Abstract
Tissue damage due to cancer, congenital anomalies, and injuries needs new efficient treatments that allow tissue regeneration. In this context, tissue engineering shows a great potential to restore the native architecture and function of damaged tissues, by combining cells with specific scaffolds. Scaffolds made of natural and/or synthetic polymers and sometimes ceramics play a key role in guiding cell growth and formation of the new tissues. Monolayered scaffolds, which consist of uniform material structure, are reported as not being sufficient to mimic complex biological environment of the tissues. Osteochondral, cutaneous, vascular, and many other tissues all have multilayered structures, therefore multilayered scaffolds seem more advantageous to regenerate these tissues. In this review, recent advances in bilayered scaffolds design applied to regeneration of vascular, bone, cartilage, skin, periodontal, urinary bladder, and tracheal tissues are focused on. After a short introduction on tissue anatomy, composition and fabrication techniques of bilayered scaffolds are explained. Then, experimental results obtained in vitro and in vivo are described, and their limitations are given. Finally, difficulties in scaling up production of bilayer scaffolds and reaching the stage of clinical studies are discussed when multiple scaffold components are used.
Keywords: bilayered scaffolds, biomaterials, biomimetism, material design, medical applications, tissue engineering
Scaffolds are commonly used in tissue engineering as supporting structures for cell growths. They must be appropriately designed, in terms of properties and functionality, to mimic the architecture of the native tissue of interest. Moreover, osteochondral, cutaneous, vascular, and many other native tissues are based on multilayered structures, therefore their regeneration in an appropriate manner needs multilayered scaffolds.
1. Introduction
Tissues and organs of the human body can be damaged because of such pathologies as cancer, congenital anomalies, traumas, and injuries.[ 1 ] Current clinical approaches for repairing damaged tissues refer to autografts and organ transplantation (primarily allografts), but each has limitations. Autografts, which are grafts transferred from the same individual, include additional surgical procedures at the site of tissue harvest. They increase the complexity of the surgical procedure, as well as functional and aesthetic consequences for the patient, such as donor site morbidity and post‐operative pain, not to mention the limited availability of tissue. An alternative to autografts is allografts, or cadaveric tissues. Allografts are the best alternative but show limitations because of the limited availability of donors, the risk of disease transmission, and the risk of immune rejection. To prevent the latter, long‐term immunosuppressive treatment is usually required, but the long‐term risks of such treatment are elevated (cancers, infections, cardiovascular diseases…).[ 2 ] Therefore, full regeneration of a tissue with all its functionalities remains challenging. Nowadays, tissue engineering (TE) is an actively developing field that aims to overcome the limits of conventional treatments.[ 3 ] TE refers to the combination of cells and scaffolds to build a structure that would be able to restore and maintain the native architecture and function of damaged tissues and/or organs.[ 1 ]
Scaffolds are supporting structures for the cells and can be made of natural and/or synthetic polymers, as well as ceramics in certain cases. They must be appropriately designed to mimic the architecture of the native tissue of interest, facilitate integration in host tissue, and new tissue formation in vivo. For this to happen, biomimetic scaffolds must provide structural support for the cells to adhere, spread, migrate, proliferate, and produce an extracellular matrix (ECM).[ 4 ] Therefore, parameters such as porosity, pore size, and pore structure are of the outmost importance and should be taken into consideration during early phases of the design.[ 5 ] In addition, scaffolds should be biocompatible, i.e., nonthrombogenic, non‐immunogenic, and resistant to infections.[ 6 ] Moreover, scaffolds have to be able to maintain their shape and withstand mechanical constraints during surgical operations, when they are implanted into the body. More precisely, they should not break during the procedure, fit within the target tissue, and do not cause any mechanical damage to the body.[ 7 ]
During the development of new scaffolds for TE, some studies focus on monolayered scaffolds, which have uniform material composition and structure.[ 8 ] However, monolayered scaffolds were reported as not being able to mimic the biological environment, and not to be optimal for tissue repair.[ 9 ] Hence, inspired by the natural multilayered structure of natural tissues (such as osteochondral, cutaneous, osseous, nervous, vascular tissues, and urinary bladder), more complex multilayered scaffolds were proposed, and appeared to be more advantageous for TE.[ 10 ] Indeed, 3D scaffolds, typically porous with interconnected pore networks, have gained much attention. They better mimic in vivo tissue organization, compared to conventional monolayered scaffolds. They can provide an appropriate environment for the cells by ensuring mechanical support, as well as physical and biochemical stimuli for optimal cell growth and functions.[ 11 ] They should be chemically and structurally similar to the targeted tissue to achieve the optimal regeneration.[ 12 ] For that, a wide range of materials is used, classified into three principal groups: synthetic polymers, natural polymers, and (bio)ceramics.[ 11 ] From these biomaterials, different scaffolding design strategies are reported in the literature.[ 13 ]
In this review, we focus on biomimetic bilayered scaffolds used in a wide range of TE applications. The use of bilayered scaffolds allows the creation of a tissue‐specific environment with two or more different regions resembling the stratified anatomical architecture. The fabrication of this type of scaffold can be achieved by one or more components, by similar or different techniques, by assembling two scaffolds produced separately in a final unique entity. To fabricate such biomimetic scaffolds, numerous fabrication techniques have been developed, with more or less success, and can generally be classified into two categories: conventional and advanced. The benefits and drawbacks of each one have been described in detail. The described design approaches in this review include cellular scaffold structures (cells and scaffolds) or acellular structures (scaffolds that are later colonized by the host cells). In both cases, biomolecules such as growth factors can also be incorporated. This article offers an overview of current advances in the field of bilayered scaffolds for the engineering of multilayered tissues. The multilayer tissues discussed in this review are vascular, bone/cartilage, skin, periodontal, urinary bladder, and trachea tissues. Furthermore, in the present review, we decided to focus only on newly synthesized bilayered materials, and not to mention bilayered scaffold produced from decellularized graft tissues. Decellularized materials derived from different tissues and organs have been extensively described in the literature.[ 14 , 15 ] Their advantage is the ability to provide support for the cells, comparatively good biocompatibility, weak immune response, and abundance of bioactive molecules. However, the drawbacks include the necessity to obtain quality grafts, long decellularization protocols, and the risk of the presence of toxic agents used during decellularization.
In this review, we will first present a brief description of the anatomy and the functionality of each native tissue. Second, we will focus on various scaffold design strategies, i.e., the selection of materials and fabrication techniques, used in order to build an appropriate structure with desired mechanical and biological properties existing in the native target tissues (Figure 1 ). Furthermore, this review will highlight the versatility of 3D bilayered scaffolds in achieving tissue regeneration in vitro and in vivo, with descriptions of animal models and human clinical trials. Finally, future outlooks of scaffold engineering for TE will be given.
Figure 1.
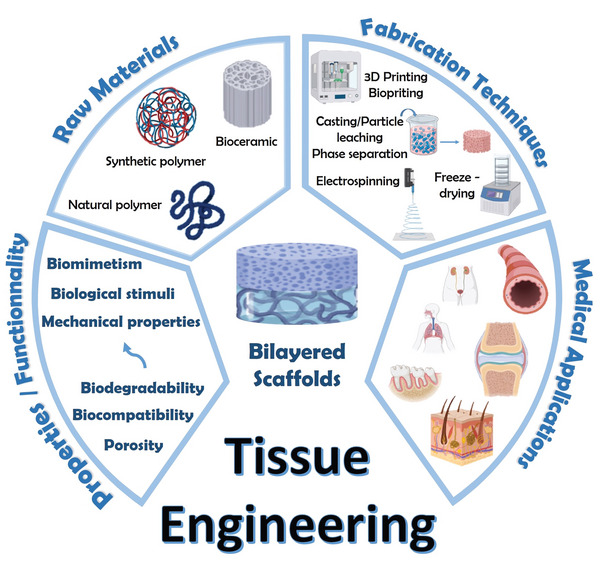
Overview of the strategies to design bilayered scaffolds for TE. TE refers to the combination of cells and scaffolds to build a structure, which would be able to restore and maintain the native architecture and function of damaged tissues and/or organs. Scaffolds are supporting structures for the cells and must be appropriately designed, in terms of properties and functionality, to mimic the architecture of the native tissue of interest. It exists various scaffold design strategies, i.e., selection of raw materials and fabrication techniques. Scaffolds can be made of natural and/or synthetic polymers, as well as bioceramics and can be built with several fabrication techniques. Selecting the appropriate scaffold design strategies is crucial.
2. Overview of Scaffold Fabrication Techniques
Designing and manufacturing are key steps is the conception process of 3D scaffolds for tissue repair. To date, there are numerous fabrication techniques that have been developed, more or less successful, to build 3D scaffolds for TE applications. They can be classified into two categories: conventional and advanced. According to the specific requirements that a scaffold should meet to mimic the target tissue (in particular in terms of matrix composition with chemical and structural similarities), selecting the appropriate fabrication technique is crucial. Indeed, various properties of tissue‐engineered scaffolds, such as the shape, porosity, and mechanical properties, are closely related to the manufacturing techniques. Conventional techniques including casting/particle leaching, freeze‐drying, phase separation method, and electrospinning can be used to build the scaffolds with interconnected porous structures, but do not provide enough control over scaffold architecture, pore network, and pore size. To overcome these limitations, the researchers are developing advanced fabrication techniques as an alternative to conventional scaffold fabrication methods such as 3D printing and bioprinting. Indeed, advanced fabrication techniques are capable of creating customized scaffolds with precise control over their structure. Both conventional and advanced techniques are described below, followed by a discussion of the advantages and drawbacks of each.
2.1. Conventional Fabrication Methods
In this section, we describe in detail conventional fabrication methods for 3D scaffolds. This description is based on the following references.[ 16 , 17 , 18 , 19 , 20 , 21 ]
2.1.1. Solvent Casing/Particle Leaching
Solvent casting/particle leaching is one of the most common methods to prepare porous scaffolds with interconnection networks. An organic solvent is used to dissolve the polymer of choice in which salt or polymer particles, which are used as porogens, are dispersed. From all porogens, sodium chloride is the best known; potassium chloride, sugar (glucose, saccharose), or gelatin also can be added to the solution in order to create pores by forming a polymer–porogen network. The polymer/porogen mixture in solvent is then cast into a mold and when the solvent evaporates, it leaves behind a solid composite material consisting of polymer matrix and porogens. This resulting matrix is submerged in water to leach out the particles and create a porous structure. It is a relatively easy‐to‐process and low‐cost approach. In a general manner, one of the main benefits is the effective control and tunability of porosity (up to 90%) and pore size (average between 100 and to 700 µm) to build appropriate structures with desired mechanical and biological characteristics. However, because of the casting and solvent evaporation step, this method is clearly limited to the fabrication of scaffolds up to 3 mm thick and is time‐consuming. Another drawback is the use of organic solvent, and the presence of its residues, which is hard to be completely removed from the scaffold during the drying step.
2.1.2. Freeze‐Drying
Freeze‐drying, also known as lyophilization, refers to a versatile method that allows to obtain 3D porous scaffolds with a high porosity (>90%) without the requirement of porogens. This method involves the use of a polymer solution that is frozen at a temperature between −20 °C and −80 °C, leading to ice crystal formation. The next step consists of the elimination of the solvent by complete sublimation with help of a lyophilizer to form a solid scaffold with various interconnecting pores. In fact, the resulting macro porosity corresponds to the empty area initially occupied by ice crystals. This technique is mostly favorable for designing scaffolds with high porosity (between 20 and 400 µm) and interconnectivity, with the considerable benefit to adjust structure and pore size by changing parameters such as the nature and the concentration of polymer and the freezing temperature. However, the pore size usually achieved with this technique corresponds to the lower limit for TE applications. On the other hand, the process is conducted at low temperatures, which can be beneficial to maintain the integrity of the biological factors embedded in the scaffold. In conclusion, the freeze‐drying technique is a suitable method for TE to fabricate a wide range of polymer‐based scaffolds, but the small pore size of the scaffold, the polydispersity in porosity, and above all the use of cytotoxic solvents for mixing the polymer limit its application. In addition, it only concerns a small group of synthetic polymers, which are water‐soluble, comprising polyglycolide (PGA) or poly(lactic‐co‐glycolic acid) (PLGA).
2.1.3. Phase Separation Method
Phase separation is a simple technique based on changes in thermal energy to induce demixing of a homogenous polymer/solvent solution: it can become thermodynamically unstable under certain conditions and tends to separate into two phases. Indeed, demixing, or phase separation, leads to the formation of two phases: the polymer‐rich phase which solidifies or precipitates allowing to obtain a nanofibrous structure in the polymer matrix, and the solvent‐rich phase which is eliminated by evaporation, sublimation, or extraction to produce porosity in the polymer matrix. Phase separation can be induced by two processes: by decreasing the temperature—thermally induced phase separation (TIPS) method, the most often used—or by adding nonsolvent to the polymer solution—diffusion induced phase separation (DIPS). Phase separation technique provides 3D scaffolds with a wide span of pore sizes and morphologies, and can be easily combined with other fabrication technologies, such as particulate leaching or 3D printing, to appropriately tune scaffolds for TE applications. However, it requires the use of organic solvents, results in poor pore interconnectivity and is limited to a small range of polymers with low melting temperatures.
2.1.4. Electrospinning
Electrospinning is electrohydrodynamic‐based process widely used for the fabrication of nanofibers from a polymer solution. The most basic set up needs an injection pump, a syringe tipped with a needle, a high voltage power source and a collector plate. Briefly, a high voltage is applied in the system to create an electric field between the tip of the needle and the collector plate. A polymer solution, contained in the syringe, is pumped to the tip of the needle. When a liquid droplet is formed out of the needle, it is electrified and this generates an electrically charged jet of polymer solution that moves toward the collector and forms fibers. This method has been extensively explored in TE because nanofibers are tunable in terms of size and spatial arrangement. Indeed, many parameters can be adjusted, among them polymer's molecular weight, conductivity, viscosity of the solution, surface tension, flow rate, voltage and distance between the nozzle tip and the collector. Another potentially interesting advantage of this technique is the possibility to use a wide range of materials such as PLGA, polycaprolactone (PCL), polylactide (PLA), silk fibroin (SF), collagen, and many other polymers. However, this process requires the use of organic solvents, and there remains a challenge to obtain electrospinning‐based 3D structures with appropriate pores size and shape to fulfill the needs in TE.
2.2. Advanced Fabrication Methods
In this section, we describe in detail advanced fabrication methods for 3D scaffolds. This description is based on the opposite references.[ 16 , 17 , 18 , 19 , 20 , 21 ]
2.2.1. 3D Printing
Over the past decades, 3D printing, also called additive manufacturing, has gained much interest and has been extensively used in TE. This computer‐aided design (CAD) technology can fabricate objects with complex structures by adding materials —ceramics, powders, plastics, metals, or liquids—by a layer‐by‐layer process with a bottom‐up approach. There are many various 3D printing techniques that can be classified into: i) laser‐based 3D printing which includes in particular stereolithography (SLA), selective laser sintering (SLS), digital laser printing (DLP); ii) extrusion‐based 3D printing which includes fused deposition modeling (FDM), and iii) ink‐based 3D printing which includes in particular ink jet printing (IJP), and aerosol jet printing (AJP). Among them, extrusion‐based 3D printing is the most commonly known printing technique in which the material is drawn through a nozzle and then selectively deposited layer by layer. 3D printing has led to considerable improvements in scaffold design and repeatability. It has the advantage to provide custom‐made porous complex scaffolds with detailed control over spatial geometry, microarchitecture, surface area‐to‐volume ratio, and porosity. It is an easy, cost‐effective process with less waste production, but requiring a high setup cost. With the rise of this technology, we will certainly be able, within a few years, to print detailed and clinically accurate scaffolds.
2.2.2. Bioprinting
Bioprinting is an advanced form of 3D printing to create structures from a bioink, a mixture of both materials and cells, or cells alone. Three main bioprinting techniques are extrusion‐based (EBB), droplet‐based (DBB), and laser‐based bioprinting (LBB). Whatever the technique is, once printed, the scaffold is commonly placed in a bioreactor to promote cell proliferation and maturation prior to implantation. Extrusion bioprinting is the most commonly used and consists of a nozzle that dispenses bioink continuously by physical force and pneumatic pressure to print scaffolds. Bioprinting provides customized/personalized 3D scaffolds with greater shape complexity, high accuracy, and high speed of printing. Cellular bioprinting allows to quickly manufacture complex 3D tissue structures with high cell viability and distribution, and ensures rapid tissue maturation for an effective tissue repair. However, 3D bioprinting is still costly and has to be further developed to reach a good control over the resulting materials/scaffolds properties.
Table 1 summarizes the advantages and disadvantages of each fabrication method that have been described in details in this section.
Table 1.
Advantages and disadvantages of different types of scaffolds fabrication techniques for tissue engineering application
| Technique | Advantages | Disadvantages |
|---|---|---|
| Conventional fabrication methods | ||
| Solvent casting/particle leaching |
High porosity (50‐90%) Controlled and tunable pore size and structure Easy‐to‐process and low cost |
Low pore interconnectivity Use of organic solvents High energy and time consuming Limited to thin membranes (<3 mm) |
| Freeze‐drying |
High porosity (>90%) and interconnectivity High interconnectivity of the porous network Tunable pore size and structure Capability of integrated bioactive molecules Capability of obviating high temperatures |
Poor control of scaffold porosity and morphology Use of organic solvents High energy and time consuming |
| Phase separation method |
Controlled and tunable pore size and structure Easily combined with other techniques Capability of integrated bioactive molecules Capability of obviating high temperatures |
Limited to a very few range of polymers Use of organic solvents Poor control scaffold morphology |
| Electrospinning |
Wide range of polymer Controllable process parameters to tune fibers Simple and low cost |
Mostly 2D scaffolds – Limited to produce 3D scaffolds Poor control of pore size and shape |
| Advanced fabrication methods | ||
| 3D printing |
Complex 3D shapes with high resolution Independent control of porosity and pore size Wide range of polymers |
Time‐consuming layer‐by‐layer processing and high cost |
| Bioprinting |
Greater shape complexity with high accuracy High speed of printing Suitable for incorporating cells into the scaffold with high cell viability (80/90%) |
High cost |
3. Vascular Tissue Engineering
Cardiovascular diseases, affecting the heart or blood vessels, are the leading causes of death worldwide.[ 22 ] In the field of vascular disease treatment, autologous vascular grafts are the most well‐established and common clinical option allowing to replace native vessels.[ 23 ] However, an ever‐persistent demand to reconstruct blood vessels, combined with the limited availability of autologous grafts (which may be of poor quality) and the complexity of the surgical procedure (which can potentially cause donor site morbidity), have motivated researchers to design scaffolds for tissue‐engineered vascular grafts (TEVGs).[ 24 ]
3.1. Architecture and Characteristics of Native Blood Vessels
The architecture of blood vessels depends on the vessel size and type, but a basic structure can be described independently of size and type, except capillaries. Blood vessels can be classified into three groups: arteries, capillaries, and veins. Arteries bring oxygenated blood to the tissues and veins return deoxygenated blood to the heart. Capillaries ensure exchanges of oxygen, nutrients, and wastes between blood and tissues, thanks to their thin walls and small diameter. The wall of the arteries and veins is formed by three layers: tunica intima, tunica media, and adventitia. Tunica intima is the inner layer, which is in contact with the blood, and consists of a monolayer of endothelial cells (ECs) and a basement membrane composed of mesh‐like substrate of type IV collagen. ECs are oriented along the blood flow direction[ 25 ] and play a key role in many biological processes such as coagulation, blood flow regulation, hemostasis, or inflammation.[ 26 ] Deeper there is a subendothelial connective tissue followed by an internal elastic membrane that separates intima and media. Tunica media is the middle layer and contains vascular smooth muscle cells (VSMCs), networks of elastin (elastic lamellae), and crimped collagen fibers (Col I and III). VSMCs are contractile cells that allow vessel contraction or dilatation, maintaining the proper blood pressure. They are arranged concentrically along the axis of the vessel. Media and adventitia are separated by an external elastic membrane,[ 27 ] and adventitia is the outmost layer, composed of fibroblasts, mainly aligned collagen fibers (Col I and III), and some elastin. It maintains the vessel's structure by preventing over‐extending or over‐retracting of the vessel.[ 26 , 27 ] The general structure of blood vessels is illustrated in Figure 2 . Finally, capillaries are the smallest vessels; they are lined by an only single layer of ECs and connect arteries and veins.[ 28 ]
Figure 2.
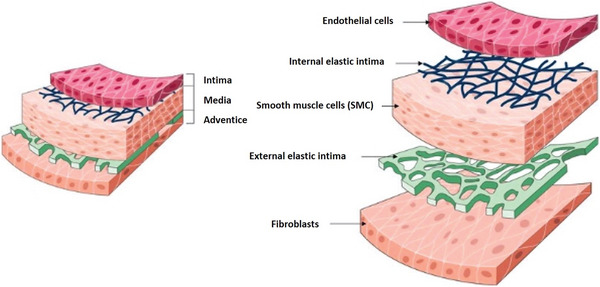
General structure of blood vessels composed of three main layers: tunica intima, tunica media, and tunica adventitia. Reproduced under the terms of the Creative Commons License.[ 27 ] Copyright 2021, the Author(s). Published by Frontiers Media S.A.
From a mechanical perspective, the vascular wall is composed of approximately 70% of water, 30% in dry mass of collagen and elastin, with complementary percentages comprising glycosaminoglycans, proteoglycans, and vascular cells.[ 29 ] Collagen is the most common structural protein in the arterial wall, providing mechanical support and strength. Elastin is the second most common structural protein in the arterial wall and is secreted by VSMCs. As a key component of arterial ECM, it provides elasticity/recoil and allows interlamellar communication. Elastin fibers are 1000 times more flexible than collagen and are found in high abundance in the aorta.[ 30 ]
The composition and thickness of blood vessels vary according to the vessel type (artery or vein) and diameter. For instance, large arteries, like the aorta, are composed of a thick media layer and a high amount of elastin. Narrow vessels (i.e., small arteries) contain less elastin, but more smooth muscle cells. On the contrary, veins have a thinner media layer and, therefore, a less amount of elastic tissue.[ 29 ]
3.2. Strategies and Approaches
TE of vascular vessels is a promising approach to compensate the lack of native graft materials and to properly induce the regeneration of the tissue in terms of its architecture and properties. Blood vessels are dynamic tissues suited to withstand both the flow of blood and pressure, therefore, good understanding of the structural architecture of native tissues—including dimensions, composition, structure, and mechanical properties—is required to develop biomimetic scaffolds for vascular TE. From a biomimetic perspective, an ideal vascular scaffold should ideally replicate the structure and the functionality of the three distinct layers of a native blood vessel: tunica intima, tunica media, and tunica adventitia.[ 31 ] Additionally, native blood vessel faces warping, stretching, and expansion in the human body. Thus, high elasticity and a high degree of mechanical strength are also the main criteria for designing vessel‐like structures.
3.2.1. Building Bilayered Scaffolds for Vascular TE with One Component
Synthetic vascular grafts made of polyethylene terephthalate (PET), polytetrafluoroethylene (PTFE), or polyurethane (PU) have been developed and are still currently considered as gold standard materials for vascular TE.[ 32 ] Even so, these existing synthetic grafts have two main limitations: i) a limited patency and ii) a lack of appropriate tissue colonization.[ 27 ] Considering this, most of the TE approaches rely now on aliphatic polyesters, such as PLA, PGA, and PLGA copolymers.[ 33 ] Aliphatic polyesters represent the largest group of synthetic biodegradable polymers commonly used in vascular TE due to their good physical and mechanical properties comparable to those of native vascular tissues such as elasticity, mechanical/tensile strength, or degradation rate.
To cite another example of aliphatic polyester, PCL is also a competitive material used for medical applications.[ 34 ] For example, based on the specific architectural feature of blood vessels, Zhu et al. designed a PCL‐based bilayered vascular graft, in which VSMCs orientation is precisely respected (Figure 3a).[ 35 ] His goal was to develop a small‐diameter vascular graft mimicking the structure of native blood vessels by inducing the regeneration of circumferentially aligned VSMCs. The inner layer is composed of circumferentially aligned PCL microfibers prepared by wet‐spinning. In the wet‐spinning technique, a polymer is dissolved in a spinning solvent, then it is extruded out through a spinneret submerged in a chemical bath, and precipitates in fiber form. On the other hand, the outer layer made of random PCL electrospun nanofibers significantly enhanced the mechanical properties of vascular grafts, preventing bleeding due to the implantation (Figure 3b). The in vitro tests demonstrated: i) alignment of the cytoskeleton of VSMCs after 3 days of culture; ii) cell alignment along the circumferentially oriented fibers; iii) functional regenerated neoartery and iv) high viability of VSMCs in the scaffold. Furthermore, in the in vivo experiments, VSMCs and ECs were first seeded on the scaffolds and cultured in vitro in a bioreactor, then implanted in rat abdominal aorta. The results showed that the VSMCs layer with circumferential orientation and longitudinally aligned ECs layer were successfully regenerated. In this regard, Zhu et al. have developed a strong candidate for vascular tissue regeneration. However, further investigations are needed to evaluate the performance of this scaffold in large animals.
Figure 3.
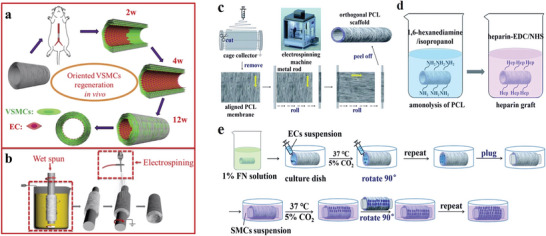
Schematic illustration of the fabrication process set up by Zhu et al. a) Hypothesis orientation of circumferentially aligned PCL microfibers which could guide VSMCs regeneration. b) The two‐step fabrication process that has been used to manufacture the inner layer with circumferentially oriented PCL fibers by wet‐spinning and the outer layer randomly with oriented nanofibers by electrospinning. Reproduced with permission.[ 35 ] Copyright 2015, Elsevier. Schematic illustration of the fabrication process set up by Li et al. c) The custom‐made electrospinning technique to develop a PCL‐based two‐layer‐tubular scaffold in which the directions of the fibers of these two layers were orthogonal. d) Heparinization of PCL to improve its hydrophilicity. e) ECs and VSMCs co‐culture: ECs were seeded on the inner layer in which nanofibers are oriented along a axial direction whereas VSMCs were seeded on the outer layer in which nanofibers are oriented with a circumferential direction. Adapted under the terms of the Creative Commons License.[ 36 ] Copyright 2021, the Authors. Published by the Royal Society of Chemistry.
With a similar strategy, Li et al. have proposed a simple method to coculture ECs/VSMCs, mimicking cellular structure of native vascular vessels: ECs grow along the direction of blood flow while VSMCs grow circumferentially along the vessel wall.[ 36 ] Via a custom‐made electrospinning technique, they developed a two‐layer tubular scaffold in which the directions of the fibers of these two layers were orthogonal (Figure 3c). In fact, nanofibers of the inner layer for ECs culture were oriented along an axial direction, whereas nanofibers of the outer layer for VSMCs culture were with a circumferential direction (Figure 3e). It should be noted that heparin, a natural anticoagulant, has been grafted onto the scaffold to improve the hydrophilicity of PCL (Figure 3d). First of all, they investigated the guiding effect of the aligned fibers for cell orientation. For that, ECs and VSMCs were separately cultured on heparinized PCL films composed of aligned fibers and compared with random nanofibers used as a control. The two main results have shown that: i) grafted‐heparin has significantly enhanced adhesion between the substrate and the cells and cell proliferation, and ii) aligned fibers had a crucial guiding effect and induced cell orientation. Afterward, ECs and VSMCs were co‐cultured on the inner and outer layers of the tubular scaffold, respectively (Figure 3e). After 10 days, ECs and VSMCs lined almost the entire inner and outer side of the scaffold and growth in the desired orientations, along their fibrous directions. Additionally, mechanical properties’ characterization showed tensile stress similar to native human coronary arteries. Using electrospinning methods, this study was the first to develop a scaffold mimicking the orientation of native blood vessels. It may bring synthetic vascular grafts closer to clinical application.
Most research today focuses on polymer blends to produce blend materials with unique structural and mechanical properties based on specific properties of each of them. In vascular TE, PCL is the most commonly used synthetic polymer, but its main disadvantage concerns slow degradation rate and low cell attachment due to its high hydrophobicity.[ 34 ] Therefore, blending PCL with other materials may be a good strategy.[ 37 ] One of them, poly(L‐lactide‐co‐ε‐caprolactone) (PLCL), a copolymer of PLA and PCL, has been investigated for its potential use in TE. It was previously reported that PLCL tubular scaffolds were fabricated for small‐diameter vessel replacement by an extrusion‐particulate leaching technique.[ 38 ] However, these extruded PLCL scaffolds presented a lack of mechanical strength (tensile strength), making them unable to resist to the stitching process during in vivo implantation, as well as to physiological blood pressure.
To overcome this problem, Kim et al. studied a PLCL‐based (molar ratio 50:50) bilayered tubular scaffold having high mechanical properties, fabricated using a custom‐made gel‐spinning technique.[ 39 ] The bilayered scaffold was composed of i) an inner porous layer as a blood barrier to block blood leakage and ii) an outer fibrous layer for mechanical strength. They reported better mechanical strength and increased cell adhesion and proliferation, as compared to the extruded PLCL tubular scaffolds. Further in vivo studies (on the canine model) to evaluate the mechanical stability have to be conducted because blood leakage pressure would change along the biodegradation of scaffold in the body. In another study, Shin et al. prepared a dual‐layered electrospun scaffold from PLCL, composed of microfibrous and nanofibrous layers. Then, gelatin was grafted on the scaffold using acrylic acid (AAc) and γ‐ray irradiation to improve cell adhesion.[ 40 ] This grafting improved scaffold hydrophilicity, VSMCs proliferation and infiltration toward the microfibrous layer. In addition, they seeded human umbilical vein endothelial cells (HUVECs) on the entire surface of the nanofibrous layer to develop the intimal layer mimicking tunica intima.
Natural biopolymers like collagen, SF or polysaccharides are very promising for various TE applications.[ 41 ] Sometimes, natural polymers exhibit better mechanical properties than synthetic polymers, making them of particular interest. It is the case of silk, a natural protein that is widely used as a potential biopolymer for designing TE scaffolds.[ 42 ] There are many different silk‐producing sources: silkworms, spiders, lacewing, glow‐worm, and mites. Bombyx mori, a mulberry‐feeding domesticated silkworm, is the most famous silk source commonly producing silk for the TE field.[ 43 ] Interestingly, a recent study reported the investigation of the endemic non‐mulberry silk from Antheraea assama. Indeed, Gupta et al. have first demonstrated the superior performance, in terms of mechanical and biological traits, of A. assama silk compared to B. mori silk, and its promising features for vascular TE applications.[ 44 ] In their previous in vitro studies, they had already suggested that A. assama silk i) supported vascular cell growth and functionality; ii) had superior mechanical/elastic properties compared to mulberry silk, and iii) reduced acute thrombosis in vivo thanks to the natural presence of RGD sequences (arginine‐glycine‐aspartic acid) on surface of non‐mulberry A. assama silk.[ 45 ] In their study, they designed a bilayered biomimetic small diameter vascular graft composed of i) an inner porous layer with interconnectivity—mimicking tunica media—to allow cellular infiltration; and ii) an outer dense electrospun layer—mimicking adventitia—to confer mechanical resistance. They adopted molding and freeze‐drying method to design the inner porous layer followed by coating of an electrospun outer nanofibrous layer (Figure 4a). With this pioneering method, parameters such as porosity, pore size, degradation rate and mechanical properties can be controlled by tuning protein percentage, freezing temperature and thickness of both layers. The results demonstrated that bilayered A. assama‐silk grafts fabricated using this method led to morphologically and mechanically biomimetic structures. In vivo short‐term implantation in rat aorta showed long‐term patency, suturability, and strength (Figure 4b). This A. assama silk is of great potential for designing vascular grafts and could be further extended for engineering other tissues as well. However, further studies are needed to improve in vivo performance and to investigate neo‐vessel formation in a long‐term in vivo implantation models.
Figure 4.
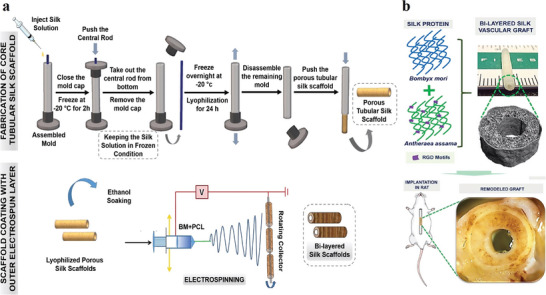
Schematic illustration of fabrication process set up by Gupta et al. a) Fabrication methodology of bilayered small vascular graft using silk‐based scaffolds. It is composed of an inner porous layer prepared by molding and freeze‐drying, followed by coating with electrospun outer nanofibrous layer. b) Graphical description of the bilayered scaffold and in vivo implantation in rat. Adapted with permission.[ 44 ] Copyright 2020, American Chemical Society.
3.2.2. Building Bilayered Scaffolds for Vascular TE with More Than One Component
Until now, a large range of biodegradable synthetic polymers has been considered as a well‐performing choice for TE of blood vessels.[ 23 ] One of the relatively new polyesters that are being increasingly explored is poly(glycerol sebacate) (PGS). Kharazi et al. aimed to combine PCL with PGS to find an appropriate balance between hydrophobic and hydrophilic characteristics.[ 46 ] Indeed, PGS has a superior cellular response, compared to PCL, and attractive blood compatibility owing to its hydrophilic nature. PGS is very fast‐degrading both in vitro and in vivo and a non‐electrospunable polymer (due to its low viscosity at low molecular weights and its rigidity due to harsh cross‐linking at high molecular weights), which limits its potential in TE.[ 47 ] That is why, by blending PGS and PCL together, Kharazi et al. have succeeded in production of electrospun nanofibers (thanks to the presence of PCL) suitable to improve cell attachment and proliferation (thanks to the presence of PGS). Regarding this, they fabricated a bilayered nanofibrous scaffold composed of i) PGS/PCL electrospun nanofibers as an inner layer which mimicked the antithrombotic features of the native intima and supported the attachment, growth, and infiltration of mesenchymal stem cells (MSCs); and ii) PCL nanofiber as an outer layer which reduced the degradation rate, reinforced and kept the integrity of the scaffold during the regeneration process (Figure 5 ).
Figure 5.

a) Entire view of the bilayered tubular scaffold composed of PGS/PCL electrospun nanofibers. b) SEM image of PGS/PCL nanofibers layer. c) SEM image of PCL layers. d) Cross section. e) SEM image of the interface between outer and inner layers. Reproduced with permission.[ 46 ] Copyright 2018, John Wiley & Sons Ltd.
More recently, on the same topic, Rekabgardan et al. mixed PGS with PU to introduce a novel fibrous scaffold which comprised two layers: i) an electrospun pure PU layer beneath another ii) electrospun PGS‐PU layer.[ 48 ] Based on the advantages and drawbacks of each, combining PU and PGS improved mechanical properties, biodegradation rate, and cell growth and proliferation on the scaffold.
Many other studies have reported polymer blends for vascular TE. Wang et al. developed a bilayered tubular scaffold with a macroporous and biomimetic nanofibrous structure: a poly(L‐lactic acid) (PLLA)/PLCL microporous inner layer, and a PLLA/PCL macroporous outer layer (Figure 6b).[ 49 ] This work focused on the production of interconnected porous architecture which provides sufficient space for cell infiltration and proliferation, thereby facilitating new tissue formation. It was the first study fabricating a porous‐heterogeneous scaffold without using porogens, but via a two‐step phase separation technique (Figure 6a). Additionally, this method allowed tuning pore size in a simple way by adjusting polymer ratio. The microporous nanofibrous inner and outer layers were respectively used for endothelialization with ECs and VSMCs infiltration. Besides, after 3 months in vivo implantation into the jugular vein of New Zealand white rabbits, the implanted scaffolds kept patency and displayed favorable repair performance for small diameter blood vessels.
Figure 6.
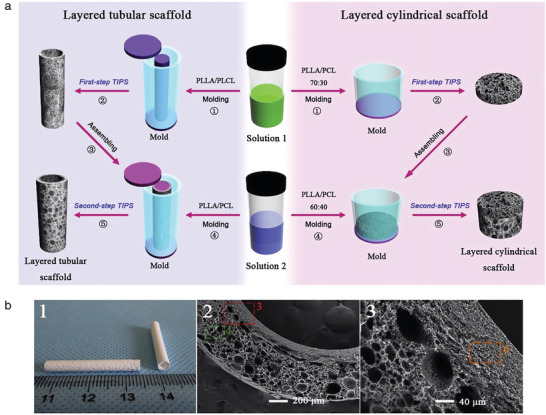
a) Scheme of the two‐steps thermal‐induced phase separation technique to fabricate heterogeneous porous bilayered nanofibrous vascular grafts. The bilayered scaffold is PLLA‐based: a PLLA/PLCL microporous as an inner layer, and a PLLA/PCL macroporous as an outer layer. b) Reproduced with permission.[ 49 ] Copyright 2018, Elsevier.
In many works, a mix of bioderived materials and high‐molecular polymer materials are used. Liu et al. demonstrated that PCL can also be used with SF as a promising candidate for vascular TE applications.[ 50 ] Indeed, the electrospun PCL/SF microfiber scaffold has shown better mechanical strength and degradability compared to those of natural SF.
Moreover, by combining two of the most popular methods—electrospinning and freeze‐drying—Norouzi and Shamloo fabricated a dual‐layer scaffold with PCL fibers as an inner layer and heparinized‐based gelatin hydrogel as an outer layer.[ 51 ] Introduction of (heparinized‐based) gelatin into the blend greatly improved HUVECs proliferation and decreased the risk of thrombosis, thanks to the presence of heparin. In order to tune biodegradability, Li et al. added PLGA into the same blend, despite lower flexibility of PLGA, as compared to PCL.[ 52 ] The mixture exhibited a fast biodegradation rate and desired mechanical properties. They also focused on the cell‐specific orientation of native blood vessels (two oriented structures with circumferentially aligned VSMCs and longitudinally aligned ECs), already pointed out in Zhu et al.[ 35 ] and Li et al.[ 36 ] in their study.
Last, Zhao et al. combined all these polymers to take advantage of their properties: spider silk for its stability, flexibility, and elasticity superior to artificial fiber; PCL for its mechanical properties; chitosan for its biocompatibility; and gelatin for its excellent biocompatibility and hydrophilicity.[ 53 ] In terms of mechanical and biological properties, the results reported by Zhao et al. are close to the studies described above.[ 50 , 51 , 52 ]
Despite lower rigidity and mechanical strength, as compared to synthetic polymers, blends of natural polymeric materials are also used by the researchers. However, biofabrication techniques (such as microfabrication techniques, fiber‐based technologies and 3D bioprinting) to design large complex tissues from natural polymers, such as blood vessels for transplantation, are limited.[ 54 ] Indeed, the resulting scaffolds clearly present lack of strength and suture retention strength. Nevertheless, a recent work proved the possibility to develop advanced multilayered tubular structures by 3D bioprinting technology, with an enhanced mechanical strength.[ 55 ] This bioprinting method has a great potential to create multitude of tubular tissue architectures and is advantageous for controlling gradient composition/porosity/strength. It can definitely be extended for the fabrication of bilayered scaffold, as has already been explored by Xu et al.[ 56 ] Another alternative is to use a hierarchical approach for scaffold fabrication, called “bottom‐up” approach, where the layers are built sequentially and allow to modify and tailor each layer's properties independently, resulting in more complex structures.
In the study of Ryan et al., this kind of approach was used, allowing highly tailorable structures.[ 57 ] The researchers created vascular grafts using combination of three fabrication techniques: casting/solvent evaporation, crosslinking, and freeze‐drying. As raw materials, they focused on ECM proteins like collagen and elastin, to mimic native blood vessel ECM composition. Thus, a collagen‐elastin‐based biomimetic bilayered tubular scaffold was developed, containing i) a porous outer layer (mimicking the tunica media) offering a suitable environment for smooth muscle cells (SMCs) infiltration and proliferation and ii) a dense inner film‐like layer (mimicking the tunica intima) providing mechanical support. With their fabrication technique, each layer can be independently optimized to create a multilayered structure with tailored properties for desired mechanical and/or biological performance. Furthermore, it displays good layer integration, uniform wall thickness, and low immunogenicity.
Few articles deal with ECM components to mimic vascular scaffolds. Collagen (type I) and glycosaminoglycans (GAGs) are the major molecules of the extracellular matrix.[ 58 ] Both enable to build scaffolds to integrate and support new tissues. They provide elasticity, tissue strength, and flexibility. Zhou et al. were the first to build up a bilayered scaffold with collagen‐heparin (Col‐HP) to manufacture the inner layer, and collagen‐hyaluronic acid (Col‐HA) for the outer layer.[ 59 ] Both of them were cross‐linked via EDC reaction (chemical reticulation between carboxylic acid and amine groups from two different molecules thanks to the presence of 1‐ethyl‐3‐(3‐dimethylaminopropyl)carbodiimide (called “EDC”)). The resulting cross‐linked scaffold exhibited more favorable physicochemical, mechanical and biological properties compared to non‐crosslinked structures. Fibroblast cells (Cos‐7) and human microvascular endothelial cells (HMECs) were seeded for 3 days on Col‐HA surface and on Col‐HP layer, respectively. As a result, the authors have demonstrated that a bilayered cross‐linked scaffold promotes vascular regeneration by inducing fibroblasts and endothelial cell growths on each layer. However, strategies using crosslinking agents have many drawbacks: very often there is a lack of cells in the central part of the scaffolds, and there is a risk of cytotoxicity due to cross‐linking agent residues. Further in vivo evaluations are under consideration and will provide information on these eventual drawbacks.
In TE, hydrogels have become an important basic biomaterial. Thus, Badhe et al. have developed a macroporous scaffold based on a chitosan/gelatin hydrogel which clearly, from a morphological and mechanical point of view, mimics blood vessels.[ 60 ] They used a modified solvent casting/particulate leaching method. However, gelatin‐based hydrogel melts easily at high temperatures (even with the body temperature), and therefore chemical crosslinking or copolymerization step is required to match the appropriated mechanical and elasticity strength. That is why a curing step in scaffold preparation was added. The resulting scaffold was composed of chitosan/gelatin‐based two layers with the following structure: i) an inner macroporous layer providing a large surface area which allowed strong cell adhesion and proliferation; and ii) an outer nonporous layer used as a barrier protection for cells and providing additional flexibility as well as elasticity. To evaluate cell proliferation, primary human dermal fibroblasts (HDFs) were seeded onto the bilayered scaffold for 20 days. SEM images clearly show cells attaching to the surface of the scaffold (Figure 7 ). On day 10, they observed extended and attached filopodia and on day 20, cells almost reached confluence and covered the scaffold surface. Thus, this result confirms the optimal biological properties of the designed bilayered scaffold, allowing fibroblast adhesion, growth, and proliferation.
Figure 7.
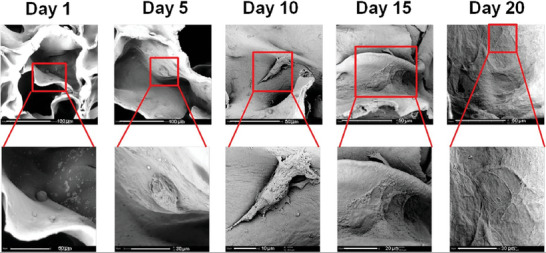
HDFas cell seeding onto the chitosan/gelatin‐based bilayered scaffold at days 1, 5, 10, 15, and 20 after culture in proliferation medium. Reproduced with permission.[ 60 ] Copyright 2016, Elsevier.
These different studies, summarized in Table 2 , showed that a wide variety of polymers, natural or synthetic, individually or mixed together, can be used to mimic human vascular tissue. In general, it is clear that electrospinning has gained popularity over the past decades in vascular TE, and has been clearly detailed in the literature.[ 23 , 37 , 61 ] Indeed, it has demonstrated a great potential to produce mimicking bilayered TEVG scaffolds. However, several challenges still remain open: use of toxics solvents, poor control over mechanical properties and degradation, problematic to obtain 3D structures with difficulty to control pore size for biomedical applications needs, insufficient cell infiltration to the scaffolds’ core, and finally inhomogeneous cell distribution.[ 37 ] To overcome the limitations, we believe that the use of advanced fabrication techniques should be a good alternative. Thus, 3D printing, and especially material extrusion additive manufacturing, is advantageous for designing structures with higher accuracy and greater shape complexity. Moreover, a fine controlling of gradient composition/porosity/strength will be possible, and combining bioprinting to incorporate cells to the scaffold is also possible. However, it is surprising that in the literature only few articles deal with 3D (bio)printing of multilayered scaffolds, including bilayered scaffolds.[ 55 , 62 , 63 ] We believe that researchers have to pay attention to this, and extensive work has to be conducted in future years. Furthermore, after many years of research, the future of TEVGs appears to be promising with arrival of new disruptive technologies, although many pre‐clinical and clinical studies will be necessary to validate these innovative solutions.
Table 2.
Summary of raw materials and fabrication methods used in vascular TE and corresponding to articles described above
| Clinical application | Raw materials | Fabrication methods | Refs. | ||
|---|---|---|---|---|---|
| Vascular TE | Building bilayered scaffolds with one component | ||||
| Natural materials |
Inner layer Outer layer |
Silk fibroin |
Molding/freeze‐drying Electrospinning |
Gupta et al. [44] | |
| Synthetic materials |
Inner layer Outer layer |
PCL |
Wet‐spinning Electrospinning |
Zhu et al. [35] | |
|
Inner layer Outer layer |
PCL | Electrospinning | Li et al. [36] | ||
|
Inner layer Outer layer |
PLCL | Gel spinning molding | Kim et al. [39] | ||
|
Inner layer Outer layer |
PLCL | Electrospinning | Shin et al. [40] | ||
| Building bilayered scaffolds with more than one component | |||||
| Blends of natural materials |
Inner layer Outer layer |
Collagen/chitosan | Solvent casting‐co‐particulate leaching | Badhe et al. [60] | |
|
Inner layer Outer layer |
Collagen Collagen/HA |
Cross‐linking | Zhou et al. [59] | ||
|
Inner layer Outer layer |
Collagen/elastin | Solvent evaporation/freeze‐drying/cross‐linking | Ryan et al. [57] | ||
| Blends of synthetic materials |
Inner layer Outer layer |
PGS/PCL PCL |
Electrospinning | Kharazi et al. [46] | |
|
Inner layer Outer layer |
PLLA/PLCL PLLA/PCL |
Dual‐phase separation | Wang et al. [49] | ||
|
Inner layer Outer layer |
PU PGS/PU |
Electrospinning | Rekabgardan et al. [48] | ||
| Blends of natural/synthetic materials |
Inner layer Outer layer |
Silk/PCL/gelatin/chitosan | Electrospinning | Zhao et al. [53] | |
|
Inner layer Outer layer |
PCL Gelatin |
Co‐electrospinning Freeze‐drying |
Norouzi et al. [51] | ||
|
Inner layer Outer layer |
PCL/PLGA/gelatin | Electrospinning | Li et al. [52] | ||
|
Inner layer Outer layer |
PGS/silk fibroin | Electrospinning | Liu et al. [50] | ||
4. Osteochondral Tissue Engineering
Osteochondral defects (OCD) refer to structural damage of the articular cartilage surface and the underlying subchondral bone, provoked by accidental injuries or osteoarthritis and resulting in severe pain, swelling and catching of the joint.[ 64 ] In the most serious cases, OCD repair is necessary, but these tissues are still hard to regenerate, because of the complexity of natural osteochondral tissue in terms of composition, structure, and function.[ 65 ] Surgical procedure is the most common current clinical approach to treat OCD injuries. It can be palliative (arthroscopic debridement, abrasion arthroplasty, and chondroplasty), reparative (microfracture, drilling, autologous or allogeneic osteochondral transplantation) or restorative (autologous chondrocyte implantation) according to the level of repair.[ 65 ] However, these approaches are not ideal because of intrinsic shortcomings, limitations, and complications in the final repair.
4.1. Osteochondral Tissue Structure
Osteochondral tissue is a cartilage–bone interface and is composed of cartilage and subchondral bone (Figure 8 ).[ 65 ] Cartilage is an avascular tissue composed of chondrocytes and ECM, containing water, collagen, and proteoglycans.[ 13 ] It can be separated into four zones, according to the composition and organization of each component.[ 13 , 64 ] The superficial/tangential zone is the 10–20% upper zone of cartilage. It is composed of densely packed collagen fibrils (type II and IX), parallel to the cartilage surface and many flattened ellipsoidal‐like aligned chondrocytes. It is an articulating surface, in contact with synovial fluid, with cells secreting proteins that maintain tissue lubrification and tensile properties.[ 8 ] The middle/transitional zone is the largest one (40–60%), with collagen fibrils being randomly oriented. In the deep/radial zone (30%–40%), cells and collagen fibrils are oriented perpendicularly to the cartilage surface. Deep and middle zones are the areas resisting against compressive forces.[ 8 , 64 ] Under these three articular cartilage zones, the tidemark is a structure that separates uncalcified cartilage from the last small region, the calcified cartilage.[ 8 , 64 ] Calcified cartilage is a transition zone between the flexible uncalcified cartilage and the rigid subchondral bone. It is permeable to small nutritional solutes, playing a role in maintaining the microenvironment between cartilage and subchondral bone.[ 8 ] The underlying subchondral bone is a vascularized tissue that can be separated into two layers. The subchondral cortical bone (or subchondral bone plate) has low porosity and few blood vessels, while the subchondral trabecular bone is highly vascularized and more porous. Bone tissue is composed of the bone ECM and osteoblasts, osteocytes, and osteoclasts (cells involved in perpetual bone breakdown and remodeling).[ 65 ] Subchondral bone transmits mechanical loads and provides nutriments for the cartilage. Moreover, it contains nerves, which explain its contribution to pain in diseases.[ 8 ]
Figure 8.
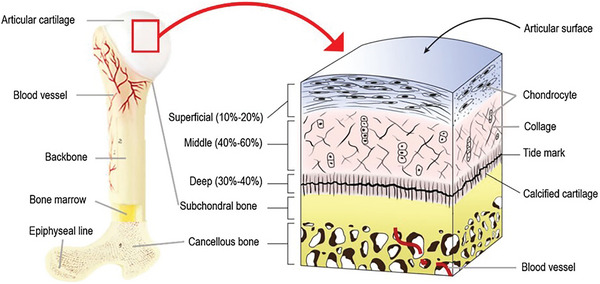
Osteochondral tissue structure. Cross section of a long bone and a schematic presentation of the osteochondral unit. Reproduced with permission.[ 66 ] Copyright 2020, Springer Nature.
4.2. Strategies and Approaches
Osteochondral tissue engineering (OCTE) is now considered as the best strategy for treating OCD and engineering such complex tissues. To design and fabricate a scaffold for OCD regeneration, several requirements have to be met:[ 8 ] i) in terms of composition, it should be biocompatible (with no rejection) and have stable physicochemical properties after implantation into the body; ii) structurally, it should have desired biomechanical properties and be a suitable environment (with an appropriate pore size and porosity) for cell attachment, proliferation, in‐growth, and neo‐tissue formation; iii) functionally, it should induce simultaneous regeneration of both cartilage and subchondral bone, maintain the cell phenotype, and be integrated into the surrounding cartilage and bone. Ideally, the aim would be to develop stratified scaffolds in order to mimic the native osteochondral tissue interface, comprising: i) a cartilage phase divided into four zones with the superficial one inducing the alignment and the morphology of chondrocytes, ii) a calcified cartilage zone, and iii) a subchondral bone zone (Figure 8).
4.2.1. Building Bilayered Scaffolds for Osteochondral TE with One Component
Synthetic polymers such as PLA, PLGA, and PCL are promising candidates for OCTE.[ 67 ] Indeed, the structure and properties of synthetic materials can be easily tailored and varied according to specific clinical applications, by altering the chemical composition, crystallinity, and molecular weight of the polymers.
It is known that regeneration of specific tissues mostly depends on the following properties of the scaffolds: porosity, pore size, pore shape, pore distribution (interconnection between the pores), and architecture (overall shape of the object). On this, Pan et al. investigated the effect of scaffold porosity on spontaneous in vivo osteochondral repair in New Zealand white rabbit models.[ 68 ] For that, PLGA was used as a scaffold matrix. By molding/particulate leaching method, they created a porous structure using salt particles as porogens, with which pore size, shape, and porosity can be easily controlled. However, this fabrication method is often criticized because it uses toxic solvents (here dichloromethane) and often leaves residues. To solve the problem, we found interesting that several authors had the idea to replace the solvent casting step by a melt‐molding step: it is called melt‐molding/particulate leaching method.[ 69 ] Their goal was to find the appropriate porosity balance: avoiding too high porosity leading to mechanically weak scaffold or too low porosity preventing interconnectivity of the pores. For that, the PLGA‐based bilayered scaffolds (Figure 9a,b) were fabricated with the same porosity or different ones (pore size: 200–300 µm) on the cartilage and bone layers, respectively, and the porosity effect was then examined in vivo. Three porosity combinations were tested on rabbit models, with pre‐seeded bone marrow‐derived mesenchymal stem cell scaffolds (BMSCs), one of the main sources of cells used for OCTE. Indeed, it should be noted that MSCs have gained considerable attention and have been explored as attractive cell source, thanks to their multipotency with the ability to easily differentiate into osteoblasts and chondrocytes.[ 8 , 70 , 71 ] The three scaffolds porosities are the following: Scaffold A: porosity = 92%–77%, Scaffold B: porosity = 85%–85%, and Scaffold C: porosity = 77%–92% (Figure 9c–e). Six and 12 weeks after surgery, the group with 92% porosity in the chondral layer and 77% porosity (Porosity A) in the subchondral layer resulted in the best efficacy in terms of repair of osteochondral defects.
Figure 9.
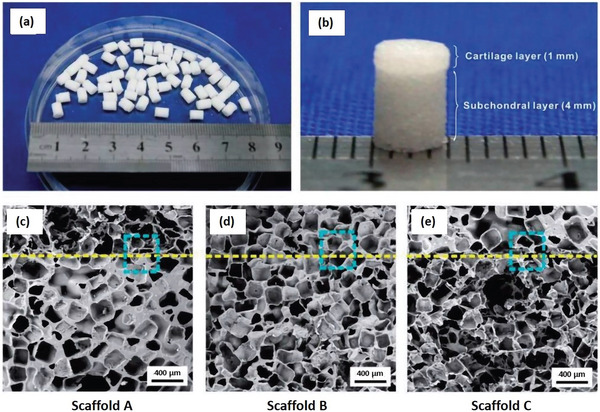
a,b) General view of the bilayered PGLA scaffold structure with the cartilage and the subchondral layer. c–e) SEM micrographs of the three groups of bilayered scaffolds porosities. The dashed line indicates the border of the two layers. Reproduced under the terms of the Creative Commons License.[ 68 ] Copyright 2015, the Author(s). Published by Oxford University Press.
4.2.2. Building Bilayered Scaffolds for Osteochondral TE with More Than One Component
Previous studies have reported that pure polymer‐based scaffolds are not always suitable and effective for the rapid treatment of osteochondral defects.[ 70 ] Therefore, incorporating bioactive factors into biomaterials may be a good strategy for accelerating osteochondral tissue regeneration.[ 72 ] In this regard, Zheng et al. decided to combine synthetic polymers with natural polymers and add bioactive factors to promote osteochondral regeneration.[ 73 ] They aimed to develop a composite scaffold with 2 layers as following: i) hydrogel chondral layer (gelatin/silk‐fibroin/dextran blend) in which a cartilage‐inducing molecule, called kartogenin (KGN), is loaded into, and ii) a porous nanofibrous subchondral layer (PLLA/PLGA/PCL blend) in which bone morphogenetic protein 2 (BMP‐2) derived peptide (osteogenic factor) is loaded into. For in vitro release study, rhodamine B was used as a model drug to test the release profile of KGN whereas the fluorescein isothiocyanate (FITC) was chosen for BMP‐2 derived peptides. The two different bioactive factors could be released from the corresponding layers for more than 28 days (cumulative release ≥ 60%), with nevertheless the observation of an initial burst within the first few days for the rhodamine B. To study the cartilage differentiation potential, they seeded BMSCs on the KGN‐loaded hydrogel cartilage layer. The expression of several chondrogenic markers (COLI, COLII, SOX9, and aggrecan), measured by RT‐PCR at 7 and 14 days of culture, was significantly higher compared to non‐KGN‐loaded hydrogel, and confirmed that KGN loading promoted cartilage differentiation of BMSCs. Similar results were found for osteogenic differentiation of BMSCs on BMP‐2‐derived peptide‐loaded nanofibrous subchondral layer (RUNX2, COLI, OPN, and OCN specific osteogenic markers). In vivo experiments in rabbit knee joint model suggested that dual‐factor‐loaded bilayered scaffold might be a successful candidate for the treatment of osteochondral defects, by simultaneously enhancing the regeneration of cartilage and subchondral bone. However, with a total of eight different products, it may be difficult to bring it to the market for end‐use, given the regulations (regulatory affairs) of implantable devices.
For the simultaneous regeneration of osteochondral tissues, Natarajan et al. also added bioactive factors into their bilayered 3D printed scaffold.[ 74 ] PCL and PLGA were used for the chondral and subchondral layers, respectively, loaded with two bio‐factors. They added chondroitin sulfate (CS) in PCL and beta‐tricalcium phosphate (β‐TCP) in PLGA. More interestingly, they focused their strategy on 3D printed bilayered scaffolds having various infill densities, in terms of pore gradients and cell arrangement. Indeed, 3D printing technology is commonly known to be advantageous for controlling gradient composition/porosity/strength. They 3D‐printed a bilayered scaffold with an infill density which supported easy cell seeding at the top of the scaffold and prevented cell leakage during the seeding at the bottom. In vitro experiments showed that the open and interconnected pore structure of the scaffold enhanced cell adhesion/attachment, spreading and proliferation of MCSs. Moreover, the bilayered scaffold greatly induced the simultaneous differentiation of rabbit adipose‐derived multipotent cells (ADMSCs) into two cell lineages: chondrocytes (on the chondral layer) and osteoblasts (on the subchondral layer). Besides, the addition of bioactive factors promoted proliferation of ADMSCs and conveniently supported their differentiation.
In another work, Chen et al. explored the possibility to design a bilayered gene‐activated composite osteochondral scaffold, by using growth factor plasmids to induce MSCs differentiation.[ 75 ] They chose hydroxyapatite (HAP), chitosan and gelatin as raw materials for the scaffold, which was fabricated by conventional methods (namely mixing, molding and freeze‐drying). Indeed, previous studies have shown that chitosan/gelatin‐based scaffolds were ideal candidates to simulate cartilage ECM formation and provide a favorable matrix for chondrogenesis.[ 76 , 77 ] On the other hand, HAP has been naturally used for its composition, similar to human bone. In more details, a mix of HAP/chitosan‐gelatin (HCG) was used for design the subchondral bone layer, whereas chitosan/gelatin (CG) was used for the hyaline cartilage layer. For gene activation, they directly incorporated transforming growth factor‐β1 (TGF‐β1) and BMP‐2 plasmids in chondral and subchondral layers, respectively, and seeded MSCs in these layers (Figure 10 ). In vitro results demonstrated that spatially controlled and localized gene delivery could induce by MSCs expression and differentiation of the specific proteins of each layer: collagen type II and aggrecan, which are cartilage‐specific markers, and osteonectin, osteopontin and collagen I, which are bone‐specific markers. This was done in vitro for 14 days before differentiation into chondrocytes and osteoblasts, respectively. In the same study, osteochondral repair was evaluated in rabbit knee defect model. The results showed simultaneous cartilage and bone regeneration, with the appropriate restoration of the osteochondral architecture. Finally, the authors developed a promising strategy, based on a gene delivery system and multidifferentiation from a single stem cell population, to promote the engineering of complex tissues.
Figure 10.

Schematic representation of the preparation of the bilayered gene activated composite osteochondral scaffold: the hyaline cartilage layer was made with a mix of chitosan‐gelatin (CG) whereas HAP/chitosan‐gelatin (HCG) were used for the subchondral bone layer. pTGF‐ β1: plasmid TGF‐ β1; pBMP‐2: plasmid BMP‐2; MSC: mesenchymal stem cell; CG: chitosan‐gelatin; HCG: hydroxyapatite/chitosan‐gelatin. Reproduced with permission.[ 75 ] Copyright 2011, Elsevier.
Human bone ECM is a complex structure that comprises an organic component based on a network of collagen fibers reinforced with an inorganic phase composed of phosphate crystals.[ 78 ] Current osteochondral‐based materials refer to natural and synthetic polymers, even metallic materials. But recently, a specific class of materials—namely bioceramics, which are made with calcium phosphate (CaP) materials—have been proposed as the main constituent of osteochondral scaffolds.[ 79 ] Indeed, they possess unique bioactive properties like osteoinductivity (capacity to induce osteogenesis) and osteoconductivity (capacity to bone grow on a surface), making them suitable candidates for bone regeneration.
In OCTE, CaP‐based materials have been investigated alone, but several studies have reported their combination with proteins.[ 80 ] Among a lot of suitable polymers, Yan et al. made the combination of SF and CaP attractive for bone and osteochondral regeneration.[ 81 ] They developed a bilayered scaffold composed of i) a SF chondral layer and ii) a SF‐nanoCaP subchondral layer. Briefly, physicochemical characterization revealed good mechanical properties (compressive modulus), homogeneous porosity distribution, and CaP distributed in the silk‐nanoCaP layer, without any migration of this CaP migration in the chondral layer. In vitro, cultivated rabbit bone marrow mesenchymal stromal cells (BMSCs) attached well and proliferated on the scaffold with a good viability. Subcutaneous implantation in rabbit knee defects showed that after 4 weeks the scaffold was well‐integrated into the host tissue and supported tissue ingrowth and angiogenesis, with no real sign of inflammation. Histological and immunohistochemical staining confirmed cartilage regeneration in the top silk layer and novo bone ingrowths and vessel formation in the silk‐nanoCaP layer.
Four types of CaP materials are commonly used in bone/osteochondral TE: namely hydroxyapatite (HAP), tricalcium phosphate (TCP), biphasic calcium phosphates (BCP), and amorphous calcium phosphates (ACP).[ 82 ] Combining natural or synthetic polymers with these calcium phosphate materials is a promising strategy for OCTE.
Seong et al. originally mixed collagen (type I) with CaP‐based materials, namely BCP, to mimic the cartilage and bone tissue, respectively.[ 83 ] They focused on the stratified design of aligned channels in a bilayered scaffold to enhance the efficiency of osteochondral tissue repair. Indeed, it is already known that the structure (pore orientation and pore size) of a scaffold can play a critical role in TE, particularly here in cell migration up to the cartilage region.[ 84 ] Aligned BCP/collagen scaffolds were successfully fabricated by sequential coextrusion and unidirectional freezing. Aligned structures exhibited significantly better mechanical properties compared to random structures. As shown in Figure 11A, cells could spread along the aligned channels which provide a driving force for their migration up to the damaged cartilage zone. In vitro evaluations demonstrated that: i) aligned channels effectively guided preosteoblast cells (MC3T3‐E1) to attach to the structure in highly stretched shapes, thereafter, migrating upward faster (Figure 11B); ii) aligned channels supported a superior osteochondral tissue regeneration compared to the random structure; and iii) smaller channels (140 µm) exhibited better cell migration and proliferation. At the same time, in vivo analysis performed on rabbit osteochondral defect model significantly revealed that bilayered scaffolds with aligned channels having an optimal channel diameter of 270 µm exhibited an accelerated BMSC migration and higher osteochondral regeneration, compared with a random porous structure. For their part, Ribeiro et al. chose β‐tricalcium phosphate (β‐TCP), and more specifically ion‐doped β‐TCP with pure zinc (Zn) and strontium (Sr).[ 85 ] Indeed, doping β‐TCP with the combination of Zn and Sr (elements existing in the bone) significantly enhances mechanical properties of the scaffold and positively affects human adipose‐derived stem cell growth and osteogenesis. More precisely, the researchers fabricated a bilayered scaffold composed of: i) horseradish peroxidase (HRP)‐cross‐linked silk fibroin (SF) as a cartilage‐like layer fully integrated into a ii) HRP‐SF/ZnSr‐doped β‐tricalcium phosphate subchondral bone‐like layer. The ion‐doped bilayered scaffolds presented high mechanical properties, controllable porosity, and TCP distribution. In terms of biological performances, in vitro coculture of human osteoblasts (HOBs) and human articular chondrocytes (HACs) was performed: the bilayered scaffold exhibited adequate properties for cell proliferation, infiltration, and ECM production. Nevertheless, in vivo evaluations need to be done.
Figure 11.
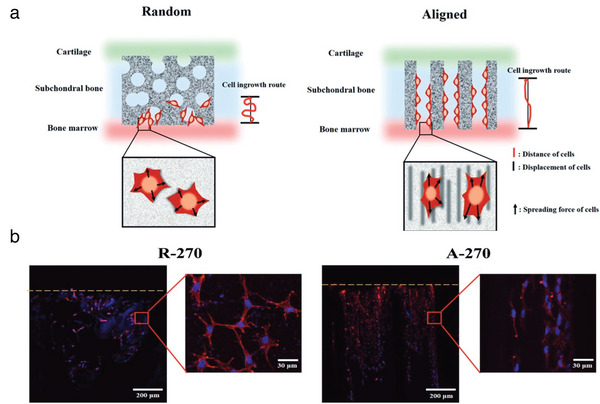
a) Schematic representation of hypothetic cell migration in the random and aligned structures randomly structure compared to the aligned structure. b) Observation of migrating cell morphology on BCP‐based bilayered scaffold through R‐270 (Random structure‐270 µm pore size) and A‐270 (Aligned structure‐270 µm pore size) after 7 d of seeding. The yellow line indicates the top surface of the bilayered scaffold. The yellow line represented the top surface of the BCP scaffold. Adapted with permission.[ 83 ] Copyright 2017, Wiley‐VCH GmbH.
Among other materials, HAP represents nearly 65% of the weight of mineral phase of human bone tissue and is thus one of the best choice as osteochondral scaffold material.[ 86 ] HAP plays an important role in cartilage and bone TE and induces a biological activity with a full biocompatibility and no toxicity. In this regard, Liu et al. proposed a biomimetic scaffold made with a mix of human‐like‐collagen (HLC), hyaluronic acid (HA), and HAP particles to optimally simulate the composition and structural characteristics of natural cartilage and bone.[ 87 ] By combining three techniques—liquid phase synthesis, chemical cross‐linking and freeze‐drying—they designed a bilayered hydrogel scaffold consisting of: i) a macroporous HLC/HA chondral layer and ii) a small‐porous HLC/HA/HAP subchondral layer. All the tested physiochemical characteristics were similar to natural osteochondral support and suitable for new tissue formation: pore size (chondral layer: 120–300 µm; subchondral layer: 20–80 µm), high levels of porosity, and excellent mechanical properties. In vitro assays indicated that the scaffold was highly biocompatible and allowed significant human BMSC adhesion and proliferation with normal morphology. Moreover, in vivo experiments on rabbit models revealed that bilayered scaffold showed an effective repair and reconstruction of subchondral bone and cartilage, with a complete closure of the defect at the end of 12 weeks after surgery (Figure 12 ).
Figure 12.
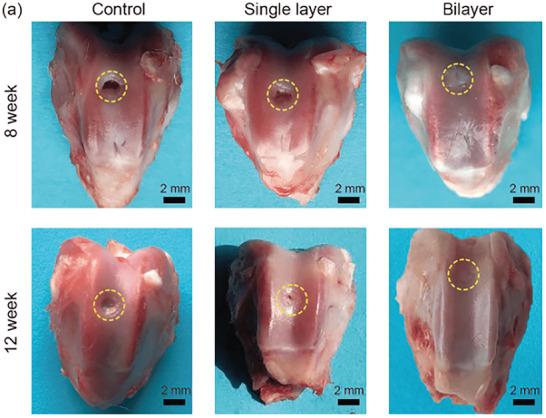
Macroscopic evaluation of defect site of the control group (left in blank without any processing), single cartilage layer (HLC‐HA) and bilayered scaffold (HLC‐HA cartilage layer and HLC‐HA‐HAP subchondral) after 8 and 12 weeks after surgery. Reproduced with permission.[ 87 ] Copyright 2020, Science China Press and Springer‐Verlag.
In another study, Zhu et al. introduced HAP particles of two different sizes into both layers of their bilayered hydrogel scaffold: i) micro‐HA in the chondral layer for promoting cartilage matrix deposition and ii) HA nanocrystals in the subchondral layer for enhancing osteogenesis.[ 88 ] Furthermore, a major innovation consists in the use of a double‐network (DN) hydrogel system with the incorporation of bacterial cellulose (BC) to solve the poor mechanical properties of biopolymer‐based hydrogels. With its hydrophilic ultrafine fibers, bacterial cellulose can easily make chemical hydrogen bonds with the DN structure, resulting in a high‐strength biohydrogel. Their DN biohydrogel consisted of two polymer networks composed of i) poly(γ‐glutamic acid), lysine, and alginate, and ii) bacterial cellulose fabricated via chemical and physical cross‐linking. All the physicochemical characterizations (mechanical/rheological/morphological properties) and swelling behavior, as well as in vivo assays on osteochondral defect model of rabbits clearly showed that these bilayered scaffolds can be promising candidates for osteochondral regeneration. Recently, Kumbhar et al. also investigated if BC can be used in the treatment of osteochondral defects.[ 89 ] BC is widely used for biomedical applications for its unique physicochemical properties[ 90 , 91 ] and has already been explored as a biomaterial scaffold for cartilage TE.[ 92 , 93 ] To closely mimic cartilage and bone, the researchers designed a bilayered scaffold with, respectively: i) a BC‐glycosaminoglycans (chondroitin‐6‐sulfate) (BC‐GAGs) layer; and ii) a BC‐hydroxyapatite (BC‐HA) layer. They claimed being the first to develop high‐performing BC‐based acellular scaffolds for the repair of OCD. In vitro, the bilayered scaffold showed good biocompatibility and supported attachment and proliferation of HOBs and HACs. In vivo, subcutaneous implantations in rat model allowed tissue ingrowth, cartilage regeneration with deposition of ECM and regeneration of subchondral bone by the host cells, without any immunological reaction.
In another study, Sartori et al. incorporated magnesium‐doped hydroxyapatite (Mg/HA) crystals coprecipitated into collagen (type I) to design the subchondral layer, whereas the chondral layer was simply made with collagen (type I).[ 94 ] Being the most abundant of human tissues, Type I‐collagen was evidently chosen for its biocompatibility, low toxicity, biodegradability, and ability to guide cell proliferation and differentiation. Magnesium, highly present in bone structure, plays a key role in bone metabolism by stimulating, among others, osteoblast proliferation.[ 95 ] Therefore, with such elementary components, the results of the study confirmed that the bilayered scaffold was able to sustain human MSC attachment, proliferation, and chondrogenic and osteogenic differentiation with deposition of ECM. In vivo experiments with nude mice have only confirmed the potential of this scaffold in TE with bone and chondral neo‐tissues formation, tissue growth, and neoangiogenesis.
Recently, biodegradable polymer/hydroxyapatite composites as bone graft substitutes have been investigated. Nano‐hydroxyapatite/polyamide6 (n‐HAP/PA6) was reported as a good choice among all synthetic bone materials, due to its close composition and mimicking structure to natural bone minerals.[ 96 ] In another study, Li et al. used n‐HA/PA6 for the subchondral bone layer, and PVA/gelatin/vanillin for the cartilage layer to fabricate a PVA/gelatin/vanillin‐n‐HA/PA6 bilayered scaffold.[ 97 ] It is pointed out that vanillin, a natural phenolic aldehyde, could be added to the blend to improve the miscibility of polyvinyl alcohol (PVA)/gelatin composite by cross‐linking reactions and may also improve antioxidant and anti‐inflammatory activities. Additionally, an intervening nonporous PVA/gelatin layer allowed to bond of the two distinct porous layers together. In vivo implantation to rabbit knees for 12 weeks revealed that the cell‐seeded bilayered scaffold, carrying chondrogenically and osteogenically induced BMSCs on both layers, respectively, was able to successfully repair the osteochondral defect with similar characteristics to the native surrounding tissue. Histological examinations also confirmed the formation of cartilage‐like and underlying bone‐like tissues at 6 and 12 weeks after implantation, with a good integration with the native osteochondral tissues (Figure 13 ).
Figure 13.
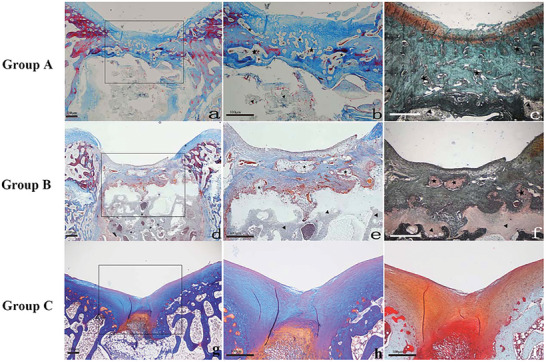
Histological examination of PVA/Gel/V‐n‐HA/PA6 scaffolds at 12 weeks. a,b,d,e,g,h) Refer to Masson's trichrome stain, whereas c,f,i) to safranin‐O stain. a) Group A—cell‐seeded bilayered scaffold (×20). b,c) Detail of the PVA/gelatin/vanillin zone (×40). d) Group B—bilayered scaffold (×20). e,f) Detail of the PVA/gelatin/vanillin zone (×40). g) Group C—control group (×20). h,i) Detail of the defect zone (×40). The triangle symbol refers to the nHA/PA6 scaffold and the star symbol refers to the PVA/gelatin/vanillin scaffold. Reproduced with permission.[ 97 ] Copyright 2015, John Wiley & Sons.
Table 3 summarizes current TE strategies and shows how OCTE with bilayered scaffolds emerged as a potential alternative for repairing OCD. In this field of TE, we reviewed many approaches in recent studies: freeze‐drying, cross‐linking, or particulate leaching, which are used to create bilayered scaffolds suitable for the regeneration of OCD with controlled porosity, interconnected pores, and proper mechanical properties. However, these conventional methods are not efficient enough to fabricate an ideal substitute mimicking the native osteochondral tissues. Scaffolds for OCTE require to meet higher standards: uniform pore size, high interconnectivity, and high porosity. We think that this drawback can be reduced by introducing some other techniques such as electrospinning, 3D printing/bioprinting, and a combination of molding techniques. Considering this, many studies have been carried out but focusing on multilayered scaffolds.[ 98 , 99 , 100 , 101 ] In the future, we suggest that it can be easily extended to the fabrication of bilayered scaffolds.
Table 3.
Brief summary of raw materials and fabrication methods used in all the articles described above in osteochondral TE and using biomimetic bilayer scaffolds
| Clinical application | Raw materials | Fabrication methods | Refs. | ||
|---|---|---|---|---|---|
| Osteochondral TE | Building bilayered scaffolds with one component | ||||
| Synthetic materials |
Chondral layer Subchondral layer |
PLGA | Molding/particulate leaching method | Pan et al. [68] | |
| Building bilayered scaffolds with more than one component | |||||
| Blend of synthetic materials |
Chondral layer Subchondral layer |
PCL/PLGA | 3D‐printing | Natarajan et al. [74] | |
| Blends of natural/synthetic/bioceramics materials |
Chondral layer Subchondral layer |
Chitosan/gelatin HAP/chitosan/gelatin |
Mixing/molding/freeze‐drying | Chen et al. [75] | |
|
Chondral layer Subchondral layer |
PVA/gelatin/vanillin n‐HAP/PA6 |
Mixing/molding/cross‐linking | Li et al. [97] | ||
|
Chondral layer Subchondral layer |
SF SF/nanoCaP |
Mixing/molding/freeze‐drying | Yan et al. [81] | ||
|
Chondral layer Subchondral layer |
BC‐GAG BC‐HAP |
Mixing | Kumbhar et al. [55] | ||
|
Chondral layer Subchondral layer |
Collagen Mg/HAP |
Mixing/cross‐linking | Sartori et al. [94] | ||
|
Chondral layer Subchondral layer |
Collagen BCP |
Coextrusion Freeze‐drying |
Seong et al. [83] | ||
|
Chondral layer Subchondral layer |
BC/HAP | Cross‐linking | Zhu et al. [88] | ||
|
Chondral layer Subchondral layer |
HRP/SF HRP‐SF/ZnSr‐doped β‐TCP |
Salt leaching/freeze‐drying | Ribeiro et al. [85] | ||
|
Chondral layer Subchondral layer |
Gelatin/SF/dextran PLLA/PLGA/PCL |
Cross‐linking Dual‐phase separation |
Zheng et al. [73] | ||
|
Chondral layer Subchondral layer |
HLC‐HA HLC‐HA‐HAP |
Liquid phase synthesis/cross‐linking/freeze‐drying | Liu et al. [87] | ||
Furthermore, we observed that bilayered scaffolds in OCTE were usually targeted to repair only cartilage and bone phases, but never the whole osteochondral tissue structure: they typically neglect the calcified cartilage zone. We think that this is one of the limitations of bilayered scaffolds in the specific field of OCTE, and it would be better to further continue developing multilayered OC scaffolds, considering each native osteochondral regions (Figure 8).[ 71 ] Some recent studies and reviews have already made considerable efforts to meet the demand.[ 66 , 102 , 103 , 104 ] However, increasing the number of phases will increase the complexity of fabrication. By the way, it has already been reported that moving to more than three phases, specifically to address the precise buildup of each 4 zones of the cartilage, results in the breakdown of the cartilage phases into its different zones.[ 98 ]
5. Skin Tissue Engineering
Skin is the largest organ of the human body, as it represents nearly 7% of the total adult body weight and 2 m2 of the surface area in adults.[ 105 ] It acts as a protective barrier against external physical, chemical, and biological agents. In more detail, the integumentary system protects the body from i) physical assaults as UV irradiation thanks to melanocytes; ii) heat/cold shock and minor cuts, scratches, or abrasions thanks to keratin; iii) chemical assaults, as irritants and allergens chemical compounds, thanks to the tough and waterproof skin structure's; and iv) microbial assaults as bacteria and fungi thanks to Langerhans cells. Skin also plays a role in thermoregulation and maintains normal hydration levels. There is a wide range of skin pathologies that lead to large defects: burns, trauma, and genetic defects.[ 106 ] In case of deep injuries or serious skin tissue damages, natural healing is not always successful, which can lead to chronic wounds. Chronic wounds are wounds that fail to heal through the normal phases of wound healing, heal slowly, or heal but tend to recur. They include diabetic foot ulcers, pressure ulcers, arterial insufficiency ulcers and venous stasis ulcers.[ 107 ]
5.1. Anatomical Structure of Skin
At the outermost layer of the body, the skin is formed by three layers: epidermis, dermis, and hypodermis. The epidermis is the thinnest and the most external layer of skin. It's an avascular epithelial tissue, composed of multiple layers of keratinocytes, which differentiate and mature as they move toward the surface of the skin.[ 108 ] The basal layer (the inner layer of the epidermis) contains a single layer of keratinocytes, which divide and move through a granular layer to the stratum corneum (the uppermost layer of the skin) composed of an association of dead keratinocytes.[ 109 , 110 ] The epidermis provides a waterproof barrier that ensures protection against pathogens and foreign substances.[ 109 , 111 ] The basal layer also contains melanocytes producing melanin, a skin color pigment that protects skin from sunlight's radiation. Epidermis also contains Langerhans cells, which are part of the skin immune system.[ 108 , 111 ] Under the basal layer, the basement membrane separates the epidermis from the dermis. Dermis is just below the epidermis and is the thickest layer of skin. It is a fibrous connective tissue made of fibroblasts, blood vessels, lymph ducts, nerves, glands, and hair roots. Fibroblasts secrete collagen and elastin, thus providing mechanical strength and elasticity to the skin.[ 111 ] Blood vessels provide nutrients. Hypodermis is the innermost layer, a subcutaneous layer containing adipose tissue, blood vessels, and nerve. It has a thermoregulation role and connects the skin with the internal organ system.[ 108 ]
The structure and major components of normal human skin are presented in Figure 14 .
Figure 14.

Anatomical structure of the skin. a) Cross‐section through the skin. b) Layers of skin. Adapted with permission.[ 112 ] Copyright 2019, EMAP Publishing Ltd.
5.2. Strategies and Approaches
Skin grafts can be a solution, but remain limited in donor availability.[ 113 ] Moreover, currently, no bioengineered skin completely simulating the complex skin tissue, either in form or function, has been reported. Thus, a strong demand for skin substitutes encourages researchers to look for an ideal skin substitute mimicking native skin, for regeneration and wound healing.
5.2.1. Building Bilayered Scaffolds for Skin TE with More Than One Component
Synthetic biodegradable polymers, such as PLA, PGA, PLGA, PCL, poly(glycolide‐co‐caprolactone) (PGCL), and PLCL have been commonly used for skin TE. Among them, PLCL has been reported as a good candidate for skin tissue repair.[ 114 ] However, PLCL degradation contributes to local acidification of the environment, which is not in favor of skin regeneration. To overcome this, Pan et al. have demonstrated the advantage of blended PLCL with poloxamer, a nonionic surfactant widely used in the biomedical field. This poloxamer can neutralize the production of lactic acid during PLCL degradation in the body.[ 114 ] In their study, they fabricated the “epidermal layer” of their bilayered scaffold with electrospun PLCL/poloxamer nanofibers membrane. A dextran/gelatin hydrogel matrix was used for the dermal layer. Mechanical performances (tensile strength and modulus) of PLCL/poloxamer nanofiber membranes demonstrated good resilience and compliance properties useful to build the protective barrier. Satisfactory biocompatibility was also demonstrated and allowed to support adipose‐derived stem cell proliferation. Additionally, the dextran/gelatin hydrogel as dermal layer appeared favorable for applications in skin tissue repair with high swelling property, good compressive strength, and adequate surface area, allowing adipose‐derived stem cell proliferation and ECM production.
On the other hand, synthetic polymers can also be combined with natural polymers to form a composite material and to improve cellular compatibility.[ 115 ] Monteiro et al. have designed an in situ forming dermal‐epidermal scaffold to treat full‐thickness skin defects.[ 116 ] The dermal component was made of fibrin and cross‐linked hyaluronic acid (HAX) gel. Interestingly, this gelling dermal layer, containing human dermal fibroblasts, can be directly applied onto the lesion and can suit differing lesion shapes (Figure 15A). Then, the epidermal component composed of a rugged hyaluronic acid membrane preseeded with keratinocytes has to be placed on top of the dermal component once it is jellified (Figure 15B). The epidermal component was also combined with poly‐L‐lysine (PLL) to provide anchoring to the dermal layer with covalent imine bonds (between free amines of PLL and aldehydes from HA in the dermal component) (Figure 15C). Additionally, they also coated it with laminin‐5, an adhesion protein to significantly enhance cell attachment of keratinocytes (Figure 15B). Finally, the dermal component had the desired mechanical properties and allowed 3D spreading of human fibroblasts, forming a dermal matrix. The epidermal component showed the right robustness and allowed the creation of a monolayer of keratinocytes, with proliferation and a rapid re‐epithelialization of a full‐thickness skin defect. Moreover, the epidermal component also protected the dermal component from dehydration, mechanical disruption and infection. This bilayered substitute forming a single composite scaffold in situ is promising to mimic the skin environment and to optimize the skin regeneration. However, with a total of five different products and two cell models, it remains a complex strategy that will be hard to scale‐up at the industrial level. Further investigations are needed to evaluate the performance of the scaffold in animal models.
Figure 15.

Schematic representation of the bilayered epidermal‐dermal scaffold. a) In a clinical context, the dermal component (hydrogel), containing dermal human fibroblasts, would be injected directly into the lesion. It would instantly cross‐link in situ and adapt to the shape of lesion. b) Then, the epidermal component, pre‐seeded with keratinocytes is applied on top of the dermal layer. c) Fibroblast‐containing dermal matrix (blue) and keratinocyte‐containing epidermal membrane (pink) are linked together by covalent imine bonding (amine‐aldehyde interactions). PLL and HAX abbreviations refer to poly‐L‐lysine and cross‐linked hyaluronic acid, respectively. Reproduced with permission.[ 116 ] Copyright 2014, Elsevier.
In another study, Ghafari et al. used cellulose nanofiber (CNF)/PVA blend (as dermal/epidermal layer respectively) for mimicking the complex bilayered structures of natural skin tissue.[ 117 ] The innovative part of this project lies in the set‐up of a one‐step freeze‐drying method to fabricate a bilayered porous scaffold with different porosities and pore sizes. Briefly, the procedure of fabrication comprised: i) a first step of CNF/PVA‐cross‐linked mixtures at different concentrations followed by ii) a one‐step freeze‐drying for assembling the dermal and the epidermal layers. Freeze‐drying appears as an appropriate method to adapt porosity and pore size by modulating different factors such as volume fraction of the dispersed phase, polymer concentration, molecular weight of polymers, and growth of ice crystals. PVA was chosen for its elasticity and flexibility properties. Cellulose has always demonstrated excellent mechanical properties (high tensile strength), water‐insolubility, high swelling capacities, hydrophilicity, and nontoxicity.[ 118 ] Results have demonstrated that polymer concentration was one of the factors affecting the most the porosity and the pore sizes: i) porosities of 95.32% and 88.53% were observed for dermal and epidermal respectively, with a highly interconnected porosity (SEM analysis), and ii) pore size of 90.71 ± 2.4 and 19.72 ± 3.6 µm were measured for dermal and epidermal layers, respectively. Fibroblasts and keratinocytes were seeded on both layers of the scaffold to evaluate the biocompatibility and it demonstrated a high cell viability. This study was the first to set up a one‐step freeze‐drying technique having the ability to control material composition, pore size and porosity in each layer by adjusting polymer concentration, and to use this technique to obtain a completely integrated TE to host tissue scaffold for skin repair.
Using a similar technique, Ooi et al. designed a bilayered hybrid scaffold only made of natural origin components to produce a wound dressing: originally, it was composed of ovine collagen and plant nanocellulose blend, in which genipin (a natural cross‐linker) has been added to improve mechanical strength and enhance fibroblast attachment.[ 119 , 120 ] First, the authors mentioned that the resulting composite scaffold of collagen and nanocellulose exhibited the beneficial properties from each of them: that is to say, low densities, high porosities, strong water absorption, and good mechanical strength. Afterwards, they have shown good water absorption capacity to maintain a moist environment and swelling properties to provide essential nutrients for the cells, and high porosity with interconnectivity to facilitate cell growth, cell proliferation and thus provide neovascularization. Nevertheless, cell adhesion, proliferation, as well as biocompatibility needed to be further investigated.[ 121 ]
In another study, Bektas et al. observed similar results for their bilayered mimicking skin graft, such as high‐water content, an interconnected porosity, and an appropriate stability, as in terms of degradation rate samples remained intact after 14 days of incubation and an appropriate elastic modulus.[ 122 ] Differentiating from Ooi et al., the bilayered scaffold was composed of: i) a collagen‐based sponge alone (BLColl) or in combination with chondroitin sulfate (BLCollCS) as an epidermis layer; and ii) a sodium‐carboxymethyl‐cellulose‐based (NaCMC) sponge for the dermis layer of the skin. Coculture on the bilayered scaffold was tested by seeding fibroblasts on the dermis layer and keratinocytes on the epidermis layer (BLColl and BLCollCS). This cell culture study demonstrated a better keratinocytes attachment (with stronger collagen type I and III expression) and proliferation on BLCollCS support than BLColl, and unsuccessful expected fibroblastic attachment on the NaCMC dermis layer, probably due to the high‐water content and to the chemical composition. Based on these observations, they did not offer any other alternative as they explain their difficulties to modify the NaCMC layer by grafting or blending with cell attracting molecules.
When the skin is extensively injured, preventing bacterial contamination is one of the major challenges in skin TE. Indeed, bacterial infections can appear months to years after a surgical graft causing implant failure and patient suffering. Thus, novel strategies are focused on the integration into biomaterials of bioactive ingredients, such as antibacterial agents including antibiotics, nanoparticles, cationic organic agents, and others.[ 123 ]
In addition to their antibacterial effect, it is known that bioactive ingredients can also improve cell behavior and promote wound healing. In the case of the use of nanoparticles as antibacterial agents, metallic nanoparticles with various metals (copper, gold, titanium, or zinc among others) have been widely used, but silver exhibited the strongest antibacterial activity against anaerobic, aerobic, Gram‐positive and Gram‐negative bacterial strains.[ 124 ] Cakir et al. aimed to design an antibacterial scaffold by adding silver sulfadiazine (AgSD) into their blend.[ 125 ] In fact, they combined: i) a silk fibroin‐based nanofibrous layer as an epidermis layer; and ii) a freeze‐dried porous silk fibroin spongy layer as a dermis layer. Furthermore, heparin was added to the dermal layer for the stimulation of fibroblast adhesion and growth, whereas antibacterial activity was emphasized by adding AgSD to the epidermal layer. The antibacterial properties of the bilayered scaffold were tested against Staphylococcus aureus bacteria, the most common pathogen involved in skin infections.[ 126 ] Bacterial growth was inhibited for AgSD concentrations of more than 10% loaded into the nanofibrous layer.
In order to design innovative materials, Shaik et al. have reported a bi‐functional—antioxidant and antibacterial—bilayered scaffold to manage infections and oxidative stress wound healing. Oxidative stress plays an important role in all phases of wound healing: free radicals are generated in the wound by the inflammatory cells themselves, and they are able to regulate the healing process. However, in some cases, an excess of oxidative stress could lead to tissue damaging.[ 127 ] The authors have hypothesized that the simultaneous incorporation of an antibacterial agent (namely silver) and an antioxidant compound (namely silymarin (SM)) into the composition could enhance healing in case of chronic wounds. They combined layer upon layer: i) a chitosan‐silver (CS‐Ag) layer with ii) a chitosan‐collagen‐silymarin layer (CS‐CO‐SM) to mimic the dermal and epidermal nature of the skin, respectively. In vivo experiments on rats demonstrated that the bilayered scaffold accelerated the process of wound healing with the absence of inflammatory cells, proliferation of fibroblasts and neovascularization. Ten days post injury, a complete re‐epithelization of tissue sections was observed, showing a structure similar to normal skin.
On the same topic, Wang et al. mimicked the bilayered structures of the dermis (papillary dermis and reticular dermis) by developing a collagen/chitosan‐based scaffold loaded with active ingredients to deliver two supplementary biological activities: a rapid angiogenesis based on the use of recombinant human vascular endothelial growth factor (rhVEGF), and an antibacterial activity from gentamicin.[ 128 ] rhVEGF and gentamicin were loaded into PLGA microspheres, which have been added into the collagen/chitosan blend in low (lower layer) and high (upper layer) concentrations. In fact, microspheres were pertinently used to prolong drug release, with a long‐term release exceeding 28 and 49 days for gentamicin and rhVEGF, respectively. Then, an additional molding step was required to build the bilayered scaffold. To evaluate the antibacterial property of the dermal scaffold, S. aureus and Serratia marcescens were cultured with the dermal scaffold for 1 day to 7 days. With an inhibition zone of 1.84 cm in diameter on S. marcescens, the dermal scaffold (“test group”: bilayered dermal scaffold with gentamicin‐loaded PLGA microspheres) demonstrated a notable antibacterial effect (Figure 16A) in comparison to negative control (Figure 16C). Positive control (collagen/chitosan/gentamicin complex) showed a stronger antibacterial effect (inhibition zone: 2.98 cm in diameter) (Figure 16B), but probably due to the presence of “free” gentamicin in the complex more rapidly released, compared to sustained release of gentamicin‐loaded microspheres (Figure 16D). However, S. marcescens and S. aureus seemed to be sensitive to gentamicin‐loaded PLGA microspheres incorporated into the dermal scaffold. Additionally, in vitro release kinetics demonstrated a long release period close to two months for rhVEGF‐loaded PLGA microsphere, helping vascular regeneration and skin repair during the remodeling periods. Moreover, cocultured fibroblast behavior on the lower layer and upper layer were separately examined. After 3 days of incubation, results indicated that both layers were suitable for mouse fibroblast adhesion and proliferation.
Figure 16.
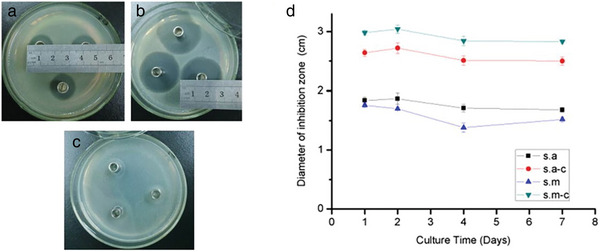
Petri‐dish showing the antibacterial activity of gentamicin after 1 d of culture with: a) Serratia marcescens test group (bilayered dermal scaffold with gentamicin‐loaded PLGA microspheres); b) Serratia marcescens positive control group (collagen/chitosan/gentamicin complex); c) Serratia marcescens negative control group (collagen/chitosan scaffold). d) Diameters of inhibition zone with the culture time. s.a refers to Staphylococcus aureus; s.m refers to Serratia marcescens; s.a‐c and s.m‐c refer to their positive control group (referring to (collagen/chitosan/gentamicin complex). Adapted with permission.[ 128 ] Copyright 2015, Elsevier.
All these studies, summarized in Table 4 , describe different ways to develop innovative bilayered biomimetic scaffolds in the field of skin TE. Globally, reported scaffolds can become potential candidates for skin tissue repair and wound healing. However, too complex systems with many different components/structures are risky, as they can fail to reach an application due to difficulties encountered in the industrial process, i.e., in regulation and also production. For example, a risk could be difficult to manage during the regulation process: a scaffold composed of two layers of different material compositions can be subject to delamination during its implantation, and all the benefits of the transplant may be lost. At the moment, most of the skin scaffolds are monolayers, such as hydrogels and nanofibers that can promote wound healing.[ 129 , 130 ]
Table 4.
Brief summary of raw materials and fabrication methods used in all the articles described above in skin TE
| Clinical application | Raw materials | Fabrication methods | Refs. | ||
|---|---|---|---|---|---|
| Skin TE | Building bilayer scaffolds with more than one component | ||||
| Blends of natural materials |
Upper layer Lower layer |
Collagen/chitosan | Mixing/molding | Wang et al. [128] | |
|
Dermal layer Epidermal layer |
Collagen/nanocellulose | Cross‐linking | Ooi et al. [121] | ||
| Blends of natural/synthetic/mineral materials |
Dermal layer Epidermal layer |
PLCL/poloxamer Detran/gelatin |
Electrospinning Cross‐linking |
Pan et al. [114] | |
|
Dermal layer Epidermal layer |
Fibrin/HA HA/PLL |
Cross‐linking | Monteiro et al. [116] | ||
|
Dermal layer Epidermal layer |
NaCMC Collagen |
Cross‐linking/freeze‐drying | Bektas et al. [122] | ||
|
Dermal layer Epidermal layer |
Silk fibroin Silk fibroin/AgSD |
Freeze‐drying Electrospinning |
Cakir et al. [125] | ||
|
Dermal layer Epidermal layer |
Chitosan/Ag Chitosan/collagen/silymarin |
Mixing/freeze‐drying | Shaik et al. [131] | ||
|
Dermal layer Epidermal layer |
Cellulose/PVA | Freeze‐drying | Ghafari et al. [117] | ||
6. Other Tissue Engineering Medical Applications
A few other TE approaches for medical applications reported the fabrication of bilayered scaffolds. It is the case for periodontal, urethral, and tracheal TE.
6.1. Periodontal Tissue Engineering
Periodontitis is an oral inflammatory disease characterized by destroying tooth‐supporting tissues, progressive teeth damage, and in the most serious cases, and tooth loss.[ 132 ] The main cause of periodontitis is poor oral hygiene, because it leads to the formation of bacterial plaque and tartar on the teeth surface, causing inflammation. It can also be caused by other risk factors such as smoking, diabetes, or stress. Periodontitis has also been closely associated with the occurrence of various systemic diseases, including cardiovascular diseases, cancer, obesity, or diabetes.[ 133 ] Therefore, an urgent public health need encourages researchers to find methods for treating periodontal defects. Several approaches for periodontal TE have been developed in the last years, based on the regeneration of multiple tissues: periodontium made up of hard tissues (cementum and alveolar bone) and soft tissues (gingiva and periodontal ligament).[ 134 ]
Sundaram et al. developed a bilayered construct to fully restore the architecture and the functionality of the native periodontium.[ 135 ] By using PCL‐based electrospun membrane and a chitosan‐calcium sulfate (CaSO4) scaffold, they succeeded in the simultaneous regeneration of the periodontal ligament and alveolar bone. They have shown that their bilayered constructs, consisting of a scaffold layer with osteoconductive material (CaSO4), can enhance the attachment, infiltration, and proliferation of human dental follicle stem cells (HDFCs). It can also induce differentiation into osteoblastic and fibroblastic cell types. More recently, on the same topic, Yu et al. combined self‐assembly and microstamping techniques to construct an intrafibrillarly mineralized collagen (IMC) and unmineralized parallel‐aligned fibrils, mimicking natural periodontal hard/soft tissues.[ 136 ] Results showed that their bilayered scaffold exhibited excellent bone regeneration potential. For the regeneration of the periodontal ligament, which corresponds to a soft tissue, they added concentrated growth factor (CGF) into the fibrin network, which potently reconstructed native periodontium.[ 137 ]
Periodontal diseases may also result in small bone defects, which imperatively need treatments based on guided bone regeneration (GBR) from bone substitute biomaterials or membranes.[ 138 ] One of the roles of GBR biomaterials is to prevent the invasion of epithelial cells. Regarding this, Martin‐Thomé et al. recently developed a GBR PLGA‐based dental membrane with two distinct layers: i) a dense film layer to prevent gingival epithelial cell invasion (growth impeding bone formation with its upper layer) and ii) a microfibrous layer to guide bone regeneration.[ 139 ] All the conducted multicentric clinical trials done between February 2015 and November 2015 (with 7 dental practices involving 26 patients) demonstrated the safety of the clinical use of this bilayered membrane for guided bone tissue regeneration in various dental surgery applications. A similar study was performed by Zahid et al. However, PU and PCL/bioactive glass (BG) were used as a nonporous lower layer and porous upper layer respectively (and having the same functionality as the two layers described by Martin‐Thomé et al.).[ 140 ] In vitro and in vivo studies revealed that the bilayered structure permitted fast healing without any inflammatory response and was enhanced by the bioactivity of BG nanoparticles. It can be used as a potential biomaterial for guided bone regeneration in periodontal applications. Another possibility to design bioactive GBR membranes was found by Tamburaci and Tihminlioglu,[ 141 ] who focused on the development of resorbable natural polymer‐based scaffold. For that, they used chitosan and Si‐doped nanohydroxyapatite particles for the microporous sublayer and chitosan/polyethylene oxide (PEO) nanofiber for the upper layer. All the results have clearly shown that their designed bilayer nanocomposite membranes have the potential for use in periodontal tissue regeneration.
6.2. Urethral Tissue Engineering
Urethra is a distensible tube that carries urine from the bladder out of the body. It not only plays the role of a urinary duct but, in males, also serves as a conduit for sperm during sexual acts.[ 142 ] It is composed of an epithelium lining on the lumen, a spongy submucosa and, finally, a smooth muscle layer. Several congenital birth defects (hypospadias, epispadias) or/and acquired pathologies (urethral strictures) can compromise the normal functionality of the urethra.[ 143 ] These abnormalities must require an extensive urethral reconstruction, because with time, it can lead to complications (e.g., stricture formation, graft failure).[ 144 ] TE may solve these problems and be a promising approach for urethra repair, even if urethral reconstruction continues to be a challenging field for urologists.[ 144 ]
Reconstruction by using scaffolds is one of the main approaches for urethral repair, with the use of various synthetic or natural biomaterials. The most common cell types used for urethral reconstruction are autologous urothelial cells, buccal mucosa cells, keratinocytes, fibroblasts, and smooth muscle cells.[ 145 ] In response to the lack of appropriate scaffolds that would support coculture, Lv et al. developed a bilayered scaffold comprising a microporous network of SF and a nanoporous BC layer.[ 146 ] It should be pointed out that epithelial cells and muscle cells are typically used to reconstruct urethral tissues.[ 147 ] The feasibility and potential of urethral reconstruction with these bilayered scaffolds were evaluated in dog urethral defect models. Coculture of lingual keratinocytes and lingual muscle cells confirmed the suitability (in terms of cell distribution, viability and cell morphology) of the SF‐BC scaffolds to provide urethral regeneration. In a previous report, Lv et al. had already tried to replace SF with potato starch (PS) (and combined it with BC) to build biomaterials for hollow organ reconstruction.[ 148 ] The bilayered BC/PS nanofibrous scaffolds led to enhanced wound healing and improved vessel formation when tested on dog urethral defects models. Lv et al. clearly claimed that these both scaffolds could be also used for other types of tissue‐engineered hollow‐organ including vascular, bladder, ureteral, bowel, and intestinal.
Biomaterials are widely used in tissue regeneration of the urinary bladder, which has a structure similar to the urethra with the adventitia, the muscular layer, the submucosa layer, and, finally, the urothelium. It is the case of the study performed by Zhao et al., in which they developed a bilayered scaffold for urinary bladder regeneration in a rat model.[ 149 ] The bilayered scaffold is composed of a i) silk fibroin‐based porous network and ii) an underlying natural acellular matrix (bladder acellular matrix graft). To better fit urethral requirements, this bilayered scaffold acts as a natural waterproof barrier and supports the needs of various cell types. With a such structure of scaffold, that could address the lack of appropriate scaffolding in this field. Briefly, all in vivo results demonstrated that the bilayered scaffolds may be a promising scaffold for bladder regeneration in the rat bladder augmentation model, with a fast regeneration of smooth muscle, blood vessels, and nerves.
6.3. Tracheal Tissue Engineering
The cases of airway cancers have been increasing in recent years. Nowadays, surgical procedure is considered the only curative treatment for extensive cancers of the trachea and larynx.[ 150 ] Total laryngectomy in patients with advanced (tracheo)laryngeal cancer causes postoperative complications such as swallowing difficulties and respiratory embarrassment, leading to a substantial loss of quality of life. To address this problem, an artificial larynx based on titanium restoring sphincter function and extending the remaining trachea was first implanted in human in 2012, but there was a lack of mechanical flexibility and a limited tissue integration.[ 151 ] Considering this, development of fully functional tissue‐engineered tracheal implants (with the formation of cartilage tissue, epithelium, and neovascularization) has emerged as a promising future for repairing tracheal defects.[ 152 ]
Few studies have reported preparation of a bilayered system for tissue‐engineered trachea. In one of these, Wu et al. discussed the fabrication of a bilayered tubular scaffold via traditional electrospinning and dynamic liquid electrospinning methods based on i) electrospun poly(L‐lactide‐co‐ε‐caprolactone) P(LLA‐CL)/collagen fibers for the inner layer and ii) P(LLA‐CL)/collagen yarns for the outer layer.[ 153 ] The in vitro analysis showed that the collagen matrix incorporated into the scaffold exhibited a better result in biocompatibility, tracheal epithelial adhesion, migration, and proliferation on both layers, compared to those without collagen matrix. Then, scaffolds were seeded with autologous tracheal cells, epithelial cells, and chondrocytes, on their specific layer respectively before being implanted in rat tracheal fascia for prevascularization. After processing of cellularization and prevascularization, scaffolds were then implanted in situ in rat trachea. After being implanted in vivo, these scaffolds induced regeneration and reconstruction of the tracheal tissue, as well as capillary neogenesis network growth. Romanova et al. also developed a well‐differentiated human airway epithelium by seeding primary human cells on a bilayered non‐woven scaffold.[ 154 ] From a common blend of polymers (chitosan/Gelatin/PLA), they fabricated a bilayered woven scaffold by electrospinning showing structural similarity to the natural ECM and with adequate mechanical properties. Lastly, O'Leary et al. combined collagen and hyaluronate to design a bilayered tracheobronchial epithelial scaffold to treat chronic respiratory disease.[ 155 ] They aimed to develop an in vitro physiologically representative tracheobronchial epithelial coculture model for a better understanding of native epithelial tissue. Briefly, scaffolds were made with collage‐hyaluronate copolymer by integrating: i) a thin film top layer for the epithelial cell culture into a ii) 3D porous (lyophilized) sublayer for coculturing. All the results have shown that the bilayered scaffolds supported the co‐culture of the epithelium (Calu‐3 cells) with fibroblasts cells (Wi38 lung cells) and thus it allowed to regenerate a submucosal tissue analog of the respiratory tract.
7. Conclusions and Future Perspectives
In this review, we offered an overview of recent strategy designs in the fabrication of bilayered scaffolds for the field of multilayer TE. The multilayer tissues described in this review are vascular, bone/cartilage, skin, periodontal, urinary bladder, and trachea tissues. In this field, scaffolds are key components aiming to fully restore the complex architecture and functionality of the targeted damaged tissue(s). For that, scaffolds should fill several requirements, they have to: i) provide a 3D appropriate environment for the cell adhesion, spreading, proliferation, and production of ECM; ii) provide sufficient mechanical strength to resist the stitching process during in vivo implantation and maintain its shape during the healing process (considering physiological constraints) while being easy for surgeons to handle; iii) possess a sufficient porosity to provide nutrients and oxygen to cells (and also waste removal) for a better ingrowth and regeneration of autologous tissue, and in obvious manners; iv) being biocompatible, biodegradable (with non‐toxicity of side products), non‐thrombogenic, resistant to infections and inducing healing response without inflammation.
We detailed many different techniques, divided into conventional and advanced fabrication techniques, to build biomimetic bilayered scaffolds providing the principle, procedure, and advantages/disadvantages of each technique. Conventional techniques include casting/particle leaching, freeze‐drying, phase separation method, and electrospinning for building 3D scaffolds with interconnected porous structures, but with certain limitations such as poor control of scaffold architecture, pore network, and pore size. To overcome these limitations, advanced fabrication techniques like 3D printing and bioprinting are the most widely used and have been developed as great alternatives to produce high porosity and a variety of achievable pore sizes with a wide range of material choices and easy process. Each technique has its own advantages and controllable variables allowing to modify the overall scaffold properties, but sooner or later becomes limited by its own drawbacks. It is quite hard to offer a unique perspective because some strategies like bioprinting are emerging but not yet fully mature. On the other hand, conventional methods like electrospinning are already studied and could provide some new solutions to solve clinical problems. There is no grail that currently stands out, and it is hard to imagine that one unique strategy will provide all the solutions needed in various domains of TE. That is why the combination of several manufacturing techniques has gained more attention, and we think that is the best strategy to mimic various layers of a single tissue. Through a detailed understanding of both tissue composition and properties, we look forward to future fabrication methods that can mutually strengthen different techniques to overcome all known limitations and improve the current function of biomedical scaffolds.
The selection of appropriate raw materials, such as commercially available natural polymers, synthetic polymers or bioceramics, and the modification of their properties, used individually or mixed together for taking advantage of each other's properties, is challenging. By the way, because of the tissue complexity, we observe a trend for increasing complexity in the manufacture of scaffolds to respond to clinical issues, in terms of manufacturing strategies and particularly in composition. We believe that scaffold manufacturing must be a subtle balance between simplicity and sophistication to mimic the architecture of the native tissue of interest. The development of the scaling‐up and manufacturing can be a long and complex process when several components and/or several methods are used. Therefore, the ratio between simplicity of the designed scaffold and clinical efficacy must be high enough, but to match all the following criteria: an easy scale‐up, a low‐cost production, optimized composition including raw materials and additive biomolecules, and a simple regulation stage with a significant benefit for the patient.
Finally, we not only discussed the in vitro results of the described bilayered scaffolds, but also in vivo studies in common animal models for multilayer TE, and we noted a gap between in vitro and in vivo research. We think that future articles need to focus more on animal studies to illuminate the bilayer scaffold performances in vivo and in clinical situations. In addition, most of the references did not address the immune reaction related to the material used, whereas it is necessary that the scaffold avoid a strong host immune response. We suggest for future research to concentrate also on the evaluation of the immune reaction of the designed materials; and why not to introduce the innovative immune‐inert biomaterials concept to bilayered scaffolds.[ 156 ]
According to all the articles described above, bilayered scaffolds seem to have promising future outlooks in TE. However, one of the challenges limiting the application of bilayered scaffolds for TE could be the impossibility to perfectly mimic certain targeted tissues (for instance in OCTE). It remains difficult to mimic the tissue in integrality and not to be limited to two distinct layers. For many applications, multilayer materials would be more appropriate, although some questions remain open and are still to be considered: the complexity of fabrication and, above all, tissue regeneration. Indeed, poor integration, reinforced by non‐sufficient adhesive strength between adjacent nonhomogeneous layers, can lead to the delamination and final failure in tissue regeneration. We found interesting to further focus on continuous‐gradient scaffolds, composed of a single matrix with gradient properties and do not exhibit individual layers, for problem solving.[ 65 , 157 , 158 , 159 ]
Another important parameter regarding bilayered scaffolds is their final clinical application. All scaffolds described in this review are currently not commercially available for medical healthcare, because they are only in the earlier stages of development (in vitro/in vivo preclinical studies) and need further validations before starting clinical trials (testing in humans). Very few bilayered scaffold designs have made it to human clinical trials in the field of TE. Some of them are on the way to markets or already commercially available for current clinical use in osteochondral repair[ 8 , 160 , 161 , 162 , 163 ]: i) MaioRegen (Med&Care) composed of (type I equine) collagen and magnesium‐enriched hydroxyapatite; ii) Agili‐C (CartiHeal), a porous and resorbable scaffold made up with aragonite and HA; iii) ChondroMimetic (Collagen Solution PLC) based on collagen, GAGs, and calcium phosphate; iv) TruFit (Smith & Nephew) which consists of calcium phosphate and PLGA/PGA, and v) Chondro‐Gide (Geistlich) a bilayer (smooth and compact top layer/rough and porous bottom layer) collagen I/III membrane. By contrast, there are a variety of commercial skin substitutes that are available in the market designed for use with specific clinical issues, but scaffolds that simultaneously bio‐mimic the dermal and the epidermal layers are limited in two products: Biobrane (Mylan Bertek Pharmaceuticals Inc.) made of nylon mesh and a silicone membrane implanted in porcine collagen, and Integra (Integra LifeSciences Corp.) which consists of a silicone membrane and matrix nylon fibers of cross‐linked bovine tendon collagen and a glycosaminoglycan (chondroitin‐6‐sulfate).[ 111 , 164 ] No supplementary commercial products have been reported in other TE fields but in view of the large amount of current research in this field, but we are certain that many new products on the market will certainly be available soon.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Acknowledgements
The authors acknowledge the Cancéropôle Est and the Institut Carnot MICA for their fundings.
Biographies
Christelle Bertsch is currently a Ph.D. candidate in the lab Inserm U1121 “Biomaterials and Bioengineering” under the direction of Prof. Christian Debry. She obtained her engineering diploma in materials and polymers from the Mulhouse National College of Chemistry (France) in 2021. Her Ph.D. work consists of finding natural‐based biomaterials as scaffolds for the laryngeal reconstruction using tissue engineering.

Hélène Maréchal is resident in ENT and head and neck surgery in the department of Prof. Christian Debry at the University of Strasbourg (France) since 2020. During her master of science, she is going to work in the lab Inserm U1121 “Biomaterials and Bioengineering” on tracheal and laryngeal reconstruction using tissue engineering.

Philippe Lavalle obtained a Ph.D. degree in biophysics in 1998 from the University of Strasbourg (France). Then he moved to the Biozentrum in Basel (Switzerland). He got the position of research director at Institut National de la Recherche Médicale (Inserm) in 2011. Since 2013, he is deputy director of the Inserm lab “Biomaterials and Bioengineering” in Strasbourg. He focuses his research on the design of new materials/coatings for health and has applied for seven patents and published more than 130 peer‐reviewed publications. He co‐founded the company SPARTHA Medical, devoted to producing antimicrobial personalized coatings.

Léa Fath obtained an M.D. degree in 2020 after completing an internship in ENT and head and neck surgery in the department of Prof. Christian Debry at the University of Strasbourg (France) and a Ph.D. degree in 2021 performed within the Inserm unit U1121 “Biomaterials and Bioengineering.” She is currently a physician in the ENT department of the University Hospital of Strasbourg (France). Her research work focuses on laryngeal and tracheal rehabilitation using aortic allografts and natural‐based biomaterial.

Bertsch C., Maréchal H., Gribova V., Lévy B., Debry C., Lavalle P., Fath L., Biomimetic Bilayered Scaffolds for Tissue Engineering: From Current Design Strategies to Medical Applications. Adv. Healthcare Mater. 2023, 12, 2203115. 10.1002/adhm.202203115
References
- 1. Shafiee A., Atala A., Annu. Rev. Med. 2017, 68, 29. [DOI] [PubMed] [Google Scholar]
- 2. Khan Y., Yaszemski M. J., Mikos A. G., Laurencin C. T., J. Bone. Jt. Surg. Am. 2008, 90, 36. [DOI] [PubMed] [Google Scholar]
- 3. Berthiaume F., Maguire T. J., Yarmush M. L., Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403. [DOI] [PubMed] [Google Scholar]
- 4. Chan B. P., Leong K. W., Eur. Spine J. 2008, 17, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loh Q. L., Choong C., Tissue Eng., Part B 2013, 19, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarkar S., Schmitz‐Rixen T., Hamilton G., Seifalian A. M., Med. Biol. Eng. Comput. 2007, 45, 327. [DOI] [PubMed] [Google Scholar]
- 7. Mao Z., Fan B., Wang X., Huang X., Guan J., Sun Z., Xu B., Yang M., Chen Z., Jiang D., Yu J., Front. Bioeng. Biotechnol. 2021, 9, 621483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wei W., Dai H., Bioact. Mater. 2021, 6, 4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ai C., Lee Y. H. D., Tan X. H., Tan S. H. S., Hui J. H. P., Goh J. C.‐H., J. Orthop. Transl. 2021, 30, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liverani L., Boccaccini A. R., Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices: Principles and Advances, Elsevier, Amsterdam: 2018. [Google Scholar]
- 11. Nikolova M. P., Chavali M. S., Bioact. Mater. 2019, 4, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ivanovski S., Vaquette C., Gronthos S., Hutmacher D. W., Bartold P. M., J. Dent. Res. 2014, 93, 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng C., Zhu H., Li J., Feng C., Yao Q., Wang L., Chang J., Wu C., Theranostics 2018, 8, 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neishabouri A., Soltani Khaboushan A., Daghigh F., Kajbafzadeh A.‐M., Majidi Zolbin M., Front. Bioeng. Biotechnol. 2022, 10, 805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang X., Chen X., Hong H., Hu R., Liu J., Liu C., Bioact. Mater. 2022, 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhushan S., Singh S., Maiti T. K., Sharma C., Dutt D., Sharma S., Li C., Tag Eldin E. M., Bioengineering 2022, 9, 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eltom A., Zhong G., Muhammad A., Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar]
- 18. Kumar A., Jacob A., J. Appl. Biol. Biotechnol. 2022, 10, 163. [Google Scholar]
- 19. Zhao P., Gu H., Mi H., Rao C., Fu J., Turng L., Front. Mech. Eng. 2018, 13, 107. [Google Scholar]
- 20. Perić Kačarević Ž., Rider P., Alkildani S., Retnasingh S., Pejakić M., Schnettler R., Gosau M., Smeets R., Jung O., Barbeck M., Int. J. Artif. Organs 2020, 43, 69. [DOI] [PubMed] [Google Scholar]
- 21. Vesvoranan O., Anup A., Hixon K. R., Biomimetics 2022, 7, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roth G. A., Mensah G. A., Johnson C. O., Addolorato G., Ammirati E., Baddour L. M., Barengo N. C., Beaton A. Z., Benjamin E. J., Brodmann M., Cahill T. J., Carapetis J., Catapano A. L., Chugh S. S., Cooper L. T., Coresh J., Criqui M., DeCleene N., Eagle K. A., Emmons‐Bell S., Feigin V. L., Fernández‐Solà J., Fowkes G., Gakidou E., Grundy S. M., He F. J., Howard G., Hu F., Inker L., Karthikeyan G., J. Am. Coll. Cardiol. 2020, 76, 2982.33309175 [Google Scholar]
- 23. Leal B., Wakabayashi N., Oyama K., Kamiya H., Braghirolli D. I., Pranke P., Front. Cardiovasc. Med. 2021, 7, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang S., Ellman D. G., Andersen D. C., Cells 2021, 10, 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang A. H., Niklason L. E., Cell. Mol. Life Sci. 2014, 71, 2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tennant M., McGeachie J. K., Aust. N. Z. J. Surg. 1990, 60, 747. [DOI] [PubMed] [Google Scholar]
- 27. Devillard C. D., Marquette C. A., Front. Bioeng. Biotechnol. 2021, 9, 721843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Augustin H. G., Koh G. Y., Science 2017, 357, eaal2379. [DOI] [PubMed] [Google Scholar]
- 29. Camasão D. B., Mantovani D., Mater. Today Bio 2021, 10, 100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Organ Tissue Engineering, (Eds: Eberli D., Lee S. J., Traweger A.), Springer International Publishing, Cham, Switzerland: 2021. [Google Scholar]
- 31. Pashneh‐Tala S., MacNeil S., Claeyssens F., Tissue Eng., Part B 2016, 22, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chlupáč J., Filová E., Bačáková L., Physiol. Res. 2009, 58, S119. [DOI] [PubMed] [Google Scholar]
- 33. Ye H., Zhang K., Kai D., Li Z., Loh X. Jun, Chem. Soc. Rev. 2018, 47, 4545. [DOI] [PubMed] [Google Scholar]
- 34. Malikmammadov E., Tanir T. E., Kiziltay A., Hasirci V., Hasirci N., J. Biomater. Sci., Polym. Ed. 2018, 29, 863. [DOI] [PubMed] [Google Scholar]
- 35. Zhu M., Wang Z., Zhang J., Wang L., Yang X., Chen J., Fan G., Ji S., Xing C., Wang K., Zhao Q., Zhu Y., Kong D., Wang L., Biomaterials 2015, 61, 85. [DOI] [PubMed] [Google Scholar]
- 36. Li M.‐X., Li L., Zhou S.‐Y., Cao J.‐H., Liang W.‐H., Tian Y., Shi X.‐T., Yang X.‐B., Wu D.‐Y., RSC Adv. 2021, 11, 31783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rickel A. P., Deng X., Engebretson D., Hong Z., Mater. Sci. Eng. C 2021, 129, 112373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeong S. I., Kim S. H., Kim Y. H., Jung Y., Kwon J. H., Kim B.‐S., Lee Y. M., J. Biomater. Sci., Polym. Ed. 2004, 15, 645. [DOI] [PubMed] [Google Scholar]
- 39. Kim S.‐H., Chung E., Kim S.‐H., Jung Y., Kim Y. H., Kim S. H., J. Biomater. Sci., Polym. Ed. 2010, 21, 289. [DOI] [PubMed] [Google Scholar]
- 40. Shin Y. M., Lim J.‐Y., Park J.‐S., Gwon H.‐J., Jeong S. I., Lim Y.‐M., Biotechnol. Bioprocess. Eng. 2014, 19, 118. [Google Scholar]
- 41. Schmidt D., Hoerstrup S. P., Strategies in Regenerative Medicine, Springer, New York, NY: 2009. [Google Scholar]
- 42. Sun W., Gregory D. A., Tomeh M. A., Zhao X., Int. J. Mol. Sci. 2021, 22, 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fazal N., Latief N., Osteoarthritis Cartilage 2018, 26, 1583. [DOI] [PubMed] [Google Scholar]
- 44. Gupta P., Lorentz K. L., Haskett D. G., Cunnane E. M., Ramaswamy A. K., Weinbaum J. S., Vorp D. A., Mandal B. B., Acta Biomater. 2020, 105, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gupta P., Kumar M., Bhardwaj N., Kumar J. P., Krishnamurthy C. S., Nandi S. K., Mandal B. B., ACS Appl. Mater. Interfaces 2016, 8, 15874. [DOI] [PubMed] [Google Scholar]
- 46. Kharazi A. Z., Atari M., Vatankhah E., Javanmard S. H., Polym. Adv. Technol. 2018, 29, 3151. [Google Scholar]
- 47. Vogt L., Ruther F., Salehi S., Boccaccini A. R., Adv. Healthcare Mater. 2021, 10, 2002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rekabgardan M., Rahmani M., Soleimani M., HosSein Zadeh S., Roozafzoon R., Parandakh A., Khani M.‐M., ASAIO J. 2022, 68, 123. [DOI] [PubMed] [Google Scholar]
- 49. Wang W., Nie W., Zhou X., Feng W., Chen L., Zhang Q., You Z., Shi Q., Peng C., He C., Acta Biomater. 2018, 79, 168. [DOI] [PubMed] [Google Scholar]
- 50. Liu X., Chen B., Li Y., Kong Y., Gao M., Zhang L. Z., Gu N., J. Bioact. Compat. Polym. 2021, 36, 59. [Google Scholar]
- 51. Norouzi S. K., Shamloo A., Mater. Sci. Eng. C 2019, 94, 1067. [DOI] [PubMed] [Google Scholar]
- 52. Li X., Huang L., Li L., Tang Y., Liu Q., Xie H., Tian J., Zhou S., Tang G., J. Biomater. Sci., Polym. Ed. 2020, 31, 439. [DOI] [PubMed] [Google Scholar]
- 53. Zhao L., Xu Y., He M., Zhang W., Li M., Compos. Interfaces 2014, 21, 869. [Google Scholar]
- 54. Elkhoury K., Morsink M., Sanchez‐Gonzalez L., Kahn C., Tamayol A., Arab‐Tehrany E., Bioact. Mater. 2021, 6, 3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cao X., Maharjan S., Ashfaq R., Shin J., Zhang Y. S., Engineering 2021, 7, 832. [Google Scholar]
- 56. Xu L., Varkey M., Jorgensen A., Ju J., Jin Q., Park J. H., Fu Y., Zhang G., Ke D., Zhao W., Hou R., Atala A., Biofabrication 2020, 12, 045012. [DOI] [PubMed] [Google Scholar]
- 57. Ryan A. J., Ryan E. J., Cameron A. R., O'Brien F. J., Acta Biomater. 2020, 112, 52. [DOI] [PubMed] [Google Scholar]
- 58. Sell S. A., Wolfe P. S., Garg K., McCool J. M., Rodriguez, Bowlin G. L., Polymers 2010, 2, 522. [Google Scholar]
- 59. Zhou J., Ying H., Wang M., Su D., Lu G., Chen J., Mater. Sci. Eng. C 2018, 92, 447. [DOI] [PubMed] [Google Scholar]
- 60. Badhe R. V., Bijukumar D., Chejara D. R., Mabrouk M., Choonara Y. E., Kumar P., du Toit L. C., Kondiah P. P. D., Pillay V., Carbohydr. Polym. 2017, 157, 1215. [DOI] [PubMed] [Google Scholar]
- 61. Yao T., Baker M. B., Moroni L., Nanomaterials 2020, 10, 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fazal F., Raghav S., Callanan A., Koutsos V., Radacsi N., Biofabrication 2021, 13, 032003. [DOI] [PubMed] [Google Scholar]
- 63. Potere F., Belgio B., Croci G. A., Tabano S., Petrini P., Dubini G., Boschetti F., Mantero S., Front. Bioeng. Biotechnol. 2022, 10, 918690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nukavarapu S. P., Dorcemus D. L., Biotechnol. Adv. 2013, 31, 706. [DOI] [PubMed] [Google Scholar]
- 65. Niu X., Li N., Du Z., Li X., Bioact. Mater. 2023, 20, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hu X., Xu J., Li W., Li L., Parungao R., Wang Y., Zheng S., Nie Y., Liu T., Song K., Appl. Biochem. Biotechnol. 2020, 191, 785. [DOI] [PubMed] [Google Scholar]
- 67. Donnaloja F., Jacchetti E., Soncini M., Raimondi M. T., Polymers 2020, 12, 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pan Z., Duan P., Liu X., Wang H., Cao L., He Y., Dong J., Ding J., Regener. Biomater. 2015, 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reignier J., Huneault M. A., Polymer 2006, 47, 4703. [Google Scholar]
- 70. Nooeaid P., Salih V., Beier J. P., Boccaccini A. R., J. Cell. Mol. Med. 2012, 16, 2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fu J.‐N., Wang X., Yang M., Chen Y.‐R., Zhang J.‐Y., Deng R.‐H., Zhang Z.‐N., Yu J.‐K., Yuan F.‐Z., Front. Bioeng. Biotechnol. 2022, 9, 812383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Deng C., Chang J., Wu C., J. Orthop. Transl. 2019, 17, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zheng L., Li D., Wang W., Zhang Q., Zhou X., Liu D., Zhang J., You Z., Zhang J., He C., ACS Biomater. Sci. Eng. 2019, 5, 4564. [DOI] [PubMed] [Google Scholar]
- 74. Natarajan A., Sivadas V., Nair P. D., Biomed. Mater. 2021, 16, 054102. [DOI] [PubMed] [Google Scholar]
- 75. Chen J., Chen H., Li P., Diao H., Zhu S., Dong L., Wang R., Guo T., Zhao J., Zhang J., Biomaterials 2011, 32, 4793. [DOI] [PubMed] [Google Scholar]
- 76. Guo T., Zhao J., Chang J., Ding Z., Hong H., Chen J., Zhang J., Biomaterials 2006, 27, 1095. [DOI] [PubMed] [Google Scholar]
- 77. Diao H., Wang J., Shen C., Xia S., Guo T., Dong L., Zhang C., Chen J., Zhao J., Zhang J., Tissue Eng., Part A 2009, 15, 2687. [DOI] [PubMed] [Google Scholar]
- 78. Carvalho M. S., Cabral J. M. S., da Silva C. L., Vashishth D., Polymers 2021, 13, 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pina S., Rebelo V. M., Oliveira J. M., Reis R. L., in Osteochondral Tissue Engineering, (Eds: Oliveira J. M., Pina S., Reis R. L., San Roman J.), Springer, Cham, Switzerland: 2018, 35. [Google Scholar]
- 80. Baino F., Novajra G., Vitale‐Brovarone C., Front. Bioeng. Biotechnol. 2015, 3, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yan L.‐P., Silva‐Correia J., Oliveira M. B., Vilela C., Pereira H., Sousa R. A., Mano J. F., Oliveira A. L., Oliveira J. M., Reis R. L., Acta Biomater. 2015, 12, 227. [DOI] [PubMed] [Google Scholar]
- 82. Samavedi S., Whittington A. R., Goldstein A. S., Acta Biomater. 2013, 9, 8037. [DOI] [PubMed] [Google Scholar]
- 83. Seong Y.‐J., Kang I.‐G., Song E.‐H., Kim H.‐E., Jeong S.‐H., Adv. Healthcare Mater. 2017, 6, 1700966. [DOI] [PubMed] [Google Scholar]
- 84. Chen G., Kawazoe N., in Osteochondral Tissue Engineering, (Eds: Oliveira J. M., Pina S., Reis R. L., San Roman J.), Springer, Cham, Switzerland: 2018, 171. [Google Scholar]
- 85. Ribeiro V. P., Pina S., Costa J. B., Cengiz I. F., García‐Fernández L., del Mar Fernández‐Gutiérrez M., Paiva O. C., Oliveira A. L., San‐Román J., Oliveira J. M., Reis R. L., ACS Appl. Mater. Interfaces 2019, 11, 3781. [DOI] [PubMed] [Google Scholar]
- 86. Ginebra M.‐P., Espanol M., Maazouz Y., Bergez V., Pastorino D., EFORT Open Rev. 2018, 3, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu K., Liu Y., Duan Z., Ma X., Fan D., Sci. China: Technol. Sci. 2021, 64, 793. [Google Scholar]
- 88. Zhu X., Chen T., Feng B., Weng J., Duan K., Wang J., Lu X., ACS Biomater. Sci. Eng. 2018, 4, 3534. [DOI] [PubMed] [Google Scholar]
- 89. Kumbhar J., Jadhav S., Bodas D., Barhanpurkar‐Naik A., Wani M., Paknikar K., Rajwade J., Int. J. Nanomed. 2017, 12, 6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Betlej I., Zakaria S., Krajewski K. J., Boruszewski P., Sains Malays. 2021, 50, 493. [Google Scholar]
- 91. Swingler S., Gupta A., Gibson H., Kowalczuk M., Heaselgrave W., Radecka I., Polymers 2021, 13, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yadav V., Sun L., Panilaitis B., Kaplan D. L., J. Tissue Eng. Regener. Med. 2013, 9, E276. [DOI] [PubMed] [Google Scholar]
- 93. Yin N., Stilwell M. D., Santos T. M. A., Wang H., Weibel D. B., Acta Biomater. 2014, 12, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sartori M., Pagani S., Ferrari A., Costa V., Carina V., Figallo E., Maltarello M. C., Martini L., Fini M., Giavaresi G., Mater. Sci. Eng. C 2017, 70, 101. [DOI] [PubMed] [Google Scholar]
- 95. Uppal G., Thakur A., Chauhan A., Bala S., J. Magnesium Alloys 2022, 10, 356. [Google Scholar]
- 96. Cheng L., Li Y., Zuo Y., Li J., Wang H., Mater. Res. Innovations 2008, 12, 192. [Google Scholar]
- 97. Li X., Li Y., Zuo Y., Qu D., Liu Y., Chen T., Jiang N., Li H., Li J., J. Biomed. Mater. Res. 2015, 103, 3226. [DOI] [PubMed] [Google Scholar]
- 98. Doyle S. E., Snow F., Duchi S., O'Connell C. D., Onofrillo C., Di Bella C., Pirogova E., Int. J. Mol. Sci. 2021, 22, 12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu J., Li L., Suo H., Yan M., Yin J., Fu J., Mater. Des. 2019, 171, 107708. [Google Scholar]
- 100. Chainani A., Hippensteel K. J., Kishan A., Garrigues N. W., Ruch D. S., Guilak F., Little D., Tissue Eng., Part A 2013, 19, 2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kilian D., Ahlfeld T., Akkineni A. R., Bernhardt A., Gelinsky M., Lode A., Sci. Rep. 2020, 10, 8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jia S., Wang J., Zhang T., Pan W., Li Z., He X., Yang C., Wu Q., Sun W., Xiong Z., Hao D., ACS Appl. Mater. Interfaces 2018, 10, 20296. [DOI] [PubMed] [Google Scholar]
- 103. Levingstone T. J., Thompson E., Matsiko A., Schepens A., Gleeson J. P., O'Brien F. J., Acta Biomater. 2016, 32, 149. [DOI] [PubMed] [Google Scholar]
- 104. Amadori S., Torricelli P., Panzavolta S., Parrilli A., Fini M., Bigi A., Macromol. Biosci. 2015, 15, 1535. [DOI] [PubMed] [Google Scholar]
- 105. Dhasmana A., Singh S., Kadian S., Singh L., J. Dermatol. Skin Care 2018, 1, 1. [Google Scholar]
- 106. Rezaie F., Momeni‐Moghaddam M., Naderi‐Meshkin H., Int. J. Lower Extremity Wounds 2019, 18, 247. [DOI] [PubMed] [Google Scholar]
- 107. Bowers S., Franco E., Am Fam Physician 2020, 101, 8. [PubMed] [Google Scholar]
- 108. Galli M., Yao Y., Giannobile W. V., Wang H.‐L., Plast. Aesthetic Res. 2021, 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nourian Dehkordi A., Mirahmadi Babaheydari F., Chehelgerdi M., Raeisi Dehkordi S., Stem Cell Res. Ther. 2019, 10, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Candi E., Schmidt R., Melino G., Nat. Rev. Mol. Cell Biol. 2005, 6, 328. [DOI] [PubMed] [Google Scholar]
- 111. Vig K., Chaudhari A., Tripathi S., Dixit S., Sahu R., Pillai S., Dennis V., Singh S., Int. J. Mol. Sci. 2017, 18, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lawton S., Skin 1: The structure and functions of the skin, nursingtimes.net (accessed: September 2022). 2019, 115. [Google Scholar]
- 113. Yu J. R., Navarro J., Coburn J. C., Mahadik B., Molnar J., Holmes J. H., Nam A. J., Fisher J. P., Adv. Healthcare Mater. 2019, 8, 1801471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pan J., Liu N., Sun H., Xu F., PLoS One 2014, 9, e112885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kohli N., Sharma V., Brown S. J., García‐Gareta E., Biomaterials for Skin Repair and Regeneration, Elsevier, Amsterdam: 2019. [Google Scholar]
- 116. Monteiro I. P., Gabriel D., Timko B. P., Hashimoto M., Karajanagi S., Tong R., Marques A. P., Reis R. L., Kohane D. S., Acta Biomater. 2014, 10, 4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ghafari R., Jonoobi M., Amirabad L. M., Oksman K., Taheri A. R., Int. J. Biol. Macromol. 2019, 136, 796. [DOI] [PubMed] [Google Scholar]
- 118. Hickey R. J., Pelling A. E., Front. Bioeng. Biotechnol. 2019, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mathew A. P., Oksman K., Pierron D., Harmand M.‐F., Macromol. Biosci. 2013, 13, 289. [DOI] [PubMed] [Google Scholar]
- 120. Shah R., Stodulka P., Skopalova K., Saha P., Polymers 2019, 11, 2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ooi K. S., Haszman S., Wong Y. N., Soidin E., Hesham N., Mior M. A. A., Tabata Y., Ahmad I., Fauzi M. B., Mohd Yunus M. H., Materials 2020, 13, 4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kilic Bektas C., Kimiz I., Sendemir A., Hasirci V., Hasirci N., J. Biomater. Sci., Polym. Ed. 2018, 29, 1764. [DOI] [PubMed] [Google Scholar]
- 123. Liang Y., Liang Y., Zhang H., Guo B., Asian J. Pharm. Sci. 2022, 17, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Konop M., Damps T., Misicka A., Rudnicka L., J. Nanomater. 2016, 2016, 7614753. [Google Scholar]
- 125. Cakir C. O., Ozturk M. T., Tuzlakoglu K., Mater. Technol. 2018, 33, 651. [Google Scholar]
- 126. Bouvet C., Gjoni S., Zenelaj B., Lipsky B. A., Hakko E., Uçkay I., Int. J. Infect. Dis. 2017, 60, 44. [DOI] [PubMed] [Google Scholar]
- 127. Hokynková A., Babula P., Pokorná A., Nováková M., Nártová L., Šín P., Čes. Slov. Neurol. Neurochir. 2019, 82, S37. [Google Scholar]
- 128. Wang F., Wang M., She Z., Fan K., Xu C., Chu B., Chen C., Shi S., Tan R., Mater. Sci. Eng. C 2015, 52, 155. [DOI] [PubMed] [Google Scholar]
- 129. Farokhi M., Mottaghitalab F., Fatahi Y., Khademhosseini A., Kaplan D. L., Trends Biotechnol. 2018, 36, 907. [DOI] [PubMed] [Google Scholar]
- 130. Nilforoushzadeh M. A., Amirkhani M. A., Zarrintaj P., Salehi Moghaddam A., Mehrabi T., Alavi S., Sisakht M. M., J. Cosmet. Dermatol. 2018, 17, 693. [DOI] [PubMed] [Google Scholar]
- 131. Shaik M. M., Dapkekar A., Rajwade J. M., Jadhav S. H., Kowshik M., J. Mater. Sci.: Mater. Med. 2019, 30, 13. [DOI] [PubMed] [Google Scholar]
- 132. Li B., Jin Y., Stem Cell Biology and Tissue Engineering in Dental Sciences, Elsevier, Amsterdam: 2015. [Google Scholar]
- 133. Bui F. Q., Almeida‐da‐Silva C. L. C., Huynh B., Trinh A., Liu J., Woodward J., Asadi H., Ojcius D. M., Biomed. J. 2019, 42, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dabra S., Chhina K., Soni N., Bhatnagar R., J. Dent. Res. 2012, 9, 41. [PMC free article] [PubMed] [Google Scholar]
- 135. Sundaram M. N., Sowmya S., Deepthi S., Bumgardener J. D., Jayakumar R., J. Biomed. Mater. Res., Part B 2016, 104, 761. [DOI] [PubMed] [Google Scholar]
- 136. Yu M., Luo D., Qiao J., Guo J., He D., Jin S., Tang L., Wang Y., Shi X., Mao J., Cui S., Fu Y., Li Z., Liu D., Zhang T., Zhang C., Li Z., Zhou Y., Liu Y., Bioact. Mater. 2022, 10, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nityasri, Aromal S., Pradeep K., Kalaivani V., Pandian R., J. Dent. Health Oral Disord. Ther. 2018, 9, 350. [Google Scholar]
- 138. Liu J., Kerns D. G., Open Dent. J. 2014, 8, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Martin‐Thomé H., Bourdin D., Strube N., Saffarzadeh A., Morlock J.‐F., Campard G., Evanno C., Hoornaert A., Layrolle P., J. Oral Implantol. 2018, 44, 138. [DOI] [PubMed] [Google Scholar]
- 140. Zahid S., Khan A. S., Chaudhry A. A., Ghafoor S., Ain Q. U., Raza A., Rahim M. I., Goerke O., Rehman I. U., Tufail A., J. Biomater. Appl. 2019, 33, 967. [DOI] [PubMed] [Google Scholar]
- 141. Tamburaci S., Tihminlioglu F., Mater. Sci. Eng. C 2021, 128, 112298. [DOI] [PubMed] [Google Scholar]
- 142. Rashidbenam Z., Jasman M. H., Hafez P., Tan G. H., Goh E. H., Fam X. I., Ho C. C. K., Zainuddin Z. M., Rajan R., Nor F. M., Shuhaili M. A., Kosai N. R., Imran F. H., Ng M. H., Tissue Eng. Regener. Med. 2019, 16, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pastorek D., Culenova M., Csobonyeiova M., Skuciova V., Danisovic L., Ziaran S., Biomedicines 2021, 9, 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. de Kemp V., de Graaf P., Fledderus J. O., Ruud Bosch J. L. H., de Kort L. M. O., PLoS One 2015, 10, e0118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Amesty M. V., Chamorro C. I., López‐Pereira P., Martínez‐Urrutia M. J., Sanz B., Rivas S., Lobato R., Fossum M., Front. Pediatr. 2021, 9, 691131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lv X., Feng C., Liu Y., Peng X., Chen S., Xiao D., Wang H., Li Z., Xu Y., Lu M., Theranostics 2018, 8, 3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Orabi H., Bouhout S., Morissette A., Rousseau A., Chabaud S., Bolduc S., Sci. World J. 2013, 2013, 154564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lv X., Yang J., Feng C., Li Z., Chen S., Xie M., Huang J., Li H., Wang H., Xu Y., ACS Biomater. Sci. Eng. 2016, 2, 19. [DOI] [PubMed] [Google Scholar]
- 149. Zhao Y., He Y., Guo J., Wu J., Zhou Z., Zhang M., Li W., Zhou J., Xiao D., Wang Z., Sun K., Zhu Y‐j., Lu M‐j., Acta Biomater. 2015, 23, 91. [DOI] [PubMed] [Google Scholar]
- 150. Ott L. M., Weatherly R. A., Detamore M. S., Ann. Biomed. Eng. 2011, 39, 2091. [DOI] [PubMed] [Google Scholar]
- 151. Debry C., Vrana N. E., Dupret‐Bories A., N. Engl. J. Med. 2017, 376, 97. [DOI] [PubMed] [Google Scholar]
- 152. Kojima K., Vacanti C. A., Anat. Rec. 2014, 297, 44. [DOI] [PubMed] [Google Scholar]
- 153. Wu T., Zheng H., Chen J., Wang Y., Sun B., Morsi Y., El‐Hamshary H., Al‐Deyab S. S., Chen C., Mo X., J. Mater. Chem. B 2017, 5, 139. [DOI] [PubMed] [Google Scholar]
- 154. Romanova O. A., Tenchurin T. H., Demina T. S., Sytina E. V., Shepelev A. D., Rudyak S. G., Klein O. I., Krasheninnikov S. V., Safronova E. I., Kamyshinsky R. A., Mamagulashvili V. G., Akopova T. A., Chvalun S. N., Panteleyev A. A., Cell Proliferation 2019, 52, e12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. O'Leary C., Cavanagh B., Unger R. E., Kirkpatrick C. J., O'Dea S., O'Brien F. J., Cryan S.‐A., Biomaterials 2016, 85, 111. [DOI] [PubMed] [Google Scholar]
- 156. Roseti L., Parisi V., Petretta M., Cavallo C., Desando G., Bartolotti I., Grigolo B., Mater. Sci. Eng. C 2017, 78, 1246. [DOI] [PubMed] [Google Scholar]
- 157. Vrana N. E., Dupret A., Coraux C., Vautier D., Debry C., Lavalle P., PLoS One 2011, 6, e20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cross L. M., Thakur A., Jalili N. A., Detamore M., Gaharwar A. K., Acta Biomater. 2016, 42, 2. [DOI] [PubMed] [Google Scholar]
- 159. Zhang B., Huang J., Narayan R. J., J. Mater. Chem. B 2020, 8, 8149. [DOI] [PubMed] [Google Scholar]
- 160. Longley R., Ferreira A. M., Gentile P., Int. J. Mol. Sci. 2018, 19, 1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lopa S., Madry H., Tissue Eng., Part A 2014, 20, 2052. [DOI] [PubMed] [Google Scholar]
- 162. Abdel‐Sayed P., Pioletti D. P., Nanomedicine 2015, 10, 2893. [DOI] [PubMed] [Google Scholar]
- 163. Bicho D., Pina S., Reis R. L., Oliveira J. M., in Osteochondral Tissue Engineering, (Eds: Oliveira J. M., Pina S., Reis R. L., San Roman J.), Springer, Cham, Switzerland: 2018. [Google Scholar]
- 164. Perez‐Favila A., Martinez‐Fierro M. L., Rodriguez‐Lazalde J. G., Cid‐Baez M. A., de J Zamudio‐Osuna M., Martinez‐Blanco Ma. del R., Mollinedo‐Montaño F. E., Rodriguez‐Sanchez I. P., Castañeda‐Miranda R., Garza‐Veloz I., Medicina 2019, 55, 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


