Abstract
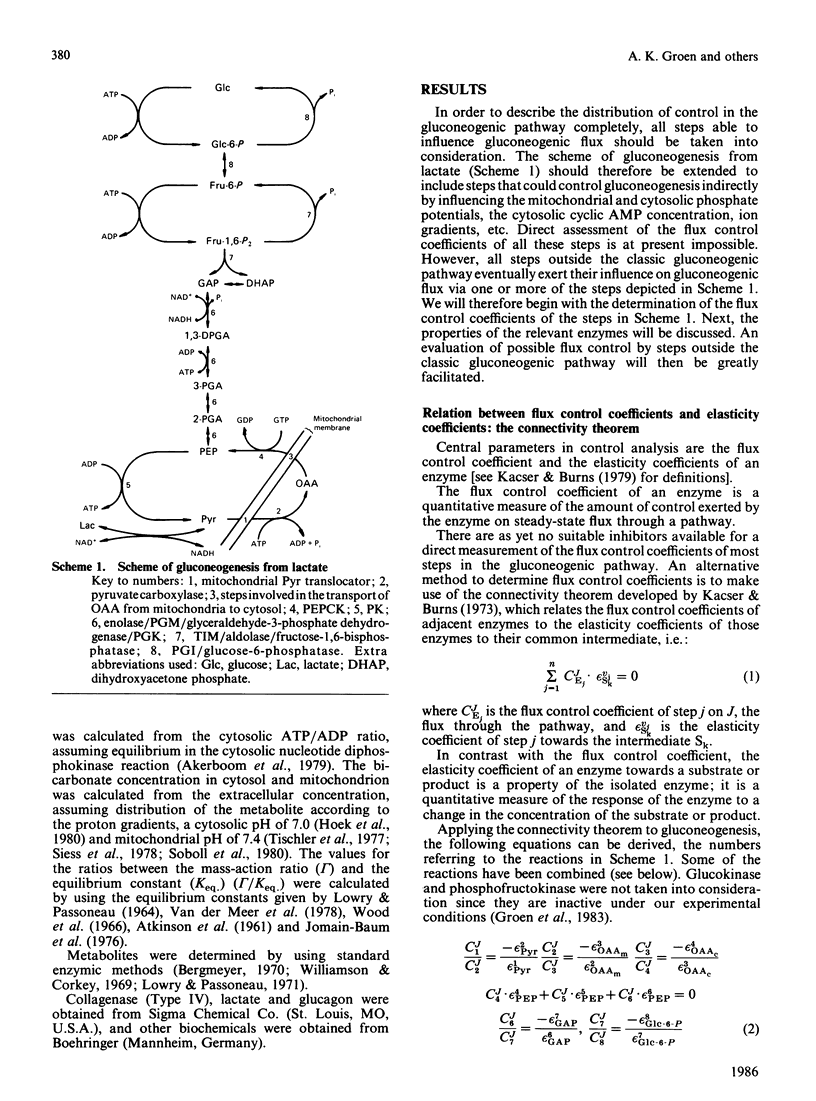
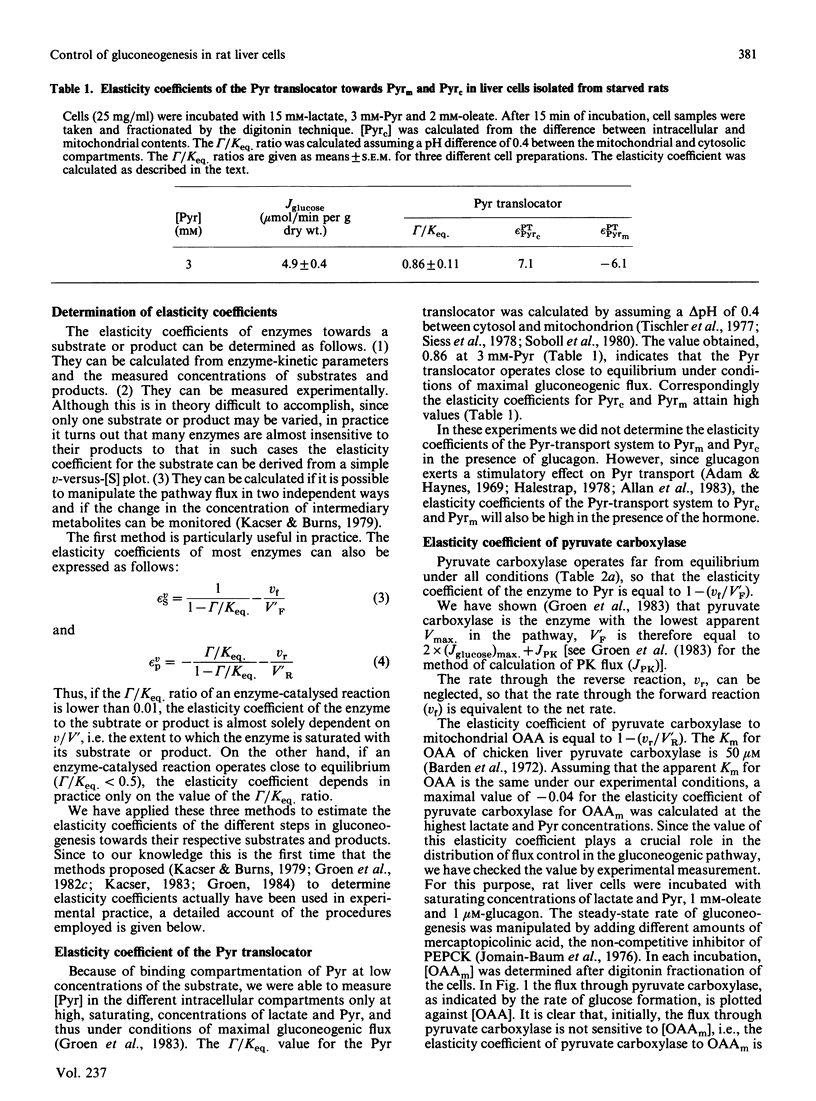
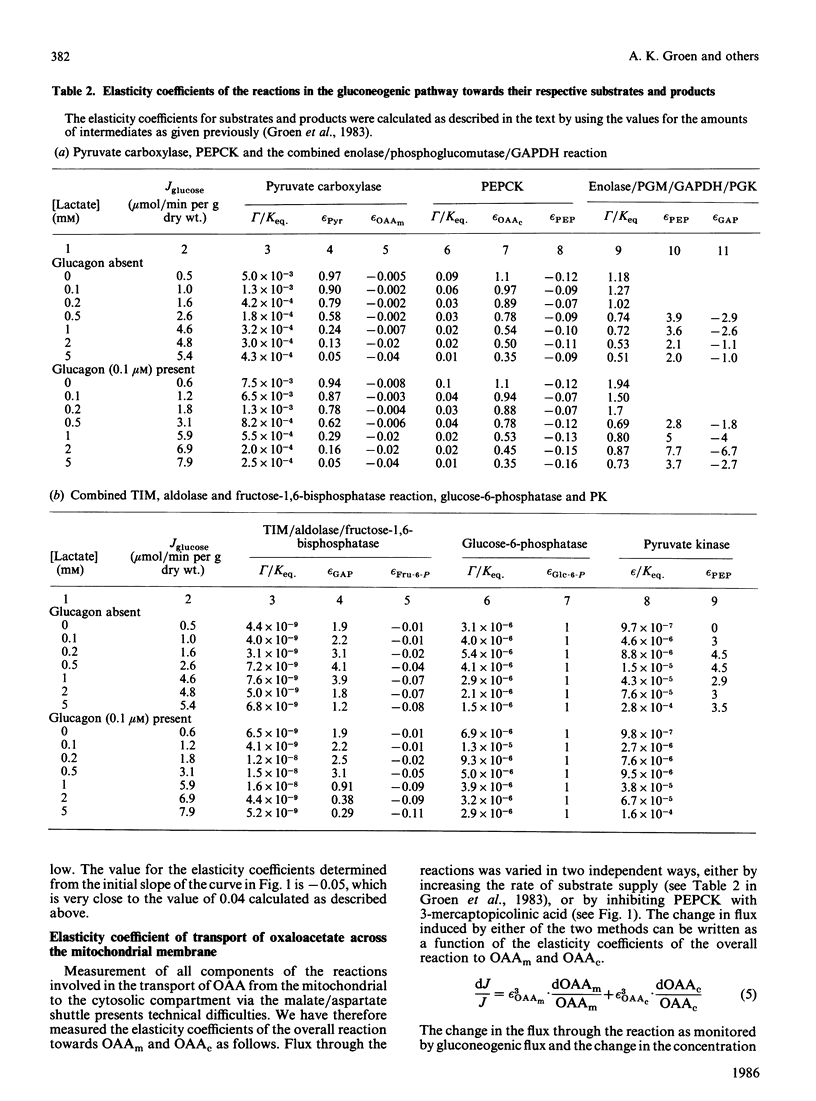
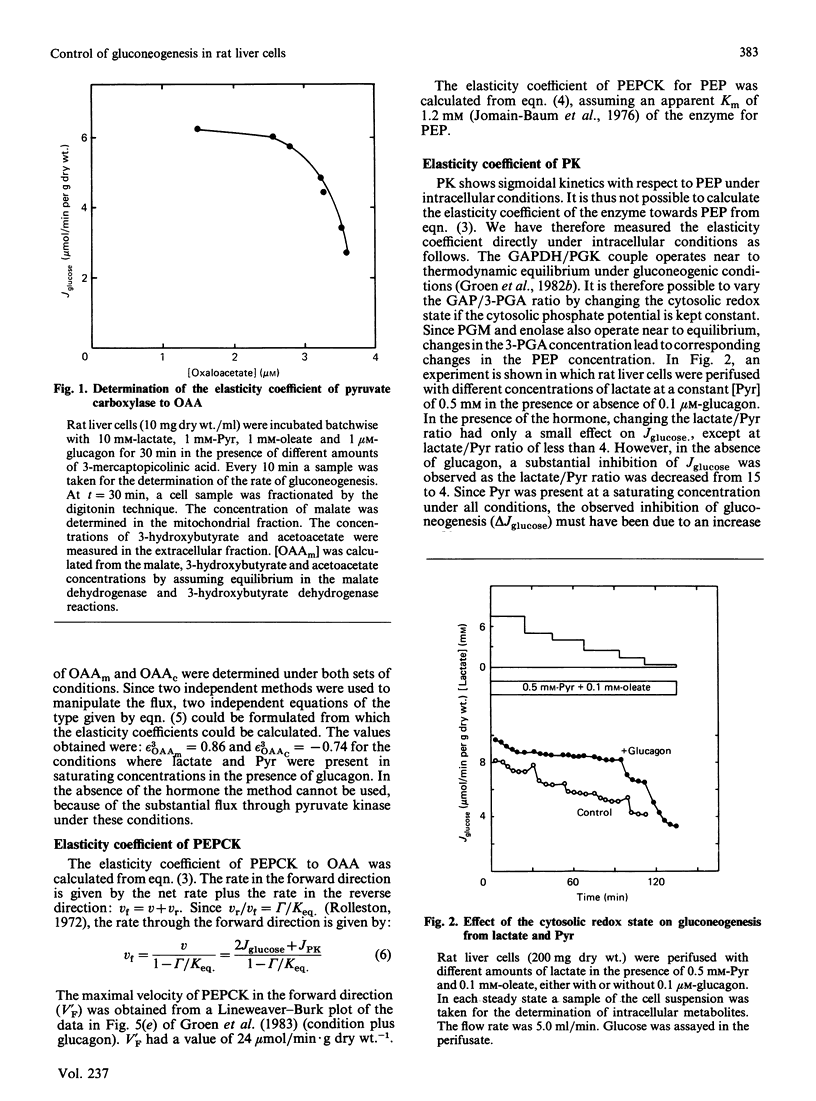
We have used control analysis to quantify the distribution of control in the gluconeogenic pathway in liver cells from starved rats. Lactate and pyruvate were used as gluconeogenic substrates. The flux control coefficients of the various enzymes in the gluconeogenic pathway were calculated from the elasticity coefficients of the enzymes towards their substrates and products and the fluxes through the different branches in the pathway. The elasticity coefficients were either calculated from gamma/Keq. ratios (where gamma is the mass-action ratio and Keq. is the equilibrium constant) and enzyme-kinetic data or measured experimentally. It is concluded that the gluconeogenic enzyme pyruvate carboxylase and the glycolytic enzyme pyruvate kinase play a central role in control of gluconeogenesis. If pyruvate kinase is inactive, gluconeogenic flux from lactate is largely controlled by pyruvate carboxylase. The low elasticity coefficient of pyruvate carboxylase towards its product oxaloacetate minimizes control by steps in the gluconeogenic pathway located after pyruvate carboxylase. This situation occurs when maximal gluconeogenic flux is required, i.e. in the presence of glucagon. In the absence of the hormone, when pyruvate kinase is active, control of gluconeogenesis is distributed among many steps, including pyruvate carboxylase, pyruvate kinase, fructose-1,6-bisphosphatase and also steps outside the classic gluconeogenic pathway such as the adenine-nucleotide translocator.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON M. R., JOHNSON E., MORTON R. K. Equilibrium constants of phosphoryl transfer from C-1 to C-6 of alpha-D-glucose 1-phosphate and from glucose 6-phosphate to water. Biochem J. 1961 Apr;79:12–15. doi: 10.1042/bj0790012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam P. A., Haynes R. C., Jr Control of hepatic mitochondrial CO2 fixation by glucagon, epinephrine, and cortisol. J Biol Chem. 1969 Dec 10;244(23):6444–6450. [PubMed] [Google Scholar]
- Akerboom T. P., Krietsch W. K., Kuntz G., Sies H. Enzymatic measurement of adenine and guanine (plus inosine) triphosphates and diphosphates in isolated cells and the mitochondrial matrix compartment obtained from rat liver. FEBS Lett. 1979 Sep 1;105(1):90–94. doi: 10.1016/0014-5793(79)80893-5. [DOI] [PubMed] [Google Scholar]
- Allan E. H., Chisholm A. B., Titheradge M. A. Hormonal stimulation of mitochondrial pyruvate carboxylation in filipin-treated hepatocytes. Biochem J. 1983 May 15;212(2):417–426. doi: 10.1042/bj2120417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden R. E., Fung C. H., Utter M. F., Scrutton M. C. Pyruvate carboxylase from chicken liver. Steady state kinetic studies indicate a "two-site" ping-pong mechanism. J Biol Chem. 1972 Feb 25;247(4):1323–1333. [PubMed] [Google Scholar]
- Casazza J. P., Stone S. R., Fromm H. J. Kinetic studies of bovine liver fructose-1,6-bisphosphatase. J Biol Chem. 1979 Jun 10;254(11):4661–4665. [PubMed] [Google Scholar]
- Claus T. H., Pilkis S. J. Effect of dichloroacetate and glucagon on the incorporation of labeled substrates into glucose and on pyruvate dehydrogenase in hepatocytes from fed and starved rats. Arch Biochem Biophys. 1977 Jul;182(1):52–63. doi: 10.1016/0003-9861(77)90282-x. [DOI] [PubMed] [Google Scholar]
- Feliú J. E., Hue L., Hers H. G. Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2762–2766. doi: 10.1073/pnas.73.8.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell D. A., Sauro H. M. Metabolic control and its analysis. Additional relationships between elasticities and control coefficients. Eur J Biochem. 1985 May 2;148(3):555–561. doi: 10.1111/j.1432-1033.1985.tb08876.x. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Sips H. J., Vervoorn R. C., Tager J. M. Intracellular compartmentation and control of alanine metabolism in rat liver parenchymal cells. Eur J Biochem. 1982 Feb;122(1):87–93. doi: 10.1111/j.1432-1033.1982.tb05851.x. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Vervoorn R. C., Van der Meer R., Tager J. M. Control of gluconeogenesis in rat liver cells. I. Kinetics of the individual enzymes and the effect of glucagon. J Biol Chem. 1983 Dec 10;258(23):14346–14353. [PubMed] [Google Scholar]
- Groen A. K., Vervoorn R. C., Wanders R. J., Van der Meer R., Tager J. M. An evaluation of the metabolite indicator method for determining the cytosolic phosphate potential in rat liver cells. Biochim Biophys Acta. 1982 Oct 11;721(2):172–177. doi: 10.1016/0167-4889(82)90065-9. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P., Armston A. E. A re-evaluation of the role of mitochondrial pyruvate transport in the hormonal control of rat liver mitochondrial pyruvate metabolism. Biochem J. 1984 Nov 1;223(3):677–685. doi: 10.1042/bj2230677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. Pyruvate and ketone-body transport across the mitochondrial membrane. Exchange properties, pH-dependence and mechanism of the carrier. Biochem J. 1978 Jun 15;172(3):377–387. doi: 10.1042/bj1720377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974 Feb 15;42(1):89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Hers H. G., Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980 Feb 25;255(4):1458–1464. [PubMed] [Google Scholar]
- Hue L. The role of futile cycles in the regulation of carbohydrate metabolism in the liver. Adv Enzymol Relat Areas Mol Biol. 1981;52:247–331. doi: 10.1002/9780470122976.ch4. [DOI] [PubMed] [Google Scholar]
- Jomain-Baum M., Schramm V. L., Hanson R. W. Mechanism of 3-mercaptopicolinic acid inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1976 Jan 10;251(1):37–44. [PubMed] [Google Scholar]
- Kacser H., Burns J. A. MOlecular democracy: who shares the controls? Biochem Soc Trans. 1979 Oct;7(5):1149–1160. doi: 10.1042/bst0071149. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kacser H. The control of enzyme systems in vivo: elasticity analysis of the steady state. Biochem Soc Trans. 1983 Jan;11(1):35–40. doi: 10.1042/bst0110035. [DOI] [PubMed] [Google Scholar]
- Kraus-Friedmann N. Hormonal regulation of hepatic gluconeogenesis. Physiol Rev. 1984 Jan;64(1):170–259. doi: 10.1152/physrev.1984.64.1.170. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V. THE RELATIONSHIPS BETWEEN SUBSTRATES AND ENZYMES OF GLYCOLYSIS IN BRAIN. J Biol Chem. 1964 Jan;239:31–42. [PubMed] [Google Scholar]
- Mapes J. P., Harris R. A. Inhibition of gluconeogenesis and lactate formation from pyruvate by N6, O2'-dibutyryl adenosine 3':5'-monophosphate. J Biol Chem. 1976 Oct 25;251(20):6189–6196. [PubMed] [Google Scholar]
- Regen D. M., Pilkis S. J. Sensitivity of pathway rate to activities of substrate-cycle enzymes: application to gluconeogenesis and glycolysis. J Theor Biol. 1984 Dec 21;111(4):635–658. doi: 10.1016/s0022-5193(84)80259-3. [DOI] [PubMed] [Google Scholar]
- Riou J. P., Claus T. H., Pilkis S. J. Control of pyruvate kinase activity by glucagon in isolated hepatocytes. Biochem Biophys Res Commun. 1976 Dec 6;73(3):591–599. doi: 10.1016/0006-291x(76)90851-2. [DOI] [PubMed] [Google Scholar]
- Rognstad R. Cyclic AMP induced inhibition of pyruvate kinase flux in the intact liver cell. Biochem Biophys Res Commun. 1975 Apr 21;63(4):900–905. doi: 10.1016/0006-291x(75)90653-1. [DOI] [PubMed] [Google Scholar]
- Rolleston F. S., Newsholme E. A. Control of glycolysis in cerebral cortex slices. Biochem J. 1967 Aug;104(2):524–533. doi: 10.1042/bj1040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Wieland O. H. Distribution of metabolites between the cytosolic and mitochondrial compartments of hepatocytes isolated from fed rats. Hoppe Seylers Z Physiol Chem. 1978 Jul;359(7):785–798. doi: 10.1515/bchm2.1978.359.2.785. [DOI] [PubMed] [Google Scholar]
- Sips H. J., Groen A. K., Tager J. M. Plasma-membrane transport of alanine is rate-limiting for its metabolism in rat-liver parenchymal cells. FEBS Lett. 1980 Oct 6;119(2):271–274. doi: 10.1016/0014-5793(80)80269-9. [DOI] [PubMed] [Google Scholar]
- Sistare F. D., Haynes R. C., Jr The interaction between the cytosolic pyridine nucleotide redox potential and gluconeogenesis from lactate/pyruvate in isolated rat hepatocytes. Implications for investigations of hormone action. J Biol Chem. 1985 Oct 15;260(23):12748–12753. [PubMed] [Google Scholar]
- Soboll S., Elbers R., Scholz R., Heldt H. W. Subcellular distribution of di- and tricarboxylates and pH gradients in perfused rat liver. Hoppe Seylers Z Physiol Chem. 1980 Jan;361(1):69–76. doi: 10.1515/bchm2.1980.361.1.69. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Vignais P. V., Krebs H. A. Is the adenine nucleotide translocator rate-limiting for oxidative phosphorylation? Biochem J. 1978 May 15;172(2):333–342. doi: 10.1042/bj1720333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler M. E., Hecht P., Williamson J. R. Determination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Arch Biochem Biophys. 1977 May;181(1):278–293. doi: 10.1016/0003-9861(77)90506-9. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- Williamson J. R., Browning E. T., Scholz R. Control mechanisms of gluconeogenesis and ketogenesis. I. Effects of oleate on gluconeogenesis in perfused rat liver. J Biol Chem. 1969 Sep 10;244(17):4607–4616. [PubMed] [Google Scholar]
- Wood H. G., Davis J. J., Lochmüller H. The equilibria of reactions catalyzed by carboxytransphosphorylase, carboxykinase, and pyruvate carboxylase and the synthesis of phosphoendolpyruvate. J Biol Chem. 1966 Dec 10;241(23):5692–5704. [PubMed] [Google Scholar]
- van Berkel T. J., Kruijt J. K., Koster J. F., Hülsmann W. C. Cyclic nucleotide-, pyruvate- and hormone-induced changes in pyruvate kinase activity in isolated rat hepatocytes. Biochem Biophys Res Commun. 1976 Oct 4;72(3):917–925. doi: 10.1016/s0006-291x(76)80219-7. [DOI] [PubMed] [Google Scholar]
- van der Meer R., Akerboom T. P., Groen A. K., Tager J. M. Relationship between oxygen uptake of perifused rat-liver cells and the cytosolic phosphorylation state calculated from indicator metabolites and a redetermined equilibrium constant. Eur J Biochem. 1978 Mar 15;84(2):421–428. doi: 10.1111/j.1432-1033.1978.tb12183.x. [DOI] [PubMed] [Google Scholar]


