4W) in patients with advanced solid malignancies: The phase Ib PALOMA study (ID 369) Presented at European Lung Cancer Congress (ELCC), Prague, Czech Republic, March 20-23, 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.24.01001.