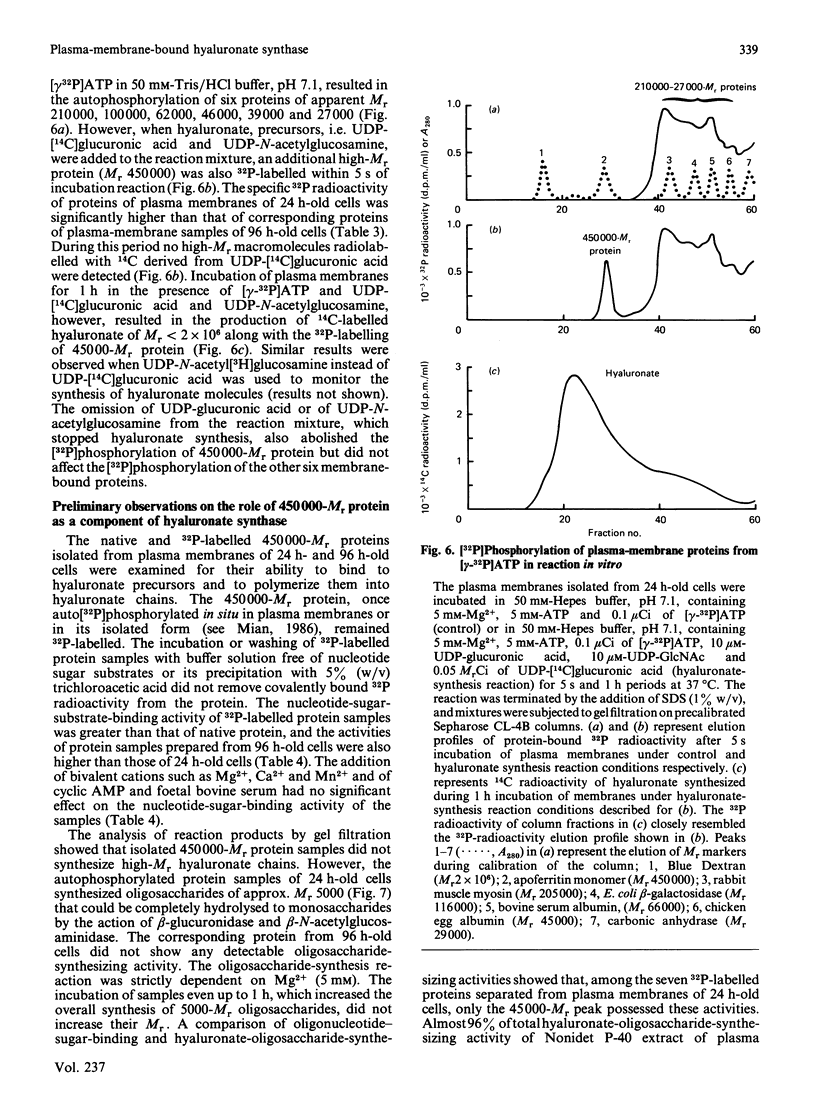
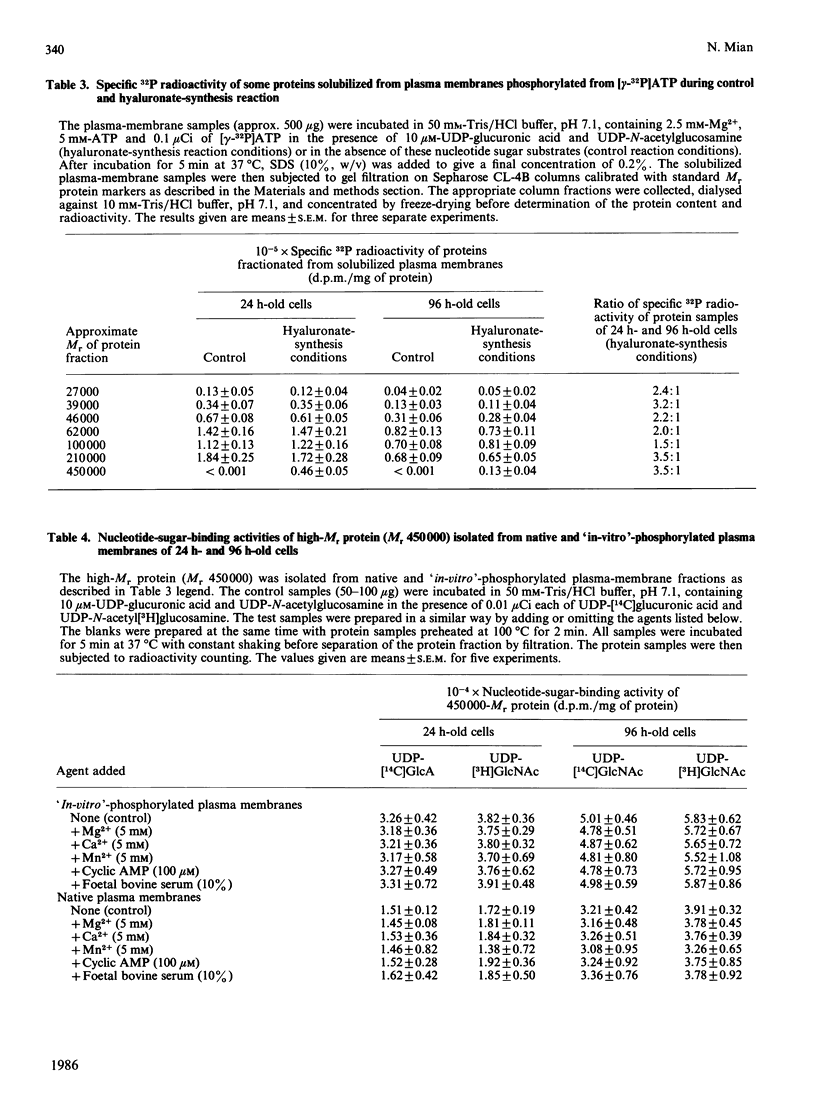
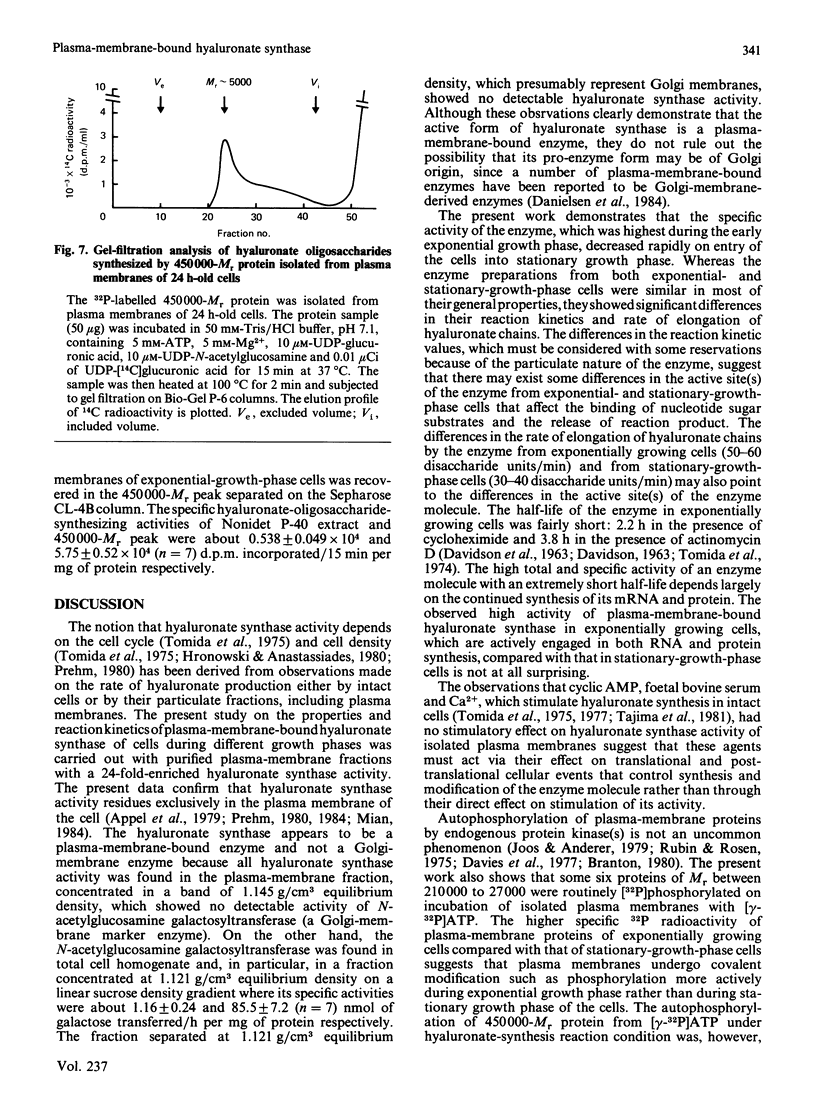
Abstract
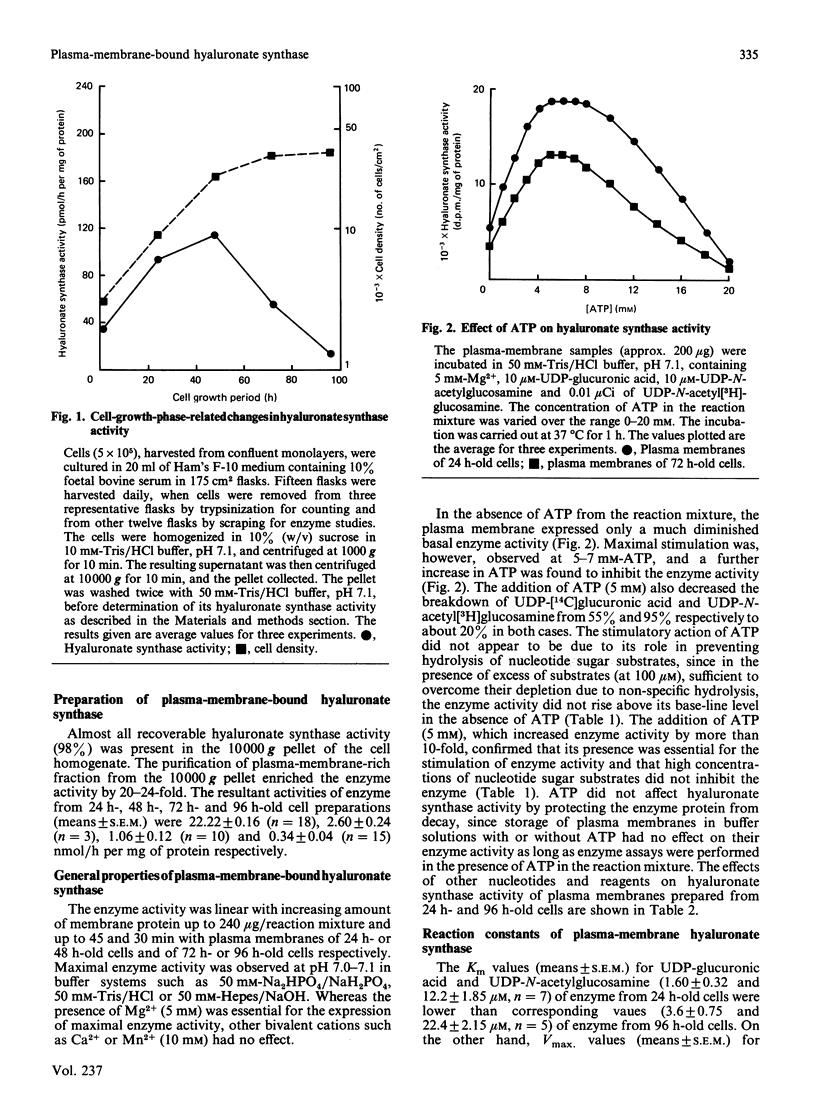
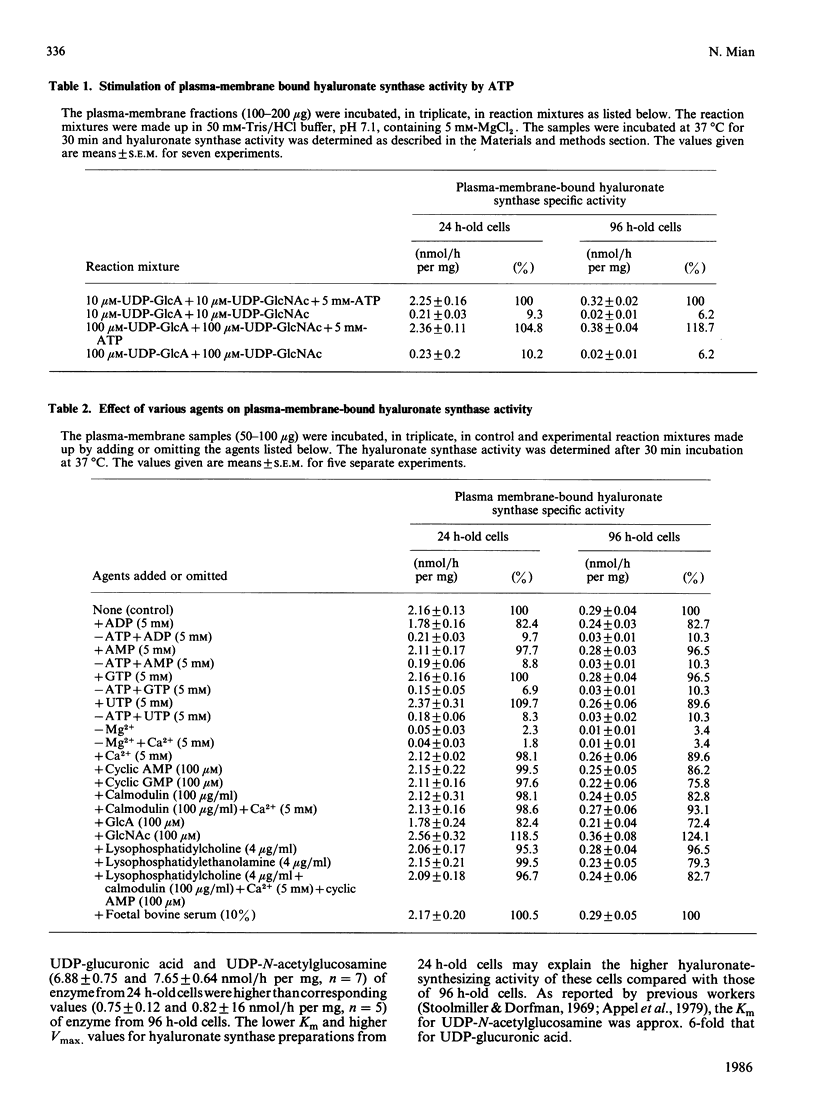
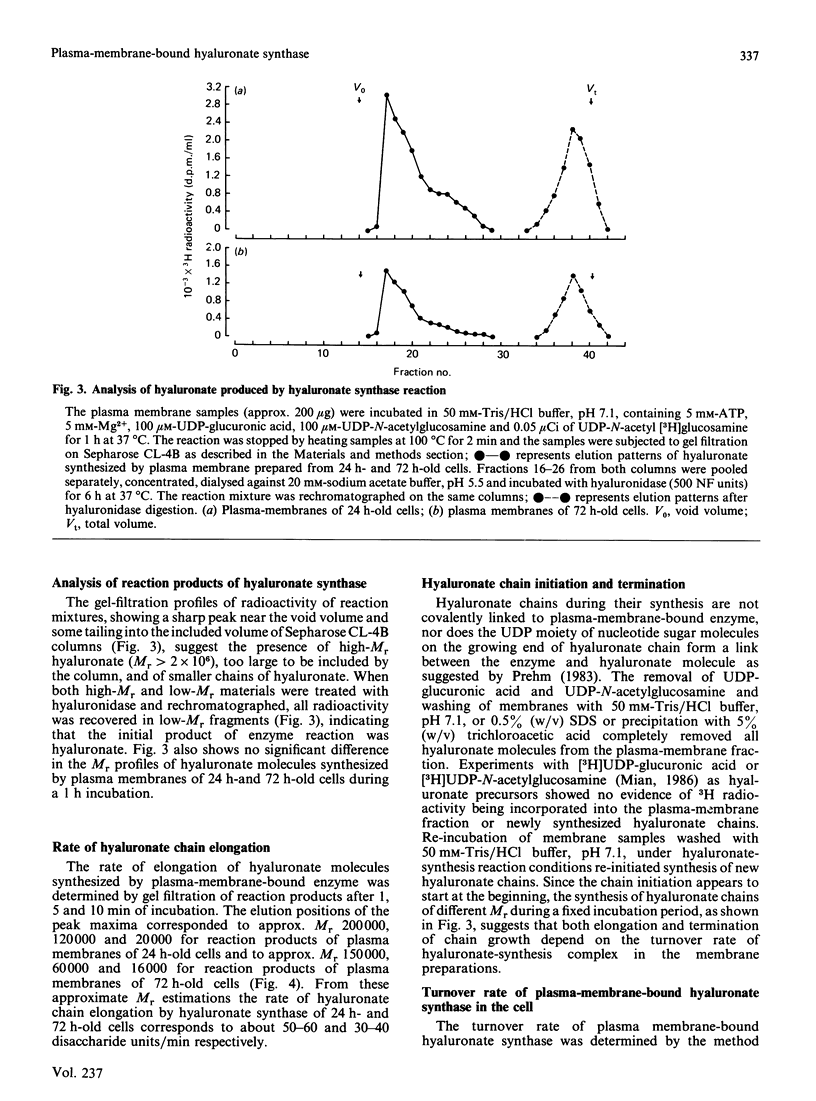
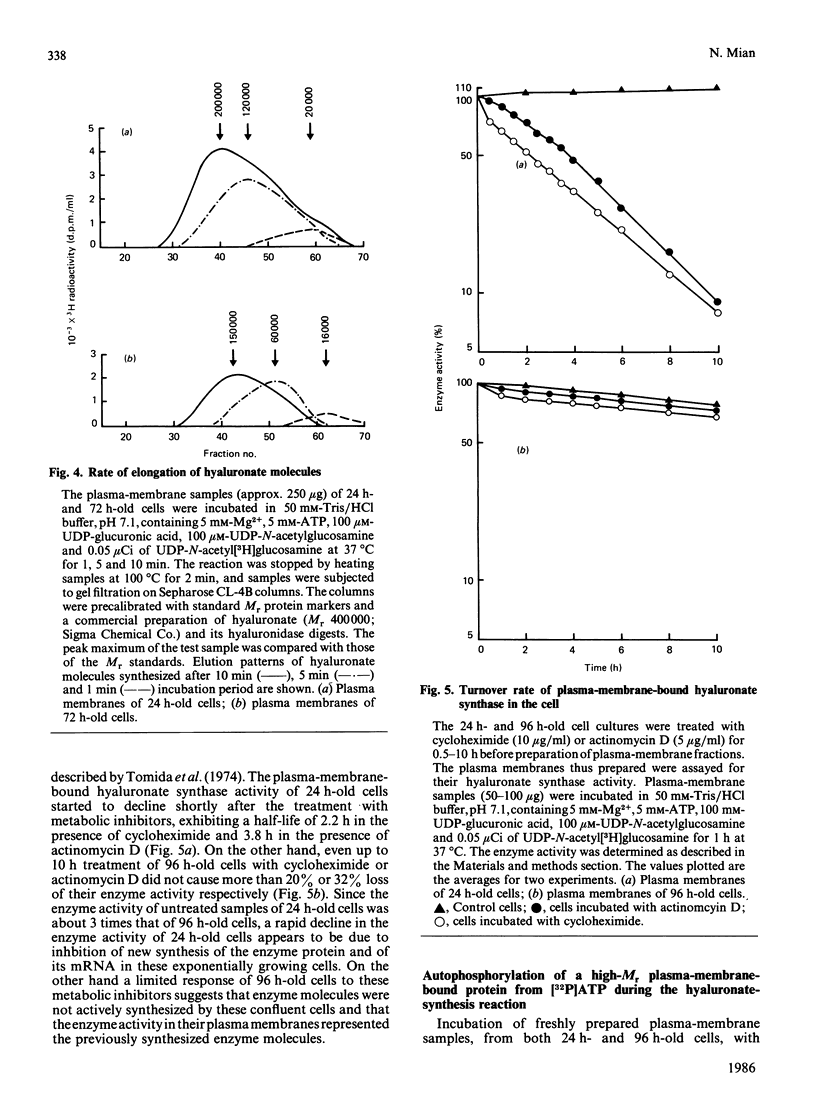
Hyaluronate synthase activity is localized exclusively in plasma-membrane fractions of cultured human skin fibroblasts. The enzyme activity of plasma membranes prepared from exponential-growth-phase cells was about 6.5 times that of stationary-growth-phase cells. Hyaluronate synthase from exponential-growth-phase cells exhibited lower Km and higher Vmax. values for both UDP-N-acetylglucosamine and UDP-glucuronic acid and higher rate of elongation of hyaluronate chains compared with the enzyme from stationary-growth-phase cells. Hyaluronate synthase exhibited an extremely short half-life, 2.2 h and 3.8 h respectively when cells were treated with cycloheximide and actinomycin D. The cell-growth-phase-dependent variations in hyaluronate synthase activity appear to be due to its high turnover rate as well as due to some post-translational modification of the enzyme protein as cells progress from early exponential to stationary growth phase. The isolated plasma membranes contained a protein (Mr approx. 450,000) that was selectively autophosphorylated from [gamma-32P]ATP in vitro in the presence of hyaluronate precursors in the reaction mixture and that also exhibited some hyaluronate-synthesis-related properties. The 32P-labelled protein isolated from plasma membranes of exponentially growing cells expressed an efficient UDP-[14C]glucuronic acid- and UDP-N-acetyl[3H]glucosamine-binding activity and was able to synthesize oligosaccharides (Mr 5000) of [14C]glucuronic acid and N-acetyl[3H]glucosamine residues. The corresponding protein of stationary-growth-phase cells, which expressed much higher nucleotide-sugar-precursor-binding activity, appeared to have lost its oligosaccharide-synthesizing activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel A., Horwitz A. L., Dorfman A. Cell-free synthesis of hyaluronic acid in Marfan syndrome. J Biol Chem. 1979 Dec 10;254(23):12199–12203. [PubMed] [Google Scholar]
- BURTON K. The relation between the synthesis of deoxyribonucleic acid and the synthesis of protein in the multiplication of bacteriophage T2. Biochem J. 1955 Nov;61(3):473–483. doi: 10.1042/bj0610473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton P. E. Protein phosphorylation and the proliferation of chick embryo fibroblasts: analysis by in vitro phosphorylation using isolated plasma membranes and whole cell homogenates. Can J Biochem. 1980 Jan;58(1):30–39. doi: 10.1139/o80-005. [DOI] [PubMed] [Google Scholar]
- DAVIDSON E. H., ALLFREY V. G., MIRSKY A. E. Gene expression in differentiated cells. Proc Natl Acad Sci U S A. 1963 Jan 15;49:53–60. doi: 10.1073/pnas.49.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON E. H. Heritability and control of differentiated function in cultured cells. J Gen Physiol. 1963 May;46:983–998. doi: 10.1085/jgp.46.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M., Norén O., Sjöström H. Biosynthesis of microvillar proteins. Biochem J. 1984 Jul 1;221(1):1–14. doi: 10.1042/bj2210001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Shizuta Y., Olden K., Gallo M., Pastan I. Phosphorylation of filamin and other proteins in cultured fibroblasts. Biochem Biophys Res Commun. 1977 Jan 10;74(1):300–307. doi: 10.1016/0006-291x(77)91408-5. [DOI] [PubMed] [Google Scholar]
- EARL D. C., KORNER A. THE ISOLATION AND PROPERTIES OF CARDIAC RIBOSOMES AND POLYSOMES. Biochem J. 1965 Mar;94:721–734. doi: 10.1042/bj0940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Dorfman A. Glycosaminoglycan synthesis by cultured human skin fibroblasts after transformation with simian virus 40. J Biol Chem. 1977 Jul 25;252(14):4777–4785. [PubMed] [Google Scholar]
- Hronowski L., Anastassiades T. P. The effect of cell density on net rates of glycosaminoglycan synthesis and secretion by cultured rat fibroblasts. J Biol Chem. 1980 Nov 10;255(21):10091–10099. [PubMed] [Google Scholar]
- Ipata P. L. Sheep brain 5'-nucleotidase. Some enzymic properties and allosteric inhibition by nucleoside triphosphates. Biochemistry. 1968 Feb;7(2):507–515. doi: 10.1021/bi00842a004. [DOI] [PubMed] [Google Scholar]
- Joos T. F., Anderer A. Characterization of specific differences in protein phosphorylation of the plasma membrane and the endoplasmic reticulum of mouse fibroblasts. Biochim Biophys Acta. 1979 Apr 18;584(1):104–115. doi: 10.1016/0304-4165(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Kittlick P. D., Neupert G., Bolck F. Acid mucopolysaccharides in fibroblast cultures. 1. Influence of cell density, pH-value and lactate concentration on the MPS distribution pattern. Exp Pathol (Jena) 1976;12(3-4):149–158. [PubMed] [Google Scholar]
- Kittlick P. D., Neupert G. Hypoxia in fibroblast cultures. 2. Influence of pH on the distribution pattern of acid mucopolysaccharides. Exp Pathol (Jena) 1975;10(5-6):229–240. [PubMed] [Google Scholar]
- Koyama D., Tomida M., Ono T. The enhanced production of hyaluronic acid by cultured rat fibroblast cells treated with cyclic AMP and its dibutyryl derivative. J Cell Physiol. 1975 Dec;87(2):189–197. doi: 10.1002/jcp.1040870207. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Acid mucopolysaccharides in cultured fibroblasts of cystic fibrosis of the pancreas. Biochem Biophys Res Commun. 1968 Dec 30;33(6):954–958. doi: 10.1016/0006-291x(68)90405-1. [DOI] [PubMed] [Google Scholar]
- Mian N. Characterization of a high-Mr plasma-membrane-bound protein and assessment of its role as a constituent of hyaluronate synthase complex. Biochem J. 1986 Jul 15;237(2):343–357. doi: 10.1042/bj2370343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarthy J. M., Short J., Lansing A. I., Lieberman I. Function and control of liver alkaline phosphatase. J Biol Chem. 1972 Mar 25;247(6):1767–1774. [PubMed] [Google Scholar]
- Prehm P. Hyaluronate is synthesized at plasma membranes. Biochem J. 1984 Jun 1;220(2):597–600. doi: 10.1042/bj2200597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Induction of hyaluronic acid synthesis in teratocarcinoma stem cells by retinoic acid. FEBS Lett. 1980 Mar 10;111(2):295–298. doi: 10.1016/0014-5793(80)80813-1. [DOI] [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Mechanism of chain growth. Biochem J. 1983 Apr 1;211(1):191–198. doi: 10.1042/bj2110191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolmiller A. C., Dorfman A. The biosynthesis of hyaluronic acid by Streptococcus. J Biol Chem. 1969 Jan 25;244(2):236–246. [PubMed] [Google Scholar]
- Tajima T., Iijima K., Watanabe T., Yamaguchi H. The influence of calcium ions on the synthesis of collagen and glycosaminoglycans in human diploid cells in culture. Exp Pathol. 1981;19(4):219–225. doi: 10.1016/s0232-1513(81)80067-9. [DOI] [PubMed] [Google Scholar]
- Tomida M., Koyama H., Ono T. Effects of adenosine 3':5'-cyclic monophosphate and serum on synthesis of hyaluronic acid in confluent rat fibroblasts. Biochem J. 1977 Mar 15;162(3):539–543. doi: 10.1042/bj1620539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida M., Koyama H., Ono T. Induction of hyaluronic acid synthetase activity in rat fibroblasts by medium change of confluent cultures. J Cell Physiol. 1975 Aug;86(1):121–130. doi: 10.1002/jcp.1040860114. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Biswas C., Gross J. Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6299–6303. doi: 10.1073/pnas.76.12.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P. Hyaluronate turnover during chondrogenesis in the developing chick limb and axial skeleton. Dev Biol. 1972 Nov;29(3):321–329. doi: 10.1016/0012-1606(72)90071-1. [DOI] [PubMed] [Google Scholar]



