Abstract
We recently demonstrated that CD8+ T cells could block herpes simplex virus type 1 (HSV-1) reactivation from latency in ex vivo trigeminal ganglion (TG) cultures without destroying the infected neurons. Here we establish that CD8+ T-cell prevention of HSV-1 reactivation from latency is mediated at least in part by gamma interferon (IFN-γ). We demonstrate that IFN-γ was produced in ex vivo cultures of dissociated latently infected TG by CD8+ T cells that were present in the TG at the time of excision. Depletion of CD8+ T cells or neutralization of IFN-γ significantly enhanced the rate of HSV-1 reactivation from latency in TG cultures. When TG cultures were treated with acyclovir for 4 days to insure uniform latency, supplementation with recombinant IFN-γ blocked HSV-1 reactivation in 80% of cultures when endogenous CD8+ T cells were present and significantly reduced and delayed HSV-1 reactivation when CD8+ T cells or CD45+ cells were depleted from the TG cultures. The effectiveness of recombinant IFN-γ in blocking HSV-1 reactivation was lost when its addition to TG cultures was delayed by more than 24 h after acyclovir removal. We propose that when the intrinsic ability of neurons to inhibit HSV-1 gene expression is compromised, HSV-specific CD8+ T cells are rapidly mobilized to produce IFN-γ and perhaps other antiviral cytokines that block the viral replication cycle and maintain the viral genome in a latent state.
Following primary infection of epithelial surfaces, herpes simplex virus type 1 (HSV-1) gains access to the termini of sensory neurons, is transported in a retrograde direction to the neuron cell bodies in sensory ganglia, replicates, spreads to other neurons, and establishes a lifelong latent infection in a portion of the neurons. Recurrent HSV-1 disease results from reactivation of latent HSV-1 in sensory neurons followed by anterograde axonal transport to epithelial or epidermal surfaces. Therefore, treatments that block reactivation of HSV-1 from latency could effectively prevent recurrent disease in the face of latent infection. The long-term retention of CD8+ T cells and production of the cytokine gamma interferon (IFN-γ) in the latently infected trigeminal ganglion (TG) suggest a possible role for the immune system in controlling HSV-1 reactivation from latency (4, 10, 16, 20).
Ganglionic latency is classically defined as retention of HSV-1 genomes in neurons in the absence of virion production. The definition of latency at the molecular level is currently evolving. Clearly, a family of transcripts called latency-associated transcripts is produced in abundance in at least a portion of latently infected neurons (6, 7). In addition, transcripts for the HSV-1 α (immediate-early) gene, infected cell protein 4 (ICP4), and β (early) gene thymidine kinase were detected in low abundance in latently infected murine sensory ganglia (13). Most studies have failed to detect latency-associated transcript translation products, and the production of any viral proteins in latently infected neurons is currently uncertain. However, the presence of viral transcripts in latently infected neurons suggests the potential for protein synthesis. Further, in the appropriate context, T cells are exquisitely sensitive to minute quantities of antigenic proteins (8), and it is plausible that T-cell activation could occur in response to antigens present at levels below the sensitivity of normal detection methods. Indeed, there is evidence that virus-specific CD8+ T cells are retained and persistently activated at sites of infection where viral proteins are no longer detectable and viral transcripts are in low abundance (1, 11).
HSV-1 infection of mice results in a latent infection in sensory ganglia, but the virus does not normally reactivate from latency (24, 25). Interestingly, leukocytes, including CD4+ and CD8+ T lymphocytes and macrophages, have been detected in mouse TG up to 9 months after a primary HSV-1 infection (4, 16, 20). Moreover, leukocytic infiltration of the latently infected mouse TG is associated with the presence of the cytokines IFN-γ, tumor necrosis factor alpha, and interleukin-6, suggesting persistent activation of the infiltrating cells (4, 10, 16). Cytokine production in the TG was inhibited by treatment of latently infected mice with the antiviral drug acyclovir (ACV), consistent with the involvement of viral proteins in T-cell activation (9). These findings are consistent with the notion that individual latently infected neurons may periodically lose the capacity to inhibit expression of HSV-1 genes, resulting in low-level and perhaps intermittent production of viral proteins. These viral proteins might stimulate T cells in the ganglion to produce cytokines that prevent further progression through the viral life cycle to the stage of virion production. In this hypothetical scenario, T cells supplement the intrinsic ability of neurons to inhibit HSV gene expression and maintain the virus in a latent state.
We are testing the capacity of CD8+ T cells to maintain HSV-1 in a latent state in sensory neurons in an ex vivo model. Latently infected mouse TG are excised, dissociated into single-cell suspensions, and cultured in the presence or absence of reagents that block certain T-cell functions. Using this approach, it was recently demonstrated that CD8+ T cells that were present in latently infected TG 14 days after corneal infection were capable of blocking HSV-1 reactivation from latency in ex vivo cultures (15). When TG were obtained 34 days after corneal infection, the endogenous CD8+ T cells could delay, but could not prevent, reactivation, unless supplemented with exogenous CD8+ T cells obtained from the lymph nodes of HSV-1-infected, but not noninfected, mice. The failure of the endogenous CD8+ T cells to block reactivation in day 34 TG cultures might reflect their reduced numbers (compared to those of day 14 TG cultures), but it might also reflect functional changes in the CD8+ T cells that remain in the TG between 14 and 34 days after HSV-1 infection. Importantly, both the exogenous and endogenous (day 14) CD8+ T cells were able to prevent HSV-1 reactivation without eliminating the pool of latently infected neurons in the TG cultures. The latter observation suggested the involvement of a nonlytic mechanism of suppression of HSV-1 reactivation from latency.
Another important observation to emerge from previous studies was that a small portion of latently infected neurons in cultures that were protected by CD8+ T cells expressed the HSV-1 α protein ICP4 and the β protein ICP8, but failed to express detectable γ2 (glycoprotein C) gene transcripts or proteins. A recent study demonstrated transient expression of major histocompatibility complex (MHC) class I molecules on neurons in HSV-1-infected sensory ganglia (18). We hypothesized that early in the reactivation process, expression of a limited array of HSV-1 genes in conjunction with MHC class I genes might provide the epitopes that stimulate CD8+ T cells, leading to their production of antiviral cytokines. Our present findings are consistent with this hypothesis and demonstrate the capacity of IFN-γ to prevent HSV-1 reactivation from latency in TG neurons.
MATERIALS AND METHODS
HSV-1 infection.
Female BALB/c mice (Frederick Cancer Research Center, Frederick, Md.), 6 to 8 weeks old, were anesthetized by intramuscular injection of 2.0 mg of ketamine hydrochloride (Phoenix Pharmaceutical, Inc., St. Joseph, Mo.) and 0.2 mg of xylazine (Phoenix Pharmaceutical, Inc.) in 0.2 ml of Hanks' balanced salt solution. The RE strain of HSV-1 was grown in Vero cells, and intact virions were purified on Percoll (Pharmacia LKB Biotechnology, Inc., Piscataway, N.J.) as described previously (19). Corneas of anesthetized mice were scarified 10 times in a crisscross fashion with a sterile 30-gauge needle, and the eyes were infected topically with 3 μl of RPMI containing 1 × 105 PFU of HSV-1.
Preparation of TG cultures.
At 35 days after HSV-1 corneal infection, the ipsilateral TG was excised and treated with collagenase type I (3 mg/ml; Sigma, St. Louis, Mo.) for 1.5 h at 37°C and dispersed into single cells by triturating as previously described (15). The cells from multiple TG were pooled, and the neurons were counted. The equivalent number of cells from one TG (approximately 10,000 neurons) was added to each well of a 24-well tissue culture plate (catalog. no. 353047; Falcon), and the cells were incubated in culture medium consisting of Dulbecco's modified Eagle's medium, 10% fetal calf serum (HyClone, Logan, Utah), and recombinant murine interleukin-2 (10 U/ml; R & D Systems, Inc., Minneapolis, Minn.). Some cultures received ACV (50 μg/ml; Glaxo Wellcome, Inc., Research Triangle Park, N.C.) during the first 4 days of incubation. This treatment was shown to be optimal in preliminary experiments. After 4 days, the ACV-containing medium was removed and the cultures were rinsed with culture medium twice, and then they were incubated for an additional 10 days in culture medium alone or culture medium supplemented with monoclonal antibody (MAb) to IFN-γ (20 μg/ml, clone R4-6A2) or CD8α (100 μg/ml, clone 2.43) or with mouse recombinant IFN-γ (rIFN-γ) (1,000 World Health Organization units/ml; R & D Systems, Inc.). Where indicated, TG cell suspensions were depleted of CD8+ T cells or CD45+ cells by treatment with anti-CD8α MAb- or anti-CD45 MAb-coated magnetic beads (6 beads/cell, Dynabeads; Dynal) followed by seven rounds of magnetic separation. The resulting TG cell suspensions contained less than 1% of the depleted cell population as assessed by immunofluorescent staining with phycoerythrin-conjugated anti-CD8α MAb (clone 53-6.7; PharMingen) or biotinylated anti-CD45 MAb (anti-T200, clone m1/9.3.4.HL.2) and fluorescein isothiocyanate-conjugated Streptavidin followed by flow cytometric analysis. Some neurons and supporting cells were lost from the TG cell suspensions during immunomagnetic depletion, but adjustments were made so that the depleted and nondepleted TG cultures contained comparable numbers of neurons. Preliminary studies established that mock depletion with uncoated beads did not influence assay results, so this control was not included in the studies described herein.
HSV-1 titration in culture supernatant fluids.
At various times after culture initiation, 150 μl of medium was removed from each culture and the number of released infectious virions was determined in a standard virus plaque assay on monolayers of Vero cells (22). After each sampling, the medium was replaced with an equal volume of fresh medium of the same composition.
IFN-γ titration.
At various times after culture initiation, 150 μl of medium was removed from each culture and tested for IFN-γ content using a standard enzyme-linked immunosorbent assay (ELISA) incorporating anti-IFN-γ MAb (clone R4-6A2) as the capture antibody and biotinylated polyclonal anti-mouse IFN-γ antibody (R & D Systems, Inc.) as the detection antibody. The concentration of IFN-γ was determined from a standard curve obtained with a mouse rIFN-γ standard (1 IU per 1.19 ng, catalog no. 485-MI; R&D Systems). The sensitivity of the assay was 15.6 pg/ml.
IFN-γ production by CD8+ T cells.
The cytotoxic T-lymphocyte clone 2D5 (kindly provided by Robert H. Bonneau, Milton S. Hershey Medical Center) is H-2Kb restricted and recognizes the epitope (residues 498 to 505) within the HSV-1 structural protein glycoprotein B (gB) (2). B6/WT3 cells (kindly provided by Robert H. Bonneau) were infected with HSV-1 RE at a multiplicity of infection of 5 for 6 h, removed with trypsin (Gibco, Gaithersburg, Md.), treated with mitomycin C (400 μg/ml; Sigma), and used as stimulator cells in the cytokine release assay. To determine the effects of ACV on the production of IFN-γ by CD8+ T cells, 2 × 105 2D5 cells were cultured in the presence of 1 × 105 stimulator cells in medium alone or medium containing 25, 50, 100, or 200 μg of ACV/ml. At 2 and 4 days after culture initiation, 200 μl of supernatant was collected and assayed for IFN-γ by ELISA.
Statistics.
The significances of difference in reactivation rates were assessed by survival analysis, differences in viral titers were analyzed by an unpaired t test, and differences in IFN-γ production were analyzed by one-way analysis of variance.
RESULTS
IFN-γ is produced in latently infected TG cultures.
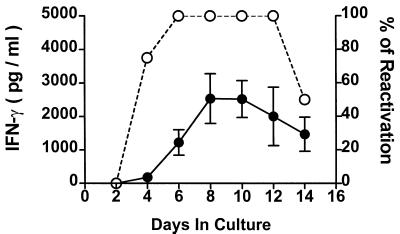
TG were excised 35 days after HSV-1 corneal infection (day 35 TG), and dissociated into single-cell suspensions. The cultures were incubated in medium alone, and the supernatant fluids were sampled every other day and assayed for IFN-γ in an ELISA and for infectious virus in a plaque assay. The cultures developed monolayers of fibroblast-like cells with multiple neurons (identifiable morphologically) and lymphocyte-like cells resting on the monolayer. IFN-γ was detectable in culture supernatants 4 days after culture initiation, reached peak levels by 8 to 10 days, and declined thereafter (Fig. 1). HSV-1 was reactivated from latency in 100% of these cultures by day 6 after culture initiation, as assessed by the presence of infectious virus in culture supernatants and detection of viral cytopathic effect in neurons and surrounding cells. Interestingly, only one plaque (area of viral cytopathic effect in fibroblasts surrounding a neuron) was observed in each culture. Virus titers in these cultures remained low (not shown) and became undetectable (i.e., negative for reactivation) in 50% of the cultures between 12 and 14 days (Fig. 1). When virus titers became undetectable, no further viral cytopathic effect was observed, and fibroblasts began to grow back into the cleared areas of the plaques (not shown). In a previous study, addition of anti-CD8α MAb to day 34 TG cultures resulted in rapid HSV-1 spread and destruction of the cultures (15). Taken together, these studies demonstrate that CD8+ T cells that are present in the TG more than 30 days after HSV-1 corneal infection can restrict the spread of HSV-1 within TG cultures and suggest a possible role for IFN-γ in this protective effect.
FIG. 1.
IFN-γ is produced in day 35 TG cultures. TG were excised 35 days after HSV-1 corneal infection, and single-cell suspensions were prepared and incubated in culture medium. Culture supernatant fluids were sampled every other day and assayed for IFN-γ content by a standard ELISA and for infectious HSV-1 (indicating HSV-1 reactivation from latency) in a plaque assay. The data (n = 8) are expressed as means ± standard errors of the mean in picograms of IFN-γ per milliliter (●) and percent reactivation from latency (○).
IFN-γ alone cannot block HSV-1 reactivation in day 35 TG cultures.
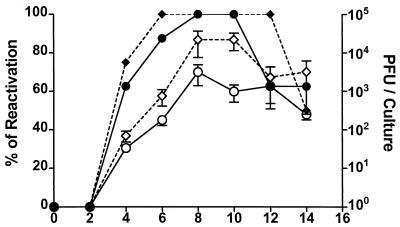
As illustrated in Fig. 1, HSV-1 was reactivated from latency in all TG cultures before IFN-γ titers reached peak levels. We reasoned, therefore, that addition of rIFN-γ at the initiation of cultures would completely block HSV-1 reactivation in cultured neurons. The data in Fig. 2 negate that hypothesis. HSV-1 was reactivated from latency in 100% of cultures in the presence or absence of rIFN-γ. The rate of reactivation was slightly delayed, the viral titers were slightly reduced, and reversion to undetectable virus titers occurred 2 days earlier in rIFN-γ-treated cultures. However, none of these differences were statistically significant at a P value of <0.05.
FIG. 2.
rIFN-γ alone cannot block HSV-1 reactivation in day 35 TG cultures. TG were excised 35 days after HSV-1 corneal infection. Single-cell suspensions were prepared, and replicate cultures (n = 8) were incubated in culture medium alone (diamonds) or medium containing rIFN-γ (1,000 U/ml) (circles). The supernatant fluids were sampled every other day and assayed for infectious virus titer in a plaque assay. The data are expressed as means ± standard errors of the mean in PFU per culture (open symbols) and percentage of cultures with detectable virus (percent reactivation) (solid symbols). Observed differences in the reactivation frequency and the viral titer between IFN-γ-treated and control cultures were not significant (P > 0.05).
Transient ACV treatment alone cannot block HSV-1 reactivation from latency in day 35 TG cultures.
The findings described above led us to propose that latent HSV-1 began to reactivate early after TG excision, and by the time of culture initiation, it had progressed too far into the viral life cycle to be controlled by IFN-γ. Therefore, we determined if 4 days of exposure to the antiherpetic drug ACV would reestablish latency in all neurons and enhance the effectiveness of CD8+ T cells in blocking HSV-1 reactivation from latency. TG were excised 35 days after HSV-1 corneal infection and pooled, and single-cell suspensions were prepared. Half of the TG cells were depleted of CD8+ T cells by immunomagnetic separation. The CD8+ T-cell-depleted and nondepleted TG cells were incubated for 4 days with ACV, the ACV was removed, and the cultures were incubated for an additional 10 days in culture medium. The supernatant fluids were sampled on alternate days and assayed for IFN-γ and for HSV-1 titers.
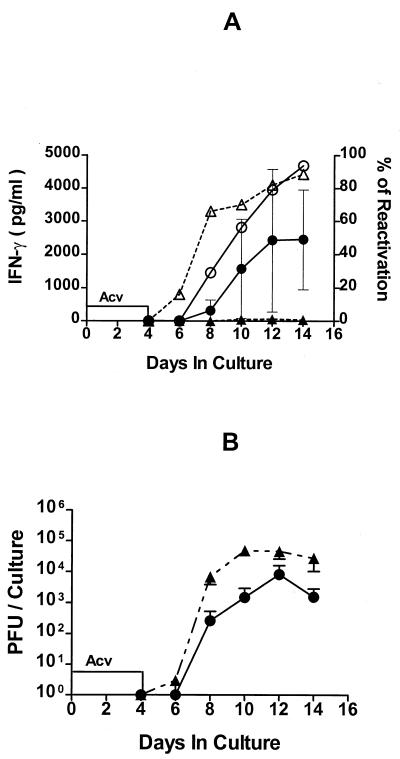
As shown in Fig. 3A, IFN-γ was produced in TG cultures containing CD8+ T cells. Interestingly, IFN-γ production was not detectable during ACV treatment, and following ACV removal, it was produced with similar kinetics to that seen in cultures that were not treated with ACV (compare Fig. 3A and Fig. 1). Depletion of CD8+ T cells from the TG cell suspension eliminated IFN-γ production. Thus, CD8+ T cells were the main source of IFN-γ in cultures of latently infected TG, and ACV delayed IFN-γ production. ACV at concentrations of up to 200 μg/ml did not affect IFN-γ production by the HSV-1-specific CD8+ T-cell clone (2D5) in response to stimulator cells that already expressed the appropriate viral epitopes (data not shown). Thus, a direct ACV block of IFN-γ production by CD8+ T cells cannot account for the delay in IFN-γ production observed in ACV-treated TG cultures.
FIG. 3.
Transient ACV treatment cannot block HSV-1 reactivation from latency in day 35 TG cultures. Day 35 TG single-cell suspensions were prepared and pooled; half of the TG cells were depleted of CD8+ T cells by immunomagnetic separation. CD8+ T-cell-depleted (triangles) and nondepleted (circles) TG cells were incubated in culture medium with ACV for 4 days. ACV was removed, and the cultures were incubated with medium alone for an additional 10 days. Culture supernatant fluids were sampled every other day and assayed for IFN-γ content by a standard ELISA and for infectious HSV-1 (indicating HSV-1 reactivation from latency) in a plaque assay. The data are expressed as means ± standard errors of the mean in picograms of IFN-γ per milliliter (panel A, closed symbols), percent reactivation from latency (panel A, open symbols), and PFU per culture (panel B). For CD8+ T-cell-depleted cultures, n = 23, and for nondepleted cultures, n = 26, except in IFN-γ determination, where n = 5. Depletion of CD8+ T cells significantly accelerated the reactivation rate (P < 0.05) and increased viral titer on day 10 (P < 0.05).
HSV-1 reactivation was accelerated in CD8+ T-cell-depleted cultures (P < 0.05), but reactivation was ultimately observed in 90% of both CD8+ T-cell-depleted and nondepleted cultures (Fig. 3A). The HSV-1 titers were somewhat higher (P < 0.05, day 10) in CD8+ T-cell-depleted cultures (Fig. 3B), and the virus destroyed these cultures within 10 days of culture (not shown). Cultures containing CD8+ T cells showed only one or two plaques (not shown) and were not destroyed. Again, we noted that HSV-1 reactivation from latency preceded peak levels of IFN-γ in most cultures (Fig. 3A).
IFN-γ can block HSV-1 reactivation if present shortly after ACV removal.
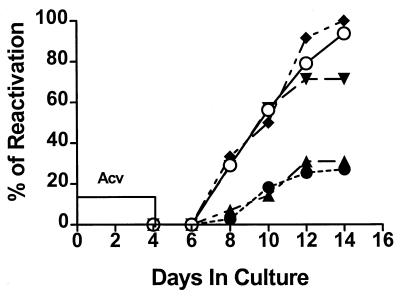
We considered that IFN-γ might effectively inhibit HSV-1 reactivation if HSV-1 latency was uniformly established through transient ACV treatment and if IFN-γ was present prior to initiation of the reactivation event. To test this possibility, day 35 TG cultures were incubated for 4 days with ACV. The cultures were then incubated in culture medium alone or culture medium that was supplemented with rIFN-γ at 0, 24, 48, or 72 h after removal of ACV. The culture supernatant fluids were sampled on alternate days, and HSV-1 titers were determined. Addition of rIFN-γ within 24 h after removal of ACV dramatically reduced HSV-1 reactivation from latency in TG cultures (P < 0.001) (Fig. 4). However, rIFN-γ had no effect on HSV-1 reactivation from latency when treatment was delayed until 48 or 72 h after removal of ACV.
FIG. 4.
IFN-γ can block HSV-1 reactivation from latency. Day 35 TG cell cultures were treated with ACV for 4 days, rinsed, and then incubated for an additional 10 days in medium alone (○, n = 26) or culture medium that was supplemented with rIFN-γ at 0 h (●, n = 25), 24 h (▴, n = 10), 48 h (▾, n = 10), or 72 h (♦, n = 10) after removal of ACV. Culture supernatant fluids were sampled every other day and assayed for infectious HSV-1 (indicating HSV-1 reactivation from latency) in a plaque assay. Reactivation frequency was significantly reduced (P < 0.001) (relative to medium-only controls) by IFN-γ treatment at 0 and 24 h only.
Does IFN-γ directly inhibit HSV-1 reactivation or indirectly augment a protective response of inflammatory cells in the ganglion?
The data in Fig. 4 clearly establish that IFN-γ could block HSV-1 reactivation from latency when present early in the reactivation process. However, these cultures contained CD8+ T cells and other inflammatory cells that were present in the TG at the time of excision. Thus, it was not clear if the IFN-γ directly blocked HSV-1 reactivation from latency in neurons or acted indirectly by enhancing a protective response of CD8+ T cells or other inflammatory cells. To address this issue, day 35 TG cell suspensions were depleted of CD8+ T cells and incubated with ACV for 4 days. After removal of ACV, medium alone or medium supplemented with rIFN-γ was added, and HSV-1 titers in culture supernatants were measured on alternate days. HSV-1 reactivation was delayed and significantly reduced in cultures that were treated with rIFN-γ (Fig. 5A) (P < 0.001). It is noteworthy that rIFN-γ did appear to be somewhat less effective at blocking HSV-1 reactivation in the absence of endogenous CD8+ T cells (60% versus 20%, compare Fig. 4 and 5A). Moreover, rIFN-γ-treated CD8+ T-cell-depleted TG cultures that reactivated were ultimately destroyed by the virus. As noted above, the virus did not destroy TG cultures that contained CD8+ T cells. These data demonstrate that IFN-γ can block HSV-1 reactivation from latency by a mechanism that is at least partially CD8+ T cell independent but is ineffective at blocking the spread of HSV-1 following a reactivation event.
FIG. 5.
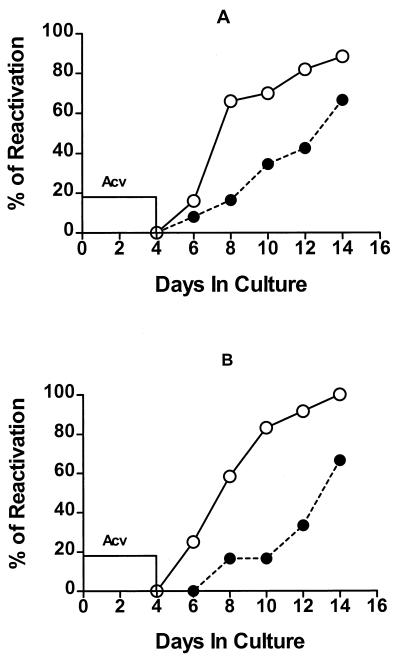
IFN-γ can directly and indirectly inhibit HSV-1 reactivation from latency in day 35 TG cultures. TG were excised 35 days after HSV-1 corneal infection and single cell suspensions were pooled, depleted of either CD8+ T cells (n = 23) (A) or CD45+ cells (n = 12) (B), and incubated with culture medium containing ACV for 4 days. After removal of ACV, cultures were incubated with medium alone (○) or medium supplemented with rIFN-γ (●) for an additional 10 days. Culture supernatant fluids were sampled every other day and assayed for infectious HSV-1 (indicating HSV-1 reactivation from latency) in a plaque assay. IFN-γ significantly reduced the reactivation frequency (P < 0.001) in both CD8+ T-cell-depleted and CD45+ cell-depleted TG cultures.
To further support the concept that IFN-γ can directly block HSV-1 reactivation from latency in neurons, day 35 TG cells were prepared and depleted of CD45+ (bone marrow-derived) cells. Cultures were prepared and treated with ACV for 4 days followed by addition of medium alone or medium supplemented with rIFN-γ. As shown in Fig. 5B, rIFN-γ significantly delayed and reduced HSV-1 reactivation from latency in TG cultures lacking any detectable CD45+ cells (P < 0.001). The effect of depleting CD45+ cells was similar to that of depleting CD8+ cells (compare Fig. 5A and B). Taken together, these data suggest that IFN-γ can act directly on latently infected neurons to inhibit HSV-1 reactivation from latency, but it can also augment a protective response that appears to be mediated primarily by CD8+ T cells.
DISCUSSION
The nervous system is a preferred site of viral persistence. The susceptibility of neurons to persistent viral infections has been attributed to the fact that neurons are poor targets for T-cell surveillance due to low expression of MHC molecules. Conversely, there is growing evidence that T cells might play an important role in maintaining certain viruses in a persistent or latent state in the central or peripheral nervous system (4, 10, 11, 15–17). A recent study involving persistent mouse hepatitis virus infection of the central nervous system demonstrated that antigen-specific CD8+ T cells were retained for prolonged periods in the central nervous system (1). The CD8+ T cells that were retained in the brain after clearance of infectious virus were functionally distinct from those present during the acute phase of infection, in that the former exhibited dramatically reduced lytic function, maintained IFN-γ production, and demonstrated an epitope shift. The CD8+ T cells that were present in the persistently infected tissue expressed the CD69 activation marker in the absence of detectable replicating virus. These apparently T-cell receptor-dependent changes occurred in the absence of detectable viral proteins. This shift in CD8+ T-cell function might represent an adaptation designed to accommodate the changing needs of the tissue as the infection progresses from the acute to the latent or persistent stage. During the acute stage of infection both lytic and nonlytic mechanisms might be required to eliminate replicating virus in the infected tissue. In contrast, nonlytic, cytokine-mediated mechanisms might be sufficient to maintain the virus in a latent or persistent state, while avoiding unnecessary tissue destruction. It is not clear what drives the retention and activation of CD8+ T cells in nervous tissue that is persistently infected with viruses. The inversion of the dominant epitope in the mouse hepatitis virus model suggests an antigen-driven process.
CD8+ T cells and other inflammatory cells are retained in the peripheral nervous system of mice for prolonged periods after HSV-1 infection, and they rapidly surround neurons following in vivo induced reactivation of HSV-1 from latency (4, 15, 16, 20, 21). Studies have demonstrated that CD8+ T cells that were present in the TG 14 days after HSV-1 corneal infection could completely block HSV-1 reactivation from latency for at least 2 weeks in explanted TG cultures (15). When anti-CD8α MAb was added to cultures 5 days after initiation, HSV-1 was promptly reactivated in multiple neurons. Although destruction of some neurons in these ex vivo cultures could not be ruled out, our findings clearly established that CD8+ T cells could prevent HSV-1 reactivation in a portion of latently infected neurons by a nonlytic mechanism.
The endogenous CD8+ T cells present in TG obtained 34 days after HSV-1 corneal infection delayed, but could not prevent, HSV-1 reactivation from latency in ex vivo cultures. HSV-1 spread and rapidly destroyed day 34 TG cultures that were treated with anti-CD8α MAb (15). In the present study, we observed a similar effect of depleting CD8+ T cells from day 35 TG cultures. The addition of rIFN-γ inhibited the spread of HSV-1 following reactivation from latency in TG cultures that contained CD8+ T cells, but it was ineffective at blocking the spread of HSV-1 in CD8+ T-cell-depleted TG. These findings establish that the CD8+ T cells that are retained in the latently infected ganglion can inhibit HSV-1 spread within the ganglion through a process that is augmented by, but not directly mediated by, IFN-γ. The fact that IFN-γ is produced in TG for at least 120 days after HSV-1 corneal infection (4, 9, 10, 16) suggests that this function of CD8+ T cells might have in vivo relevance.
An important question is whether or not IFN-γ can also block HSV-1 reactivation from latency. Our present findings demonstrate that rIFN-γ cannot block HSV-1 reactivation from latency when added at the initiation of day 35 TG cultures. Clearly, the TG cultures differ in many ways from the in vivo TG. For instance, the neurons are undoubtedly stressed during excision, which might compromise their intrinsic ability to inhibit HSV-1 gene expression. In addition, the CD8+ T cells are separated from the latently infected neurons until contact can be reestablished in culture. Moreover, the cytokines that are produced by the CD8+ T cells may be diluted and washed away more rapidly in ex vivo cultures than they are in the TG in situ.
We believe the inability of endogenous CD8+ T cells in day 35 TG or rIFN-γ to block HSV-1 reactivation might reflect some of these changes that occur in the TG upon excision. We proposed that a brief treatment of TG cultures with ACV might alleviate these problems by pushing the reactivating HSV-1 genomes back into the latent state that existed prior to TG excision and providing the CD8+ T cells time to reestablish contact with infected neurons and to be activated. It appears that this approach was only partially successful. The activation of CD8+ T cells to produce IFN-γ apparently did not occur during the course of ACV treatment. In cultures that were not treated with ACV, IFN-γ production was first detectable after 4 days in culture and was readily detectable after 6 days. In ACV-treated cultures, IFN-γ was first detectable after 8 days in culture (i.e., 4 days after removal of ACV) and was readily detectable after 10 days. Thus, the kinetics of IFN-γ production appeared to be the same in ACV-treated and nontreated cultures, but it was not initiated until after ACV removal. ACV does not directly block IFN-γ production since HSV-1-reactive CD8+ T-cell clones produced comparable amounts of IFN-γ in the presence or absence of ACV when stimulated with cells that were infected in the absence of ACV.
A possible explanation is that the antigenic epitopes that stimulate IFN-γ production by the CD8+ T cells that are retained in the latently infected ganglion are not produced in the presence of ACV. This could be explained by an ACV block in production of the HSV-1 protein that is recognized by the CD8+ T cells. When phosphorylated by HSV-1 thymidine kinase, ACV is incorporated into viral DNA and terminates chain elongation. Blocking viral DNA synthesis prevents expression of HSV-1 γ2 genes and reduces expression of γ1 genes, but it tends to enhance expression of HSV-1 α and β genes. In vivo ACV treatment of mice with latent HSV-1 infections resulted in a gradual reduction in IFN-γ production in the TG and a reduction of serum antibodies to HSV-1 gB (a γ1 gene product) (9). Moreover, in C57BL/6 mice gB contains an immunodominant epitope recognized by CD8+ T cells. Thus, HSV-1 gB might be a good candidate for a protein that is recognized by CD8+ T cells that are retained in the latently infected TG. ACV might also directly or indirectly block MHC class I up-regulation on neurons with reactivating HSV-1 genomes. Neurons in sensory ganglia have been shown to express MHC class I molecules during the acute stage of HSV-1 ganglionic infection, suggesting concordant expression of HSV-1 and MHC class I genes. It is reasonable to propose that MHC class I gene expression is up-regulated on neurons during HSV-1 reactivation from latency, and this process might be blocked by ACV. An alternative explanation could be that ACV blocks production of chemokines that are responsible for drawing the CD8+ T cells to neurons with reactivating HSV-1 genomes. These possibilities are being investigated.
ACV treatment did render latently infected neurons susceptible to IFN-γ inhibition of HSV-1 reactivation. Inhibition of HSV-1 reactivation was only observed when latently infected neurons were exposed to IFN-γ within 24 h after ACV removal from cultures. This observation suggests that IFN-γ blocks an early step in HSV-1 reactivation from latency. The rIFN-γ was less effective at blocking HSV-1 reactivation when endogenous CD8+ T cells were depleted from the day 35 TG cells. However, the effectiveness of IFN-γ was not further compromised by depletion of all bone marrow-derived cells from the TG cultures. Thus, IFN-γ appears to inhibit HSV-1 reactivation in part through augmentation of a CD8+ T-cell response. It was not determined if rIFN-γ accelerated or augmented CD8+ T-cell production of IFN-γ or if the proximal inhibitor of reactivation was an unrelated molecule.
However, IFN-γ delayed and reduced HSV-1 reactivation in day 35 TG cultures that were depleted of all detectable CD45+ cells. The latter observation suggested that IFN-γ can also directly inhibit HSV-1 reactivation in neurons. To our knowledge, this is the first direct demonstration that IFN-γ can block HSV-1 reactivation from latency in neurons. In vivo studies comparing wild-type and IFN-γ knockout (GKO) mice on a BALB/c background demonstrated more rapid reactivation of HSV-1 from latency following induction by hyperthermic stress (3) or UV-B corneal irradiation (14) in GKO mice. In the former study the overall incidence of reactivation was also increased in IFN-γ-deficient mice, whereas this difference was not observed in the latter study. Thus, our findings with the ex vivo model of HSV-1 reactivation from latency in sensory neurons are in good general agreement with in vivo studies using mice with targeted disruption of the IFN-γ gene. Moreover, our model avoids the complication of differences in control of the acute infection, establishment of latency, and possible compensatory mechanisms in GKO mice, and should facilitate studies of latency at the molecular level.
Our understanding of the mechanisms by which IFN-γ inhibits HSV-1 replication is probably incomplete, and the mechanisms may vary in different cell types. During lytic infections, IFN-γ has been shown to inhibit expression of ICP4, a potent transactivator of HSV gene expression, destabilize HSV mRNA, and stabilize HSV-1 association with nuclear protein aggregates called ND10 bodies, which inhibit HSV-1 gene expression (12, 23). Any of these mechanisms could contribute to the effect of IFN-γ on HSV-1 reactivation in neurons, although ND10 bodies have been difficult to detect in neurons (5).
It is noteworthy that endogenous CD8+ T cells in TG obtained 35 days after HSV-1 corneal infection were relatively ineffective at blocking HSV-1 reactivation in ex vivo cultures, whereas CD8+ T cells present in latently infected TG 14 days after infection completely blocked reactivation (15). Moreover, HSV-1 reactivation was completely blocked in day 34 TG cultures when supplemented with exogenous CD8+ T cells obtained from draining lymph nodes 7 days after HSV-1 corneal infection. It is not clear if the exogenous cells merely provided a critical density of HSV-reactive CD8+ T cells in the culture, or if the CD8+ T cells that are generated during acute infection possess a different functional program than those retained in the ganglion during latency. The CD8+ T cells that are generated during acute infection might be capable of blocking HSV-1 replication at a later stage in the viral life cycle. We are currently investigating the phenotypic and functional characteristics of the CD8+ T cells that are retained in the latently infected ganglion. Our preliminary findings reveal that these cells all express an αβ T-cell receptor (data not shown).
At the moment there is little prospect for eradicating the HSV-1 genome from latently infected neurons. However, approaches to inhibit reactivation of the latent virus and prevent recurrent disease appear more promising. A better understanding of the regulation of HSV-1 gene expression in neurons could lead to new approaches to preventing the loss of the intrinsic ability of neurons to inhibit HSV gene expression following stimuli such as stress, UV-B exposure, and hormonal changes. The prophylactic use of antiviral drugs such as ACV may reduce the frequency of viral shedding and recurrent disease, although this might involve a lifelong commitment to treatment. The recent evidence that CD8+ T cells can block HSV-1 reactivation from latency in part through the production of IFN-γ suggests that new approaches to vaccination might provide an additional avenue of intervention.
ACKNOWLEDGMENTS
Support for this work was provided by NIH grants EY05945 (R.L.H.) and 5 P30 EY08098 (R.L.H.), an unrestricted grant from Research to Prevent Blindness, New York, N.Y., and the Eye and Ear Foundation of Pittsburgh.
We thank JoAnne Flynn for critically reading the manuscript.
REFERENCES
- 1.Bergmann C C, Altman J D, Hinton D, Stohlman S A. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- 2.Bonneau R H, Salvucci L A, Johnson D C, Tevethia S S. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 3.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantin E M, Hinton D R, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho Y, Lee I, Maul G G, Yu E. A novel nuclear substructure, ND10: distribution in normal and neoplastic human tissues. Int J Mol Med. 1998;1:717–724. doi: 10.3892/ijmm.1.4.717. [DOI] [PubMed] [Google Scholar]
- 6.Croen K D, Ostrove J M, Dragovic L J, Smialek J E, Straus S E. Detection of an immediate early gene “anti-sense” transcript by in situ hybridization. N Engl J Med. 1987;317:1427–1432. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- 7.Deatly A M, Spivack J G, Lavi E, Fraser N W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci USA. 1987;84:3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demotz S, Grey H M, Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990;249:1028–1030. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- 9.Halford W P, Gebhardt B M, Carr D J. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- 10.Halford W P, Gebhardt B M, Carr D J J. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 11.Hawke S, Stevenson P G, Freeman S, Bangham C R. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr I M, Brown R E. pppA2′p5′A2′p5′A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci USA. 1978;75:256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer M F, Coen D M. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lekstrom-Himes J A, LeBlanc R A, Pesnicak L, Godleski M, Straus S E. Gamma interferon impedes the establishment of herpes simplex virus type 1 latent infection but has no impact on its maintenance or reactivation in mice. J Virol. 2000;74:6680–6683. doi: 10.1128/jvi.74.14.6680-6683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Khanna K M, Chen X, Fink D J, Hendricks R L. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T, Tang Q, Hendricks R L. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noisakran S, Carr D J J. Lymphocytes delay kinetics of HSV-1 reactivation from in vitro explants of latent infected trigeminal ganglia. J Neuroimmunol. 1999;95:126–135. doi: 10.1016/s0165-5728(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 18.Pereira R A, Tscharke D C, Simmons A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells, and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. J Exp Med. 1994;180:841–850. doi: 10.1084/jem.180.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pertoft H. Purification of herpes simplex virus using Percoll. Sep News. 1980;3:2. [Google Scholar]
- 20.Shimeld C, Whiteland J L, Nicholls S M, Grinfeld E, Easty D L, Gao H, Hill T J. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- 21.Shimeld C, Whiteland J L, Williams N A, Easty D, Hill T J. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and immune cell infiltration. J Gen Virol. 1996;77:2583–2590. doi: 10.1099/0022-1317-77-10-2583. [DOI] [PubMed] [Google Scholar]
- 22.Spear P G, Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpes virion. J Virol. 1972;9:143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor J L, Unverrich D, O'Brien W J, Wilcox K W. Interferon coordinately inhibits the disruption of PML-positive ND10 and immediate-early gene expression by herpes simplex virus. J Interferon Cytokine Res. 2000;20:805–815. doi: 10.1089/10799900050151076. [DOI] [PubMed] [Google Scholar]
- 24.Tullo A B, Shimeld C, Blyth W A, Hill T J, Easty D L. Spread of virus and distribution of latent infection following ocular herpes simplex in the non-immune and immune mouse. J Gen Virol. 1982;63:95–101. doi: 10.1099/0022-1317-63-1-95. [DOI] [PubMed] [Google Scholar]
- 25.Willey D E, Trousdale M D, Nesburn A B. Reactivation of murine latent HSV infection by epinephrine iontophoresis. Investig Ophthalmol Vis Sci. 1984;25:945–950. [PubMed] [Google Scholar]