Abstract
Backgrounds.
Pancreatic beta cell function and islet autoantibodies classically distinguish types of diabetes (type 1 diabetes mellitus [DM] or type 2 DM). Here, we sought to evaluate simultaneous pancreas-kidney (SPK) transplant outcomes stratified by the presence or absence of beta cell function and autoantibodies.
Methods.
SPK recipients were eligible if pretransplant autoantibodies were measured against insulin, islet cell, or glutamic acid decarboxylase 65-kD isoform. Recipients were categorized as A+ or A– based on the detection of ≥1 autoantibodies. Recipients were similarly categorized on the basis of detectable pretransplant fasting C-peptide of ≥2 ng/mL (β+) or <2 ng/mL (β–). Thus, recipients were categorized into 4 groups: A+β–, A–β–, A–β+, and A+β+. Outcomes of interest were overall pancreas graft failure (non–death-censored), death-censored pancreas, or kidney graft failure (death-censored pancreas graft failure [DCGF]; kidney DCGF), composite outcomes with any of the 3 outcomes as pancreas DCGF, use of an antidiabetic agent, or hemoglobin A1c >6.5.
Results.
One hundred eighty-three SPK recipients were included: A+β– (n = 72), A–β– (n = 42), A–β+ (n = 49), and A+β+ (n = 20). We did not detect a statistical difference in non–death-censored pancreas graft failure for A+β– recipients compared with other groups: A–β– (adjusted hazard ratio [aHR]: 0.44; 95% confidence interval [CI], 0.14-1.42), A–β+ (aHR: 1.02; 95% CI, 0.37-2.85), and A+β+ (aHR: 0.67; 95% CI, 0.13-3.33) in adjusted analyses. Similar outcomes were observed for other outcomes.
Conclusions.
In SPK recipients, outcomes were similar among recipients with classic features of type 1 DM and various other types of DM.
Diabetes mellitus (DM) has been typically classified as type 1 DM (T1DM) or type 2 DM (T2DM). T1DM is mainly due to immune-mediated or idiopathic β-cell destruction, usually leading to absolute insulin deficiency. At the same time, T2DM ranges from predominantly insulin resistance with relative insulin deficiency to a predominantly secretory defect with insulin resistance.1 Other forms of diabetes with variable pathophysiology are emerging that do not fit the typical phenotypes of T1DM or T2DM and are collectively termed “atypical diabetes.”2 However, the diagnostic criteria set by the American Diabetes Association remain the same for any diabetes that includes hemoglobin A1c (HbA1c) ≥6.5%, fasting blood glucose ≥126 mg/dL, oral glucose tolerance test 2-h glucose ≥ 200 mg/dL, or random glucose ≥200 mg/dL in a patient with classic symptoms of hyperglycemia.3
The presence of pancreatic islet autoantibodies, such as islet cell antibodies, glutamic acid decarboxylase antibodies (GADA65), and islet antigen-2 antibodies, which confirms the destructive process of β-cell, along with insulin deficiency, as assessed by C-peptide level, which is the best marker of the endogenous insulin production, is a classic feature of T1DM.4 However, prior endocrinology literature and our own clinical experience identify multiple subgroups of diabetes, such as those with autoantibodies and detectable fasting C-peptide.5,6 When autoimmune markers are analyzed, they are found in about 10% of patients clinically classified as T2DM, indicating that the frequency of T1DM could be underestimated.4 Latent autoimmune diabetes of adults is a form of diabetes with features of both T1DM and T2DM and has therefore been termed type 1.5 DM. Latent autoimmune diabetes in adults is the most frequent form of adult-onset autoimmune diabetes and the most prevalent form of autoimmune diabetes.7 Simultaneous pancreas-kidney (SPK) transplant outcomes among patients with T1DM and T2DM have similar outcomes.8 However, posttransplant outcomes among SPK recipients with various types of diabetes as assessed by the presence or absence of pretransplant C-peptide levels and pancreatic autoantibodies are unknown. Here, we categorized patients into 4 types based on the presence or absence of detectable pretransplant C-peptide and islet autoantibodies and evaluated various posttransplant outcomes. We hypothesized that outcomes may vary based on the presence or absence of pretransplant autoantibodies along with C-peptide levels. For this, we took a novel approach to assess posttransplant outcomes by comparing these groups.
MATERIALS AND METHODS
Population Selection and Study Design
We evaluated all adult SPK recipients who underwent SPK transplants between January 1, 2012, and March 31, 2022, at the University of Wisconsin. SPK recipients were included if pretransplant autoantibodies were measured against insulin, islet cells, or GAD65. Recipients were categorized as A+ or A– based on the detection of ≥1 pretransplant autoantibodies. Recipients were similarly categorized on the basis of detectable pretransplant fasting C-peptide of ≥2 ng/mL (β+) or <2 ng/mL (β–). Thus, recipients were categorized into 4 groups: A+β–, A–β–, A–β+, and A+β+. The exclusion criteria consisted of patients who were younger than 18 y at the time of the transplant, pancreas-after-kidney recipients, or pancreas transplant-alone recipients. Furthermore, we excluded recipients whose pancreas graft failed within 30 d posttransplant. Risk factors for non–death-censored pancreas graft failure (non-P-DCGF), P-DCGF, composite outcomes with any of the 3 outcomes as P-DCGF, use of an antidiabetic agent, or HbA1c >6.5; and kidney DCGF (K-DCGF) were outcomes of interest. Recipients were followed until P-DCGF or K-DCGF or death or until the end of follow-up (10/2023). This study was approved by the University of Wisconsin School of Medicine and Public Health Institutional Review Board (protocol No.: 2014-1072). This study was in adherence to the Declaration of Helsinki. The clinical and research activities reported were consistent with the Principles of the Declaration of Istanbul as outlined in “The Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
P-DCGF was defined on the basis of the current United Network for Organ Sharing criteria for pancreas graft failure, which include removal of the pancreas graft, re-registration for a pancreas transplant, registration for an islet transplant after receiving pancreas, or an insulin requirement that is ≥0.5 units/kg/d for 90 consecutive days.9 K-DCGF was defined as initiating dialysis or retransplantation before the end of the data analysis. Recipients were categorized as T1DM or T2DM based on both an independent clinical assessment (based on clinical parameters and labs) and a retrospective patient chart review independent of referring physician and or patient-determined diabetes type, based on the 18-point novel scoring system (ranging from +9 to –9) as described before.8
Selection Criteria for SPK Transplant
SPK selection criteria were based on physical, psychological, medical, and surgical aspects of the patient’s condition and are similar for T1DM or T2DM candidates at our center. All patients with T2DM were on insulin pretransplant, with detectable fasting C-peptide levels of at least ≥2 ng/mL with minimal cardiac and other comorbidities. All potential recipients were extensively discussed in the multidisciplinary selection meeting before approving or disapproving their SPK candidacy. At no time during this series or currently in our program was there a protocolized criterion for pretransplant autoantibodies level or C-peptide level to approve or disapprove SPK transplant eligibility among SPK candidates. The cutoff for body mass index among T1DM was <32 kg/m2 and for T2DM was <35 kg/m2. Contraindications for SPK transplantation parallel other solid organ transplant criteria (cardiovascular disease, active infection, cancer, noncompliance, and poor social support).10
SPK Transplant Procedure and Pretransplant Autoantibodies Monitoring
All pancreas transplants were accomplished using enteric drainage, side-to-side duodenojejunostomy to the proximal jejunum without Roux-en-Y, and systemic venous drainage to the proximal right common iliac vein or distal inferior vena cava. In most cases, the kidney was placed contralateral to the left iliac vessels.
All SPK recipients had pretransplant fasting C-peptide levels monitored during the study period. We started monitoring pretransplant autoantibodies in 2012 and have been monitoring them more consistently only since the recent past. Posttransplant monitoring of autoantibodies has not been our routine clinical practice. At no time during this series or currently in our program was there a protocolized criterion for pretransplant autoantibodies or C-peptide level to approve or disapprove SPK transplant eligibility.
Immunosuppression
Patients undergoing SPK transplants received induction immunosuppression with a depleting agent (antithymocyte globulin or alemtuzumab) or a nondepleting agent (basiliximab) based on immunological risk factors.11 Patients having pretransplant donor-specific antibodies, recipients of a secondary SPK,12 experiencing previous pancreas graft failure due to rejection, and those patients in whom an early steroid withdrawal was planned were more likely to receive depleting agents for induction. Patients were typically maintained on a triple immunosuppressive regimen, with tacrolimus, mycophenolate mofetil or mycophenolic acid, and steroids. Some patients underwent early steroid withdrawal based on clinical judgment and the patient’s request. Doses and drug levels were individually adjusted on the basis of the patient’s clinical condition, including infection, malignancy, and rejection. Most SPK recipients were maintained on tacrolimus with a trough goal of 10–12 ng/mL in the first 3 mo posttransplant, 8–10 ng/mL from months 3–12, and 6–8 ng/mL after 1 y. For patients in whom steroids were continued, prednisone was tapered to 10 mg daily by 8 wk posttransplant, with further taper determined by the managing provider. Patients undergoing early steroid withdrawal stopped taking steroids after postoperative day 4.
Statistical Analysis
Continuous data were compared using the Student t test or the Wilcoxon rank-sum test, as appropriate, whereas categorical data were analyzed using the Fisher exact test or chi-square test. P values of ≤0.05 were considered statistically significant. Risk factors associated with outcomes of interest with reference to A+β– were studied using univariate and multivariate stepwise Cox regression analyses. Variables considered to be associated with outcomes of interest from baseline characteristics were included in the adjusted model. Outcomes of interest were also analyzed using the Kaplan-Meier survival analysis. All analyses were performed using the MedCalc Statistical Software version 16.4.3 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2016).
RESULTS
A total of 183 SPK recipients fulfilled our selection criteria and were included in the study. Seventy-two recipients (39%) were in the A+β– group, 42 (23%) were in the A–β– group, 49 (27%) were in the A–β+ group, and the remaining 20 (11%) were in the A+β+ group. The mean pretransplant fasting C-peptide levels in the A+β– group was 0.25 ± 0.42 ng/mL, in the A–β– group was 0.39 ± 0.49 ng/mL, in the A–β+group was 6.1 ± 3.1 ng/mL, and in the A+β+ group was 6.9 ± 4.3 ng/mL. Among the entire cohort, 60 had an insulin antibody, 1 had an islet cell antibody, and 59 had a GAD65 antibody, whereas 28 had both insulin and GAD65 antibodies present. Among the A+β– group, 50 had insulin antibodies, none had islet cell antibodies, and 48 had GAD65 antibodies, whereas 26 had both insulin and GAD65 antibodies present. Similarly, among the A+β+ group, 10 had insulin antibodies, 1 had islet cell antibodies, and 11 had GAD65 antibodies, whereas 2 had both insulin and GAD65 antibodies present.
The baseline characteristics among the 4 groups are summarized in Table 1. There were no significant baseline characteristic differences in donor factors or immunological factors among the groups. However, a higher proportion of recipients in the A–β+ and A+β+ groups were men, non-White, and labeled as T2DM or other types of diabetes. Eleven percent of the SPK recipients in the A+β– group, 21% in the A–β– group, 94% in the A–β+ group, and 85% in the A+β+ group had T2DM/other. Also, there were differences in induction immunosuppression and rate of preemptive transplant across the groups.
TABLE 1.
Baseline characteristics
| Variables | A+β– (N = 72) |
A–β– (N = 42) |
A–β+ (N = 49) |
A+β+ (N = 20) |
P |
|---|---|---|---|---|---|
| Donor factors | |||||
| Mean age, y | 23.4 ± 11.5 | 28.8 ± 13.8 | 27.6 ± 9.4 | 26.6 ± 10.5 | 0.07 |
| Male, n (%) | 48 (67) | 25 (60) | 32 (65) | 13 (65) | 0.89 |
| Non-White, n (%) | 12 (17) | 9 (21) | 9 (18) | 2 (10) | 0.73 |
| Mean body mass index, kg/m2 | 23.0 ± 4.1 | 22.8 ± 3.8 | 23.9 ± 3.4 | 22.9 ± 3.5 | 0.45 |
| Cause of death: cardiovascular, n (%) | 7 (10) | 6 (14) | 4 (8) | 5 (25) | 0.22 |
| Terminal serum creatinine, mg/dL | 0.89 ± 0.49 | 0.83 ± 0.32 | 0.85 ± 0.36 | 0.84 ± 0.32 | 0.87 |
| Mean kidney donor profile index % | 25.0 ± 20.4 | 30.5 ± 19.6 | 23.8 ± 15.9 | 17.9 ± 11.8 | 0.08 |
| Donation after circulatory death, n (%) | 20 (28) | 11 (26) | 17 (35) | 1 (5) | 0.09 |
| Pancreas donor risk index | 1.23 ± 0.48 | 1.29 ± 0.46 | 1.20 ± 0.41 | 1.15 ± 0.37 | 0.70 |
| Pancreas cold ischemia time, h | 11.7 ± 4.2 | 13.2 ± 4.2 | 11.4 ± 3.6 | 13.0 ± 4.2 | 0.12 |
| Kidney cold ischemia time, h | 13.8 ± 4.1 | 15.1 ± 4.3 | 13.7 ± 3.9 | 15.0 ± 4.1 | 0.26 |
| Immunologic factors | |||||
| cPRA >20%, n (%) | 11 (15) | 8 (19) | 1 (2) | 3 (15) | 0.07 |
| Mean HLA mismatch (of 6) | 4.4 ± 1.2 | 4.4 ± 1.4 | 4.6 ± 1.2 | 5.0 ± 0.8 | 0.28 |
| Previous transplant, n (%) | 4 (6) | 5 (12) | 1 (2) | 0 | 0.13 |
| Mean age, y | 47.1 ± 10.3 | 46.3 ± 10.0 | 48.3 ± 8.3 | 47.1 ± 9.4 | 0.80 |
| Male, n (%) | 42 (58) | 26 (62) | 40 (82) | 16 (80) | 0.03 |
| Non-White, n (%) | 9 (13) | 7 (17) | 21 (43) | 9 (45) | <0.001 |
| Mean body mass index, kg/m2 | 26.4 ± 3.9 | 26.1 ± 3.7 | 27.4 ± 2.9 | 28.2 ± 2.4 | 0.06 |
| Diabetes type | |||||
| Type 1 | 64 (89) | 33 (79) | 3 (6) | 3 (15) | <0.001 |
| Type 2/other | 8 (11) | 9 (21) | 46 (94) | 17 (85) | |
| Recipients factors | |||||
| Induction immunosuppression, n (%) | |||||
| Alemtuzumab | 22 (31) | 15 (36) | 20 (41) | 20 (41) | 0.01 |
| Antithymocyte globulin | 44(61) | 17 (41) | 26 (53) | 26 (53) | |
| Basiliximab | 6 (8) | 10 (24) | 3 (6) | 3 (6) | |
| Early steroid withdrawal, n (%) | 6 (8) | 3 (7) | 6 (12) | 2 (10) | 0.84 |
| Preemptive transplant, n (%) | 25 (35) | 6 (14) | 0 | 6 (30) | <0.001 |
| Kidney-delayed graft function, n (%) | 5 (7) | 3 (7) | 9 (18) | 1 (5) | 0.13 |
Bold P values represent statistically significant value.
cPRA, calculated panel-reactive antibody.
There were differences in mean posttransplant follow-up; however, all 4 groups had >50 mo of follow-up (Table 2). Recipients in A–β+ and A+β+ groups had significantly higher HbA1c at the last follow-up. None of the outcomes of interest were significantly different among the groups at the last follow-up. However, at 2 y, composite outcomes were significantly higher in the A–β+ and A+β+ groups.
TABLE 2.
Outcomes at 2 y and at past follow-up
| Characteristics | A+β– (N = 72) |
A–β– (N = 42) |
A–β+ (N = 49) |
A+β+ (N = 20) |
P |
|---|---|---|---|---|---|
| Mean follow-up post-SPK, mo | 59.1 ± 24.1 | 67.8 ± 27.8 | 50.8 ± 24.8 | 62.0 ± 30.4 | 0.02 |
| Mean HbA1c with graft survival at 2 y, g/dL | 5.4 ± 0.06 | 5.5 ± 0.09 | 5.7 ± 0.08 | 6.0 ± 0.13 | 0.14 |
| Mean HbA1c among those with graft survival, g/dL | 5.5 ± 0.53 | 5.5 ± 0.50 | 5.9 ± 1.3 | 5.9 ± 0.8 | 0.009 |
| No. of patients with a HbA1c >6.5 % at 2 y | 1 (1) | 1 (2) | 5 (10) | 6 (30) | 0.09 |
| No. of patients with a HbA1c >6.5% | 1 (1) | 2 (5) | 1 (2) | 1 (5) | 0.66 |
| No. of patients on antidiabetic agents at 2 y, n (%) | 2 (3) | 0 | 5 (10) | 2 (10) | 0.31 |
| No. of patients on antidiabetic agents, n (%) | 2 (3) | 1 (2) | 6 (12) | 2 (10) | 0.24 |
| Mean serum creatinine at 2 y, mg/dL | 1.27 ± 0.60 | 1.41 ± 0.09 | 1.33 ± 0.8 | 1.24 ± 0.13 | 0.06 |
| Mean serum creatinine, mg/dL | 1.29 ± 0.40 | 1.21 ± 0.26 | 1.30 ± 0.71 | 1.22 ± 0.25 | 0.81 |
| Mean serum eGFR at 2 y, mL/min/1.73 m2 | 62.9 ± 22.6 | 59.8 ± 29.9 | 66.9 ± 26.9 | 67.2 ± 44.4 | 0.08 |
| Mean serum eGFR, mL/min/1.73 m2 | 64.8 ± 20.7 | 65.8 ± 16.7 | 72.7 ± 23.1 | 71.2 ± 19.2 | 0.21 |
| Pancreas non–death-censored graft failure at 2 y, n (%) | 4 (6) | 2 (5) | 3 (6) | 0 | 0.55 |
| Pancreas non–death-censored graft failure, n (%) | 12 (17) | 4 (10) | 6 (12) | 2 (10) | 0.69 |
| Pancreas death-censored graft failure at 2 y, n (%) | 1 (1) | 0 | 2 (4) | 0 | 0.68 |
| Pancreas death-censored graft failure, n (%) | 5 (7) | 1 (2) | 2 (4) | 0 | 0.48 |
| Pancreas composite outcomes of DCGF or use of antidiabetic agents or HbA1c >6.5% (%) at 2 y, n (%) | 4 (6) | 1 (2) | 8 (16) | 6 (30) | 0.001 |
| Pancreas composite outcomes of DCGF or use of antidiabetic agents or HbA1c > 6.5%, n (%) | 8 (11) | 4 (10) | 9 (18) | 4 (20) | 0.46 |
| Kidney non–death-censored graft failure at 2 y, n (%) | 4 (6) | 2 (5) | 2 (4) | 0 | 0.34 |
| Kidney non–death-censored graft failure, n (%) | 11 (15) | 8 (19) | 5 (10) | 3 (15) | 0.70 |
| Kidney death-censored graft failure, n (%) | 1 | 0 | 2 | 0 | 0.16 |
| Kidney death-censored graft failure, n (%) | 5 (7) | 6 (14) | 2 (4) | 2 (10) | 0.33 |
Bold P values represent statistically significant value.
eGFR, estimated glomerular filtration rate; DCGF, death-censored graft failure; HbA1c, hemoglobin A1c; SPK, simultaneous pancreas and kidney.
Similarly, with reference to A+β– recipients, recipients in none of the other groups were significantly associated with increased or decreased risk for non-P-DCGF, P-DCGF, pancreas composite outcomes, or K-DCGF (Table 3). This was further confirmed by the Kaplan-Meier survival analyses (Figure 1). Even after adjustment for multiple baseline characteristics, there were no statistically significant differences in the outcomes.
TABLE 3.
Risk for outcomes of interest
| Complications | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Pancreases non–death-censored graft failure | ||||||
| A+β– | Reference | Reference | Reference | Reference | Reference | Reference |
| A–β– | 0.47 | 0.15-1.46 | 0.19 | 0.44 | 0.14-1.42 | 0.17 |
| A–β+ | 0.92 | 0.35-2.55 | 0.92 | 1.02 | 0.37-2.85 | 0.96 |
| A+β+ | 0.54 | 0.14-2.79 | 0.54 | 0.67 | 0.13-3.33 | 0.62 |
| Pancreas DCGF | ||||||
| A+β– | Reference | Reference | Reference | Reference | Reference | Reference |
| A–β– | 0.29 | 0.03-2.52 | 0.26 | 0.30 | 0.03-3.21 | 0.31 |
| A–β+ | 0.75 | 0.14-3.88 | 0.72 | 0.84 | 0.12-5.76 | 0.86 |
| A+β+ | – | – | – | – | – | – |
| Pancreas composite outcomes | ||||||
| A+β– | Reference | Reference | Reference | Reference | Reference | Reference |
| A–β– | 0.60 | 0.18-1.99 | 0.40 | 0.49 | 0.13-1.76 | 0.27 |
| A–β+ | 2.17 | 0.82-5.70 | 0.12 | 2.14 | 0.73-6.21 | 0.16 |
| A+β+ | 1.46 | 0.43-4.96 | 0.55 | 2.35 | 0.53-10.53 | 0.26 |
| Kidney DCGF | ||||||
| A+β– | Reference | Reference | Reference | Reference | Reference | Reference |
| A–β– | 1.30 | 0.38-4.41 | 0.67 | 2.03 | 0.53-7.83 | 0.30 |
| A–β+ | 0.76 | 0.15-3.93 | 0.74 | 1.37 | 0.24-7.84 | 0.72 |
| A+β+ | 1.04 | 0.19-5.77 | 0.97 | 4.41 | 0.67-34.05 | 0.15 |
aAdjusted for: donor: age, race, DCD donor; immunological: cPRA, HLA mismatch, previous transplant; recipient: age, BMI, depleting induction, and kidney-delayed graft function.
BMI, body mass index; CI, confidence interval; cPRA, calculated panel-reactive antibody; DCD, donation after circulatory death; DCGF, death-censored graft failure; HR, hazard ratio.
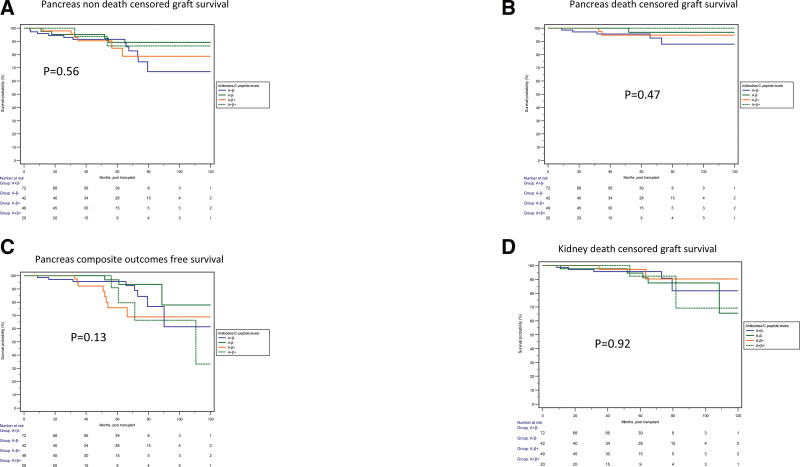
FIGURE 1.
Comparison of outcomes among 4 SPK recipient groups stratified by pretransplant fasting C-peptide and presence or absence of autoantibodies. No significant difference was observed in the pancreas non–death-censored graft survival (A; P = 0.56), pancreas death-censored graft survival (B; P = 0.47), pancreas compositive outcome-free survival (C; P = 0.13), or kidney death-censored graft survival (D; P = 0.92). SPK, simultaneous pancreas and kidney.
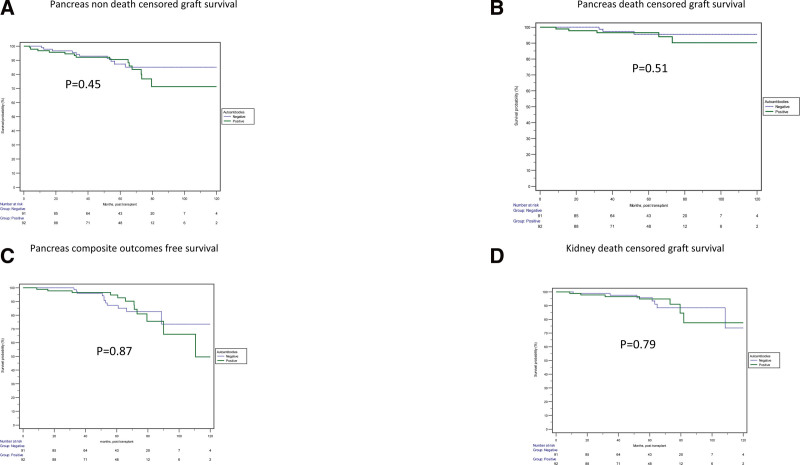
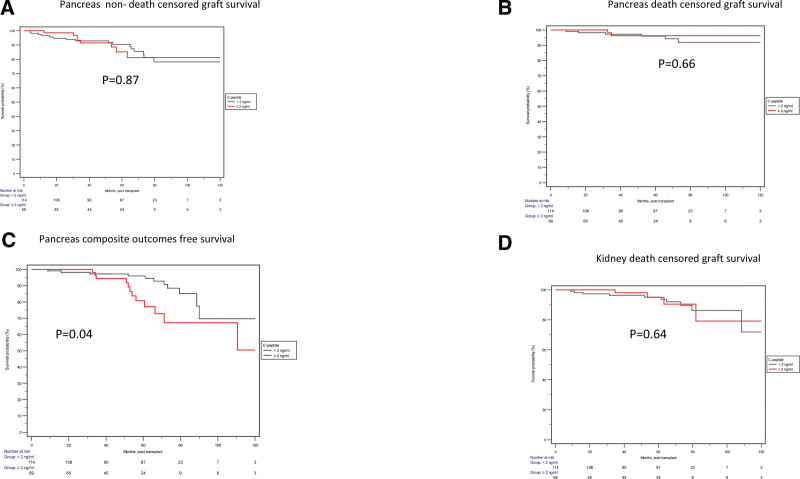
The Kaplan-Meier survival analyses, when categorized based on the A+ and A–, were not significantly associated with increased or decreased risk for outcomes of interest (Figure 2). However, when categorizing based on the pretransplant C-peptide levels, almost similar outcomes were seen except pancreas composite outcome-free survival, which was statistically inferior in the group with high C-peptide levels (Figure 3).
FIGURE 2.
Comparison of outcomes among SPK recipient groups stratified by the presence or absence of autoantibodies. No significant difference was observed in the pancreas non–death-censored graft survival (A; P = 0.45), pancreas death-censored graft survival (B; P = 0.51), pancreas compositive outcome-free survival (C; P = 0.87), or kidney death-censored graft survival (D; P = 0.79). SPK, simultaneous pancreas and kidney.
FIGURE 3.
Comparison of outcomes among SPK recipients stratified by pretransplant fasting C-peptide. No significant difference was observed in the pancreas non–death-censored graft survival (A; P = 0.87), pancreas death-censored graft survival (B; P = 0.66), or kidney death-censored graft survival (D; P = 0.64). However, pancreas compositive outcome-free survival was significantly lower in the high C-peptide group (C; P = 0.04). SPK, simultaneous pancreas and kidney.
DISCUSSION
In this large cohort of 183 SPK recipients categorized on the basis of the β-cell function and pancreatic autoantibodies, outcomes were similar among recipients with classic features of T1DM and various other types of diabetes. Recipients with the presence of pretransplant β-cell function had a significantly higher rate of composite outcomes at 2 y posttransplant, along with the higher HbA1c at the last follow-up, indicating peripheral resistance to the insulin. However, both pancreas and kidney outcomes were similar across the groups. As the numbers of recipients with non-T1DM are rising, these data support offering an SPK transplant to selected patients, irrespective of autoantibody and pretransplant C-peptide status.
The diagnosis of the types of DM is complex. There are 4 distinctive groups built on the pathogenetic features and diagnostic implications based on the autoantibodies and β-cell function. Four groups based on 2 important features commonly used to distinguish T1DM and T2DM are the presence or absence of biological markers of β-cell autoimmunity and the presence or complete absence of β-cell functional reserve. The lack of β-cell function has the highest accuracy and predictive value in classifying ketosis-prone diabetic syndromes.13,14 However, these should not be rigid classifications; rather, a scheme to differentiate etiologically and clinically distinct forms that are used to diagnose ketosis-prone diabetic syndromes is more valuable.15 Even ketosis-prone diabetic syndromes are distinct syndrome and could vary based on sex, HLA types, and whether ketosis was provoked or unprovoked. In one study, Nalini et al16 reported A–β+ ketosis-prone diabetes as a reversible beta cell dysfunction with male predominance and increased frequency of DQB1*0602. Provoked A–β+ ketosis-prone diabetes is characterized by progressive loss of beta cell reserve and increased frequency of DQB1*0302 and DRB1*04.16 In the same study, authors report unprovoked ketosis predicts long-term β-cell functional reserve, insulin-independence, and better glycemic control compared with the provoked ketosis.16 Also, even among patients with T2DM, it is not uncommon to have coexisting islet autoimmunity. The development of islet cell autoantibodies has been associated with a significantly rapid decline in β-cell function.17 We report similar findings in this study, where recipients had autoantibodies or lack of β-cell function despite being T2DM, whereas some recipients did not have autoantibodies and had a β-cell function despite being T1DM.
Historically, pancreas transplantation was offered only among patients with T1DM due to the restrictive listing criteria and uncertainty regarding outcomes among patients with T2DM.18 Even recently, in one survey among the US pancreas transplant centers, only 80% reported offering SPK transplants to patients with T2DM, and the rate was even lower in low-volume pancreas transplant programs at 60%.19 However, numerous published articles are comparing T1DM and T2DM with similar posttransplant outcomes, including one from our group by Pham et al.8,20-23 Similar to the previous studies, in a recent study from our group, where we stratified T2DM SPK recipients, based on the pretransplant C-peptide levels, we reported excellent outcomes among SPK recipients with T2DM; however, higher levels of pretransplant C-peptide levels were associated with increased risk of worse glycemic control.10
There are few studies assessing the impact of pancreatic autoantibodies and they are mainly focused on the posttransplant autoantibodies among SPK recipients. In one study, Pestana et al24 reported the presence of GAD65 antibodies “after” SPK transplant to be associated with an increased risk for pancreas dysfunction and inferior pancreas graft survival, particularly for de novo GAD65 antibodies after transplant. Similarly, in another study, Martins et al25 categorized SPK recipients based on the presence or absence of islet autoantibodies pretransplant and prospectively monitored autoantibodies after the transplant. The presence of autoantibodies (either persistent or de novo) at the last follow-up was significantly associated with increased risk for pancreas graft dysfunction and more so among the de novo group. Similar to our study, positivity for these autoantibodies pretransplantation did not influence pancreas graft survival.25 In another study, Rodelo-Haad et al26 reported that de novo development of tyrosine-phosphatases (IA-2) is associated with an increased risk for pancreas graft failure rather than GAD65. Also, in another study, guiding immunosuppression in one randomized control trial, Ringers et al27 reported a significantly higher rate of acute rejection among GAD65-positive SPK recipients receiving daclizumab for induction compared with GAD65-positive recipients with antithymocyte globulin induction or negative GAD65 antibody pretransplant. However, there was no significant difference in the other pancreas outcomes, including graft function.27 Similarly, in another study among islet cell transplants, there was no association between pretransplant autoantibodies and outcomes.28
Given the inherent inaccuracy of categorizing the type of diabetes in prospective recipients based on clinical features alone, we believe an assessment of outcomes based on categorization based on easily measured objective quantitative metrics would be valuable to providers in the modern era of pancreas transplantation. Nonetheless, this study has the expected limitations of a single-center observational study, reflecting our specific population and clinical approach. Our findings are reflective of the practices at our center, and this should be factored into the interpretation. Despite the large sample size of 183 patients, we acknowledge limitations relative to sample size and statistical power, which may mask differences in outcomes. Also, we assessed risk factors and outcomes based on pretransplant autoantibodies. As monitoring posttransplant pancreatic autoantibodies is not routine clinical practice, it was impossible to evaluate the outcomes based on the persistent or de novo pancreatic autoantibodies. However, this substantial data set with more granular data provides useful information for estimating risks and outcomes. Also, to our knowledge, this study is the largest of its kind, from a single center in the modern era with consistent surgical techniques and immunosuppressive agents. In summary, among SPK recipients, outcomes were similar among recipients irrespective of pretransplant β-cell function or autoantibodies. As the numbers of non-T1DM recipients are rising, these data support offering an SPK transplant to selected patients, irrespective of autoantibody and pretransplant C-peptide status.
Footnotes
This study was supported by an unrestricted research grant from the Virginia Lee Cook Foundation to Dr Mandelbrot.
The authors declare no conflicts of interest.
S.P. participated in data collection, design, analysis, article preparation. R.T., F.A., and B.D. participated in data collection and editing. B.C.A. participated in analysis and editing. D.M. and D.K. participated in editing. J.O. contributed in original idea and editing.
Contributor Information
Riccardo Tamburrini, Email: tamburrini@wisc.edu.
Fahad Aziz, Email: faziz@wisc.edu.
Ban Dodin, Email: ban.dodin@uchicagomedicine.org.
Brad C. Astor, Email: bcastor@medicine.wisc.edu.
Didier Mandelbrot, Email: damandel@medicine.wisc.edu.
Dixon Kaufman, Email: kaufman@surgery.wisc.edu.
Jon Odorico, Email: jon@surgery.wisc.edu.
REFERENCES
- 1.American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of care in diabetes—2024. Diabetes Care. 2024;47(Suppl 1):S20–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamaroff J, Kilberg M, Pinney SE, et al. Overview of atypical diabetes. Endocrinol Metab Clin North Am. 2020;49:695–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S14–S31. doi:10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 4.Törn C. C-peptide and autoimmune markers in diabetes. Clin Lab. 2003;49:1–10. [PubMed] [Google Scholar]
- 5.Canivell S, Gomis R. Diagnosis and classification of autoimmune diabetes mellitus. Autoimmun Rev. 2014;13:403–407. [DOI] [PubMed] [Google Scholar]
- 6.Landin-Olsson M, Nilsson KO, Lernmark A, et al. Islet cell antibodies and fasting C-peptide predict insulin requirement at diagnosis of diabetes mellitus. Diabetologia. 1990;33:561–568. [DOI] [PubMed] [Google Scholar]
- 7.Laugesen E, Østergaard JA, Leslie RD; Danish Diabetes Academy Workshop and Workshop Speakers. Latent autoimmune diabetes of the adult: current knowledge and uncertainty. Diabet Med. 2015;32:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pham PH, Stalter LN, Martinez EJ, et al. Single center results of simultaneous pancreas-kidney transplantation in patients with type 2 diabetes. Am J Transplant. 2021;21:2810–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Definition of pancreas graft failure: pancreas committee June 2015. Available at https://unos.org/wp-content/uploads/Definition-of-Pancreas-Graft-Failure.pdf#:~:text=Define%20pancreas%20graft%20failure%20as%20any%20of%20the%20following:%20Recipient%E2%80%99s. Accessed June 6, 2024. [Google Scholar]
- 10.Parajuli S, Mandelbrot D, Aufhauser D, et al. Higher fasting pretransplant C-peptide levels in type 2 diabetics undergoing simultaneous pancreas-kidney transplantation are associated with posttransplant pancreatic graft dysfunction. Transplantation. 2023;107:e109–e121. [DOI] [PubMed] [Google Scholar]
- 11.Aziz F, Parajuli S, Kaufman D, et al. Induction in pancreas transplantation: T-cell depletion versus IL-2 receptor blockade. Transplant Direct. 2022;8:e1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niederhaus SV, Leverson GE, Lorentzen DF, et al. Acute cellular and antibody-mediated rejection of the pancreas allograft: incidence, risk factors and outcomes. Am J Transplant. 2013;13:2945–2955. [DOI] [PubMed] [Google Scholar]
- 13.Balasubramanyam A, Garza G, Rodriguez L, et al. Accuracy and predictive value of classification schemes for ketosis-prone diabetes. Diabetes Care. 2006;29:2575–2579. [DOI] [PubMed] [Google Scholar]
- 14.Balasubramanyam A, Nalini R, Hampe CS, et al. Syndromes of ketosis-prone diabetes mellitus. Endocr Rev. 2008;29:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldonado M, Hampe CS, Gaur LK, et al. Ketosis-prone diabetes: dissection of a heterogeneous syndrome using an immunogenetic and beta-cell functional classification, prospective analysis, and clinical outcomes. J Clin Endocrinol Metab. 2003;88:5090–5098. [DOI] [PubMed] [Google Scholar]
- 16.Nalini R, Ozer K, Maldonado M, et al. Presence or absence of a known diabetic ketoacidosis precipitant defines distinct syndromes of “A-β+” ketosis-prone diabetes based on long-term β-cell function, human leukocyte antigen class II alleles, and sex predilection. Metab Clin Exp. 2010;59:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks-Worrell BM, Boyko EJ, Palmer JP. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care. 2014;37:3286–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papageorge CM, Bolognese AC, Odorico JS. Expanding access to pancreas transplantation for type 2 diabetes mellitus. Curr Opin Organ Transplant. 2021;26:390–396. [DOI] [PubMed] [Google Scholar]
- 19.Parsons RF, Matar A, Lentine KL, et al. Pancreas transplantation perceptions and practice: results from a national US survey. Clin Transplant. 2021;35:e14432. [DOI] [PubMed] [Google Scholar]
- 20.Light J, Tucker M. Simultaneous pancreas kidney transplants in diabetic patients with end-stage renal disease: the 20-yr experience. Clin Transplant. 2013;27:E256–E263. [DOI] [PubMed] [Google Scholar]
- 21.Gruessner AC, Laftavi MR, Pankewycz O, et al. Simultaneous pancreas and kidney transplantation-is it a treatment option for patients with type 2 diabetes mellitus? An analysis of the international pancreas transplant registry. Curr Diab Rep. 2017;17:44. [DOI] [PubMed] [Google Scholar]
- 22.Sampaio MS, Kuo HT, Bunnapradist S. Outcomes of simultaneous pancreas-kidney transplantation in type 2 diabetic recipients. Clin J Am Soc Nephrol. 2011;6:1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratta RJ, Rogers J, Farney AC, et al. Pancreas transplantation in C-peptide positive patients: does “type” of diabetes really matter? J Am Coll Surg. 2015;220:716–727. [DOI] [PubMed] [Google Scholar]
- 24.Pestana N, Malheiro J, Silva F, et al. Impact of pancreatic autoantibodies in pancreas graft survival after pancreas-kidney transplantation. Transplant Proc. 2020;52:1370–1375. [DOI] [PubMed] [Google Scholar]
- 25.Martins LS, Henriques AC, Fonseca IM, et al. Pancreatic autoantibodies after pancreas-kidney transplantation—do they matter? Clin Transplant. 2014;28:462–469. [DOI] [PubMed] [Google Scholar]
- 26.Rodelo-Haad C, Aguera ML, Martinez-Vaquera S, et al. Tyrosine-phosphatase and glutamate-decarboxylase antibodies after simultaneous pancreas kidney transplantation: do they have an impact on pancreas graft survival? Transplant Proc. 2015;47:107–111. [DOI] [PubMed] [Google Scholar]
- 27.Ringers J, van der Torren CR, van de Linde P, et al. Pretransplantation GAD-autoantibody status to guide prophylactic antibody induction therapy in simultaneous pancreas and kidney transplantation. Transplantation. 2013;96:745–752. [DOI] [PubMed] [Google Scholar]
- 28.Anteby R, Lucander A, Bachul PJ, et al. Evaluating the prognostic value of islet autoantibody monitoring in islet transplant recipients with long-standing type 1 diabetes mellitus. J Clin Med. 2021;10:2708. [DOI] [PMC free article] [PubMed] [Google Scholar]