Abstract
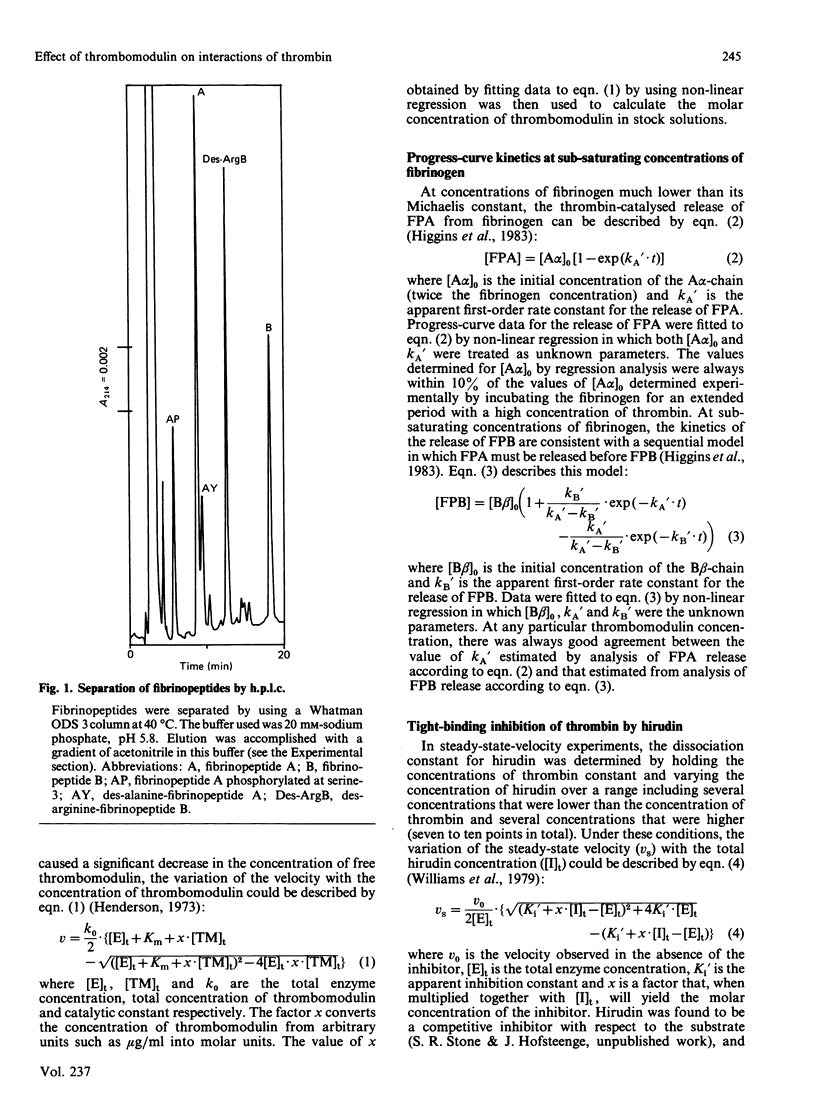
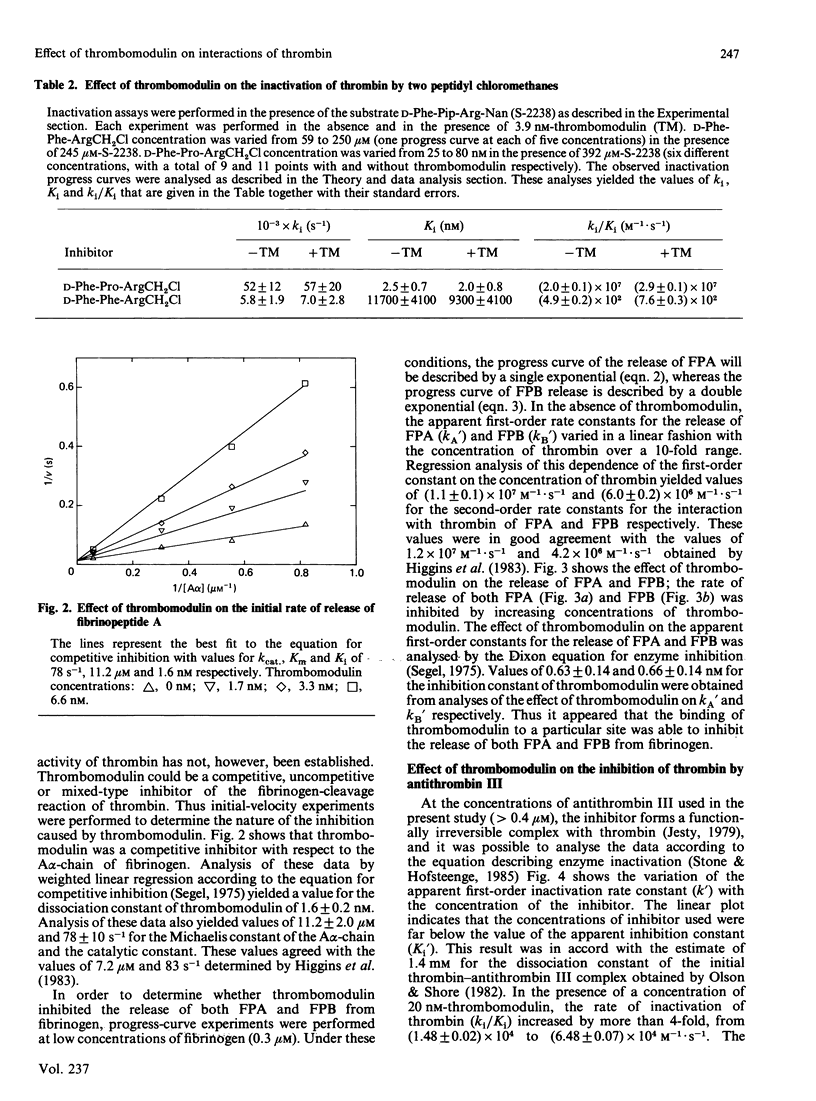
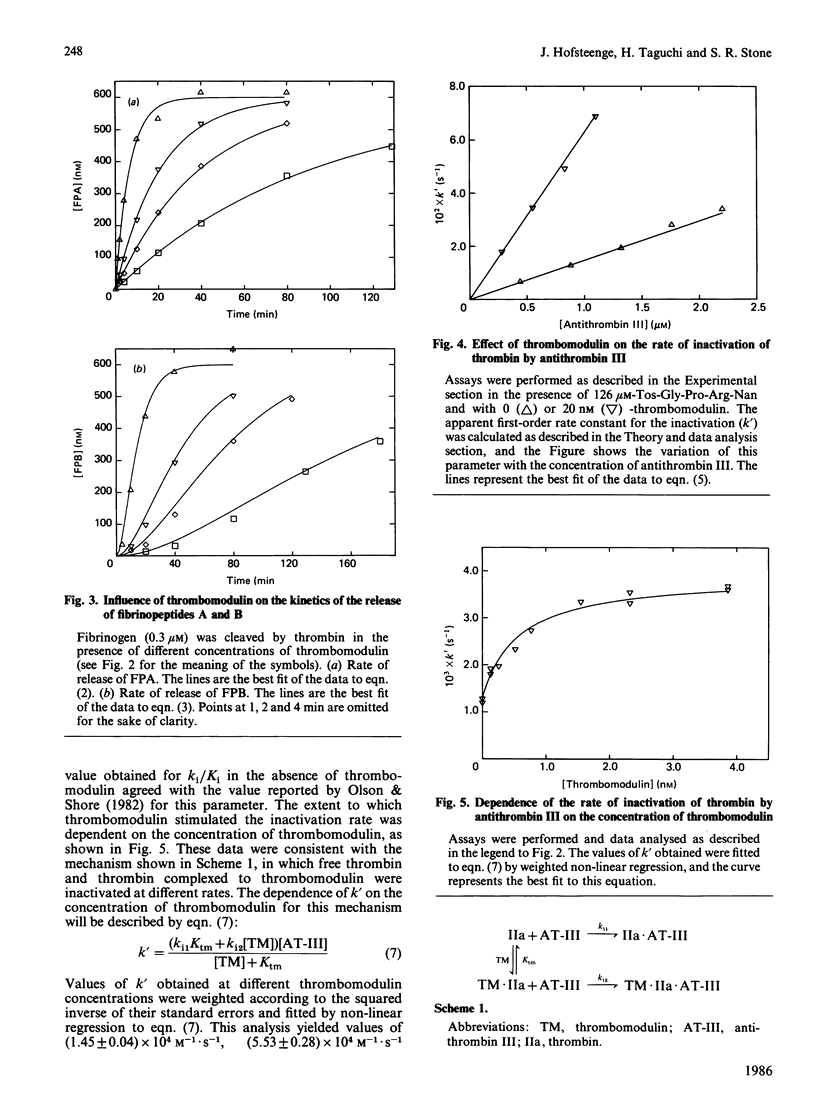
Thrombomodulin decreased by 20-30% the Michaelis constant of two tripeptidyl p-nitroanilide substrates of thrombin. Thrombomodulin increased the rate of inactivation of thrombin by two peptidyl chloromethane inhibitors by a similar amount. This effect appeared to be due to a decrease in the dissociation constants of the inhibitors. An improved method for the separation of fibrinopeptides A and B by h.p.l.c. was developed, and this method was used to study the effect of thrombomodulin on the thrombin-catalysed cleavage of fibrinogen. In this reaction, thrombomodulin was a competitive inhibitor with respect to the A alpha-chain of fibrinogen. The release of fibrinopeptide B was also inhibited by thrombomodulin. Analysis of the inhibition caused by thrombomodulin with respect to fibrinopeptides A and B yielded the same dissociation constant for the thrombin-thrombomodulin complex. In the presence of thrombomodulin, the rate of inactivation of thrombin by antithrombin III was stimulated 4-fold. This stimulation showed saturation kinetics with respect to thrombomodulin. Thrombomodulin was found to compete with hirudin for a binding site on thrombin. As a result of this competition, hirudin became a slow-binding inhibitor of thrombin at high thrombomodulin concentrations. Estimates of the dissociation constant for thrombomodulin were obtained in several of the above experiments, and the weighted mean value was 0.7 nM.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOMBACK B., BLOMBACK M., SEARLE J. On the occurrence of phosphorus in fibrinogen. Biochim Biophys Acta. 1963 Jul 2;74:148–151. doi: 10.1016/0006-3002(63)91345-3. [DOI] [PubMed] [Google Scholar]
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Björk I., Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982 Oct 29;48(3):161–182. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Iwanaga S., Reuterby J., Blombäck M. Primary structure of human fibrinogen and fibrin. I. Clevage of fibrinogen with cyanogen bromide. Isolation and characterization of NH 2 -terminal fragments of the ("A") chain. J Biol Chem. 1972 Mar 10;247(5):1496–1512. [PubMed] [Google Scholar]
- Boissel J. P., Le Bonniec B., Rabiet M. J., Labie D., Elion J. Covalent structures of beta and gamma autolytic derivatives of human alpha-thrombin. J Biol Chem. 1984 May 10;259(9):5691–5697. [PubMed] [Google Scholar]
- Cha S. Tight-binding inhibitors--III. A new approach for the determination of competition between tight-binding inhibitors and substrates--inhibition of adenosine deaminase by coformycin. Biochem Pharmacol. 1976 Dec 15;25(24):2695–2702. doi: 10.1016/0006-2952(76)90259-8. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A., Endrenyi L. Fitting of enzyme kinetic data without prior knowledge of weights. Biochem J. 1981 Mar 1;193(3):1005–1008. doi: 10.1042/bj1931005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson A., Björk I. Properties of antithrombin-thrombin complex formed in the presence and in the absence of heparin. Biochem J. 1983 Aug 1;213(2):345–353. doi: 10.1042/bj2130345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellenback R. J., Chien S. The extinction coefficient of fibrinogen from man, dog, elephant, sheep, and goat at 280 mmu. Proc Soc Exp Biol Med. 1970 May;134(1):353–355. doi: 10.3181/00379727-134-34792. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Esmon N. L., Harris K. W. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982 Jul 25;257(14):7944–7947. [PubMed] [Google Scholar]
- Esmon C. T. Protein-C: biochemistry, physiology, and clinical implications. Blood. 1983 Dec;62(6):1155–1158. [PubMed] [Google Scholar]
- Esmon N. L., Carroll R. C., Esmon C. T. Thrombomodulin blocks the ability of thrombin to activate platelets. J Biol Chem. 1983 Oct 25;258(20):12238–12242. [PubMed] [Google Scholar]
- Esmon N. L., Owen W. G., Esmon C. T. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein C. J Biol Chem. 1982 Jan 25;257(2):859–864. [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin specificity. Ann N Y Acad Sci. 1981;370:468–495. doi: 10.1111/j.1749-6632.1981.tb29757.x. [DOI] [PubMed] [Google Scholar]
- Henderson P. J. Steady-state enzyme kinetics with high-affinity substrates or inhibitors. A statistical treatment of dose-response curves. Biochem J. 1973 Sep;135(1):101–107. doi: 10.1042/bj1350101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. L., Lewis S. D., Shafer J. A. Steady state kinetic parameters for the thrombin-catalyzed conversion of human fibrinogen to fibrin. J Biol Chem. 1983 Aug 10;258(15):9276–9282. [PubMed] [Google Scholar]
- Higgins D. L., Shafer J. A. Fibrinogen Petoskey, a dysfibrinogenemia characterized by replacement of Arg-A alpha 16 by a histidyl residue. Evidence for thrombin-catalyzed hydrolysis at a histidyl residue. J Biol Chem. 1981 Dec 10;256(23):12013–12017. [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Jameson G. W., Roberts D. V., Adams R. W., Kyle W. S., Elmore D. T. Determination of the operational molarity of solutions of bovine alpha-chymotrypsin, trypsin, thrombin and factor Xa by spectrofluorimetric titration. Biochem J. 1973 Jan;131(1):107–117. doi: 10.1042/bj1310107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesty J. The kinetics of formation and dissociation of the bovine thrombin.antithrombin III complex. J Biol Chem. 1979 Oct 25;254(20):10044–10050. [PubMed] [Google Scholar]
- Kehl M., Lottspeich F., Henschen A. Analysis of human fibrinopeptides by high-performance liquid chromatography. Hoppe Seylers Z Physiol Chem. 1981 Dec;362(12):1661–1664. [PubMed] [Google Scholar]
- Lottenberg R., Christensen U., Jackson C. M., Coleman P. L. Assay of coagulation proteases using peptide chromogenic and fluorogenic substrates. Methods Enzymol. 1981;80(Pt 100):341–361. doi: 10.1016/s0076-6879(81)80030-4. [DOI] [PubMed] [Google Scholar]
- Lottenberg R., Jackson C. M. Solution composition dependent variation in extinction coefficients for p-nitroaniline. Biochim Biophys Acta. 1983 Feb 15;742(3):558–564. doi: 10.1016/0167-4838(83)90273-x. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L. A rapid method for the purification of bovine thrombin and the inhibition of the purified enzyme wtih phenylmethylsulfonyl fluoride. Biochemistry. 1971 Jun 22;10(13):2501–2506. doi: 10.1021/bi00789a012. [DOI] [PubMed] [Google Scholar]
- Maruyama I., Salem H. H., Ishii H., Majerus P. W. Human thrombomodulin is not an efficient inhibitor of the procoagulant activity of thrombin. J Clin Invest. 1985 Mar;75(3):987–991. doi: 10.1172/JCI111800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr, Majerus P. W. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980 Jul 1;105(2):304–310. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Nyström C., Björk I. The size and shape of human and bovine antithrombin III. Eur J Biochem. 1977 Aug 15;78(1):195–203. doi: 10.1111/j.1432-1033.1977.tb11730.x. [DOI] [PubMed] [Google Scholar]
- Olson S. T., Shore J. D. Demonstration of a two-step reaction mechanism for inhibition of alpha-thrombin by antithrombin III and identification of the step affected by heparin. J Biol Chem. 1982 Dec 25;257(24):14891–14895. [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Salem H. H., Maruyama I., Ishii H., Majerus P. W. Isolation and characterization of thrombomodulin from human placenta. J Biol Chem. 1984 Oct 10;259(19):12246–12251. [PubMed] [Google Scholar]
- Selwyn M. J. A simple test for inactivation of an enzyme during assay. Biochim Biophys Acta. 1965 Jul 29;105(1):193–195. doi: 10.1016/s0926-6593(65)80190-4. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J. Specificity of activated human protein C. Biochem J. 1985 Sep 1;230(2):497–502. doi: 10.1042/bj2300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Stenflo J., Dahlbäck B., Teodorsson B. Inactivation of human coagulation factor V by activated protein C. J Biol Chem. 1983 Feb 10;258(3):1914–1920. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F., Duggleby R. G. Methotrexate, a high-affinity pseudosubstrate of dihydrofolate reductase. Biochemistry. 1979 Jun 12;18(12):2567–2573. doi: 10.1021/bi00579a021. [DOI] [PubMed] [Google Scholar]


