Abstract
One step in the process of herpes simplex virus (HSV) entry into cells is the binding of viral glycoprotein D (gD) to a cellular receptor. Human nectin-2 (also known as HveB and Prr2), a member of the immunoglobulin (Ig) superfamily, serves as a gD receptor for the entry of HSV-2, variant forms of HSV-1 that have amino acid substitutions at position 25 or 27 of gD (for example, HSV-1/Rid), and porcine pseudorabies virus (PRV). The gD binding region of nectin-2 is believed to be localized to the N-terminal variable-like (V) Ig domain. In order to identify specific amino acid sequences in nectin-2 that are important for HSV entry activity, chimeric molecules were constructed by exchange of sequences between human nectin-2 and its mouse homolog, mouse nectin-2, which mediates entry of PRV but not HSV-1 or HSV-2. The nectin-2 chimeric molecules were expressed in Chinese hamster ovary cells, which normally lack a gD receptor, and tested for cell surface expression and viral entry activity. As expected, chimeric molecules containing the V domain of human nectin-2 exhibited HSV entry activity. Replacement of either of two small regions in the V domain of mouse nectin-2 with amino acids from the equivalent positions in human nectin-2 (amino acids 75 to 81 or 89) transferred HSV-1/Rid entry activity to mouse nectin-2. The resulting chimeras also exhibited enhanced HSV-2 entry activity and gained the ability to mediate wild-type HSV-1 entry. Replacement of amino acid 89 of human nectin-2 with the corresponding mouse amino acid (M89F) eliminated HSV entry activity. These results identify two different amino acid sequences, predicted to lie adjacent to the C′ and C" beta-strands of the V domain, that are critical for HSV entry activity. This region is homologous to the human immunodeficiency virus binding region of CD4 and to the poliovirus binding region of CD155.
The entry of herpes simplex virus (HSV) into cells is a multistep process that requires the interaction of several viral glycoproteins with various cell surface receptors. Initial attachment occurs via the binding of viral glycoprotein C (gC) or glycoprotein B (gB) to cell surface heparan sulfate. Subsequently, glycoprotein D (gD) interacts with one of its several cellular receptors. This somehow triggers fusion of the viral and cellular membranes, a step that requires glycoprotein H (gH), glycoprotein L (gL), gB, gD, a cellular gD receptor, and possibly additional cellular molecules (reviewed in references 40 and 41). Other members of the alphaherpesvirus family, such as porcine pseudorabies virus (PRV) and bovine herpesvirus-1 (BHV-1), encode homologous sets of viral glycoproteins and enter cells through a very similar mechanism. These animal herpesviruses are able to utilize some of the human receptors for entry, which partly explains their ability to infect cultured human cells (41).
Two of the four gD receptors identified to date are nectin-1 (44), previously named HveC (13), HIgR (8), and Prr1 (22), and nectin-2, previously named HveB (45) and Prr2 (10). The mouse homologs of these human receptors have considerable sequence identity with their human counterparts, 95% for nectin-1 and 72% for nectin-2, and also exhibit viral entry activity (25, 38, 39). Both the mouse and human forms of nectin-1 can serve as entry receptors for HSV-1, HSV-2, PRV, and BHV-1 (8, 13, 25, 26, 38). On the other hand, the mouse and human forms of nectin-2 have a more limited entry activity and different specificities. Mouse nectin-2 is an entry receptor for PRV but not BHV-1 or HSV strains (39), whereas human nectin-2 can mediate the entry of PRV and variant forms of HSV-1 that have amino acid substitutions at position 25 or 27 of gD (21, 45). HSV-1 strains expressing gDs with the Q27P or Q27R amino acid substitution have been designated Rid variants (9). Human nectin-2 can also serve as a weak receptor for HSV-2 entry but has very little entry activity for wild-type HSV-1 strains (21, 45).
Nectin-1 and nectin-2 belong to a subgroup of the immunoglobulin (Ig) superfamily, based on structural and sequence similarities. Other members of this subgroup include nectin-3 (34, 36) and the poliovirus receptor (CD155) (24). Nectin-3 has no reported viral entry activity, but CD155 is able to mediate entry of PRV and BHV-1 (13). Each of these proteins can be expressed as multiple isoforms that are secreted or membrane bound due to the use of different C-terminal exons (6, 16, 20, 36, 41). All members of this Ig subfamily contain three homologous Ig domains, an N-terminal variable-like (V) domain and two constant-like (C2) domains. The membrane-bound forms have the most efficient viral entry activity, apparently independent of the sequences of the transmembrane region or cytoplasmic tail (8, 13, 21). A secreted form of nectin-1 was reported to bind to cells and to have detectable HSV entry activity (20).
The cellular role of the nectins includes participation in cell-to-cell adhesion. These proteins localize to cadherin-based adherens junctions in polarized epithelium or to cell junctions in nonpolarized cells and link neighboring cells through trans-homophilic or heterophilic interactions (36, 43, 44). Certain isoforms of the nectins containing specific carboxy-terminal sequences can associate with the actin cytoskeleton through interactions with afadin, an F-actin binding protein (15, 23, 36). The binding of nectin to afadin is important for localization of the nectins to cadherin-based junctions (15, 23, 44). Although binding of nectin-1 to afadin is not necessary for HSV entry (8, 13, 35), this interaction does facilitate the efficient cell-to-cell spread of HSV infection (35). Since nectin-1 and nectin-2 are probably coexpressed in a variety of cell types, they may serve redundant roles in certain cell types. However, mutations that abolish expression of full-length nectin-1 in humans result in autosomal recessive cleft lip or palate ectodermal dysplasia syndrome (42). Knockout of the nectin-2 gene in mice resulted in male sterility through defects in spermatogenesis, without obvious effects on females (5).
Various lines of evidence suggest that the interaction of HSV with nectin-1 or nectin-2 occurs through the V domain. Soluble nectin-1 consisting of only the V domain blocks the entry of HSV into nectin-1-expressing cells and binds soluble gD (7, 19). Similar results were observed with a truncated nectin-2 protein containing only the V domain when tested with the HSV-1 variant U21 and soluble U21 gD (21). Also, a nectin-1 protein deleted for the two C2-like domains can mediate HSV entry, albeit inefficiently (7). Epitope mapping of anti-nectin-1 antibodies that can inhibit gD binding and HSV entry suggests that amino acids (a.a.) 80 to 104 of the V domain of nectin-1 are critical for the gD interaction (17).
The aim of this study was to identify specific regions and amino acids within the V domain of nectin-2 that are important for HSV entry. We chose to study nectin-2 to take advantage of the difference in entry specificity of the human and murine homologs. A chimeric approach was used to identify human nectin-2 sequences that are able to confer HSV entry activity on mouse nectin-2 and therefore are critical for viral entry activity. Various sets of nectin-2 chimeras were constructed by replacing regions of mouse nectin-2 with the corresponding amino acids of human nectin-2. The chimeric proteins were tested for ability to mediate entry of several alphaherpesviruses, including wild-type HSV-1, HSV-1/Rid1, HSV-2, PRV, and BHV-1.
As expected, the V domain of human nectin-2 was shown to contain the critical determinants for HSV entry. Two short regions (a.a. 75 to 81 and a.a. 89) within the V domain of human nectin-2 were identified that could independently convert mouse nectin-2 into an entry receptor for HSV-1/Rid1. The resulting chimeras also exhibited HSV-1 and improved HSV-2 entry activity, neither characteristic of the parental molecules. A human nectin-2 molecule containing the substitution M89F lost HSV but not PRV entry activity. Thus, we have identified two regions and specific amino acid residues within the V domain of nectin-2 that are critical for HSV entry activity. These amino acid residues map to one side of the V domain within loops adjacent to the C′ and C" beta-strands. This region is homologous to that of CD4 and CD155 that is critical for human immunodeficiency virus (HIV) and poliovirus entry, respectively. This region is also adjacent to the homologous region in nectin-1 that contains epitopes for monoclonal antibodies (MAbs) that can inhibit gD binding. In light of the homology between nectin-1 and -2, these studies will aid in the identification and characterization of specific sequences in nectin-1 that are important for viral entry.
MATERIALS AND METHODS
Cells and viruses.
CHO-K1 cells were provided by J. Esko (University of California, San Diego). β-Galactosidase (β-gal) reporter viruses used were wild-type HSV-1 (strain KOS) and HSV-1/Rid1, described previously as KOS/tk12 and KOS-Rid1/tk12, respectively (45); gH-negative PRV(Kaplan) (3), provided by T. Mettenleiter (Federal Research Center for Virus Diseases of Animals, Insel Reims, Germany); and BHV-1(Cooper)TK-bgal+v4a (27), provided by L. Bello (University of Pennsylvania). The reporter virus HSV-2(333)gJ−, engineered to contain a cytomegalovirus-lacZ cassette in place of part of the glycoprotein J (gJ) gene, will be described in detail elsewhere (C. Rowe and P. G. Spear, unpublished results). PRV was propagated and titered on gH-expressing Vero SW78 cells (3), and BHV-1 was propagated and titered on MDBK cells. All other viruses were propagated and titered on Vero cells.
Plasmids.
Plasmid pMW20, containing the human nectin-2 open reading frame (ORF) (GenBank AF058448) in pcDNA3, has been described previously (45). This plasmid was used for the expression of human nectin-2 in all assays. Plasmid Mph-pcDNA3, containing the mouse nectin-2 ORF, was provided by D. Shukla (University of Missouri). To generate this plasmid, primers 5′-CTGAAGCTTCCCATGGCCCGGGCCGCAGTC and 5′-GTCTCTAGAGTAGGGTCACACGTAAACTGC were used to amplify mouse nectin-2 coding sequences from a 15.5-day-old embryonic mouse (C57BL/6J) cDNA library (Gibco-BRL). The amplified segment was digested with HindIII and XbaI and cloned into pcDNA3 (D. Shukla and P. G. Spear, unpublished results). The coding sequence was identical to that described before (GenBank M12197) (32).
(i) Plasmids expressing chimeric or mutated receptors.
All sequences were first cloned into pUC19 (New England Biolabs) for mutagenesis and then cloned into pcDNA3.1 (Invitrogen) for mammalian expression. Site-directed mutagenesis was performed according to the manufacturer's instructions using a PCR-based system (QuikChange site-directed mutagenesis kit; Stratagene).
(ii) Plasmids expressing Ch 1 to Ch 5.
Plasmid pWM31 contains the mouse nectin-2 ORF altered by the introduction of a SacII restriction site between the V and C2 domains (5′-AACGGTACCCGCCGCGGGGTGACCTGG) and an EcoRV site between the C2-C2 domains (5′-CCCTCCAGAAGTATCGATATCCGGCTATGATGAC). Upon sequencing, a nucleotide change was detected in this plasmid which resulted in an amino acid substitution (R463W) in the fifth amino acid from the carboxyl-terminal end of mouse nectin-2. The nectin-2 protein expressed from pWM31 is indistinguishable from that expressed from Mph-pcDNA3 in viral entry assays (data not shown). pWM31 was used in all experiments as the wild-type mouse nectin-2 expression plasmid. pWM33 contains the ORF of human nectin-2 (SacI fragment of pMW20) in pUC19 altered by the addition of a SacII site between domains V and C2 (5′-GGTCCGTCCGCGGGATGACCTGGCTC) and an EcoRV site between domains C2 and C2 (5′-CCTCCTGAAGTGTCGATATCCGGCTATGAT). Plasmid pWM39 (Ch 1) contains the ectodomain of human nectin-2 (SrfI-BlpI fragment of pWM33) fused to the transmembrane (TM) and cytoplasmic domains of mouse nectin-2 (BlpI-SrfI fragment of pWM31). pWM41 (Ch 2) contains the V and first C2 domain of human nectin-2 (SrfI-EcoRV fragment of pWM39) followed by the C2 domain, TM, and cytoplasmic sequences of mouse nectin-2 (EcoRV-SrfI fragment of pWM31). pWM38 (Ch 3) contains the V domain of human nectin-2 (SrfI-SacII of pWM33) followed by mouse nectin-2 sequences (SacII-SrfI fragment of pWM31). pWM36 (Ch 4) contains the two C2 domains of human nectin-2 (SacII-BlpI fragment of pWM28, a plasmid similar to pWM33) ligated between the V domain and TM sequences of mouse nectin-2 (BlpI-SacII fragment of pWM31). pWM20 (Ch 5) contains the ectodomain of mouse nectin-2 (HindIII-BlpI fragment from Mph-pcDNA3) fused to the TM and cytoplasmic domains of human nectin-2 (BlpI-HindIII fragment of pMW20).
(iii) Plasmids expressing Ch 7 to Ch 20, F84 mutants, and M89 mutants.
Plasmids were constructed by performing site-directed mutagenesis on pWM29, which contains the mouse nectin-2 ORF (SacI fragment of pWM33) in pUC19. For the M89 mutants, site-directed mutagenesis was performed on pWM68, which contains the ORF of human nectin-2 (BamHI fragment of pMW20) in pUC19. Mutated plasmids were sequenced to ensure that there were no unintended mutations and the mutagenized coding region was cloned into the HindIII site or BamHI site (for human nectin-2 mutants) of pcDNA3.1 for mammalian expression. The nectin-2 molecules are encoded by the following plasmids: Ch 6, pWM61; Ch 7, pWM62; Ch 8, pWM63; Ch 9, pWM64; Ch 10, pWM65; Ch 11, pWM66; Ch 12, pWM75; Ch 13, pWM74; Ch 14, pWM77; Ch 15, pWM78; Ch 16, pWM71; Ch 17, pWM70; Ch 18, pWM72; Ch 19, pWM79; Ch 20, pWM76; F84A, pWM87; F84I, pWM89; F84T, pWM88; F84K, pWM85; F84E, pWM86; F84Y, pWM84; M89I, pWM100; and M89F, pWM101.
CELISA for detection of proteins on cell surfaces.
A cell enzyme-linked immunosorbent assay (CELISA) was performed as described previously (11, 12). Briefly, transfections were performed on subconfluent CHO-K1 cell monolayers using Lipofectamine (Gibco-BRL). Each well of a six-well plate received 1.5 μg of plasmid, 5 μl of Lipofectamine, and Opti-MEM (Gibco-BRL) in 1 ml. Twenty-four hours later, cells were replated into 96-well dishes. The next day, cells were incubated with PBS-BSA (phosphate-buffered saline [PBS] plus 0.5 mM MgCl2, 1 mM CaCl2, and 3% bovine serum albumin [BSA]). This solution was also used for all washes and for antibody dilutions. After 30 min, the appropriate primary antibody was added to cells. Antibodies used included anti-human nectin-2 R146 (45), provided by G. Cohen and R. Eisenberg (University of Pennsylvania), anti-mouse nectin-2 6B3 [α-mouse nectin-2 (V)], and anti-mouse nectin-2 18C12 [α-mouse nectin-2 (C2)] (1), provided by A. Nomoto (University of Tokyo). Cells were washed four times and fixed using 2% formaldehyde–0.2% glutaraldehyde in PBS. Then, cells were incubated for 30 min with biotin-conjugated secondary antibody (1:500 dilution) (Sigma), washed as before, and incubated with Amdex streptavidin-conjugated horseradish peroxidase at 1:20,000 dilution (Amersham) for 30 more min. Cells were then washed and reacted with substrate solution containing 3,3′,5,5′-tetramethylbenzidine (BioFX). At various times after substrate addition, plates were read at 370 nm. Alternatively, the reaction was stopped by the addition of stopping solution (BioFX), and plates were read at 410 nm.
Virus entry assays.
Virus entry assays were performed as previously described (30). Briefly, CHO-K1 cells were transfected as described above and replated into 96-well plates after 24 h. Cells were then exposed to serial dilutions of β-gal-expressing virus diluted in PBS plus 0.1% glucose and 1% heat-inactivated serum. After 6 h, cells were washed, incubated with the β-gal substrate ONPG (o-nitrophenyl-β-d-galactopyranoside) and analyzed as described previously (30).
RESULTS
Critical determinants for HSV entry map to the V domain of human nectin-2.
To generate the first set of human-mouse nectin-2 chimeric molecules, identical restriction enzyme sites were engineered between the V-C2 and C2-C2 domains of the cloned human and mouse nectin-2 genes by site-directed mutagenesis. These sites were used, together with existing shared enzyme sites, to exchange the V, first C2, or second C2 domain gene segments between the human and mouse nectin-2 genes in different combinations. Five different chimeric molecules were constructed (Fig. 1).
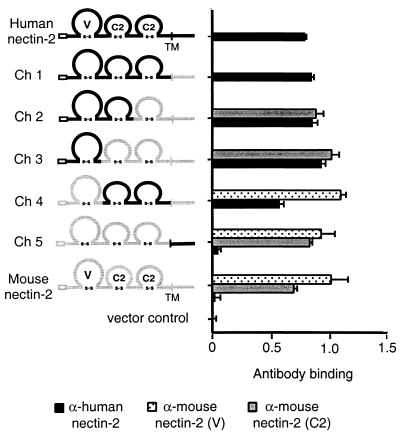
FIG. 1.
Nectin-2 chimeric molecules and cell surface expression. A schematic representation of human, mouse, and chimeric nectin-2 molecules is shown. Human nectin-2 sequences are represented by black lines, and mouse nectin-2 by gray lines. Empty boxes correspond to the proposed signal peptides, and the transmembrane region (TM) is marked by a vertical line. Ig-like domains (V, C2, and C2) are drawn as loops, connected by the predicted disulfide linkage. The name of each molecule is shown to the left of the drawing. To determine cell surface expression of the various nectin-2 molecules, CHO-K1 cells were transfected with plasmids expressing human or mouse nectin-2 or chimeric molecules or with control plasmid. Forty-eight hours later cells were exposed to the appropriate primary antibody and fixed. Antibody binding was detected with a biotinylated secondary antibody, followed by streptavidin-horseradish peroxidase. Peroxidase activity was used as a measure of antibody binding. Values shown are the means and standard deviations of quadruplicate determinations. Data for each molecule are shown directly to its right. Antibodies used were polyclonal rabbit anti-human nectin-2 antibody (α-human nectin-2) and monoclonal anti-mouse nectin-2 antibodies recognizing an epitope in the V domain or second C2 domain.
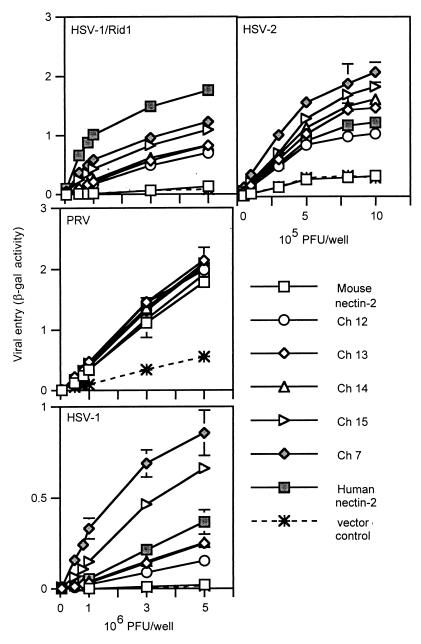
To determine whether the chimeric molecules retained proper conformation and were expressed on the cell surface, Chinese hamster ovary cells (CHO-K1) were transfected with plasmids expressing the wild-type nectin-2 molecules or chimeras or with control plasmid and tested, by CELISA, for the binding of anti-human or anti-mouse nectin-2 antibodies (Fig. 1). The antibodies used were a polyclonal anti-human nectin-2 antibody and two monoclonal anti-mouse nectin-2 antibodies that recognize the mouse nectin-2 V domain and second C2 domain, respectively. Results shown in Fig. 1 demonstrate that the polyclonal anti-human nectin-2 antibodies bound, at nearly equivalent levels, to all chimeras containing some portion of the ectodomain of human nectin-2. The monoclonal anti-mouse nectin-2 antibodies detected, at similar levels, cell surface expression of all chimeric molecules containing the mouse nectin-2 V and C2 domains. These results indicate that all chimeric molecules were expressed on the cell surface as efficiently as the parental molecules and retained at least some antigenic determinants.
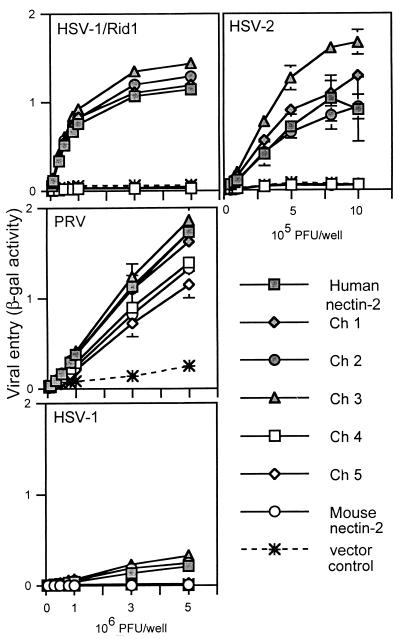
To determine which chimeric molecules were able to mediate HSV entry, viral entry assays were performed. CHO-K1 cells, normally resistant to the entry of HSV, were transfected with plasmids expressing wild-type human or mouse nectin-2 or the chimeric molecules or with control plasmid and then exposed to serial dilutions of reporter viruses HSV-1/Rid1 (an HSV-1 variant expressing gD with the amino acid substitution Q27P), HSV-2, and PRV. These reporter viruses contain a lacZ cassette in the viral genome and express β-gal upon entry into cells. β-Gal activity was used as a measure of viral entry. Results from a representative experiment are shown in Fig. 2. Wild-type human and mouse nectin-2 and all the chimeras were functional for PRV entry, which confirms cell surface expression and retention of functional activity by the chimeric molecules. Only chimeric molecules that contained the V domain of human nectin-2 (Ch 1, Ch 2, and Ch 3) were able to mediate entry of HSV-2 or HSV-1/Rid1 at efficiencies similar to that of wild-type human nectin-2. A very low level of entry activity was also observed with human nectin-2 and Ch 1, Ch 2, and Ch 3 for wild-type HSV-1. Conversely, chimeric molecules containing the V domain of mouse nectin-2 (Ch 4 and Ch 5) behaved similarly to wild-type mouse nectin-2 and were not able to mediate HSV entry. Exchange of the other domains (first C2, second C2, or TM-cytoplasmic) did not significantly affect the entry ability of the molecules, which was determined solely by the V domain present. We noted, however, that Ch 3 was reproducibly more efficient than human nectin-2 at mediating HSV-2 entry. These experiments indicate that the V domain of human nectin-2 is sufficient to convert mouse nectin-2 into a functional HSV entry receptor and support data by others (21) indicating that the V domain of human nectin-2 contains critical determinants for HSV entry into cells.
FIG. 2.
Alphaherpesvirus entry activities of wild-type and chimeric nectin-2 molecules. CHO-K1 cells transfected with plasmids expressing human nectin-2, mouse nectin-2, or chimeric molecules or with control plasmid were replated onto 96 wells and exposed to serial dilutions of reporter viruses (HSV-1/Rid1, PRV, HSV-1, and HSV-2) for 6 h. Infected cells were then washed, and β-gal substrate was added. β-Gal activity was used as a measure of viral entry. Values shown are the means and standard deviations of triplicate determinations. Similar results were obtained in two other experiments.
Identification of amino acids within V domain of human nectin-2 that can confer HSV entry activity on mouse nectin-2.
To identify amino acid residues within the V domain of human nectin-2 required for HSV entry activity, new chimeric molecules were constructed in which single or multiple amino acids in the V domain of mouse nectin-2 were replaced with the corresponding residues from human nectin-2, as explained below. HSV entry activity of all chimeras was determined, in viral entry assays, by exposing transfected CHO-K1 cells to serial dilutions of reporter alphaherpesviruses HSV-1, HSV-1/Rid1, HSV-2, PRV, and BHV-1 and measuring β-gal activity 6 h after infection. The chimeric molecules were also tested for cell surface expression, in a CELISA, by measuring the binding of anti-mouse nectin-2 (C2) antibody to CHO-K1 cells transfected with the various molecules.
(i) V domain chimeras.
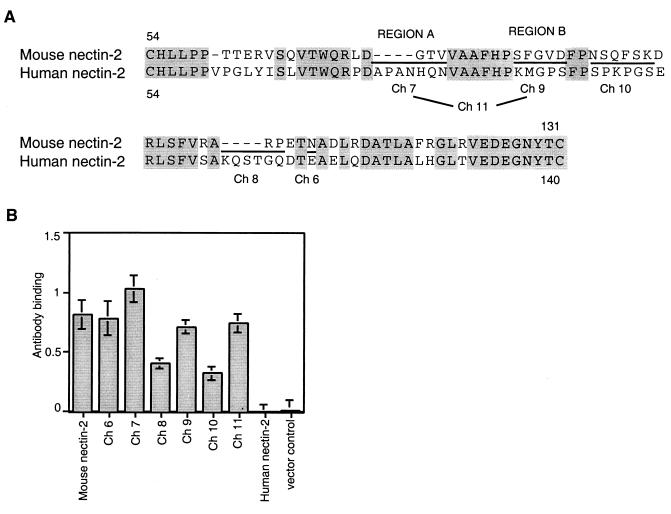
Alignment of sequences between the two cysteines of the V domain of human and mouse nectin-2 identified discrete regions of divergence between these molecules (Fig. 3A). Six new chimeric molecules were constructed in which these regions in mouse nectin-2 were replaced by the corresponding sequences from human nectin-2 using site-directed mutagenesis. Note that Ch 11 has both of the substitutions made individually for Ch 7 and Ch 9. As shown in Fig. 3B, the anti-mouse nectin-2 (C2) antibody bound to CHO-K1 cells expressing mouse nectin-2 and all the chimeric molecules, indicating that the chimeras were expressed on the cell surface. The finding that reduced levels of antibody bound to cells expressing Ch 8 and Ch 10 was reproducible and suggests that these chimeras were not expressed or transported to the cell surface as efficiently as wild-type mouse nectin-2 or that the substitutions in the V domain somehow altered the conformation of the membrane-proximal C2 domain, which seems unlikely.
FIG. 3.
Alignment of human and mouse nectin-2 sequences in the V domain and cell surface expression of V domain chimeric molecules. (A) The sequence between the two cysteines of the V domain of mouse nectin-2 is aligned above the corresponding sequence of human nectin-2. Amino acids are numbered from the initial methionine. Gray shading indicates identity between the sequences. New chimeric molecules were constructed by replacing the underlined mouse nectin-2 sequences with the sequences at equivalent positions in human nectin-2 and given the names shown below the human sequence. Ch 11 has both of the substitutions shown for Ch 7 and Ch 9. The amino acids exchanged in Ch 7 and Ch 9 are referred to in the text and later figures as regions A and B, respectively. (B) Cell surface expression of wild-type and V domain chimeric molecules. CHO-K1 cells were transfected with plasmids expressing human nectin-2, mouse nectin-2, or chimeric molecules or with control plasmid and 48 h later tested for the binding of anti-mouse nectin-2 (C2) antibody as explained for Fig. 1. Values shown are the means and standard deviations of triplicate determinations. The same transfected cell populations were used to obtain the results shown in panel B and in Fig. 4.
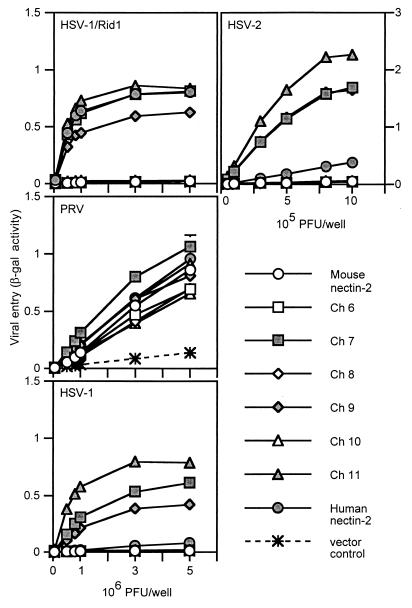
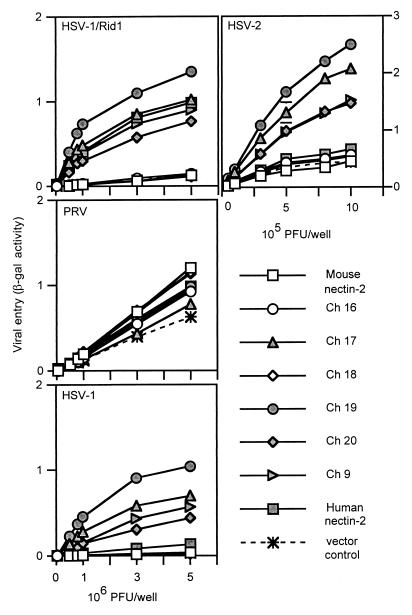
Results of a representative experiment to test the viral entry activity of these chimeric molecules are shown in Fig. 4. All wild-type and chimeric molecules were able to mediate entry of PRV despite the apparently reduced levels of Ch 8 and Ch 10, confirming their cell surface expression and retention of some functional activity. Ch 6, 8, and 10 behaved like mouse nectin-2 and were not able to mediate HSV entry. However, Ch 7, 9, and 11 gained the ability to mediate entry of HSV-1/Rid1 to levels similar to that of human nectin-2. These chimeras were also able to mediate HSV-2 entry but much more efficiently than did human nectin-2. Since the chimeric molecules exhibited enhanced HSV-2 entry activity, we tested whether the chimeras could mediate entry of other alphaherpesviruses (HSV-1 and BHV-1). Chimeras 7, 9, and 11 were able to mediate entry of wild-type HSV-1 into cells much more efficiently than human nectin-2. None of the molecules exhibited BHV-1 entry activity (data not shown). The results from these experiments show that two regions from human nectin-2, regions A (a.a. 75 to 81) and B (a.a. 88 to 92) (Fig. 3A), could independently or jointly transfer HSV-1/Rid1 entry activity to mouse nectin-2. The resulting chimeras also exhibited enhanced HSV-1 and HSV-2 entry activity compared to wild-type human nectin-2.
FIG. 4.
Alphaherpesvirus entry activities of V domain nectin-2 chimeric molecules. CHO-K1 cells transfected with plasmids expressing human nectin-2, mouse nectin-2, or V domain chimeric molecules or with control plasmid were infected with reporter alphaherpesviruses (HSV-1/Rid1, PRV, HSV-1, and HSV-2) as described for Fig. 2. Values shown are the means and standard deviations of triplicate determinations. Similar results were obtained in two other experiments.
(ii) Single amino acid substitutions.
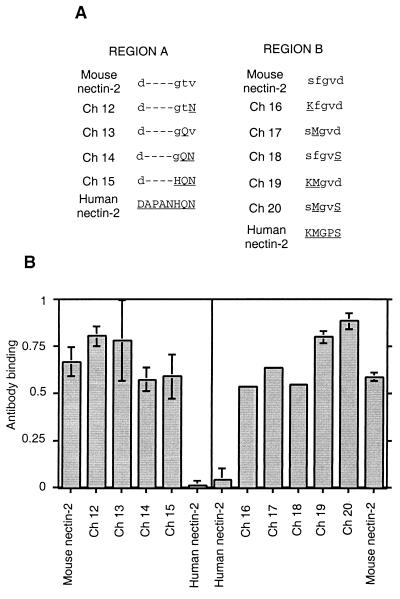
To determine whether specific amino acids within region A or B of human nectin-2 could transfer HSV entry activity to mouse nectin-2, new chimeric molecules were constructed in which one or a few amino acids in these regions in mouse nectin-2 were replaced with the corresponding amino acids from human nectin-2. The amino acid sequences of regions A and B of the new chimeras and the wild-type nectin-2 molecules are shown in Fig. 5A.
FIG. 5.
Amino acid sequences of regions A and B in nectin-2 chimeras Ch 12 to Ch 20 and cell surface expression. (A) The amino acid sequences of regions A and B in nectin-2 chimeric molecules are shown between the mouse nectin-2 (lowercase letters) and human nectin-2 (uppercase letters and underlined) sequences. The name given to each chimera is shown to the left of the sequence. (B) Cell surface expression of region A and B nectin-2 chimeric molecules. CHO-K1 cells were transfected with plasmids encoding human nectin-2, mouse nectin-2, or region A or B nectin-2 chimeric molecules and tested for the binding of anti-mouse nectin-2 (C2) antibody as described for Fig. 1. Values are the means and standard deviations of quadruplicate determinations.
All the region A and B chimeras were expressed on the cell surface, as shown by CELISA using the anti-nectin-2 (C2) antibody (Fig. 5B). All the chimeras were also able to mediate entry of PRV, although with different efficiencies, which confirms cell surface expression and retention of some functional entry activity for the chimeric molecules (Fig. 6 and 7).
FIG. 6.
Alphaherpesvirus entry activities of region A nectin-2 chimeric molecules. CHO-K1 cells were transfected with plasmids expressing wild-type and region A chimeric molecules, Ch 7, or control plasmid. Forty-eight hours later, cells were exposed to serial dilutions of reporter alphaherpesviruses (HSV-1/Rid1, PRV, HSV-1, or HSV-2) as described for Fig. 2. β-Gal activity was used as a measure of virus entry. The means and standard deviations of triplicate determinations are shown for a representative experiment. The assays were repeated three times with similar results.
FIG. 7.
Alphaherpesvirus entry activities of region B nectin-2 chimeric molecules. CHO-K1 cells were transfected with plasmids expressing wild-type and region B chimeric molecules, Ch 9, or control plasmid. Forty-eight hours later, cells were exposed to serial dilutions of reporter alphaherpesviruses (HSV-1/Rid1, PRV, HSV-1, or HSV-2) as described for Fig. 2. β-Gal activity was used as a measure of virus entry. The means and standard deviations of triplicate determinations are shown for a representative experiment. Similar results were obtained in two other experiments.
A representative experiment for the viral entry assays using region A chimeras is shown in Fig. 6. Ch 7, which contains the entire region A of human nectin-2 (Fig. 3A), was included for comparison. All of the region A chimeric molecules were able to mediate entry of HSV-1/Rid1, but not as efficiently as did human nectin-2 (Fig. 6). These chimeras also gained the ability to mediate entry of HSV-2 to levels similar to, or greater than that of human nectin-2. Only Ch 15 exhibited wild-type HSV-1 entry activity to a level approaching that of Ch 7. These results show that substitutions in region A have different effects on entry of the four alphaherpesviruses tested. None of the substitutions affected PRV entry activity. Any of the substitutions in region A resulted in acquisition of HSV-1/Rid1 entry activity but not to the levels observed with wild-type human nectin-2. For HSV-2, any of the substitutions in region A is enough for acquisition of entry activity; the more region A amino acids derived from human nectin-2, the more enhanced the entry activity. For wild-type HSV-1, at least the three amino acids substituted in Ch 15 are required for significant levels of entry activity.
A representative experiment for the viral entry assays using region B chimeras is shown in Fig. 7. Ch 9, which contains the entire region B of human nectin-2 (Fig. 3A), was included for comparison. All chimeric molecules that contained a Met instead of Phe at position 84 (Ch 17, Ch 19, and Ch 20) gained the ability to mediate entry of HSV-1/Rid1 and had enhanced entry activity for HSV-1 and HSV-2. Ch 19, containing substitution of two amino acids (S83K and F84M) exhibited the highest levels of entry activity for all three HSV strains tested. Other substitutions in this region (Ch 16 and Ch 18) did not confer HSV entry activity on mouse nectin-2. Interestingly, the single amino acid substitution at position 84 (F84M) actually reduced PRV entry activity (Ch 17).
Effect of various substitutions in position 84 of mouse nectin-2 on alphaherpesvirus entry.
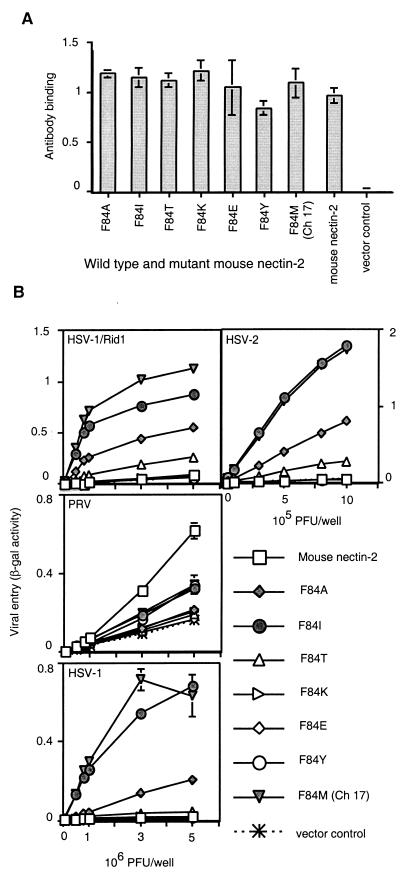
Ch 17, which contains the F84M mutation, was functional for HSV entry. The Phe at that position in mouse nectin-2 may cause steric hindrance (or a different conformational structure in that area) to prevent HSV entry. Alternatively, a Met, as present in human nectin-2, may be specifically required for entry activity. To determine the effect on HSV entry of other amino acid substitutions in position 84 of mouse nectin-2, we constructed mouse nectin-2 molecules containing different amino acid residues in that position (Fig. 8). All mouse nectin-2 mutants were expressed on the cell surface at levels similar to mouse nectin-2, as detected by the binding of the anti-mouse nectin-2 (C2) antibody to transfected CHO-K1 cells (Fig. 8A).
FIG. 8.
Cell surface expression and alphaherpesvirus entry activities of mouse nectin-2 mutants containing amino acid substitutions at position 84. (A) Six mouse nectin-2 mutants were constructed in which the amino acid at position 84 was replaced with another amino acid (Ala, Ile, Thr, Lys, Glu, or Tyr) and given the name of the substitution as shown on the x axis. To assess cell surface expression, CHO-K1 cells transfected with plasmids expressing the mouse nectin-2 position 84 mutants, wild-type mouse nectin-2, or control plasmid were tested for the binding of anti-mouse nectin-2 (C2) antibody as described for Fig. 1. Results shown are the means and standard deviations of triplicate determinations. (B) CHO-K1 cells transfected as above were infected with reporter alphaherpesviruses (HSV-1/Rid1, PRV, HSV-1, and HSV-2) as described for Fig. 2. The means and standard deviations of triplicate determinations are shown for a representative experiment. The assays were repeated twice with similar results.
The mutant mouse nectin-2 molecules were tested for viral entry activity, and a representative experiment is shown in Fig. 8B. In addition to Ch 17 (F84M), mutants containing either an Ile (F84I) or an Ala (F84A) gained the ability to mediate entry of HSV-1, HSV-1/Rid1, and HSV-2. The activity of F84I was very similar to that of Ch 17 (F84M), indicating that an Ile can substitute very well for Met in that position to allow HSV entry. F84T exhibited a somewhat reduced but detectable entry activity for HSV-1/Rid1 and HSV-2. Mutants having a charged residue (F84K and F84E) or an aromatic amino acid (F84Y) were inactive for viral entry. Some mutants exhibited significantly reduced ability to mediate PRV entry (F84E, F84T, F84K, and F84A).
Altering the Met at position 89 of human nectin-2 influences HSV entry activity.
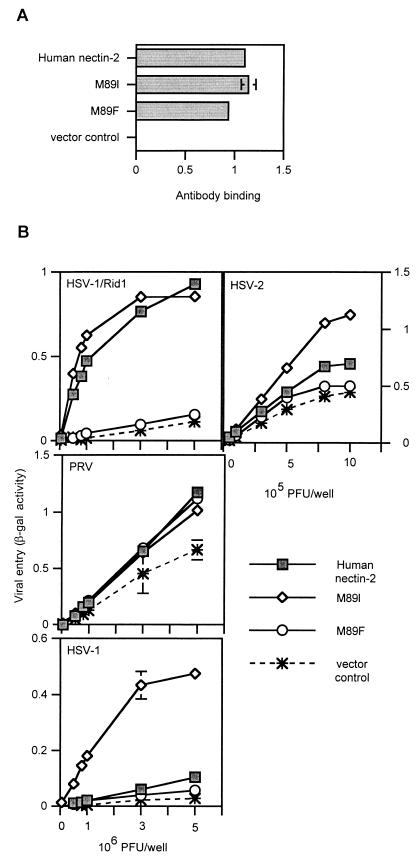
Because changing the Phe at position 84 of mouse nectin-2 to Met or Ile confers HSV entry activity, we tested the effect of substituting the Met at the equivalent position (a.a. 89) in human nectin-2 with other amino acids. Human nectin-2 mutants were constructed in which the Met at position 89 was replaced by a Phe, as in mouse nectin-2, or by Ile (Fig. 9). We hypothesized that the M89F mutation would eliminate human nectin-2 HSV entry activity. Also, because the F84I substitution in mouse nectin-2 conferred HSV entry activity, the M89I mutation should not affect the ability of human nectin-2 to mediate HSV entry.
FIG. 9.
Cell surface expression and alphaherpesvirus entry activities of human nectin-2 mutants containing amino acid substitutions at position 89. (A) Two human nectin-2 mutants were constructed in which the amino acid at position 89 was exchanged for an Ile or a Phe, as shown on the y axis. To assess cell surface expression, CHO-K1 cells transfected with plasmids expressing wild-type human nectin-2, M89I, M89F, or control plasmid were tested for the binding of anti-human nectin-2 antibody, as described for Fig. 1. Results shown are the means and standard deviations of triplicate determinations. (B) Alphaherpesvirus entry activities of wild-type human nectin-2 and position 89 mutants. CHO-K1 cells were transfected as above and 48 h later exposed to serial dilutions of reporter alphaherpesviruses (HSV-1/Rid1, PRV, HSV-1, and HSV-2) as described for Fig. 2. Results shown are the means and standard deviations of triplicate determinations. The assays were repeated three times with similar results.
The human nectin-2 mutants were tested for cell surface expression using the polyclonal anti-human nectin-2 antibody. As shown in Fig. 9A, the mutants were detected at the cell surface at levels similar to wild-type human nectin-2. When tested for viral entry activity (Fig. 9B), all molecules were able to mediate PRV entry. However, the M89F mutant lost the ability to mediate entry of HSV-1/Rid1, HSV-1, and HSV-2. As hypothesized, substitution of an Ile in this position (M89I) did not affect HSV-1/Rid1 entry. Interestingly, the M89I mutant exhibited enhanced HSV-1 and HSV-2 entry activity. Thus, replacing the Met at position 89 with Phe alters the herpesvirus entry activity of human nectin-2, eliminating HSV but not PRV entry activity, yielding an activity profile similar to that of mouse nectin-2. An Ile in the equivalent position is associated with enhanced HSV-1 and HSV-2 entry activity, as when an Ile is present in mouse nectin-2. Clearly the particular amino acid at position 84 in mouse nectin-2 or 89 in human nectin-2 is crucial for the ability of these cell surface molecules to mediate HSV and PRV entry.
DISCUSSION
In this study our goal was to identify structural differences between human and mouse forms of nectin-2 that accounted for ability of the human form but not the mouse form to mediate HSV entry. Our results confirmed the previous finding that determinants for HSV entry reside in the N-terminal V-like Ig domain of human nectin-2 (21). More importantly, they demonstrated that the particular amino acid residues present in two small regions of the V domain determined whether either mouse or human nectin-2 could serve as an HSV entry receptor. Moreover, we found that changes in amino acid sequence in these regions had different effects on the ability of several alphaherpesviruses tested to utilize these receptors. This indicates that the various forms of alphaherpesvirus gD must differ in their interactions with each of the various wild-type and mutated forms of nectin-2.
The exchange of Ig domains between human and mouse forms of nectin-2 gave straightforward results. All chimeras with the V domain of human nectin-2 had the entry activities characteristic of human nectin-2, and all chimeras with the V domain of mouse nectin-2 had the more restricted entry activity characteristic of mouse nectin-2. This confirms that the V domain contains the critical determinants for HSV entry (21). The only anomaly was the reproducible enhancement of entry activity for HSV-2 when the V domain of human nectin-2 was fused to the remainder of the mouse nectin-2 molecule (Ch 3 in Fig. 2). Possibly, the C2 domains of mouse nectin-2 provide a better scaffold for presentation of the human V domain to HSV-2 than do the C2 domains of human nectin-2.
When short stretches of mouse sequence within the V domain were replaced with human sequences from equivalent positions, replacements in two of the five regions of divergence tested gave remarkable results. Replacements within regions A or B or both (Fig. 3 to 7) conferred ability to mediate entry of HSV-1/Rid1 and also conferred ability to mediate entry of HSV-1 and HSV-2 much more efficiently than is characteristic of human nectin-2. Further dissection of region A revealed that replacement of mouse amino acids GTV with human amino acids HQN (Ch 15) was almost as effective as substituting with APANHQN (the entire sequence of human nectin-2 present at this position) (Ch 7). Further dissection of region B revealed that all mouse chimeras having Met at position 84 (as in human nectin-2 in the equivalent position) had acquired HSV-1/Rid1 entry and also new entry activities, whereas all those that retained the Phe at position 84 had acquired no new entry activities, irrespective of other changes made in this region. The optimal replacement in region B, even better than replacing the entire region, was the substitution of mouse amino acids SF at positions 83 and 84 with the human amino acids KM from the equivalent positions 88 and 89.
Replacement of amino acids in region A or B of mouse nectin-2 with human nectin-2 sequences conferred normal (for HSV-1/Rid1) or enhanced (HSV-1 and HSV-2) entry activities. However, the replacement of one amino acid in region B of human nectin-2 with the corresponding mouse amino acid (M89F) eliminated all HSV entry activities, even though region A was unchanged. Thus, the presence of a Phe at position 89 in the context of the human V domain or at position 84 in the context of the mouse V domain is associated, in both cases, with absence of any HSV entry activity. The presence of a Phe at position 84 in the context of a mouse V domain containing human sequences in region A is associated with HSV entry activity. Whether the presence of a Phe at position 84 in mouse nectin-2 permits HSV entry activity depends on the sequence in region A. It might be possible to alter human nectin-2 so that presence of a Phe at position 89 would also permit HSV entry activity.
The amino acid present at position 84 in the mouse V domain or at position 89 in the human V domain is clearly critical for entry of all the viruses tested, but the particular amino acid preferred at this position for optimal entry activity is different for PRV and the HSV strains. The only molecules that exhibited reduced entry activity for PRV were Ch 9 (which has the entire region B replaced with human sequences) and various mouse nectin-2 mutants containing substitutions in position 84 in region B, including F84M (Ch 7). For PRV entry, the preferred amino acid at position 84 was Phe, at least for mouse nectin-2. The results obtained with the HSV strains provide a clear contrast. Phe at position 84 in mouse (wild-type mouse nectin-2) or 89 in human (M89F) was associated with absence of HSV entry activity, suggesting that PRV and HSV strains respond differently to the presence of Phe at this position. The substitutions F84M and F84I in mouse nectin-2 conferred entry activity for HSV-1/Rid1 to levels similar to that observed for human nectin-2. The same substitutions conferred enhanced entry activity for wild-type HSV-1 and HSV-2, highlighting differences in entry requirements for HSV-1/Rid and wild-type HSV-1 and HSV-2. Also, the M89I substitution in human nectin-2 was without effect on HSV-1/Rid1 entry, whereas the M89F substitution eliminated this entry activity. As expected, the M89F substitution also eliminated the entry activity observed for wild-type HSV-1 and HSV-2, but surprisingly, the M89I substitution enhanced entry activity for both wild-type HSV-1 and HSV-2. Assuming that these variations in sequence of the nectin-2 V domain influence gD binding, the gDs encoded by PRV and HSV-1/Rid1 must each differ from those encoded by HSV-1 and HSV-2 in their interactions with each form of nectin-2 tested. The differences observed between HSV-1 and HSV-2 entry seem to be principally quantitative and not qualitative.
Although the direct binding of alphaherpesvirus gDs to nectin-1 and other entry receptors has been observed (7, 12, 18, 19, 25, 38, 46), it has been difficult to detect the binding of gD to nectin-2. A previous report demonstrated by ELISA the weak binding of the variant form of gD U21 (amino acid substitution at position 25) to soluble forms of human nectin-2 (21). We were able to detect low levels of binding of a soluble form of HSV-1/Rid1 gD to cells expressing a variant form of mouse nectin-2 with high entry activity (Ch 19), but not to wild-type human nectin-2 or some of the other active chimeras. The failure to detect HSV gD binding to human nectin-2 is probably due to low affinity of the interaction. Alterations made here, resulting in chimeric molecules with enhanced entry activity, may also enhance the affinity of gD-nectin-2 interactions enough to begin to detect binding of soluble gD to cells or binding of gD to receptor by ELISA. Other methods will have to be devised to obtain quantitative comparisons of the gD-receptor interactions, in order to determine whether the apparent affinity of the interaction correlates with entry activity of the parental and chimeric receptors described here.
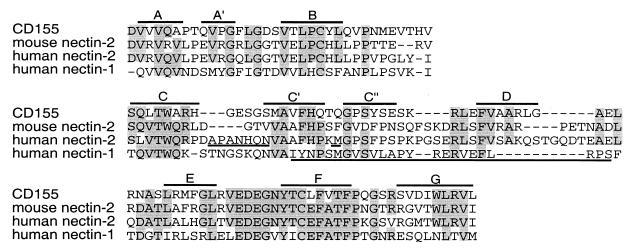
Alignment of mouse and human nectin-2 sequences with that of CD155 allowed us to make rough predictions about the location of the amino acids in regions A and B relative to the beta-strands that form the V domain (Fig. 10). Loop regions between the beta-strands, specifically B-C, C-C′, C′-C", C"-D, and D-E loops, showed the most variability in sequence among the molecules. Region A appears to localize to an area encompassing the C-C′ loop and region B to the C′-C" loop. The C′-C" region is predicted to be exposed and available for interactions with virus, and in fact, equivalent regions of other members of the Ig superfamily have been shown to be contact points for viruses. Studies on CD155 have identified the C′-C"-D region as an important component of the binding site for poliovirus. Specifically, amino acids localized to the C′-C" loop are crucial for viral binding to receptor (4, 31). Substitution of a single amino acid to a bulkier residue (Q55F), in the position equivalent to the Met in region B of human nectin-2, abolished poliovirus binding (31). The region in CD4 encompassing the C′-C"-D beta-strands and intervening loops has been identified as the binding site for HIV (2, 29). Transfer of this region of human CD4 to the rat homolog can confer HIV entry activity on the hybrid molecule (37). In addition, substitutions of amino acids in the C′-C" loop affect virus binding (33). Thus, it seems that the equivalent region of the V domains of different Ig molecules can be utilized by several viruses for binding to the cell surface and for entry.
FIG. 10.
Alignment of nectin-2, nectin-1, and poliovirus receptor (CD155) V domain sequences. The sequence of the V domain of CD155 (24) was aligned with the corresponding sequences of mouse and human nectin-2 and human nectin-1. The proposed location of the beta-strands of CD155 (14) is indicated above the CD155 sequences. Gray shading represents identity in amino acid of at least three of the molecules. Regions A and B of human nectin-2 are underlined. The proposed epitopes for MAbs that inhibit gD binding to nectin-1 are underlined (17).
Because nectin-2 is believed to be a gD receptor, as are all the other HSV entry receptors, regions A and B in human nectin-2 could form part or all of the contact site with gD or could otherwise influence the conformation of the contact site. In a recent study of human nectin-1, three monoclonal antibodies were shown to inhibit the binding of gD to nectin-1. The epitopes of two of these antibodies were mapped using overlapping peptides (17). These epitopes were localized to sequences homologous to region B in nectin-2 and extending downstream to the D-E loop (Fig. 10). Taken together, these results suggest that the region in nectin-2 including the C′ and C" beta-strands and surrounding loops is the site to which HSV gD binds.
It has been shown that soluble forms of HSV-1 gD can inhibit cell adhesion mediated by nectin-1 (35), suggesting that the interface for trans-homophilic interactions between nectin-1 ectodomains may overlap with the gD-binding domain. We have preliminary evidence, however, that the M89F mutation in human nectin-2 has little or no effect on trans-homophilic interactions between nectin-2 ectodomains despite the absence of HSV entry activity. A mutation at position 136 in mouse nectin-2 was reported to abolish the trans-homophilic interaction (28). Work is in progress to assess the effects of an equivalent mutation in human nectin-2 and other mutations on both alphaherpesvirus entry and trans-homophilic interactions.
Because of the homology between nectin-1 and nectin-2, the information obtained in this study on nectin-2 will facilitate identification of the regions in nectin-1 that are critical for alphaherpesvirus entry activity. Also, the nectin-2 chimeras and mutants generated in this study will be useful for high-resolution structural studies designed to determine how the variations in primary sequence influence three-dimensional structure, interactions with various forms of alphaherpesvirus gD, and entry activity.
ACKNOWLEDGMENTS
We thank N. Susmarski and M. L. Parish for excellent technical assistance and C. Rowe, G. Cohen, R. Eisenberg, and A. Nomoto for reagents.
This work was supported by Public Health Service grants R37 AI36293 and U19 AI31494 from the National Institute for Allergy and Infectious Diseases. W.M.M. was supported by Public Health Service fellowship F31 GM 19765.
REFERENCES
- 1.Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235:374–384. doi: 10.1006/excr.1997.3685. [DOI] [PubMed] [Google Scholar]
- 2.Arthos J, Deen K C, Chaikin M A, Fornwald J A, Sathe G, Sattentau Q J, Clapham P R, Weiss R A, McDougal J S, Pietropaolo C, Axel R, Truneh A, Maddon P J, Sweet R W. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989;57:469–481. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- 3.Babic N, Klupp B G, Makoschey B, Karger A, Flamand A, Mettenleiter T C. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J Gen Virol. 1996;77:2277–2285. doi: 10.1099/0022-1317-77-9-2277. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt G, Harber J, Zibert A, de Crombrugghe M, Wimmer E. The poliovirus receptor: identification of domains and amino acid residues critical for virus binding. Virology. 1994;203:344–356. doi: 10.1006/viro.1994.1493. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard M J, Dong Y, McDermott B M, Jr, Lam D-H, Brown K R, Shelanski M, Bellve A R, Racaniello V R. Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell-cell adherent junctions. Mol Cell Biol. 2000;20:2865–2873. doi: 10.1128/mcb.20.8.2865-2873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol. 2000;10:305–319. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi F, Lopez M, Menotti L, Aoubala M, Dubreuil P, Campadelli-Fiume G. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc Natl Acad Sci USA. 1998;95:15700–15705. doi: 10.1073/pnas.95.26.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean H J, Terhune S, Shieh M-T, Susmarski N, Spear P G. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell type-dependent alterations in infectivity. Virology. 1994;199:67–80. doi: 10.1006/viro.1994.1098. [DOI] [PubMed] [Google Scholar]
- 10.Eberlé F, Dubreuil P, Mattei M G, Devilard E, Lopez M. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene. 1995;159:267–272. doi: 10.1016/0378-1119(95)00180-e. [DOI] [PubMed] [Google Scholar]
- 11.Geraghty R J, Fridberg A, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Use of chimeric nectin-1 (HveC)-related receptors to demonstrate that ability to bind alphaherpesvirus gD is not necessarily sufficient for viral entry. Virology. 2001;285:366–375. doi: 10.1006/viro.2001.0989. [DOI] [PubMed] [Google Scholar]
- 12.Geraghty R J, Jogger C R, Spear P G. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology. 2000;268:147–158. doi: 10.1006/viro.1999.0157. [DOI] [PubMed] [Google Scholar]
- 13.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Bowman V D, Mueller S, Bator C M, Bella J, Peng X, Baker T S, Wimmer E, Kuhn R J, Rossmann M G. Interaction of the poliovirus receptor with poliovirus. Proc Natl Acad Sci USA. 2000;97:79–84. doi: 10.1073/pnas.97.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda W, Nakanishi H, Miyoshi J, Mandai K, Ishizaki H, Tanaka M, Togawa A, Takahashi K, Nishioka H, Yoshida H, Mizoguchi A, Nishikawa S-I, Takai Y. Afadin: a key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J Cell Biol. 1999;146:1117–1131. doi: 10.1083/jcb.146.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koike S, Horie H, Ise I, Okitsu A, Yoshida M, Iizuka N, Takeuchi K, Takegami T, Nomoto A. The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J. 1990;9:3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummenacher C, Baribaud I, Ponce de Leon M, Whitbeck J C, Lou H, Cohen G H, Eisenberg R J. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J Virol. 2000;74:10863–10872. doi: 10.1128/jvi.74.23.10863-10872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krummenacher C, Nicola A V, Whitbeck J C, Lou H, Hou W, Lambris J D, Geraghty R J, Spear P G, Cohen G H, Eisenberg R J. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72:7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krummenacher C, Rux A H, Whitbeck J C, Ponce de Leon M, Lou H, Baribaud I, Hou W, Zou C, Geraghty R J, Spear P G, Eisenberg R J, Cohen G H. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J Virol. 1999;73:8127–8137. doi: 10.1128/jvi.73.10.8127-8137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez M, Cocchi F, Avitabile E, LeClerc A, Adelaide J, Campadelli-Fiume G, Dubreuil P. Novel, soluble isoform of the herpes simplex virus (HSV) receptor nectin-1 (or PRR1-HIgR-HveC) modulates positively and negatively susceptibility to HSV infection. J Virol. 2001;75:5684–5691. doi: 10.1128/JVI.75.12.5684-5691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez M, Cocchi F, Menotti L, Avitabile E, Dubreuil P, Campadelli-Fiume G. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J Virol. 2000;74:1267–1274. doi: 10.1128/jvi.74.3.1267-1274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez M, Eberlé F, Mattei M G, Gabert J, Birg F, Bardin F, Maroc C, Dubreuil P. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene. 1995;155:261–265. doi: 10.1016/0378-1119(94)00842-g. [DOI] [PubMed] [Google Scholar]
- 23.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 25.Menotti L, Avitabile E, Dubreuil P, Lopez M, Campadelli-Fiume G. Comparison of murine and human nectin-1 binding to herpes simplex virus glycoprotein D (gD) reveals a weak interaction of murine nectin-1 to gD and a gD-dependent pathway of entry. Virology. 2001;282:256–266. doi: 10.1006/viro.2001.0850. [DOI] [PubMed] [Google Scholar]
- 26.Menotti L, Lopez M, Avitabile E, Stefan A, Cocchi F, Adelaide J, Lecocq E, Dubreuil P, Campadelli-Fiume G. The murine homolog of human Nectin1δ serves as a species nonspecific mediator for entry of human and animal αherpesviruses in a pathway independent of a detectable binding to gD. Proc Natl Acad Sci USA. 2000;97:4867–4872. doi: 10.1073/pnas.97.9.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller J M, Whetstone C A, Bello L J, Lawrence W C, Whitbeck J C. Abortion in heifers inoculated with a thymidine kinase-negative recombinant of bovine herpesvirus 1. Am J Vet Res. 1995;56:870–874. [PubMed] [Google Scholar]
- 28.Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cisdimerization or transinteraction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- 29.Moebius U, Clayton L K, Abraham S, Harrison S C, Reinherz E L. The human immunodeficiency virus gp120 binding site on CD4: delineation by quantitative equilibrium and kinetic binding studies of mutants in conjunction with a high-resolution CD4 atomic structure. J Exp Med. 1992;176:507–517. doi: 10.1084/jem.176.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 31.Morrison M E, He Y-J, Wien M W, Hogle J M, Racaniello V R. Homolog-scanning mutagenesis reveals poliovirus receptor residues important for virus binding and replication. J Virol. 1994;68:2578–2588. doi: 10.1128/jvi.68.4.2578-2588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison M E, Racaniello V R. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J Virol. 1992;66:2807–2813. doi: 10.1128/jvi.66.5.2807-2813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson A, Seed B. Genetic analysis of monoclonal antibody and HIV binding sites on the human lymphocyte antigen CD4. Cell. 1988;54:65–72. doi: 10.1016/0092-8674(88)90180-8. [DOI] [PubMed] [Google Scholar]
- 34.Reymond N, Borg J P, Lecocq E, Adelaide J, Campadelli-Fiume G, Dubreuil P, Lopez M. Human nectin3/PRR3: a novel member of the PVR/PRR/nectin family that interacts with afadin. Gene. 2000;255:347–355. doi: 10.1016/s0378-1119(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 35.Sakisaka T, Taniguchi T, Nakanishi H, Takahashi K, Miyahara M, Ikeda W, Yokoyama S, Peng Y F, Yamanishi K, Takai Y. Requirement of interaction of nectin-1 alpha/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1. J Virol. 2001;75:4734–4743. doi: 10.1128/JVI.75.10.4734-4743.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- 37.Schockmel G A, Somoza C, Davis S J, Williams A F, Healey D. Construction of a binding site for human immunodeficiency virus type 1 gp120 in rat CD4. J Exp Med. 1992;175:301–304. doi: 10.1084/jem.175.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla D, Dal Canto M, Rowe C L, Spear P G. Striking similarity of murine nectin-1α to human nectin-1α (HveC) in sequence and activity as a gD receptor for alphaherpesvirus entry. J Virol. 2000;74:11773–11781. doi: 10.1128/jvi.74.24.11773-11781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shukla D, Rowe C L, Dong Y, Racaniello V R, Spear P G. The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. J Virol. 1999;73:4493–4497. doi: 10.1128/jvi.73.5.4493-4497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 41.Spear P G, Eisenberg R J, Cohen G H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms J A, Spritz R A. Mutations of PVRL1: encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet. 2000;25:427–430. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- 43.Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150:1161–1175. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warner M S, Geraghty R J, Martinez W M, Montgomery R I, Whitbeck J C, Xu R, Eisenberg R J, Cohen G H, Spear P G. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 46.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]