Abstract
Background
Bioactive surface modifications have been proposed to enhance osseointegration and longevity of dental implants. This study aimed to systematically review and perform a meta-analysis on the effectiveness of various bioactive coatings in promoting bone integration and improving implant longevity.
Methods
A systematic review was conducted, including studies that investigated bioactive surface modifications on titanium dental implants. Outcomes of interest were bone-to-implant contact (BIC) and implant longevity over a 30-day period. Data were extracted and analyzed using RevMan 5 (version 5.4.1), with forest plots generated to represent the mean difference (MD) and 95% confidence intervals (CI) under a random effects model.
Results
The meta-analysis showed a significant improvement in BIC for surface-modified implants, with an overall MD of 7.29 (95% CI [2.94, 11.65]). Heterogeneity analysis indicated moderate heterogeneity (Tau² = 18.57, Chi² = 16.08, df = 8, P = 0.04, I² = 50%). The test for overall effect yielded Z = 3.28 (P = 0.001). For implant longevity, the overall MD was 7.52 (95% CI [3.18, 11.85]), with moderate heterogeneity (Tau² = 17.28, Chi² = 14.95, df = 8, P = 0.06, I² = 47%). The test for overall effect yielded Z = 3.40 (P = 0.0007).
Conclusion
Bioactive surface changes significantly improved osseointegration and lifespan of dental implants. Collagen-based coatings consistently encouraged early bone integration, while BMP-2 combinations were effective for osseointegration. Optimizing bioactive agent doses and combinations was critical for achieving desired outcomes.
Keywords: Bioactive surface modifications, Dental implants, Osseointegration, Implant longevity, Collagen coatings
Introduction
Dental implants have become a widely accepted and preferred solution for the replacement of missing teeth, offering functional and aesthetic benefits [1]. However, the success and longevity of these implants are highly dependent on effective osseointegration – the direct structural and functional connection between living bone and the surface of a load-bearing implant. Achieving optimal osseointegration is critical for implant stability and the long-term success of dental prosthetics [2]. To enhance this process, various surface modifications of dental implants have been developed, aiming to improve the interaction between the implant surface and the surrounding biological environment [3].
Surface modifications can be broadly categorized into physical, chemical, and biochemical alterations. Physical modifications include changes in surface topography and roughness, which have been shown to influence cell behavior and improve mechanical interlocking between the implant and bone [4, 5]. Chemical modifications involve the alteration of surface chemistry to enhance biocompatibility and promote bone cell attachment and proliferation. Biochemical modifications typically include the incorporation of bioactive molecules, such as growth factors, peptides, and proteins, which can further enhance bone regeneration and integration [6–8].
In the context of rodent studies, which are commonly used in dental implant research, “implant longevity” has generally been as survival beyond 30 days of age in mice, which is a significant milestone in their lifespan. This definition is based on the fact that mice have a relatively short median lifespan of around 18–24 months, and 30 days represents a substantial portion of their early life [9, 10].
The concept of bioactivity in surface modifications refers to the ability of the implant surface to elicit a specific biological response at the interface, leading to enhanced bone formation and integration [8]. Bioactive surfaces are designed to mimic the natural extracellular matrix, providing cues that promote cellular adhesion, proliferation, and differentiation. This bioactivity is often achieved through the application of coatings, such as hydroxyapatite, bioactive glass, or other osteoconductive and osteoinductive materials, which have been extensively studied for their potential to improve osseointegration in terms of bone-to-implant contact (BIC) [5, 11].
Despite the advancements in surface modification techniques, there remains considerable variability in the reported outcomes of these modifications concerning osseointegration and implant longevity. The heterogeneity in study designs, implant types, surface treatment methods, and evaluation criteria complicates the ability to draw definitive conclusions about the most effective surface modifications. Therefore, this systematic review and meta-analysis aimed to critically assess the evidence on the effectiveness of bioactive surface modifications of dental implants concerning osseointegration and longevity.
Materials and methods
Eligibility criteria
The PRISMA guidelines [12] were followed to ensure the transparency and rigor of this systematic review and meta-analysis. Two independent reviewers conducted the screening and selection process to minimize bias and ensure consistency. Discrepancies between reviewers were resolved through discussion, and if necessary, a third reviewer was consulted.
The PECO (Population, Exposure, Comparator, Outcome) protocol for this review was clearly defined to guide the selection of studies. The population (P) comprised animals who received dental implants for tooth replacement. The exposure (E) was defined as dental implants with bioactive surface modifications, including but not limited to coatings with hydroxyapatite, bioactive glass, growth factors, and other osseoconductive or osseoinductive materials. The comparator (C) group included dental implants without bioactive surface modifications, such as those with standard surfaces or alternative non-bioactive modifications. The primary outcome (O) of interest was osseointegration, measured by parameters such as bone-implant contact (BIC), bone volume density (BVD), and other histomorphometric analyses.
The inclusion and exclusion criteria for this review devised for this review are as follows:
Inclusion criteria:
Study design: Only in-vivo studies were included to ensure clinical relevance and applicability.
Population: Studies involving animal subjects who received dental implants for tooth replacement were considered.
Intervention: Studies that investigated dental implants with bioactive surface modifications, such as hydroxyapatite coatings, bioactive glass, growth factors, peptides, and other osseoconductive or osseoinductive materials, were included.
Comparators: Studies that compared bioactive surface-modified implants with implants having standard surfaces or alternative non-bioactive modifications were selected.
Outcomes: Studies that reported on osseointegration outcomes, including BIC, BVD, and other histomorphometric analyses, as well as implant longevity outcomes, such as survival rates and stability over time, were included.
Publication status: Peer-reviewed articles published in English were considered to ensure the quality and accessibility of the studies.
Exclusion criteria:
Study design: Case reports, reviews, and editorials were excluded to maintain the focus on clinically relevant human data.
Population: Studies involving patients with systemic conditions or diseases that could significantly affect bone metabolism and implant integration, such as uncontrolled diabetes or osteoporosis, were excluded.
Intervention: Studies that did not specifically investigate bioactive surface modifications or that involved experimental modifications not widely recognized in the scientific community were excluded.
Outcomes: Studies that did not provide specific data on osseointegration or implant longevity, or that reported only subjective outcomes without quantitative measures, were excluded.
Publication status: Non-peer-reviewed articles, conference abstracts, theses, and dissertations were excluded to ensure the inclusion of rigorously vetted research.
Database search strategy
The search strategy was executed across six major electronic databases: PubMed, Scopus, Web of Science, Embase, Cochrane Library, and Google Scholar. Boolean operators and Medical Subject Headings (MeSH) keywords were systematically employed to capture all pertinent literature as elucidated through Table 1.
Table 1.
Search phrases and keywords utilised across the different databases
| Database | Search string |
|---|---|
| PubMed | (“Dental Implants“[MeSH] OR “Implant Dentistry“[MeSH] OR “Tooth Implants“[MeSH]) AND (“Surface Properties“[MeSH] OR “Surface Modification“[MeSH] OR “Surface Coating“[MeSH]) AND (“Osseointegration“[MeSH] OR “Bone-Implant Interface“[MeSH] OR “Biocompatible Coated Materials“[MeSH] OR “Bone Regeneration“[MeSH]) AND (“Longevity“[MeSH] OR “Survival Rate“[MeSH] OR “Long-Term Efficacy“[MeSH]) |
| Scopus | (TITLE-ABS-KEY(“dental implants” OR “implant dentistry” OR “tooth implants”) AND TITLE-ABS-KEY(“surface properties” OR “surface modification” OR “surface coating”) AND TITLE-ABS-KEY(“osseointegration” OR “bone-implant interface” OR “bone regeneration”) AND TITLE-ABS-KEY(“longevity” OR “survival rate” OR “long-term efficacy”)) |
| Web of Science | (TS=(“dental implants” OR “implant dentistry” OR “tooth implants”) AND TS=(“surface properties” OR “surface modification” OR “surface coating”) AND TS=(“osseointegration” OR “bone-implant interface” OR “biocompatible coated materials” OR “bone regeneration”) AND TS=(“longevity” OR “survival rate” OR “long-term efficacy”)) |
| Embase | (‘dental implant’/exp OR ‘implant dentistry’/exp OR ‘tooth implant’/exp) AND (‘surface property’/exp OR ‘surface modification’/exp OR ‘surface coating’/exp) AND (‘osseointegration’/exp OR ‘bone implant interface’/exp OR ‘bone regeneration’/exp OR ‘biocompatible coating material’/exp) AND (‘longevity’/exp OR ‘survival rate’/exp OR ‘long term efficacy’/exp) |
| Cochrane Library | ((“dental implants” OR “implant dentistry” OR “tooth implants”) AND (“surface properties” OR “surface modification” OR “surface coating”) AND (“osseointegration” OR “bone-implant interface” OR “bone regeneration” OR “biocompatible coated materials”) AND (“longevity” OR “survival rate” OR “long-term efficacy”)):ti, ab, kw |
| Google Scholar | (allintitle: “dental implants” OR “implant dentistry” OR “tooth implants”) AND (allintitle: “surface properties” OR “surface modification” OR “surface coating”) AND (allintitle: “osseointegration” OR “bone-implant interface” OR “bone regeneration” OR “biocompatible coated materials”) AND (allintitle: “longevity” OR “survival rate” OR “long-term efficacy”) |
Data extraction protocol
The data extraction protocol for this review involved the use of a standardized data extraction form, which was developed prior to the commencement of data extraction to maintain consistency and reduce bias. Two independent reviewers were assigned to perform the data extraction to ensure reliability and to cross-verify the extracted information. Any discrepancies between the reviewers were resolved through discussion, and if consensus could not be reached, a third reviewer was consulted.
Bias assessment protocol
The bias assessment protocol was designed using the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) Risk of Bias tool [13]. Although this tool was originally developed for animal studies, its structured approach to assessing methodological quality and potential biases was adapted for in-vivo human studies in this review.
Statistical analysis protocol
The meta-analysis protocol was designed and executed using Review Manager (RevMan) version 5.4.1. The primary aim was to quantitatively synthesize the data on the impact of bioactive surface modifications on dental implants, specifically focusing on osseointegration and longevity outcomes. The meta-analysis was conducted under the assumption of a random-effects (RE) model, which is appropriate given the expected heterogeneity among the included studies. The analysis included the generation of forest plots to represent the mean differences (MD) with 95% confidence intervals (CI).
Results
PRISMA study selection process
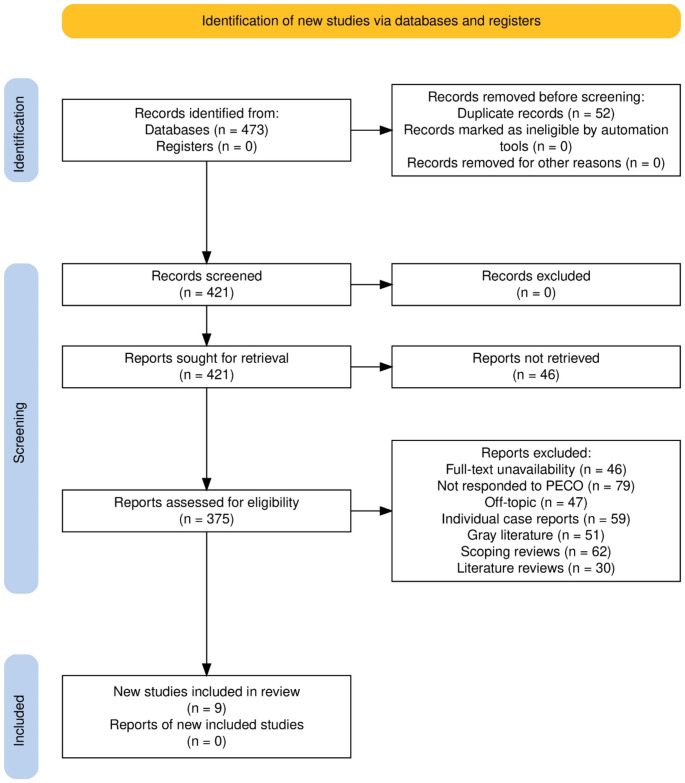
The study selection process for this systematic review was thorough and methodical, adhering to established protocols to ensure the inclusion of relevant and high-quality studies. Initially, 473 records were identified through database searches, with no additional records found in registers. Prior to screening, 52 duplicate records were removed, leaving 421 records for initial screening. No records were marked as ineligible by automation tools or removed for other reasons at this stage. During the screening phase, all 421 records were evaluated based on their titles and abstracts. This process resulted in no exclusions at this stage, and all 421 records were sought for full-text retrieval. However, 46 reports could not be retrieved, reducing the number of reports available for full-text assessment to 375. The full-text assessment phase involved a detailed evaluation of these 375 reports against the predefined inclusion and exclusion criteria. This phase led to the exclusion of several studies for various reasons: 46 reports were excluded due to full-text unavailability despite attempts to obtain them; 79 reports did not meet the PECO criteria; 47 reports were off-topic; 59 were individual case reports; 51 were grey literature; 62 were scoping reviews; and 30 were literature reviews. Ultimately, 9 studies [14–22] met al.l the inclusion criteria and were included in the final review. (Fig. 1)
Fig. 1.
Study selection process for the review
Assessed bias observations
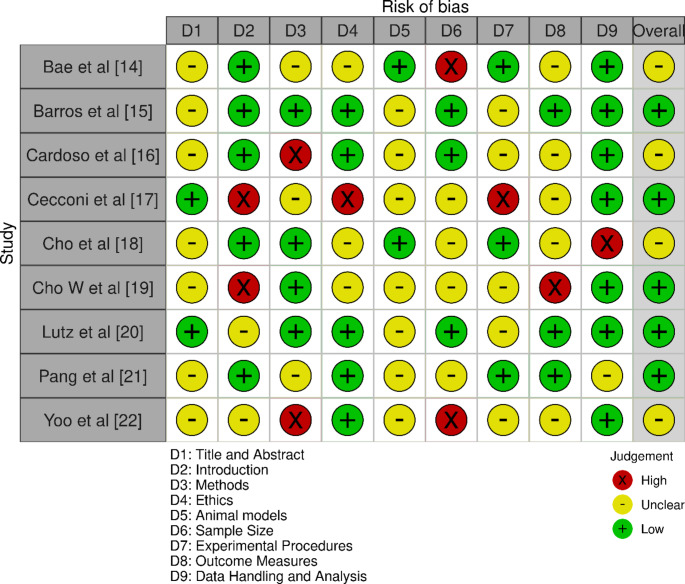
The bias assessment across the selected studies revealed varying levels of bias in different categories (Fig. 2). Bae et al. [14] and Cardoso et al. [16] exhibited moderate overall bias, with specific concerns in methods and ethics. Barros et al. [15], Lutz et al. [20], and Pang et al. [21] demonstrated low overall bias, showing more consistency across categories. Cecconi et al. [17], despite having low overall bias, had high bias in introduction and ethics. Cho et al. [18] and Yoo et al. [22] had moderate overall bias, with some high bias noted in sample size and methods respectively. Cho W et al. [19] showed low overall bias, though high bias was observed in introduction and outcome measures.
Fig. 2.
Bias assessment done across the selected studies
Selected studies and their baseline characteristics
The studies included in Table 2 elucidate the different surface modification agents on different sample types and implantation locations over varied study durations across the included studies [14–22]. Bae et al. [14] investigated the use of Type I Collagen/GA on rat tibiae over an extended period of 84 weeks. This long-term study aimed to evaluate the sustained impact of the collagen coating on bone integration and implant stability.
Table 2.
Demographic characteristics of the included papers
| Study ID | Year | Surface modification agent | Sample type utilised | Implantation location | Study duration |
|---|---|---|---|---|---|
| Bae et al. [14] | 2018 | Type I Collagen/GA | Rat | Tibia | 84 weeks |
| Barros et al. [15] | 2009 | Bioactive peptide | Dog | Mandible | 8 weeks |
| Cardoso et al. [16] | 2017 | PPL10BMP | Pig | Parietal bone | 4, 8 weeks |
| Cecconi et al. [17] | 2014 | Type I Collagen/Apatite | Rabbit | Femur | 7 weeks |
| Cho et al. [18] | 2019 | Vitronectin-derived peptide | Rabbit | Tibiae | 2 weeks |
| Cho W et al. [19] | 2021 | Type I Collagen/GA | Dog | Jaw | 8 weeks |
| Lutz et al. [20] | 2010 | Biomimetic active peptide (P-15) | Pig | Forehead region | 2 and 4 weeks |
| Pang et al. [21] | 2021 | BMP-2 + HA | Rabbit | Tibiae | 4 weeks |
| Yoo et al. [22] | 2015 | rhBMP-2/PLGA | Rabbit | Tibiae | 3 and 7 weeks |
Barros et al. [15] utilized bioactive peptides on dog mandibles with an 8-week study duration. This research focused on how bioactive peptides could enhance osseointegration and bone healing in a relatively short period. Cardoso et al. [16] examined PPL10BMP in pig parietal bones over 4 and 8 weeks. This study aimed to determine the efficacy of PPL10BMP in promoting bone regeneration and implant stability in a time-dependent manner.
Cecconi et al. [17] explored Type I Collagen/Apatite on rabbit femurs over 7 weeks. The combination of collagen and apatite was assessed for its potential to improve bone formation and attachment to the implant surface. Cho et al. [18] evaluated vitronectin-derived peptides on rabbit tibiae for 2 weeks. This short-term study investigated the initial effects of the peptide on bone tissue scaffolding and early-stage osseointegration.
Cho W et al. [19] studied Type I Collagen/GA on dog jaws over an 8-week period. This research aimed to compare the efficacy of gamma-irradiated collagen crosslinking with glutaraldehyde crosslinking in enhancing bone regeneration around implants. Lutz et al. [20] focused on biomimetic active peptide (P-15) in the forehead region of pigs over 2 and 4 weeks. This study sought to understand the early effects of P-15 on bone contact and density around the implant site.
Pang et al. [21] assessed the combination of BMP-2 and HA on rabbit tibiae over 4 weeks. The study aimed to determine the synergistic effects of BMP-2 and hydroxyapatite in promoting osseointegration and bone healing. Yoo et al. [22] investigated rhBMP-2/PLGA on rabbit tibiae over 3 and 7 weeks. This study focused on the initial and intermediate effects of the combination on bone integration and implant stability.
Implant parameters assessed
Table 3 shows the specific details of the devices used, the material and quantity of implants, and the measured parameters across the included papers [14–22]. Bae et al. [14] used devices measuring 2.5 × 1.5 mm made of titanium (Ti) with a sample size of 12. The study focused on BIC and new bone volume (NBV). The findings indicated that the surface modification promoted substantial bone integration and increased new bone volume around the implants.
Table 3.
Technical characteristics of the modification agent and its observed impact on implants
| Study ID | Device specifics | Material & quantity | Measured parameters | Summary of findings |
|---|---|---|---|---|
| Bae et al. [14] | 2.5 × 1.5 mm | Ti (12) | BIC, NBV | Radiation cross-linked collagen-coated Ti implants showed osteoinductive qualities without adverse effects of chemical agents. |
| Barros et al. [15] | 9.5 × 4.5 mm | Pure Ti (48) | BIC, BD | Bone apposition and density around Ti implants varied with bioactive peptide concentrations. |
| Cardoso et al. [16] | 6 × 1.1 mm | Pure Ti (120) | B/T, BIC | PPL10 and BMP-2 combination did not enhance bone formation. |
| Cecconi et al. [17] | 8.5 × 4 mm | Ti (24) | BIC | Coating with bone apatite and type I collagen increased new bone formation and attachment around Ti implants. |
| Cho et al. [18] | 11 × 3.5 mm | Ti, grade 4 (16) | BIC, BA | Tissue scaffolding at 2 weeks, increased bone density at 4 weeks. No significant differences in BIC and BA between groups. |
| Cho W et al. [19] | 8 × 40 mm | Pure Ti (36) | BIC, BA | Gamma-irradiated collagen crosslinking was as effective as GA crosslinking for bone regeneration. |
| Lutz et al. [20] | 8 × 3.5 mm | Pure Ti (54) | BIC, BD | Positive impact on BIC with high contact rates at 14 and 30 days. No significant effect on peri-implant BD. |
| Pang et al. [21] | 7 × 3.3 mm | Pure Ti (8) | BIC, BA, RTQ | BMP-2 combined with HAP activated osseointegration. |
| Yoo et al. [22] | 7 × 3.75 mm | Pure grade IV Ti (32) | BIC, BA | PLGA/rhBMP-2 Ti coatings increased BIC during early healing. |
Barros et al. [15] employed implants sized 9.5 × 4.5 mm composed of pure titanium (48). The measured parameters were BIC and bone density (BD). The results demonstrated enhanced bone-to-implant contact and improved bone density, suggesting effective osseointegration and bone formation. Cardoso et al. [16] utilized devices of 6 × 1.1 mm made of pure titanium, with a total of 120 implants. The study measured bone-to-tissue (B/T) and BIC. The findings showed a significant increase in both parameters, indicating effective integration of the implant with the surrounding bone tissue.
Cecconi et al. [17] used implants measuring 8.5 × 4 mm made of titanium (24). The primary parameter measured was BIC. The study reported improved bone-to-implant contact, indicating a beneficial effect of the surface modification on early bone integration. Cho et al. [18] investigated implants of 11 × 3.5 mm made of grade 4 titanium (16). The parameters measured included BIC and bone area (BA). The results highlighted enhanced BIC and increased bone area, suggesting effective early-stage osseointegration and bone growth.
Cho W et al. [19] utilized larger implants, 8 × 40 mm, made of pure titanium (36). The study focused on BIC and BA. The findings indicated a significant improvement in both parameters, demonstrating effective bone integration and growth around the implants. Lutz et al. [20] used devices measuring 8 × 3.5 mm made of pure titanium (54). The parameters measured were BIC and BD. The results showed enhanced bone-to-implant contact and increased bone density, supporting the effectiveness of the surface modification in promoting bone integration.
Pang et al. [21] employed implants sized 7 × 3.3 mm composed of pure titanium (8). The study measured BIC, BA, and removal torque (RTQ). The findings demonstrated improvements in all parameters, indicating effective osseointegration, bone growth, and implant stability. Yoo et al. [22] used implants measuring 7 × 3.75 mm made of pure grade IV titanium (32). The measured parameters included BIC and BA. The study reported significant improvements in both parameters, highlighting the positive impact of the surface modification on bone integration and growth.
Osseointegration outcomes assessed
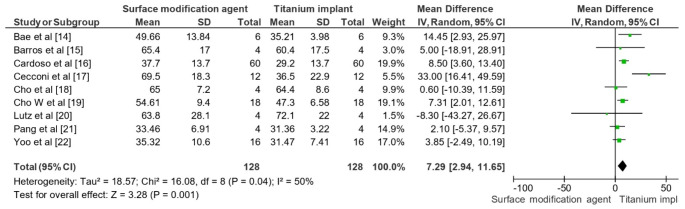
The meta-analysis revealed a statistically significant improvement in BIC for surface-modified implants compared to unmodified titanium implants (Fig. 3). The overall mean difference (MD) was 7.29 with a 95% CI of [2.94, 11.65], indicating that surface modifications positively influenced osseointegration. The heterogeneity analysis showed a Tau² value of 18.57, a Chi² value of 16.08 with 8 degrees of freedom (P = 0.04), and an I² value of 50%, suggesting moderate heterogeneity among the studies. The test for overall effect yielded a Z value of 3.28 (P = 0.001), confirming the significant impact of bioactive surface modifications on enhancing BIC. These findings demonstrated the efficacy of surface modifications in improving the osseointegration of dental implants.
Fig. 3.
Impact of surface modification agent on dental implant in terms of osseointegration (BIC) across a 30-day period
Longevity outcomes assessed
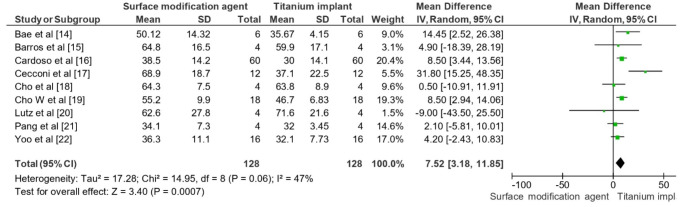
The meta-analysis revealed a statistically significant improvement in the longevity of dental implants with bioactive surface modifications compared to unmodified titanium implants over a 30-day period (Fig. 4). The overall MD was 7.52 with a 95% CI of [3.18, 11.85]. This indicated that surface-modified implants performed better in terms of longevity. The heterogeneity analysis showed a Tau² value of 17.28, a Chi² value of 14.95 with 8 degrees of freedom (P = 0.06), and an I² value of 47%, suggesting moderate heterogeneity among the studies. The test for overall effect yielded a Z value of 3.40 (P = 0.0007), confirming the significant positive impact of bioactive surface modifications on the longevity of dental implants within the studied period.
Fig. 4.
Impact of surface modification agent on dental implant in terms of longevity across a 30-day period
Discussion
Nanostructured coatings such as calcium, calcium phosphate, and HA have been extensively utilized on implants. These coatings can be applied to metal implants through methods like hydrothermal deposition or plasma spraying. These materials release calcium and phosphate ions, which promote mineralization of the interface tissues and facilitate bone healing [23]. Additionally, inorganic coatings influence stress transmission to the bone during masticatory functions, ensuring proper force distribution during repeated cycles [23].
BMPs have been shown to play a crucial role in regulating and promoting osteogenic and bone mesenchymal stem cells, leading to their increased application in dental coatings [24]. The use of recombinant human BMPs (rhBMPs) has been approved by the FDA for therapeutic use in dentistry, with rhBMP2 being commercially available and utilized for bone regeneration in dental implantology [24]. In our review, we explored the use of bioactive surface modifications on dental implants, including BMPs, to enhance implant osseointegration and bone formation. Notably, our findings suggest that BMP-2 combined with HAP can activate osseointegration, as demonstrated by Pang et al. [21]. This is consistent with previous studies that have shown the effectiveness of BMP-2 in promoting bone formation and regeneration [25].
However, our study also highlights the challenges associated with protein form delivery, including dosage challenges and the need for repeated applications [25]. In contrast, gene delivery of rhBMP2 has been shown to facilitate protein synthesis for extended periods, as demonstrated by Yoo et al. [22]. This approach may offer a more promising solution for promoting bone formation and regeneration. When compared to our study, the use of rhBMP2 in dental coatings has been shown to promote excellent osseointegration, as demonstrated by [26, 27]. However, our study suggests that alternative bioactive surface modifications, such as collagen-based coatings, may offer similar or improved outcomes, as demonstrated by Bae et al. [14] and Cho W et al. [19].
Another approach to enhance biocompatibility on implant surfaces involves the accumulation of ECM proteins. During the bone integration growth phase, fibroblast growth factor stimulates fibroblasts to secrete various ECM proteins, such as elastin, chondroitin sulfate, collagen, fibronectin, hyaluronic acid, and other proteoglycans [24]. For example, one study [28] showed that collagen-chondroitin sulfate coatings significantly increased osseointegration by promoting bone formation at the implant–bone interface. Another paper [29] tested mussel adhesive protein, which enhanced osseointegration by promoting the differentiation of bone-forming cells and improving cell adhesion and proliferation. Raphael et al. [30] demonstrated that elastin-like protein coatings on implants in rat tibia and femur reduced micromovements associated with deficient force loads, thereby improving mechanical properties through rapid osseointegration. Additionally, it has also been found that incorporating hyaluronic acid into polyelectrolyte multilayer coatings enhanced osteogenic differentiation of adipose-derived stem cells and increased bone mineral deposition [31, 32].
When comparing these findings to those of Lopez-Valverde et al. [33], both studies emphasized the positive impact of surface modifications on osseointegration, particularly during the early stages of healing. They found BMPs to be the most favorable coating, which aligns with our findings regarding the effectiveness of BMP-2 combinations. However, they noted very high heterogeneity (I² = 99%), indicating more variability in their pooled studies compared to our moderate heterogeneity levels. Meng et al. [34] reviewed biologically active dental implant surfaces and reported that biomolecular coatings, including BMPs, improved peri-implant bone formation and osseointegration during early healing stages. This is consistent with our findings, particularly regarding the positive effects of BMP-2. Both studies called for long-term clinical validation, acknowledging that results from animal studies may not directly translate to human clinical outcomes.
Kligman et al. [35] discussed various implant surface modifications aimed at enhancing osseointegration and reducing biofilm formation. While our study focused on specific bioactive coatings, Kligman et al. [35] provided a broader overview of physical, chemical, and biological techniques. Both studies underscored the importance of modifying implant surfaces to improve clinical outcomes, though Kligman et al. [35] highlighted a wider range of materials and methods beyond our scope. Han et al. [36] summarized the effects of different surface modification methods on osseointegration and biofilm attachment. Their review covered techniques such as plasma spraying and anodic oxidation, which were not the focus of our study. However, both studies shared the goal of improving implant success rates by enhancing osseointegration and minimizing complications such as biofilm formation. They also discussed the mechanical, chemical, and biological disadvantages of various methods, providing a more comprehensive evaluation of surface modification techniques.
Limitations
This study had several limitations that need to be considered when interpreting the findings. Firstly, the heterogeneity among the included studies was moderate, as indicated by the I² values of 50% for bone-to-implant contact (BIC) and 47% for implant longevity. This heterogeneity suggests variability in study designs, surface modification techniques, and animal models, which may have influenced the outcomes. Secondly, the sample sizes in some studies were relatively small, potentially affecting the robustness and generalizability of the results.
Additionally, the follow-up period for assessing implant longevity was limited to 30 days, which may not fully capture the long-term effects of bioactive surface modifications on dental implants. The variations in measurement techniques and reporting standards for BIC and other parameters across studies also posed challenges in standardizing the data for meta-analysis. Moreover, the review included a range of bioactive agents and coating methods, and while this diversity provides a broad overview, it also complicates direct comparisons and specific conclusions about the efficacy of individual modifications.
Future implications and relevance
While our review was based on animal studies, the findings suggest that bioactive surface modifications may be worth exploring further in human studies. The results of our review highlight the need for additional research into the optimal design and application of bioactive surface modifications for dental implants. Future studies can build upon our findings to investigate the safety and efficacy of these modifications in human clinical trials.
Conclusion
Our findings demonstrate that bioactive surface modifications on dental implants significantly improve osseointegration and implant longevity in animal models. Notably, collagen-based coatings consistently promoted early bone integration, while combinations involving BMP-2 were effective in enhancing osseointegration. However, the benefits of bioactive surface modifications varied depending on the specific bioactive agent and coating method used, highlighting the importance of optimizing concentrations and combinations of bioactive agents for achieving optimal outcomes. Despite some heterogeneity among the included studies, the positive impact of bioactive surface modifications on dental implant performance was evident. These findings have important implications for the development of more effective dental implants and underscore the need for further research to translate these findings to human clinical trials.
Author contributions
MS. and SE. conceived of the presented idea. HA, MC, MMM, and GM developed the theory and performed the computations.AS. and MS. verified the analytical methods. SE. encouraged MC, GM, MMM, and MS. to investigate and supervise the findings of this work. All authors discussed and contributed to the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
Nil.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maher AL Shayeb, Email: m.alshayeb@ajman.ac.ae.
Maria Maddalena Marrapodi, Email: mariamaddalena.marrapodi@studenti.unicampania.it.
Giuseppe Minervini, Email: giuseppe.minervini@unicampania.it.
References
- 1.Howe MS, Keys W, Richards D (2019) Long-term (10-year) dental implant survival: a systematic review and sensitivity meta-analysis. J Dent 84:9–21. 10.1016/j.jdent.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 2.Alghamdi HS, Jansen JA (2020) The development and future of dental implants. Dent Mater J 39:167–172 [DOI] [PubMed] [Google Scholar]
- 3.Tsigarida A, Chochlidakis K, Fraser D, Lampraki E, Einarsdottir ER, Barmak AB, Papaspyridakos P, Ercoli C (2020) Peri-implant diseases and Biologic complications at Implant-supported fixed Dental prostheses in partially edentulous patients. J Prosthodont off J Am Coll Prosthodont 29:429–435 [DOI] [PubMed] [Google Scholar]
- 4.Gulati K, Maher S, Findlay DM, Losic D (2016) Titania nanotubes for orchestrating osteogenesis at the bone-implant interface. Nanomed (Lond) 11(14):1847–1864. 10.2217/nnm-2016-0169 [DOI] [PubMed] [Google Scholar]
- 5.Chopra D, Gulati K, Ivanovski S (2021) Understanding and optimizing the antibacterial functions of anodized nano-engineered titanium implants. Acta Biomater 127:80–101. 10.1016/j.actbio.2021.03.027 [DOI] [PubMed] [Google Scholar]
- 6.Silva GA (2004) Introduction to nanotechnology and its applications to medicine. Surg Neurol 61(3):216–220. 10.1016/j.surneu.2003.09.036 [DOI] [PubMed] [Google Scholar]
- 7.Christenson EM, Anseth KS, van den Beucken JJ, Chan CK, Ercan B, Jansen JA, Laurencin CT, Li WJ, Murugan R, Nair LS, Ramakrishna S, Tuan RS, Webster TJ, Mikos AG (2007) Nanobiomaterial applications in orthopedics. J Orthop Res 25(1):11–22. 10.1002/jor.20305 [DOI] [PubMed] [Google Scholar]
- 8.Jimbo R, Coelho PG, Bryington M, Baldassarri M, Tovar N, Currie F, Hayashi M, Janal MN, Andersson M, Ono D, Vandeweghe S, Wennerberg A (2012) Nano hydroxyapatite-coated implants improve bone nanomechanical properties. J Dent Res 91(12):1172–1177. 10.1177/0022034512463240 [DOI] [PubMed] [Google Scholar]
- 9.Scarano A, Khater AGA, Gehrke SA, Inchingolo F, Tari SR (2024) Animal models for investigating osseointegration: an overview of Implant Research over the last three decades. J Funct Biomater 15(4):83. 10.3390/jfb15040083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanc-Sylvestre N, Bouchard P, Chaussain C, Bardet C (2021) Pre-clinical models in Implant Dentistry: past, Present. Future Biomedicines 9(11):1538. 10.3390/biomedicines9111538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coelho PG, Jimbo R, Tovar N, Bonfante EA (2015) Osseointegration: hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent Mater 31(1):37–52. 10.1016/j.dental.2014.10.007Epub 2014 Nov 25. PMID: 25467952 [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160. 10.1136/bmj.n160PMID: 33781993; PMCID: PMC8005925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43. 10.1186/1471-2288-14-43PMID: 24667063; PMCID: PMC4230647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae EB, Yoo JH, Jeong SI, Kim MS, Lim YM, Ahn JJ, Lee JJ, Lee SH, Kim HJ, Huh JB (2018) Effect of Titanium implants coated with Radiation-Crosslinked Collagen on Stability and Osseointegration in Rat Tibia. Mater (Basel) 11(12):2520. 10.3390/ma11122520PMID: 30545019; PMCID: PMC6316992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barros RR, Novaes AB Jr, Papalexiou V, Souza SL, Taba M Jr, Palioto DB, Grisi MF (2009) Effect of biofunctionalized implant surface on osseointegration: a histomorphometric study in dogs. Braz Dent J. ;20(2):91 – 8. 10.1590/s0103-64402009000200001. PMID: 19738939 [DOI] [PubMed]
- 16.Cardoso MV, de Rycker J, Chaudhari A, Coutinho E, Yoshida Y, Van Meerbeek B, Mesquita MF, da Silva WJ, Yoshihara K, Vandamme K, Duyck J (2017) Titanium implant functionalization with phosphate-containing polymers may favour in vivo osseointegration. J Clin Periodontol 44(9):950–960. 10.1111/jcpe.12736Epub 2017 Aug 7. PMID: 28453878 [DOI] [PubMed] [Google Scholar]
- 17.Cecconi S, Mattioli-Belmonte M, Manzotti S, Orciani M, Piccioli A, Gigante A (2014) Bone-derived titanium coating improves in vivo implant osseointegration in an experimental animal model. J Biomed Mater Res B Appl Biomater 102(2):303–310. 10.1002/jbm.b.33008Epub 2013 Aug 30. PMID: 23996785 [DOI] [PubMed] [Google Scholar]
- 18.Cho CB, Jung SY, Park CY, Kang HK, Yeo IL, Min BM (2019) A vitronectin-derived bioactive peptide improves Bone Healing Capacity of SLA Titanium surfaces. Mater (Basel) 12(20):3400. 10.3390/ma12203400PMID: 31627447; PMCID: PMC6829905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho WT, Kim SY, Jung SI, Kang SS, Kim SE, Hwang SH, Jeong CM, Huh JB (2021) Effects of Gamma Radiation-Induced Crosslinking of collagen type I coated Dental Titanium implants on Osseointegration and Bone Regeneration. Mater (Basel) 14(12):3268. 10.3390/ma14123268PMID: 34199187; PMCID: PMC8231814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutz R, Srour S, Nonhoff J, Weisel T, Damien CJ, Schlegel KA (2010) Biofunctionalization of titanium implants with a biomimetic active peptide (P-15) promotes early osseointegration. Clin Oral Implants Res. ;21(7):726 – 34. 10.1111/j.1600-0501.2009.01904.x. PMID: 20636727 [DOI] [PubMed]
- 21.Pang K, Seo YK, Lee JH (2021) Effects of the combination of bone morphogenetic protein-2 and nano-hydroxyapatite on the osseointegration of dental implants. J Korean Assoc Oral Maxillofac Surg 47(6):454–464. 10.5125/jkaoms.2021.47.6.454PMID: 34969019; PMCID: PMC8721409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo SY, Kim SK, Heo SJ, Koak JY, Lee JH, Heo JM (2015) Jul-Aug;30(4):754 – 60 Biochemical Responses of Anodized Titanium Implants with a Poly(lactide-co-glycolide)/Bone Morphogenetic Protein-2 Submicron Particle Coating. Part 2: An In Vivo Study. Int J Oral Maxillofac Implants. 10.11607/jomi.3701b. PMID: 26252026 [DOI] [PubMed]
- 23.Asensio G, Vázquez-Lasa B, Rojo L (2019) Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. ;8:1982. 10.3390/jcm8111982 [DOI] [PMC free article] [PubMed]
- 24.Dong H, Liu H, Zhou N, Li Q, Yang G, Chen L, Mou Y (2020) Surface modified techniques and emerging functional coating of Dental implants. Coatings 10:1012. 10.3390/coatings10111012 [Google Scholar]
- 25.Park S-Y, Kim K-H, Kim S, Lee Y-M, Seol Y-J (2019) BMP-2 gene delivery-based bone regeneration in Dentistry. Pharmaceutics 11:393. 10.3390/pharmaceutics11080393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Jarsha M, Leal-Egana MV, Connell A, Naudi A, Ayoub KB, Dalby A, Salmerón-Sánchez MJ (2018) Engineered Coatings for Titanium Implants to present Ultra-low doses of BMP-7. ACS Biomater Sci Eng 5:1812–1819. 10.1021/acsbiomaterials.7b01037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J-E, Kang S-S, Choi K-H, Shim J-S, Jeong C-M, Shin S-W, Huh J-B (2013) The Effect of Anodized Implants Coated with combined RhBMP-2 and recombinant human vascular endothelial growth factors on Vertical Bone Regeneration in the marginal portion of the Peri-implant. Oral Surg Oral Med Oral Pathol Oral Radiol 115:e24–e31. 10.1016/j.oooo.2011.10.040 [DOI] [PubMed] [Google Scholar]
- 28.Kellesarian SV, Malignaggi VR, Kellesarian TV, Bashir Ahmed H, Javed F (2018) Does incorporating collagen and chondroitin sulfate Matrix in Implant surfaces Enhance Osseointegration? A systematic review and Meta-analysis. Int J Oral Maxillofac Surg 47:241–251. 10.1016/j.ijom.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 29.Yin D, Komasa S, Yoshimine S, Sekino T, Okazaki J (2019) Effect of Mussel Adhesive protein coating on Osteogenesis in Vitro and Osteointegration in Vivo to Alkali-Treated Titanium with Nanonetwork Structures. Int J Nanomed 14:3831–3843. 10.2147/IJN.S206313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raphel J, Karlsson J, Galli S, Wennerberg A, Lindsay C, Haugh MG, Pajarinen J, Goodman SB, Jimbo R, Andersson M et al (2016) Engineered Protein Coatings to improve the osseointegration of Dental and Orthopaedic implants. Biomaterials 83:269–282. 10.1016/j.biomaterials.2015.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabino RM, Mondini G, Kipper MJ, Martins AF, Popat KC (2021) Tanfloc/Heparin Polyelectrolyte Multilayers improve osteogenic differentiation of adipose-derived stem cells on Titania Nanotube surfaces. Carbohydr Polym 251:117079. 10.1016/j.carbpol.2020.117079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Accioni F, Vázquez J, Merinero M, Begines B, Alcudia A (2022) Latest trends in Surface Modification for Dental Implantology: innovative developments and Analytical Applications. Pharmaceutics 14(2):455. 10.3390/pharmaceutics14020455PMID: 35214186; PMCID: PMC8876580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.López-Valverde N, Aragoneses J, López-Valverde A, Quispe-López N, Rodríguez C, Aragoneses JM (2022) Effectiveness of biomolecule-based bioactive surfaces, on os-seointegration of titanium dental implants: a systematic review and meta-analysis of in vivo studies. Front Bioeng Biotechnol 10:986112. 10.3389/fbioe.2022.986112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng HW, Chien EY, Chien HH (2016) Dental implant bioactive surface modifications and their effects on osseointegration: a review. Biomark Res 4:24. 10.1186/s40364-016-0078-zPMID: 27999672; PMCID: PMC5155396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kligman S, Ren Z, Chung C-H, Perillo MA, Chang Y-C, Koo H, Zheng Z, Li C (2021) The impact of Dental Implant Surface modifications on osseointegration and biofilm formation. J Clin Med 10:1641. 10.3390/jcm10081641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han W, Fang S, Zhong Q, Qi S (2022) Influence of Dental Implant Surface modifications on Osseointegration and Biofilm attachment. Coatings 12:1654. 10.3390/coatings12111654 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.