Abstract
Background.
Since 1998, the Swiss Organ Living-Donor Health Registry (SOL-DHR) has recorded peri- and postoperative complications of living kidney (LK) donors, as reported by all Swiss transplant centers and has collected follow-up data prospectively.
Methods.
We analyzed the early complications of 2379 consecutive individuals who donated a kidney between January 1998 and June 2022 and assessed their health-related quality of life (HRQoL) 1 y after donation.
Results.
In total, 447 early complications in 404/2379 LK donors (17.0%) were reported to the SOL-DHR. The frequency of donors with major complications (ie, Dindo-Clavien classification 3/4) was 2.4%. In total, 31 donors needed reoperation, and in 13/31 (42%), donors reoperation was necessary because of bleeding complications. Independent risk factors for major early complications were older donor age (P = 0.005) and type of surgical approach (ie, the laparoscopic retroperitoneal compared with laparoscopic transabdominal surgery; P = 0.01), but not sex. We observed a U-shaped association of body mass index, where very low/high body mass indexes had higher odds of major early complications, without reaching statistical significance. Although HRQoL was affected by kidney donation, 96.5% of donors indicated that they would donate their kidney again. The only independent risk factor for low HRQoL based on mental health scores was worsening EB after living kidney donation (P < 0.0001).
Conclusions.
Overall, living kidney donation is a safe procedure, however, donor age and type of surgical approach affect the risk of complications. A decline in emotional bonding with the recipient after donation may worsen the quality of life of the donor.
INTRODUCTION
Kidney transplantation is the therapy of choice for many patients with kidney failure (KF) and has evolved rapidly worldwide in recent decades. Despite this progress, there is still an enormous gap between organ demand and availability. Efforts to increase the number of deceased donors have been insufficient, leading to an increase in living-donor kidney transplants worldwide. In Switzerland, living donations, starting in the 1960s, now account for approximately one-third of all donations. To illustrate, in 2023 109/400 (27%) kidney transplantations were from LK donors (https://www.swisstransplant.org).
The advantages of living kidney donation (LKD) for the recipient include pre-emptive transplantation, shorter time on dialysis, shorter cold ischemia time, and improved allograft and patient long-term survival.1-3 However, LKD exposes donors to the risks of early complications and long-term physical and psychosocial consequences. Therefore, comprehensive donor information obtained from prospective cohort/registry studies is crucial.4 Over time, the selection criteria for LK donors have broadened to include elderly donors, as well as donors with comorbidities such as obesity and hypertension, if well controlled.5,6 Thus, detailed and regularly updated information regarding the risk of LKD are essential. However, prospectively collected cohort data on perioperative short- and long-term complications after LKD nephrectomy are sparse.7-11 Furthermore, HRQoL after LKD and psychosocial outcome risks from prospective studies were reported in only two articles.12,13 Because of the lack of these data in the early nineties, SOL-DHR was founded in 1993 as the first prospective registry worldwide. Since then, SOL-DHR has collected data from all LK donors of Swiss transplant centers.14
This prospective, multicenter cohort study analyzed SOL-DHR data to evaluate the incidence of complications, kidney function, metabolic parameters, HRQoL, and psychosocial outcome risks before and 12 mo after LKD.
MATERIALS AND METHODS
Prospective Cohort Study and Donor Population
SOL-DHR was launched in Switzerland to provide lifelong follow-up for kidney donors.14 Since 1993, all Swiss transplant centers have included all LK donors who have consented to participate and since 2007, it has been mandatory in accordance with the Swiss Transplantation Act.
This retrospective analysis of prospectively collected data from LK donors nested within the national registry was conducted with approval of the ethics committee of Northwestern and Central Switzerland (www.eknz.ch; project-ID 2022-01413).
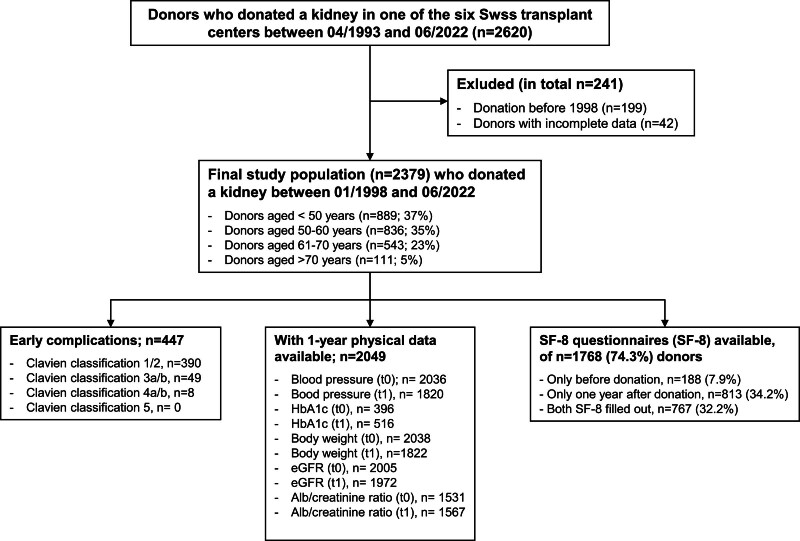
From 1993 to 2022, totally 2620 LK donors were registered in Switzerland, with 199 from before 1998 excluded because of missing data on complications. Additionally, 42 donors with incomplete datasets were excluded from the study. The final study population consisted of 2379 individuals who donated a kidney between January 1998 and June 2022. Outcomes were determined on September 30, 2023 (Figure 1).
FIGURE 1.
Study flowchart. Individuals who donated before 1998 (n = 199) as well as donors with incomplete data were excluded (n = 42). Early complications classified by Dindo-Clavien classification as well available follow-up data of donors are indicated.
Pre- and Postdonation Management
The national registry organizes a follow-up of LK donors at 1 y, and then every 2 y lifelong. Data collection principles before kidney donation, at the time of discharge from nephrectomy and thereafter have been described in detail previously.14 Briefly, transplant centers inform donors about the registry and send data on baseline characteristics, comorbidities, and the relationship with recipients to SOL-DHR. At the time of discharge from nephrectomy, a questionnaire about peri- and postoperative complications during the in-hospital stay is filled out by transplant centers and sent to SOL-DHR. In addition, early complications within the first 12 mo after nephrectomy are included in the database after thorough investigation and confirmation by SOL-DHR’s data managers.
In 2002, SOL-DHR introduced questionnaires to assess psychosocial factors and HRQoL using the validated Short-Form 8 Health Survey (SF-8). The SF-8 is a brief form of the commonly used Short-Form 36 Health Survey (SF-36), which measures eight dimensions of HRQoL and allows the calculation of two summary scales: physical (PCS) and mental health (MCS). Participants first completed the SF-8 and psychosocial factors questionnaires 12 mo after donation and then every 5 y. Additionally, since 2006, these questionnaires have been completed before donations. Between January 2002 and June 2022, n = 955 predonation and n = 1580 1-y postdonation SF-8 questionnaires from 1768/2379 donors (74.3%) were transmitted to the SOL-DHR database. In total, 767/1768 donors completed both (predonation and 1 y after donation) SF-8 questionnaires (Figure 1).
Data Collection and Objectives
Preoperative donor characteristics include height, weight, blood pressure, body mass index (BMI), sex, age, medication, and pre-existing comorbidities, such as hypertension. Early peri- and postoperative complications include surgical and other complications (ie, need for blood transfusions, wound infections, urinary tract infections (UTI), other infections, urinary retention, deep vein thrombosis, pulmonary embolism, and psychosocial complications). The complication questionnaire includes a visual scale for pain filled out by the donors. In addition, transplant centers report the side of nephrectomy, the surgical technique used, the length of in-hospital stay, and whether donors had high-blood pressure values (>140/90 mmHg) on at least 2 consecutive days during the postoperative phase. Early complications observed before 2017 were retrospectively classified according to the Dindo-Clavien grading system of surgical complications,15 and those observed since 2017 were prospectively classified by the transplant centers. Each complication is counted separately, allowing the identification of multiple complications per donor. Since the notification of complications to the national registry, 5 different surgical techniques for LKD have been performed.16 However, in this analysis, the surgical techniques were classified as either open or laparoscopic (ie, transabdominal or retroperitoneal) nephrectomy.
Collected predonation and 1-y lab parameters include serum creatinine, eGFR (calculated using CKD-EPI formula), hemoglobin A1c (HbA1c), and urine analysis (urine dipstick analysis, urine sediment if dipstick is pathological, urine protein-creatinine ratio (UPCR), and urine albumin-creatinine ratio). Since 1993, blood/urine samples for all laboratory parameters have been sent to a core laboratory (Viollier AG, Basel, Switzerland), except for urine dipstick and sediment examinations. Furthermore, data on weight, BMI, blood pressure control, occurrence of hypertension, and any medications are collected. Concerning HbA1c, these values have been collected in the registry only since August 2017.
The objectives were to investigate the incidence of early peri- and postoperative complications after LKD as well as to identify potential risk factors for adverse outcomes. Further objectives were to analyze differences in kidney function (ie, serum creatinine/eGFR and UPCR/UACR), metabolic parameters (ie, weight, BMI, and HbA1c), and HRQoL before and 12 mo after donation as well as to explore psychosocial outcome risks.
Statistical Analysis
We used JMP software version 16.0 (SAS Institute Inc., Cary, NC) and R version 4.2.2 (www.R-project.org) for statistical analysis. Categorical data were summarized as counts and percentages and analyzed using Fisher’s exact test or Pearson’s chi-squared test, as appropriate. The distribution of continuous data was analyzed using quantile-quantile plots and summarized as mean and standard deviation. Hypothesis testing for variables following a normal distribution was performed using a t-test or paired t-test for paired data. Hypothesis testing for variables not following a normal distribution was performed using the Wilcoxon rank-sum test and data summarized as median and interquartile range (IQR). As a measure of effect, the mean difference with a 95% confidence interval (CI) and corresponding Cohen’s d were calculated. For interpretation purposes effect sizes were graded according to Cohen (Cohen’s d < 0.2 equals “very small,” between 0.2 and <0.5 “small,” between 0.5 and <0.8 “medium,” and ≥0.8 a large effect size).17
Multivariable logistic regression analysis was performed to identify potential risk factors for major early complications based on the Dindo-Clavien classification (ie, major complications were defined as Dindo-Clavien classification ≥3a). Potential risk factors were selected based on pre-existing knowledge, without a P value threshold or automated variable selection. We adhered to the 1:10 rule of thumb, which suggests including one variable in the model for every 10 events to reduce the risk of overfitting. To account for the long observation time and potential procedural changes, the year of transplantation was chosen a priori as a potential confounder and included in the model. The cutoff values for the strata were determined based on the number of complications. To relax the linearity assumption, BMI and age were modeled using restricted cubic splines. The dose-response relationship for age and BMI is visualized in the dose-response plots. Based on these results and for easier interpretation, an additional regression model was built, including BMI as a categorical variable. A BMI between 20 and 24.9 kg/m² was chosen as the reference group and compared with BMI categories of <20, 25–29.9, and >30 kg/m². Statistical significance was defined as a two-tailed P value <0.05. No imputation was used to address the missing values.
Individual values of the SF-8 as well as the sum scales (ie, PCS and MCS) are reported as Norm-Based Scores as described in.18 Low physical and mental HRQoL was defined as a component score ≤30.
RESULTS
Donor Baseline Characteristics
Data on peri- and postoperative complications were available for 2379 LK donors (98.3% of all kidney donors who were registered between January 1998 and June 2022) (Figure 1). The baseline characteristics are summarized in Table 1. The mean age at the time of donation was 53 y (SD ± 11.2 y), and 64.4% of the LK donors were female. Most of the donors were partners, parents, or siblings. During the study period, three main types of donor nephrectomy were performed. The transabdominal laparoscopic method was chosen most often. In addition, 55% of open nephrectomies were performed between 1998 and 2008 (Table S1, SDC, http://links.lww.com/TXD/A707). In 58% of cases left kidney was removed. Mean predonation creatinine was 68 µmol/L (SD ± 13.5 µmol/L), and the mean eGFR was 92 ml/min/1.73m2 (SD ± 14.1 ml/min/1.73m2). Thus, most donors had normal kidney function, but in n = 20 donors (0.9%), predonation eGFR was <60 ml/min/1.73m2 (Figure 2). Of the entire donor population, 17.4% had arterial hypertension that required treatment before donation. The mean predonation systolic and diastolic blood pressures were 127 mmHg (SD ± 13.5 mmHg) and 77 mmHg (SD ± 8.7 mmHg), respectively. The mean predonation BMI was 25.5 kg/m2 (SD ± 3.8 kg/m2) and only 1.2% of the donors had a BMI >35 kg/m2. In a supplementary table, baseline characteristics stratified according to donors with/without occurrence of any early complication are summarized (Table S2, SDC, http://links.lww.com/TXD/A707).
TABLE 1.
Baseline donor characteristics, n = 2379
| Variable | Overalla |
|---|---|
| Mean age at donation (SD), y | 52.9 (±11.2) |
| Age grouping at donation, n (%) | |
| <30 y | 64 (2.7) |
| 30–40 y | 284 (11.9) |
| 41–49 y | 541 (22.8) |
| 50–60 y | 836 (35.1) |
| 61–70 y | 543 (22.8) |
| >70 y | 111(4.7) |
| Sex, n (%) | |
| Female | 1532 (64.4) |
| Male | 847 (35.6) |
| Donor-recipient relation, n (%) | |
| Parents | 572 (24.0) |
| Siblings | 513 (21.6) |
| Other relatives | 117 (4.9) |
| Partners | 842 (35.4) |
| Other nonrelatives | 335 (14.1) |
| KPD, n (%) | 51 (2.1) |
| Surgical method (broad), n (%) | |
| Nephrectomy open | 441 (18.5) |
| Laparoscopic transabdominal | 1193 (50.2) |
| Laparoscopic retroperitoneal | 745 (31.3) |
| Site, n (%) | |
| Right kidney | 598 (25.1) |
| Left kidney | 1382 (58.1) |
| Unknown | 399 (16.8) |
| Kidney function before donation | |
| Mean creatinine (SD), µmol/l | 67.9 (±13.5) |
| Mean eGFR, CKD-EPI (SD) (ml/min/1.73m2) | 92.3 (±14.1) |
| Mean UPCR (SD), mg/mmol | 9.2 (±5.5) |
| Mean UACR (SD), mg/mmol | 1.1 (±2.5) |
| Arterial hypertension before donation, n (%) | 414 (17.4) |
| Blood pressure before donation, n (%) | |
| Mean Systolic blood pressure (SD), mmHg | 126.7 (±13.5) |
| Mean Diastolic blood pressure (SD), mmHg | 77.4 (±8.7) |
| Mean Weight (SD), kg | 71.9 (±12.9) |
| Mean BMI (IQR), kg/m2 | 25.5 (±3.8) |
| BMI <30 kg/m2, n (%) | 2085 (88.0) |
| BMI 30–35 kg/m2, n (%) | 257 (10.9) |
| BMI >35 kg/m2, n (%) | 27 (1.1) |
| Mean HbA1c (IQR), % | 5.4% (±0.4) |
aValues are indicated as mean and standard deviation (SD).
KPD and eGFR = calculated by the CKD-EPI formula; in ml/min/1.73m2 of body surface, which was available for 2327 donors. CKD-EPI, UPCR, and UACR = only calculated for donors with undiluted urine (ie, urine creatinine ≥3 mmol/l; n = 1796). BMI = available for 2369 donors before donation, HbA1c = available for 430 donors before donation since August 2017. Predonation systolic/diastolic blood pressure measurements were available for 2363 donors.
BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; KPD, kidney paired donation; UACR, urine albumin-to-creatinine ratio; UPCR, urine protein-to-creatinine ratio.
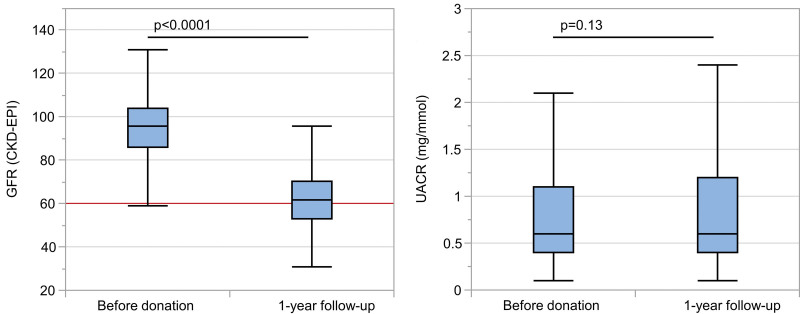
FIGURE 2.
Course of kidney function and albuminuria before and 12 mo after kidney donation. The box plots show (A) eGFR before and 12 mo after kidney donation as well as UACR at the same timepoints (B). The red line in (A) indicates an eGFR level of 60 ml/min/1.73m2. eGFR, estimated glomerular filtration rate (calculated by the CKD-EPI formula; in ml/min/1.73m2 of body surface); CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; UACR, urine albumin-to-creatinine ratio (mg/mmol).
Early Peri- and Postoperative Complications
As summarized in Table 2, there were 447 early complications in 404 LK donors (17.0%). In total, 348 (14.6%) had minor complications (Dindo-Clavien classification 1/2), and only 56 (2.4%) had major complications (ie, Dindo-Clavien classification 3/4; in detail: donors with 3a, n = 34, with 3b, n = 14, with 4a, n = 7, with 4b, n = 1). No perioperative mortality was observed during the study period. In total, 31 donors required reoperation either during hospitalization or within the first 12 mo. In 13 donors, reoperation was necessary because of bleeding complications (8 with severe retroperitoneal hematoma, 2 with arterial lesions, and 3 with diffuse venous bleeding). Other reasons for reoperation are listed in Table 2. Furthermore, 4 hemorrhages needed immediate intraoperative revision, and 2 of them were life-treating complications (Table 2). Owing to intraoperative complications, 0.7% (n = 14) of laparoscopic surgeries required conversion to laparotomy. The incidence of major early complications was slightly higher in donors who underwent laparoscopic nephrectomy than in those who underwent open surgery (2.7% versus 0.9%; P = 0.02). In detail, the frequency of major complications within the laparoscopic retroperitoneal group was 4.2%, and 1.8% within the laparoscopic transabdominal group, as well as 0.9% within the open nephrectomy group, respectively. Nevertheless, reoperations were not more frequent in donors who underwent laparoscopic surgery (P = 0.43) (data not shown). In total, 30 donors (1.3%) received blood transfusions because of significant blood loss.
TABLE 2.
Early donor complications, n = 447
| Complications | Comments | Numbers |
|---|---|---|
| Dindo-Clavien 1 | ||
| Urinary retention | 60 | |
| Other genitourinary problems | (3 hydrocele, 1 macrohematuria, 1 penis ulcer) | 5 |
| Pneumothorax | Without chest drain | 7 |
| Chyle leakage | Without further intervention | 4 |
| Severe hematoma | No blood transfusion or intervention | 16 |
| Secondary wound healing | Bedside opening | 3 |
| Psychological problems during hospitalization | 44 | |
| Pain scale >7 at dischargea | 42 | |
| Severe vomitus | ≥2 d | 14 |
| Intraoperative positioning-related complications | (5 rhabdomyolysis, 2 severe shoulder or back pain, 6 nerve lesion,1 costal fracture) | 14 |
| Anesthesia-related complications | (1 teeth damage, 1 glottis lesion, 1 keratitis, 3 severe headache >2 d postoperative) | 6 |
| Severe skin emphysema | After retroperitoneoscopic nephrectomy | 2 |
| Allergic skin reaction | Only topical treatment | 11 |
| Retained needle | Asymptomatic, no further intervention | 1 |
| Dindo-Clavien 2 | ||
| Urinary tract infection | Requiring antibiotics | 52 |
| Wound infection | Requiring antibiotics | 27 |
| Bronchopulmonary infection | Requiring antibiotics | 14 |
| Epididymitis | Requiring antibiotics | 5 |
| Septicemia | Venous line associated | 3 |
| Other infections | Requiring antimicrobial agents | 18 |
| Acute hepatitis | Drug related | 1 |
| Paralytic ileus | Prolonged ileus >2 d | 6 |
| Thrombosis | Right arm | 1 |
| Pulmonary embolism | 1 | |
| Severe hematoma | Requiring blood transfusion | 19 |
| Cardiac arrhythmia | 1 arterial fibrillation, 1 AV reentry tachycardia | 2 |
| Bronchospasm postsurgery | Known asthma bronchiale | 2 |
| Allergic reaction | 6 | |
| Other | 4 | |
| Dindo-Clavien 3a | ||
| Pulmonary edema | ICU admission, noninvasive ventilation, loop diuretics | 1 |
| Urethral stricture | Need for incision | 1 |
| Pneumothorax | Chest drainage | 5 |
| Lymphatic fistula | Percutaneous drainage | 1 |
| Chyle leakage | Percutaneous drainage | 3 |
| Iatrogenic diaphragm perforation | Chest drainage | 3 |
| Dindo-Clavien 3b | ||
| Carotid dissection | Need for reoperation | 1 |
| Benign prostatic hyperplasia | Recurrent urine retention, TURP | 1 |
| Testicular torsion | Reoperation with orchiectomy | 1 |
| Lesion of vena cava | Intraoperative revision, no blood transfusion | 2 |
| Lymphatic fistula | Reoperation | 1 |
| Hydrocele | Revision 4 mo after nephrectomy | 1 |
| Hernia | Reoperation | 4 |
| Retroperitoneal hematoma | Reoperation | 8 |
| Chyle leakage | Reoperation | 4 |
| Small bowel perforation | Reoperation | 1 |
| Mechanic ileus | Reoperation | 2 |
| Anal fistula | Reoperation | 1 |
| Arterial lesionb | Intraoperative revision 2/4, reoperation 2/4, 1/4 with mass transfusions | 4 |
| Diffuse venous bleeding | Reoperation, 2/3 needed blood transfusions | 3 |
| Need for abdominoplasty | Reoperation | 1 |
| Dindo-Clavien 4a | ||
| Chyle leakage retroperitoneal | Percutaneous drainage, need for parenteral nutrition, catheter sepsis | 1 |
| Arterial lesion (vessel with diameter 1mm in the adrenal region) | Life-threating intraoperative bleeding, successful embolization | 1 |
| Perforation of the terminal ileum | Life-threating intraoperative bleeding, need for evacuation of hematoma | 1 |
| Perforation of the jejunum | During the course development of sepsis and pulmonary embolism | 1 |
| Aortic lesion | Life-threating intraoperative bleeding | 1 |
| Severe rhabdomyolysis | Acute kidney failure, need for hemofiltration, muscle damage after surgery | 1 |
| Myocardial infarction | NSTEMI, ICU admission | 1 |
| Dindo-Clavien 4b | ||
| Pulmonary embolism | Resuscitation, ICU admission, full recovery | 1 |
| Total | n = 447 |
aPain scale information was available for 2221 donors in total.
bRenal arterial branches (n = 2), internal iliac artery (n = 1), and epigastric artery (n = 1).
ICU, intensive care unit; NSTEMI, non-ST-elevation myocardial infarction; TURP, transurethral resection of the prostate.
Early complications were most frequent genitourinary problems such as urinary retention and UTI requiring antibiotics (25.1% of all complications), followed by wound infections (6.1%), bronchopulmonary and other infections requiring antibiotics (together 9.0%), and severe hematoma requiring blood transfusions (4.3%) (Table 2). Furthermore, 42 donors indicated a pain scale >7 at discharge, which we classified as a Dindo-Clavien 1 complication regardless of whether they needed analgesics. In addition, 44 donors experienced psychological problems (depression and anxiety) during hospitalization, which was also defined as a Dindo-Clavien 1 complication (altogether 19.3% of all complications) (Table 2).
Urinary retention was more frequently observed in male compared with female donors (36/847 versus 24/1532; P = 0.0001) and in donors aged >70 y than in all other donor age categories (>70 y old: 10/111 (9%) versus 61–70 y old: 16/543 (3.0%); versus 50–60 y old: 19/836 (2.3%); versus <50 y old: 15/889 (1.7%), respectively; P ≤ 0.005). UTI was slightly more frequent in the elderly (>70 y) than in younger donors (5.4% versus 2.0%; P = 0.03). Furthermore, there was no association between wound infections and a higher BMI (P = 0.85) or between open and laparoscopic surgery (P = 1.0) (data not shown). The median hospitalization time was 5 d (IQR 4–7 d). Only 4.2% of donors were hospitalized for >10 d. In addition, the median hospitalization time for the 2 laparoscopic approaches was 5 d (IQR 4–7 d), and the median hospitalization time for the open nephrectomy approach was 6 d (IQR 4–8), respectively, indicating a statistically significant difference (P = 0.004). Further, the percentage of hospitalized donors for >10 d for the 2 laparoscopic approaches was lower (ie, 3.2%), than for the open nephrectomy approach (ie, 5.9%; P < 0.0001).
Course of Kidney Function, Blood Pressure, and Metabolic Parameters
Assessment of kidney function, blood pressure, and metabolic parameters before and 12 mo after donation only included donors with a 1-y follow-up (n = 2049, 86.1%). Concerning the evaluated outcome parameters, only eGFR showed a meaningful change from before to 12 mo after donation with an estimated mean difference of –31.74 ml/min/1.73m2 (95% CI, –32.24 to –31.24 ml/min/1.73m2), according to a mean percentage change of 33.5% (SD 11.1%).
Nevertheless, the mean eGFR after LKD was still >60 ml/min/1.73m2 and albuminuria was not higher than predonation (P = 0.13) (Table 3; Figure 2). The decrease in kidney function was dependent on donor age, with a higher risk of decrease in the elderly than in younger donors (P < 0.0001; data not shown).
TABLE 3.
Course of kidney function, blood pressure and metabolic parameters before and 12 mo after donation, n = 2049
| Parameter | Before donation mean (SD) |
12 mo after donation mean (SD) |
Mean Difference (95% CI) |
Cohen’s d | P value |
|---|---|---|---|---|---|
| Kidney function | |||||
| Creatinine (µmol/l) | 67.9 (±13.5) | 100.2 (±19.3) | 32.47 (31.86-33.08) | 2.36 | <0.0001 |
| eGFR, CKD-EPI (ml/min/1.73m2) | 95.3 (±14.2) | 62.9 (±13.7) | –31.74 (–32.24 to –31.24) | 2.84 | <0.0001 |
| UPCR (mg/mmol)a | 9.1 (±5.6) | 11.8 (±7.8) | 2.66 (2.22-3.12) | 0.13 | <0.0001 |
| UACR (mg/mmol)a | 1.1 (±2.7) | 1.2 (±3.0) | 0.13 (–0.04 to 0.31) | 0.04 | 0.13 |
| Blood pressure | |||||
| Systolic (mmHg) | 126.7 (±13.5) | 127.8 (±14.7) | 0.76 (0.07-1.46) | 0.05 | 0.03 |
| Diastolic (mmHg) | 77.4 (±8.7) | 80.5 (±9.1) | 2.76 (2.30-3.22) | 0.27 | <0.0001 |
| Metabolic variables | |||||
| HbA1c (%) | 5.4 (±0.4) | 5.4 (±0.3) | 0.05 (0.03-0.07) | 0.24 | <0.0001 |
| Body weight (kg) | 71.9 (±12.9) | 72.3 (±13.4) | 0.51 (0.31-0.70) | 0.05 | <0.0001 |
| BMI (kg/m2) | 25.5 (±3.8) | 25.7 (±4.1) | 0.26 (0.19-0.34) | 0.16 | <0.0001 |
aSamples with a urine creatinine value <3 mmol/l (n = 657) were excluded from the analysis of the UPCR and UACR as well as donors with no available urine sample (n = 471).
For all scores, the mean value (SD) is indicated. The differences between before donation and 12 mo after donation were analyzed by a paired t-test. As a measure of effect, the mean differences (95% CI) and corresponding Cohen’s d were calculated.
BMI, body mass index; CI, confidence interval; HbA1c, hemoglobin A1c; SD, standard deviation; UPCR, urine protein-to-creatinine ratio; UACR, urine albumin-to-creatinine ratio.
All other outcome parameters including UPCR showed only small mean differences with corresponding small effect sizes (ie, Cohen’s d ≤ 0.27) (Table 3). Only 0.8% of donors developed a UPCR >50 mg/mmol (ie, with a median UPCR of 55 mg/mmol; IQR, 52–63 mg/mmol; data not shown). During the postoperative phase, 111 donors (4.7%) had high-blood pressure values (>140/90 mmHg) for at least 2 consecutive days. Of these, 46 donors had been treated for hypertension before donation. In 50/111 (45%) donors, the blood pressure normalized during follow-up; however, 15 donors required continuous antihypertensive therapy. The rate of donors on antihypertensive medication increased from 17.4% before donation to 21.4% 12 mo after donation.
Course of Health-related Quality of Life
The evolution of HRQoL from before to 12 mo after kidney donation is shown in Table 4. In total, 767 donors from the original donor population completed both (pre- and 1-y postdonation) SF-8 questionnaires. Both component scale scores (ie, MCS and PCS) as well as the single scores of the eight measured items of the SF-8 questionnaire (ie, general health, physical functioning, role physical, bodily pain, vitality, social functioning, mental health, and role emotional) were in the upper range of the standard normal population before and 12 mo after LKD (Table 4).19,20 Both component scores and single quality of life items showed statistically significant differences at the two time points (P ≤ 0.002), except for mental health (P = 0.14). However, the decreases were small with corresponding small effect sizes (ie, Cohen’s d ≤ 0.38).
TABLE 4.
Course of psychosocial parameters before and 12 mo after donation, n = 767
| Parameter | Before donation mean (SD) |
12 mo after donation mean (SD) |
Mean Difference (95% CI) |
Cohen’s d | P value |
|---|---|---|---|---|---|
| Summary component scale (SF-8) | |||||
| MCS | 54.7 (±5.9) | 53.6 (±7.7) | –0.84 (–1.39 to –0.29) | 0.11 | 0.003 |
| PCS | 56.0 (±4.5) | 53.1 (±7.5) | –2.68 (–3.19 to –2.17) | 0.38 | <0.0001 |
| Quality of life questions (SF-8) | |||||
| General health | 53.4 (±4.6) | 51.4 (±6.2) | –1.64 (–2.04 to –1.24) | 0.29 | <0.0001 |
| Physical functioning | 52.7 (±3.4) | 50.8 (±5.7) | –1.69 (–2.09 to –1.30) | 0.31 | <0.0001 |
| Role physical | 53.1 (±3.7) | 50.8 (±7.3) | –2.01 (–2.51 to –1.52) | 0.29 | <0.0001 |
| Bodily pain | 58.0 (±5.7) | 56.1 (±7.5) | –1.89 (–2.44 to –1.34) | 0.24 | <0.0001 |
| Vitality | 56.2 (±5.2) | 53.2 (±7.3) | –2.71 (–3.21 to –2.21) | 0.38 | <0.0001 |
| Social functioning | 53.6 (±4.1) | 52.8 (±5.5) | –0.63 (–1.02 to –0.24) | 0.11 | 0.002 |
| Mental health | 53.1 (±5.8) | 52.5 (±7.2) | –0.42 (–0.97 to 0.14) | 0.05 | 0.14 |
| Role emotional | 51.2 (±3.6) | 50.1 (±5.2) | –0.95 (–1.33 to –0.57) | 0.18 | <0.0001 |
| Transition of health status (improved), n (%)a | 172 (22.4) | ||||
| Emotional bonding (better),b n (%) | 196 (25.6) | ||||
| “Redonation,”c yes/no, n (%) | 740 (96.5)/16 (2.1) |
aThe question evaluating the transition of health status was “Compared with last year, how would you describe your actual state of health?”
bThe question evaluating emotional bonding was “How did the relationship to the organ recipient change after donation?”
cThe question to evaluate whether a person would donate again was: “If it were possible, would you choose organ donation again?”
For all scores, the mean value (SD) is indicated if not otherwise stated. The differences between before donation and 12 mo after donation were analyzed by a paired t-test. As a measure of effect, the mean differences (95% CI) and corresponding Cohen’s d were calculated.
CI, confidence interval; MCS, mental component scale; PCS, physical component scale; SD, standard deviation; SF-8, short-form 8-item health survey.
In addition, 172/767 (22.4%) donors stated that their health status improved (Table 4) after donation, and 66.5% (510/767) described their health status as unchanged after LKD compared with before donation. In total, 96.5% (740/767) of donors indicated that they would donate their kidney again if possible (Table 4). Interestingly, 196/767 (25.6%) donors considered that their emotional bonding (EB) to the recipient improved after LKD (Table 4). Most donors (68.4%) indicated that EB remained unchanged, and only 2.1% clearly stated that EB worsened after donation.
Next, we evaluated independent risk factors of low HRQoL based on both component scores (ie, MCS and PCS) individually, taking all complete postdonation questionnaires 12 mo after LKD into account (n = 1548), as depicted in Tables S3 (SDC, http://links.lww.com/TXD/A707; PCS) and S4 (http://links.lww.com/TXD/A707; MCS). Potential confounders such as sex and age, occurrence of any early major complications after LKD, and self-reflected worsening of EB after donation were considered in the multivariable logistic regression models. Importantly, sex, age, and the occurrence of any early major complications were not independent risk factors for low HRQoL based on both component scores as shown in Tables S3 (SDC, http://links.lww.com/TXD/A707; ie, PCS; P ≥ 0.17) and S4 (SDC, http://links.lww.com/TXD/A707; ie, MCS; P ≥ 0.05). The only independent risk factor for low HRQoL based on MCS was worsening EB after LKD (odds ratio [OR], 10.42; 95% CI, 3.56-30.50; P < 0.0001) as shown in Table S4 (SDC, http://links.lww.com/TXD/A707).
Independent Risk Factors of Early Major Complications
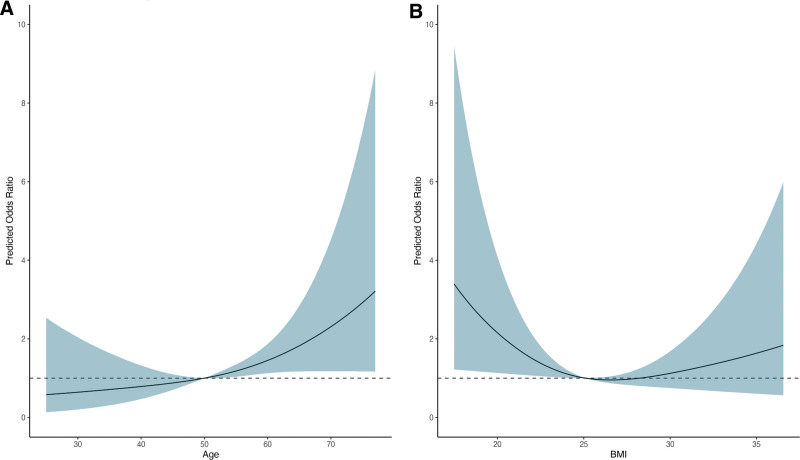
In a multivariable logistic regression analysis, donor age and the type of surgical approach were independently associated with major early complications based on the Dindo-Clavien classification (Table 5). Figure 3A and Table S5 (SDC, http://links.lww.com/TXD/A707) show the association between donor age and major classification using restricted cubic splines. When modeled continuously, the OR for donor age was 1.04 (95% CI, 1.01-1.07; P = 0.005) (Table 5). Regarding the surgical approach, laparoscopic retroperitoneal compared with laparoscopic transabdominal surgery was an independent risk factor for major early complications (OR, 2.13; 95% CI, 1.21-3.82; P = 0.01), whereas open nephrectomy compared with the laparoscopic transabdominal surgery method was not (OR, 0.50; 95% CI, 0.14-1.34; P = 0.21; Table 5). BMI showed a U-shaped association with major complications, without reaching statistical significance (Figure 3B, Table 5; Table S5, SDC, http://links.lww.com/TXD/A707). Focusing on the nowadays preferred laparoscopic surgical approach, we excluded donors with open nephrectomy within a sensitivity analysis getting similar results (Table S6, SDC, http://links.lww.com/TXD/A707).
TABLE 5.
Independent predictors of early major complications, n = 2379
| Univariate logistic regression analysis (95% CI); P value |
Multivariable logistic regression analysis (95% CI); P valuea |
|
|---|---|---|
| Risk factors of major complications* | ||
| Sex (male vs female) | 1.00 (0.57-1.73); 0.99 | 1.11 (0.62-1.93); 0.73 |
| Donor age | 1.04 (1.01-1.07); 0.002 | 1.04 (1.01-1.07); 0.005 |
| BMI | ||
| 20.0–24.9 kg/m2 | Ref | Ref |
| <20 kg/m2 | 2.19 (0.80-5.17); P = 0.09 | 2.40 (0.86-5.77); 0.07 |
| 25–29.9 kg/m2 | 0.88 (0.47-1.63); 0.69 | 0.81 (0.43-1.51); 0.51 |
| ≥30 | 1.09 (0.43-2.44); 0.84 | 1.01 (0.39-2.27); 0.99 |
| Site of nephrectomy, left vs right | 0.99 (0.54-1.83); 0.98 | |
| Year of donation | ||
| 1998-2008 | Ref | Ref |
| 2009-2018 | 1.32 (0.74-2.39); 0.36 | 0.99 (0.55-1.83); 0.98 |
| 2019-2022 | 0.92 (0.36-2.11); 0.85 | 0.63 (0.23-1.53); 0.34 |
| Surgical method | ||
| Laparoscopic transabdominal | Ref | Ref |
| Laparoscopic retroperitoneal | 2.42 (1.39-4.31); 0.002 | 2.13 (1.21-3.82); 0.01 |
| Open nephrectomy | 0.51 (0.15-1.35); 0.22 | 0.50 (0.14-1.34); 0.21 |
aThe last column represents the entire multivariable model.
Multivariable logistic regression analysis was performed to analyze the independent risk factors for major early complications*, defined as Dindo-Clavien classification ≥3a (n = 56 donors).To account for the long observation time and potential procedural changes, the years of transplantations were forced into the model as a potential confounder. Thus, the point effects of the multivariable model must be interpreted with caution. The C-statistic of the full model was 0.677.
BMI, body mass index; CI, confidence interval.
FIGURE 3.
Restricted cubic spline plot of the association between donor age and BMI with major early complications. The dark line indicates the predicted odds ratio for age (A) and BMI (B) with major complications modeled by restricted cubic splines. The blue area represents the 95% confidence interval. For age a reference value of 50 was chosen, for BMI a reference value of 25 was chosen. BMI, body mass index.
DISCUSSION
The main observation of this study was that most complications occurring in LK donors were minor, and only 2.4% had major complications. This is consistent with a previous analysis of data from the SOL-DHR16 and other studies.4 LKD-specific mortality is reported to be between 0.02% and 0.04%.10,21,22 Similar to the Norwegian registry using data from 1022 consecutively collected LK donor nephrectomies between 1997 and 2008,8 no perioperative mortality was observed in our study. The leading early complications were genitourinary problems such as urinary retention and UTI, followed by wound or other infections.
Interestingly, the independent risk factors for major early complications were older donor age and type of surgical approach, whereas sex was not. For BMI, we observed a U-shaped association, where very low and high BMIs had higher odds of major early complications, without reaching statistical significance. Despite concerns about perioperative complications and the long-term risk of metabolic syndrome, obese donors have been increasingly accepted for LKD over the last decade.23 Other registries did not find any association of early complications with obesity.8,23 In contrast, Patel et al. and Friedman et al. reported higher incidences of donor complications in obese individuals (OR 1.92 and 1.76, respectively). However, in both studies weight was dichotomized.24,25 Based on our national registry data and after thorough review of the literature, the Swiss guidelines for living donation of solid organs published by the Swiss Academy of Medical Sciences were revised and updated recently (https://www.samw.ch/en/Publications/Medical-ethical-Guidelines.html). There exists no strict national protocol for suitability of LK donors in Switzerland; however, the guidelines state that in case of an increased BMI (ie, >30 kg/m2, but <40 kg/m2) and metabolic disorders with an increased risk of diabetes (ie, impaired fasting glucose/ impaired glucose tolerance), it must be assessed on a case-by-case basis whether donation is appropriate. Donors must be informed about the additional health risks, the development of diabetes, the negative effects on the individual kidney and ultimately the occurrence of cardiovascular events.
Compared with laparoscopic transabdominal surgery, the retroperitoneal laparoscopic approach was independently associated with more major early complications, whereas open nephrectomy was not. Comparing these findings with previous literature is difficult, because many studies and meta-analysis26-28 did not differ in the types of complications after donor nephrectomy using the Dindo-Clavien classification.15 Besides, the site of nephrectomy was not a risk factor of major complications within our study. It is important to mention, that SOL-DHR does not retrieve any reasons/comments about which site was chosen for kidney nephrectomy. However, because of personal communication between the transplant centers, we know that the most important reason for choosing the right versus the left kidney is when there are anatomical vascular variances on the left kidney. Our study lacked the power to differentiate between hand-assisted and classical laparoscopic techniques, or open nephrectomy. Furthermore, the effect of the robotic approach was minimal in the donor group.
Assessing donor health values before and after donation is essential for estimating the short- and long-term health risks of living donors. Hanson et al. highlighted that kidney function is the most important outcome of living donors, underpinned by the fear of developing KF after donation.29 In the United States, a study of 193 LK donors found that 21% of donors reported anxiety about kidney injury or loss after donation.30 As expected, because of the loss of total nephron mass kidney function significantly decreased 12 mo after kidney donation. Nevertheless, the mean eGFR after LKD was still >60 ml/min/1.73m2 and albuminuria was not higher than predonation. Similar to Suwelack et al.,13 the decrease in kidney function was higher in elderly than in younger donors. Compensatory hyperfiltration of the remaining kidney helps mitigate the impact of nephrectomy, such that the net reduction in GFR early after donation is much lower than 50% (ie, approximately 30%, leading to a GFR decrease of 25–40 ml/min/1.73m231,32). This is in line with our study, which showed a mean decline of 32 ml/min/1.73m2 at 1 y after LKD. In a retrospective matched cohort study of LK donors between 2002 and 2016, Lam et al. showed that in contrast to the steady age-related decline in kidney function in nondonors, postdonation kidney function on average increased by 1 ml/min/1.73m2/y and began to plateau at 5-y postdonation.33 Kasiske et al. demonstrated within a prospective controlled study of LK donors that between 6 and 36 mo, the GFR slope declined 0.36 ml/min/y in 194 controls but increased 1.47 ml/min/y in 198 donors.32 However, to thoroughly reassure LK donors about their kidney health after donation, knowledge about the long-term outcomes of kidney function, especially the risk of KF after LKD is of interest. However, this was not within the scope of the present study.
Blood pressure and metabolic parameters showed small but statistically significant differences 12 mo after LKD compared with predonation levels, although most health parameters remained within the normal range, including blood pressure, proteinuria/albuminuria, and HbA1c. The mean BMI was slightly elevated before and after donation. Interestingly, this finding is similar to the finding of Suwelak et al.13 During the postoperative in-hospital phase, nearly 5% of donors had hypertensive values on at least 2 consecutive days, but blood pressure values were typically normalized in those not previously on medication. The percentage of donors on antihypertensive medication remained stable after donation.
LK donors may experience psychosocial benefits by improving recipients’ health. Thus, we not only wanted to focus on somatic health outcomes but also HRQoL before and 12 mo after donation as well as the willingness to donate a kidney again. Previous studies and ours have found that donors have high HRQoL before donation, often better than that of the general population,18,34 likely because donors undergo a stringent medical evaluation process for eligibility before donation, including exploration of psychosocial functioning. In the present study, 66.5% of the donors described their health status as unchanged after LKD, 20% felt improved, and 10% experienced deterioration. Nearly the same results as for the self-assessment of health status were observed for the EB of donors to their recipients after donation. Strikingly, 1 y after LKD, 96.5% of donors did not regret donating a kidney and would, if possible, do it again. Similar results were obtained by Clemens et al. who performed a multicenter study to examine the quality of life years after donation (ie, median 5.5 y).35 In this study, 97% of donors stayed firm on their decision of donating a kidney.35 The only independent risk factor for low HRQoL based on MCS was worsening EB after donation.
The strength of this multicenter cohort study is that our data reflect long-term experience with LKD in Switzerland. In addition, there is high completeness of data with detailed information collected from all donors. However, this study has some limitations. Primarily, because of the low frequency of major early complications, we were limited by the number of adjusted variables, which may result in residual confounding. Additionally, 1.7% of donors had to be excluded because of missing variables. Although this percentage is low, we cannot completely exclude the potential for selection bias. Furthermore, some analyses were restricted to patients without missing data. Caution is necessary when extrapolating our findings to other cohorts of liver kidney donors, as our population is predominantly Caucasian and drawn from a country with a universal health care system. Therefore, inferences cannot be applied to other populations.
In conclusion, our data confirm that living-donor nephrectomy is safe with a low rate of major early complications and an acceptable rate of minor complications. Although the eGFR significantly decreased after donor nephrectomy because of the loss of functioning kidney volume, there was no increase in albuminuria in living donors at 12 mo after donation. Most donors do not regret donating their kidney. However, they must be carefully educated about the potential risks, including worsening of the relationship between the donor and the recipient as a potential cause of impaired quality of life.
ACKNOWLEDGMENTS
This work is dedicated to Prof. Gilbert Thiel (born in 1934; passed away in 2012) who founded the Swiss Organ Living-Donor Health Registry in 1993 and set a milestone for comprehensive lifelong monitoring of LK donors in Switzerland. Furthermore, he was a great promoter of LK transplantation across the national border. The authors and representatives of the Swiss transplant centers thank Christa Nolte for managing the registry over many years and Lene Kraft for the current excellent administrative work on the registry and outstanding collaboration. Further, SOL-DHR thanks all transplant centers for their excellent collaboration as well as Viollier AG, Basel, Switzerland, for performing the laboratory tests.
Supplementary Material
Footnotes
There was no extra funding request necessary to conduct the study, as this study was partially performed for a master’s thesis with two master’s students.
M.D. reports a research grant from the Swiss National Science Foundation (SNF) (SNF projects P500PM_214237), and T.Z. received a grant from Innosuisse (project 40018.1 IP-LS). J.S. reports grants from the SNF to conduct the Swiss transplant cohort study (STCS) as the principal investigator (SNSF project 33CS30/201385/1). P.M.H. received grants from the Gottfried and Julia Bangerter-Rhyner Foundation. All reported grants were used outside the scope of this work.
The authors declare no conflicts of interest.
P.H.M. designed the research/study. P.H.M., C.B., Z.H., J.K., and M.D. performed research/study. All collected data. P.H.M., C.B., Z.H., J.K., and M.D. analyzed data. All wrote article.
C.B. and Z.H. contributed equally to this work as co-first authors.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
Contributor Information
Charlotte Brügger, Email: Ch.bruegger@stud.unibas.ch.
Zoé Hunkeler, Email: Zoe.hunkeler@stud.unibas.ch.
Matthias Diebold, Email: Matthias.Diebold@usb.ch.
Joana Krättli, Email: Joana.kraettli@usb.ch.
Irene Geiger, Email: Irene.geiger@usb.ch.
Caroline Wehmeier, Email: Caroline.wehmeier@usb.ch.
Thomas Wolff, Email: Thomas.Wolff@usb.ch.
Bruno Vogt, Email: Bruno.Vogt@insel.ch.
Federico Storni, Email: Federico.storni@insel.ch.
Dela Golshayan, Email: Dela.golshayan@chuv.ch.
Tobias Zingg, Email: Tobias.Zingg@chuv.ch.
Sophie de Seigneux, Email: Sophie.deseigneux@hug.ch.
Fadi Haidar, Email: Fadi.Haidar@hcuge.ch.
Isabelle Binet, Email: Francoise-Isabelle.binet@kssg.ch.
Aurelia Schnyder, Email: Schnyderaurelia@hotmail.com.
Kerstin Hübel, Email: Kerstin.Huebel@usz.ch.
Thomas Müller, Email: Thomas.Mueller@usz.ch.
Fabian Rössler, Email: fabian.roessler@usz.ch.
Jürg Steiger, Email: Juerg.steiger@usb.ch.
REFERENCES
- 1.Kasiske BL, Snyder JJ, Matas AJ, et al. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol. 2002;13:1358–1364. [DOI] [PubMed] [Google Scholar]
- 2.Abecassis M, Bartlett ST, Collins AJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol. 2008;3:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wehmeier C, Georgalis A, Hirt-Minkowski P, et al. kidney transplantations at the University Hospital Basel: A story of success and new challenges. Swiss Med Wkly. 2222;2016:w14317. [DOI] [PubMed] [Google Scholar]
- 4.Lentine KL, Lam NN, Segev DL. Risks of living kidney donation: Current state of knowledge on outcomes important to donors. Clin J Am Soc Nephrol. 2019;14:597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taler SJ, Messersmith EE, Leichtman AB, et al. ; RELIVE Study Group. Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am J Transplant. 2013;13:390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clayton PA, Saunders JR, McDonald SP, et al. Risk-factor profile of living kidney donors: The Australia and New Zealand Dialysis and Transplant Living Kidney Donor Registry 2004-2012. Transplantation. 2016;100:1278–1283. [DOI] [PubMed] [Google Scholar]
- 7.Janki S, Klop KW, Dooper IM, et al. More than a decade after live donor nephrectomy: a prospective cohort study. Transpl Int. 2015;28:1268–1275. [DOI] [PubMed] [Google Scholar]
- 8.Mjoen G, Oyen O, Holdaas H, et al. Morbidity and mortality in 1022 consecutive living donor nephrectomies: Benefits of a living donor registry. Transplantation. 2009;88:1273–1279. [DOI] [PubMed] [Google Scholar]
- 9.Minnee RC, Bemelman WA, Polle SW, et al. Older living kidney donors: Surgical outcome and quality of life. Transplantation. 2008;86:251–256. [DOI] [PubMed] [Google Scholar]
- 10.Hadjianastassiou VG, Johnson RJ, Rudge CJ, et al. 2509 living donor nephrectomies, morbidity and mortality, including the UK introduction of laparoscopic donor surgery. Am J Transplant. 2007;7:2532–2537. [DOI] [PubMed] [Google Scholar]
- 11.Janki S, Dols LF, Timman R, et al. Five-year follow-up after live donor nephrectomy—Cross-sectional and longitudinal analysis of a prospective cohort within the era of extended donor eligibility criteria. Transpl Int. 2017;30:266–276. [DOI] [PubMed] [Google Scholar]
- 12.Maple H, Chilcot J, Weinman J, et al. Psychosocial wellbeing after living kidney donation—A longitudinal, prospective study. Transpl Int. 2017;30:987–1001. [DOI] [PubMed] [Google Scholar]
- 13.Suwelack B, Berger K, Wolters H, et al. ; SoLKiD Study Group. Results of the prospective multicenter SoLKiD cohort study indicate bio-psycho-social outcome risks to kidney donors 12 months after donation. Kidney Int. 2022;101:597–606. [DOI] [PubMed] [Google Scholar]
- 14.Thiel GT, Nolte C, Tsinalis D. Prospective Swiss cohort study of living-kidney donors: study protocol. BMJ Open. 2011;1:e000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkhalter F, Huynh-Do U, Hadaya K, et al. Early complications after living donor nephrectomy: Analysis of the Swiss Organ Living Donor Health Registry. Swiss Med Wkly. 2017;147:w14497. [DOI] [PubMed] [Google Scholar]
- 17.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. L. Erlbaum Associates; 1988. [Google Scholar]
- 18.Ellert U, Lampert T, Ravens-Sieberer U. [Measuring health-related quality of life with the SF-8. Normal sample of the German population] Messung der gesundheitsbezogenen Lebensqualitat mit dem SF-8. Eine Normstichprobe fur Deutschland. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2005;48:1330–1337. [DOI] [PubMed] [Google Scholar]
- 19.Lefante JJ, Jr, Harmon GN, Ashby KM, et al. Use of the SF-8 to assess health-related quality of life for a chronically ill, low-income population participating in the Central Louisiana Medication Access Program (CMAP). Qual Life Res. 2005;14:665–673. [DOI] [PubMed] [Google Scholar]
- 20.Mjoen G, Stavem K, Westlie L, et al. Quality of life in kidney donors. Am J Transplant. 2011;11:1315–1319. [DOI] [PubMed] [Google Scholar]
- 21.Matas AJ, Bartlett ST, Leichtman AB, et al. Morbidity and mortality after living kidney donation, 1999-2001: Survey of United States transplant centers. Am J Transplant. 2003;3:830–834. [PubMed] [Google Scholar]
- 22.Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303:959–966. [DOI] [PubMed] [Google Scholar]
- 23.Schold JD, Goldfarb DA, Buccini LD, et al. Comorbidity burden and perioperative complications for living kidney donors in the United States. Clin J Am Soc Nephrol. 2013;8:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel S, Cassuto J, Orloff M, et al. Minimizing morbidity of organ donation: analysis of factors for perioperative complications after living-donor nephrectomy in the United States. Transplantation. 2008;85:561–565. [DOI] [PubMed] [Google Scholar]
- 25.Friedman AL, Cheung K, Roman SA, et al. Early clinical and economic outcomes of patients undergoing living donor nephrectomy in the United States. Arch Surg. 2010;145:356–62; discussion 362. [DOI] [PubMed] [Google Scholar]
- 26.Nanidis TG, Antcliffe D, Kokkinos C, et al. Laparoscopic versus open live donor nephrectomy in renal transplantation: A meta-analysis. Ann Surg. 2008;247:58–70. [DOI] [PubMed] [Google Scholar]
- 27.Wilson CH, Sanni A, Rix DA, et al. Laparoscopic versus open nephrectomy for live kidney donors. Cochrane Database Syst Rev. 2011;11:CD006124. doi:10.1002/14651858.CD006124.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Yuan H, Liu L, Zheng S, et al. The safety and efficacy of laparoscopic donor nephrectomy for renal transplantation: An updated meta-analysis. Transplant Proc. 2013;45:65–76. [DOI] [PubMed] [Google Scholar]
- 29.Hanson CS, Chapman JR, Gill JS, et al. Identifying outcomes that are important to living kidney donors: A nominal group technique study. Clin J Am Soc Nephrol. 2018;13:916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigue JR, Schold JD, Morrissey P, et al. ; KDOC Study Group. Mood, body image, fear of kidney failure, life satisfaction, and decisional stability following living kidney donation: Findings from the KDOC study. Am J Transplant. 2018;18:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasiske BL, Anderson-Haag T, Ibrahim HN, et al. A prospective controlled study of kidney donors: Baseline and 6-month follow-up. Am J Kidney Dis. 2013;62:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasiske BL, Anderson-Haag T, Israni AK, et al. A prospective controlled study of living kidney donors: Three-year follow-up. Am J Kidney Dis. 2015;66:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam NN, Lloyd A, Lentine KL, et al. Changes in kidney function follow living donor nephrectomy. Kidney Int. 2020;98:176–186. [DOI] [PubMed] [Google Scholar]
- 34.Wirken L, van Middendorp H, Hooghof CW, et al. The course and predictors of health-related quality of life in living kidney donors: A systematic review and meta-analysis. Am J Transplant. 2015;15:3041–3054. [DOI] [PubMed] [Google Scholar]
- 35.Clemens K, Boudville N, Dew MA, et al. ; Donor Nephrectomy Outcomes Research (DONOR) Network. The long-term quality of life of living kidney donors: A multicenter cohort study. Am J Transplant. 2011;11:463–469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.