Abstract
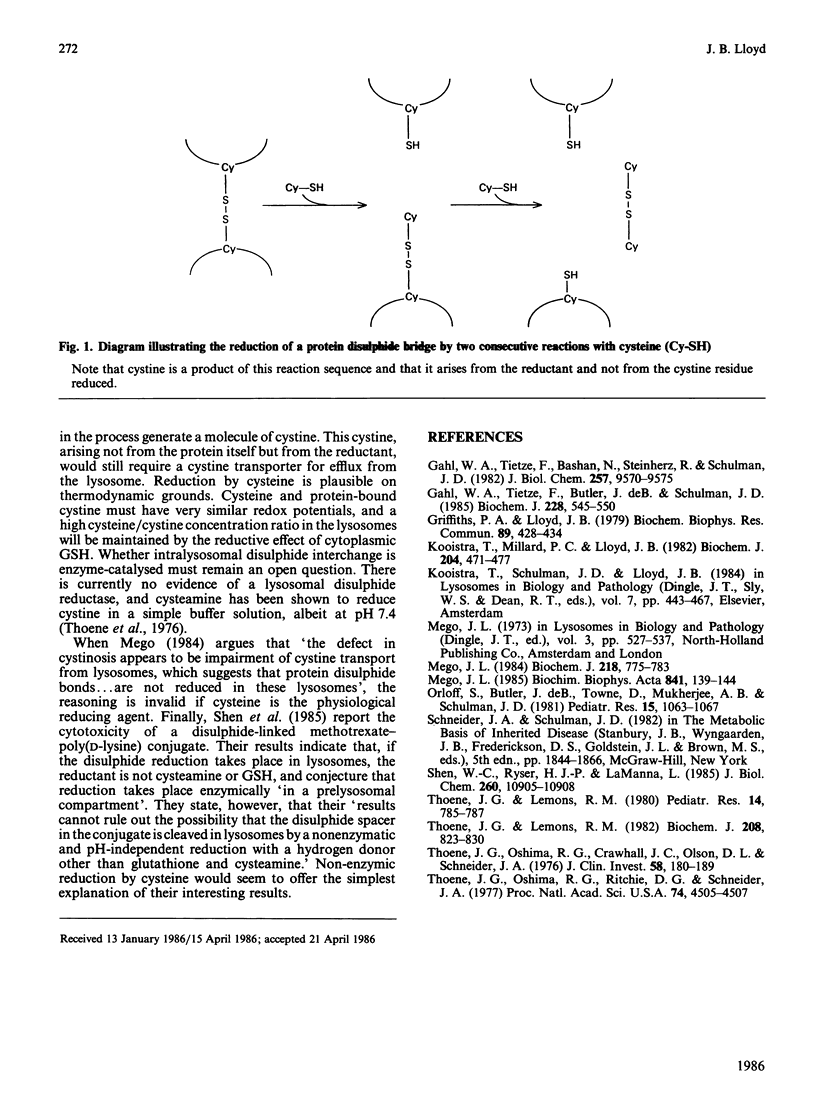
Published evidence indicates that cystine-containing proteins have their disulphide bonds reduced during proteolysis in lysosomes. However, the intralysosomal accumulation of cystine in the cells of patients with cystinosis has been seen as evidence that protein cystine residues are not reduced. The data are reconcilable and fully in harmony if it is postulated that cysteine from the cytoplasm is the physiological reducing agent.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gahl W. A., Tietze F., Bashan N., Steinherz R., Schulman J. D. Defective cystine exodus from isolated lysosome-rich fractions of cystinotic leucocytes. J Biol Chem. 1982 Aug 25;257(16):9570–9575. [PubMed] [Google Scholar]
- Gahl W. A., Tietze F., Butler J. D., Schulman J. D. Cysteamine depletes cystinotic leucocyte granular fractions of cystine by the mechanism of disulphide interchange. Biochem J. 1985 Jun 15;228(3):545–550. doi: 10.1042/bj2280545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths P. A., Lloyd J. B. Evidence for lysosomal reduction of cystine residues. Biochem Biophys Res Commun. 1979 Jul 27;89(2):428–434. doi: 10.1016/0006-291x(79)90647-8. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Millard P. C., Lloyd J. B. Role of thiols in degradation of proteins by cathepsins. Biochem J. 1982 May 15;204(2):471–477. doi: 10.1042/bj2040471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mego J. L. Role of thiols, pH and cathepsin D in the lysosomal catabolism of serum albumin. Biochem J. 1984 Mar 15;218(3):775–783. doi: 10.1042/bj2180775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mego J. L. Stimulation of intralysosomal proteolysis by cysteinyl-glycine, a product of the action of gamma-glutamyl transpeptidase on glutathione. Biochim Biophys Acta. 1985 Aug 16;841(2):139–144. doi: 10.1016/0304-4165(85)90014-5. [DOI] [PubMed] [Google Scholar]
- Orloff S., Butler J. D., Towne D., Mukherjee A. B., Schulman J. D. Pantetheinase activity and cysteamine content in cystinotic and normal fibroblasts and leukocytes. Pediatr Res. 1981 Jul;15(7):1063–1067. doi: 10.1203/00006450-198107000-00018. [DOI] [PubMed] [Google Scholar]
- Shen W. C., Ryser H. J., LaManna L. Disulfide spacer between methotrexate and poly(D-lysine). A probe for exploring the reductive process in endocytosis. J Biol Chem. 1985 Sep 15;260(20):10905–10908. [PubMed] [Google Scholar]
- Thoene J. G., Lemons R. M. Cystine accumulation in cystinotic fibroblasts from free and protein-linked cystine but not cysteine. Biochem J. 1982 Dec 15;208(3):823–830. doi: 10.1042/bj2080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoene J. G., Lemons R. Modulation of the intracellular cystine content of cystinotic fibroblasts by extracellular albumin. Pediatr Res. 1980 Jun;14(6):785–787. doi: 10.1203/00006450-198006000-00001. [DOI] [PubMed] [Google Scholar]
- Thoene J. G., Oshima R. G., Crawhall J. C., Olson D. L., Schneider J. A. Cystinosis. Intracellular cystine depletion by aminothiols in vitro and in vivo. J Clin Invest. 1976 Jul;58(1):180–189. doi: 10.1172/JCI108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoene J. G., Oshima R. G., Ritchie D. G., Schneider J. A. Cystinotic fibroblasts accumulate cystine from intracellular protein degradation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4505–4507. doi: 10.1073/pnas.74.10.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]


