Abstract
Background
Epstein-Barr virus (EBV) infection has been closely linked to the development of various types of cancer. EB nuclear antigen 1 binding protein 2 (EBNA1BP2) is a crucial molecule for stable isolation of EBV in latent infection. However, the role of EBNA1BP2 in multiple tumor types is remains unclear. In this study, we comprehensively analyzed the functional characteristics of EBNA1BP2 and investigate its potential as a prognostic biomarker in pan-cancer.
Methods
We utilized data from TCGA (The Cancer Genome Atlas) and GEO (Gene Expression Omnibus) databases and employed various bioinformatics analysis tools, including TIMER2.0, HPA, GEPIA2.0, PrognoScan, cBioPortal, CancerSEA, and BioGRID to explore the expression pattern, prognostic value, immune infiltration, and methylation level of EBNA1BP2 in pan-cancer. Additionally, we conducted enrichment analysis of genes associated with EBNA1BP2 to identify potential biological functions and pathways.
Results
Our analysis revealed that EBNA1BP2 expression was significantly higher in tumor tissues compared to tumor-adjacent tissues. We observed that lower expression of EBNA1BP2 in adrenocortical carcinoma (ACC), brain lower grade glioma (LGG), sarcoma (SARC), and uterine carcinosarcoma (UCS) was significantly associated with improved overall survival (OS) and disease-free survival (DFS). Furthermore, the promoter methylation level of EBNA1BP2 was downregulated in the majority of cancer types. At the single-cell level, EBNA1BP2 was found to be positively correlated with cell cycle and DNA repair processes, while negatively correlated with hypoxia. Additionally, EBNA1BP2 was associated with the infiltration of immune cells such as B cells, cancer-associated fibroblast cells, and CD8+ T cells. Gene enrichment analysis indicated that EBNA1BP2 was mainly involved in nucleoplasm and RNA binding pathways.
Conclusion
Our findings suggest that EBNA1BP2 may serve as a potential prognostic biomarker for survival in pan-cancer. Further experimental studies are needed to validate these findings and explore the underlying mechanisms by which EBNA1BP2 contributes to tumorigenesis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-024-01326-0.
Keywords: EBNA1BP2, Prognosis, Pan-cancer, Biomarker
Introduction
Tumors are a highly prevalent and lethal disease characterized by multifactorial involvement and complex developmental stages [1]. They are also a leading cause of death globally [2]. With advancements in research, a plethora of publicly funded databases have emerged, focusing on genomics, epigenomics, transcriptomics, proteomics, and other tumor-related fields, such as The Cancer Genome Atlas (TCGA) [3]and Gene Expression Omnibus (GEO) [4]. These resources are instrumental in identifying similarities and differences across various cancer types, and in discovering new therapeutic targets and biomarkers. Additionally, research into biological pathways and immune cell infiltration lays the groundwork for advancing treatments for malignant tumors. Consequently, analyzing the expression, pathways, prognosis, and immune infiltration of genes of interest across multiple cancer types is essential for assessing their clinical relevance.
EB nuclear antigen 1 binding protein 2 (EBNA1BP2) is a conserved eukaryotic homolog of Saccharomyces cerevisiae and is predominantly localized within the nucleolus. It has been shown to play a significant role in pre-ribosomal RNA processing, ribosomal subunit assembly, and cellular growth [5, 6]. In addition to regulating the binding of EB virus (EBV) to chromosomes, EBNA1BP2 can also regulate cellular processes such as the cell cycle and induce chromosomal instability, ultimately leading to carcinogenesis [7]. Previous study have demonstrated that EBNA1BP2 promotes proliferation in anaplastic large-cell lymphoma (ALCL) cells by regulating the tumor suppressor p53 [8]. However, there is currently a lack of research on the role and underlying mechanisms of EBNA1BP2 in multiple types of tumors.
In this study, we employed a comprehensive approach using various bioinformatics methods to analyze the expression profiles and survival outcomes associated with EBNA1BP2. Our aim was to further investigate the potential mechanisms underlying EBNA1BP2 in human tumorigenesis and identify a potential prognostic biomarker for survival.
Materials and methods
Gene expression analysis of EBNA1BP2
We utilized the "Gene DE" plate of TIMER2.0 (Tumor Immune Estimation Resource, version 2, http://timer.cistrome.org) to analyze the expression level of EBNA1BP2 in different tumor types compared to adjacent normal samples [9]. To further investigate the relationship between EBNA1BP2 expression and patients' pathological stages across all TCGA cancers, we generated box plots of complementary gene expression and performed analysis using the GEPIA2.0 (Gene Expression Profiling Interactive Analysis, version 2, http://gepia2.cancer-pku.cn/#analysis) tool [10]. Moreover, we examined the total protein expression of EBNA1BP2 in pan-cancers using the "Clinical Proteomic Tumor Analysis Consortium (CPTAC)" module of UALCAN (The University of Alabama at Birmingham CANcer data analysis Portal, http://ualcan.path.uab.edu/analysis-prot.html) [11, 12]. Additionally, we obtained promoter methylation levels of EBNA1BP2 in all cancers using this tool.
Immunohistochemistry staining
To verify the expression of EBNA1BP2 at the protein level, we utilized the HPA (Human Protein Atlas, http://www.proteinatlas.org/) tool to download immunohistochemistry (IHC) images and compare the protein expression level [13].
Survival prognosis analysis
We obtained Kaplan–Meier plots for overall survival and disease-free survival of EBNA1BP2 expression in all tumor types using the "Survival Analysis" module of GEPIA2.0. TCGA tumor patients were divided into high and low EBNA1BP2 expression groups using a cut-off value of 50%. Comparisons between these groups were performed using the log-rank test. Additionally, we utilized the Kaplan–Meier plotter tool (https://kmplot.com/analysis/) to obtain Kaplan–Meier survival curves of EBNA1BP2 in pan-cancer analysis [14].
Genetic alteration analysis
“Cancer Types Summary” module of cBioPortal (The cBio Cancer Genomics Portal) tool (https://www.cbioportal.org/) was used to acquire the alteration frequency, mutation type, mutated site information of the EBNA1BP2 protein across all TCGA tumors. Additionally, we obtained the three-dimensional (3D) structure of the protein from the "Mutations" module [15]. The "Comparison/Survival" module of cBioPortal can also be used to obtain information on the progression-free survival (PFS), disease-specific survival (DSS), disease-free survival (DFS), and overall survival (OS) of EBNA1BP2 gene mutations in various cancers.
Immune cells infiltration analysis
The correlation analysis between EBNA1BP2 expression in all tumor types and immune infiltration cells using the “Immune” module of TIMER2.0, combining various algorithms such as TIMER, EPIC, TIDE, QUANTISEQ, CIBERSORT, CIBERSORT-ABS, XCELL, MCPCOUNTER [16].
Single cell sequencing
To investigate the functional status of cancer cells at the single-cell level and explore the relationship between EBNA1BP2 expression and cancer cell function in different tumors, we utilized the CancerSEA tool (Atlas of Cancer Single Cell Status, CancerSEA-Database Commons.mhtml). Additionally, we generated t-SNE plots to visualize the EBNA1BP2 expression profiles in TCGA tumors, allowing us to observe the clustering patterns and distribution of EBNA1BP2 expression across different tumor samples [17].
Gene enrichment analysis
Protein–protein interactions network was obtained from BioGRID (Biological General Repository for Interaction Datasets, https://thebiogrid.org/). To identify genes that are correlated with EBNA1BP2 expression, we used the "Similar Gene Detection" module of GEPIA2.0 to obtain the top 100 EBNA1BP2-correlated genes from the datasets of all TCGA tumor and normal tissues [18]. We then performed pairwise gene–gene Pearson correlation analysis between EBNA1BP2 and the selected genes using the "Correlation Analysis" module of GEPIA2.0. Raw data were downloaded from the DAVID website (https://david.ncifcrf.gov), and we conducted Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses to explore the potential biological functions and signaling pathways associated with EBNA1BP2 in TCGA tumors [19].
Tissue specimen collection
Nine LUAD cases along with their paired normal lung tissue samples were collected from patients who underwent lung cancer resection surgery at the Guangdong Second Provincial General Hospital in 2023. The sample was fixed with 10% neutral formalin and embedded in paraffin for further analysis. This study has been approved by ethnic committee of Guangdong Second Provincial General Hospital (2022-KZ-295). All procedures were strictly in accordance with the appropriate version of the Declaration of Helsinki (as revised in Brazil 2013). Informed consent were obtained from each participant.
DNA extraction and real-time PCR
The total DNA of LUAD tissue and normal lung tissue was extracted with a FFPE DNA extraction kit (TianGen Biochemistry, Beijing). The real-time PCR (RT-PCR) was tested with a TaqMan probe method (ABI QuantStudio 6, Applied Biosystems, CA). The reaction was performed in a 96-well plate. Each well contained FAM-labeled probes for EBNA1BP2 and Cy5-labeled probe for reference gene GAPDH. The total volume of 10 μL of included 10 ng of tissue DNA, 7.5 μL of mix, and 0.64 μL of primer. The primer sequence was mentioned as below: EBNA1BP2 (F) 5′-3′: CGAAGCGACCCACTGATTAT and (R) 5′-3′: TCCATGGCAGCCTGTTTAG. GAPDH (F) 5′–3′: TAGGCAGCAGCAAGCATTCC and (R) 5′–3′: ACGAAGCCCTTCCAGGAGAA. EBNA1BP2 5′–3′probe: FAM-AGATGCAGAAGATTCGACAGAAGCTGC-BHQ1. GAPDH5′–3′probe: Cyc-TTGTGCCCAGACTGTGGGTGGCAGT-BHQ3. Cycling conditions included one cycle at 95 °C for 5 min, 20 cycles at 95 °C for 15 s, 64 °C for 30 s, and 40 cycles at 60 °C for 10 s. The relative expression levels of EBNA1BP2 are calculated with the 2−△△ct method.
Results
Gene expression analysis of EBNA1BP2
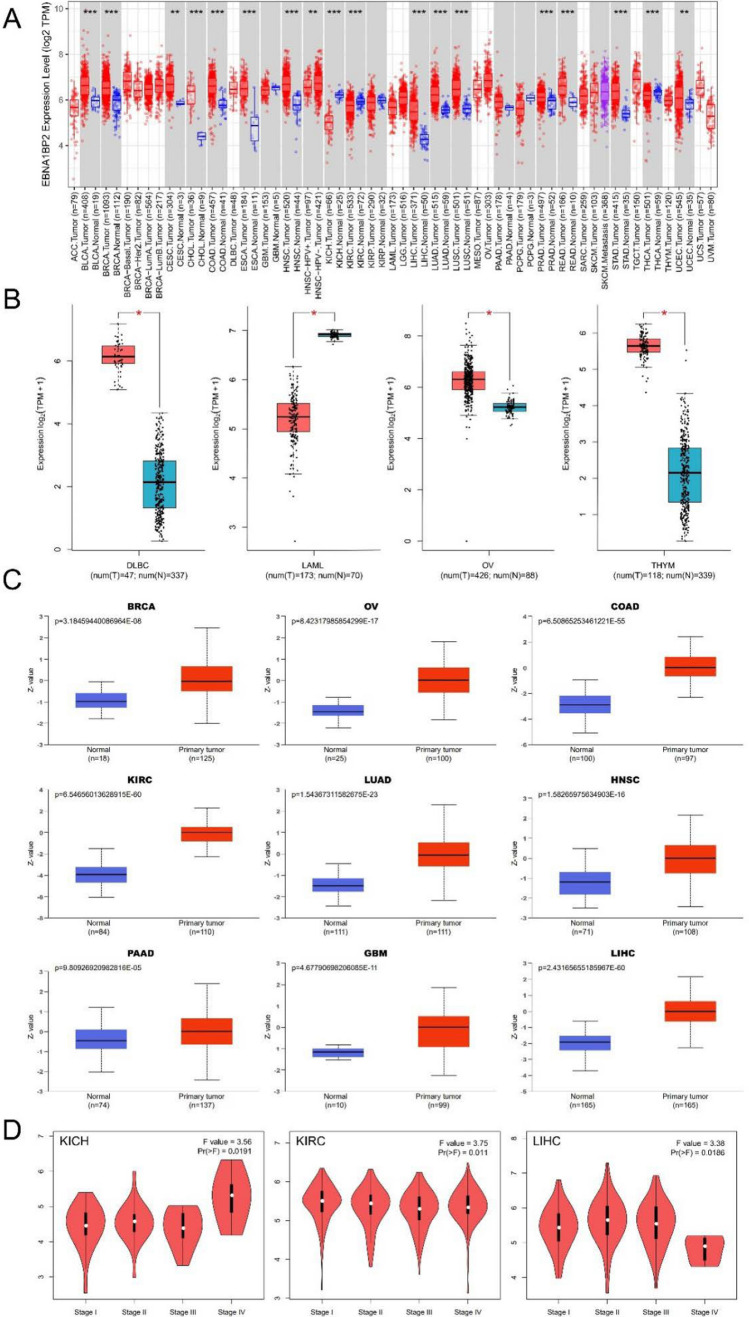
In our analysis using the TIMER2.0 database, we observed that the expression level of EBNA1BP2 was generally higher in tumor samples compared to adjacent normal tissues in most of cancer types. Specifically, we found higher expression levels of EBNA1BP2 in bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), esophageal carcinoma (ESCA), head and neck squamous cell carcinoma (HNSC), kidney renal clear cell carcinoma (KIRC), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), prostate adenocarcinoma (PRAD), rectum adenocarcinoma (READ), stomach adenocarcinoma (STAD), thyroid carcinoma (THCA), and uterine corpus endometrial carcinoma (UCEC) (Fig. 1A). However, in kidney chromophobe (KICH), we observed the opposite trend, with lower expression of EBNA1BP2 in tumor samples compared to normal tissues. To further validate our findings, we combined data from both TCGA and GTEx databases. In this analysis (Fig. 1B), we found higher expression of EBNA1BP2 in diffuse large B-cell lymphoma (DLBC), ovarian serous cystadenocarcinoma (OV), and thymoma (THYM) tumors compared to adjacent normal controls. In contrast, no significant differential expression was observed in adrenocortical carcinoma (ACC), brain lower grade glioma (LGG), sarcoma (SARC), and uterine carcinosarcoma (UCS) tumors (Supplementary Figure S1). Overall, our analysis indicates that EBNA1BP2 is highly expressed in most tumor types, suggesting its potential role in cancer development and progression.
Fig. 1.
Expression and protein levels of EBNA1BP2 in pan-cancer. A Expression level of EBNA1BP2 in human tumors from TIMER2.0. B Box plot representation the expression level of EBNA1BP2 in DLBC, LAML, OV and THYM from GTEx and TCGA database. C Total protein level of EBNA1BP2 in BRCA, OV, COAD, KIRC, LUAD, HNSC, PAAD, GBM and LIHC were analyzed using CPTAC. D GEPIA2.0 was used to compare EBNA1BP2 expression levels in different pathological stages of KICH, KIRC and LIHC. *p < 0.05; **p < 0.01; ***p < 0.001. EBNA1BP2: EB nuclear antigen 1 binding protein 2; GTEx: Genotype-Tissue Expression; TCGA: The Cancer Genome Atlas; CPTAC: Clinical Proteomic Tumor Analysis Consortium; DLBC: lymphoid neoplasm diffuse large B-cell lymphoma; LAML: acute myeloid leukemia; OV: ovarian serous cystadenocarcinoma; THYM: thymoma; BRCA: breast invasive carcinoma; COAD: colon adenocarcinoma; KIRC, kidney renal clear cell carcinoma; LUAD: lung adenocarcinoma; HNSC: head and neck squamous cell carcinoma; PAAD: pancreatic cancer; GBM: polymorphous glioblastoma; LIHC, liver hepatocellular carcinoma; KICH, kidney chromophobe
To analyze EBNA1BP2 expression at the protein level, we utilized the National Cancer Institute's CPTAC tool. Our analysis revealed that the total protein expression of EBNA1BP2 significantly increased in BRCA, OV, COAD, KIRC, LUAD, HNSC, pancreatic adenocarcinoma (PAAD), polymorphous glioblastoma (GBM), and LIHC (Fig. 1C). Furthermore, we examined the relationship between EBNA1BP2 expression and clinical tumor pathological stages using the GEPIA2.0 tool. Our analysis indicated that the expression level of EBNA1BP2 in KICH, KIRC, and LIHC showed significant differences across different pathological stages (Fig. 1D; Supplementary Figure S2).
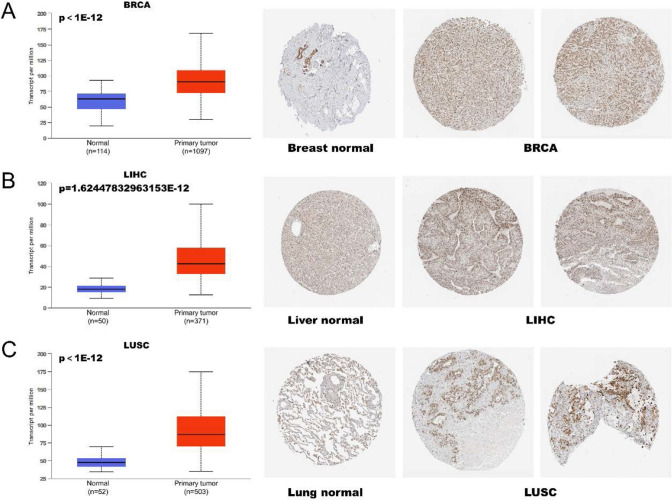
In order to further validate the expression of EBNA1BP2, IHC results of the tumors were acquired using the HPA database. The IHC staining of EBNA1BP2 was found to be moderately or strongly expressed in BRCA, LICH, and LUSC tumors. This analysis was consistent with previous EBNA1BP2 gene expression results from the TCGA database (Fig. 2A–C).
Fig. 2.
Comparison of EBNA1BP2 expression in normal tissue and tumor tissue. A–C The expression level of EBNA1BP2 obtained by UALCAN and the corresponding immunohistochemical images obtained by HAP platform both showed up-regulated expression of EBNA1BP2 in breast, liver and lung derived tumor tissues. UALCAN: The University of ALabama at Birmingham CANcer data analysis Portal; HPA: Human Protein Atlas
Survival analysis data
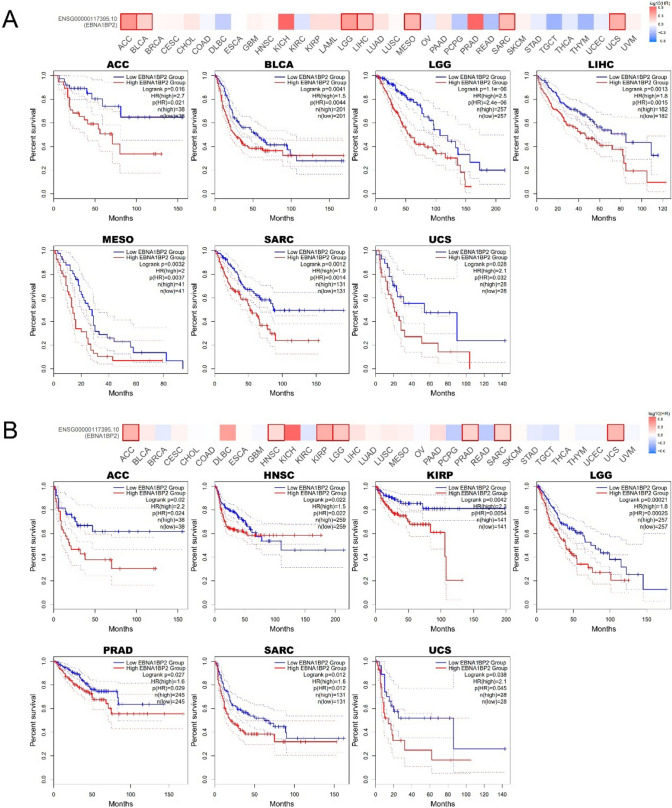
Next, we explored the relationship between the expression of EBNA1BP2 and the prognosis of survival. The result of GEPIA2.0 showed that low levels of EBNA1BP2 expression in ACC, BLCA, LGG, LIHC, MESO, SARC, UCS were significantly associated with longer OS (Fig. 3A). In ACC, HNSC, KIRP, LGG, PRAD, SARC and UCS, low EBNA1BP2 expression was also associated with better DFS (Fig. 3B).
Fig. 3.
The relationship between the expression level of EBNA1BP2 and prognosis of pan-cancer. A, B Survival charts and Kaplan–Meier curves show the relationship between the expression level of EBNA1BP2 and OS (A) and DFS (B) in human tumors were obtained by GEPIA2.0. OS: overall survival; DFS: disease-free survival; GEPIA2.0: Gene Expression Profiling Interactive Analysis, version 2
As shown in Supplementary Figure S3, our analysis revealed that lower expression of EBNA1BP2 in BLCA, LIHC, LUAD, SARC, and UCEC was associated with improved OS. However, the results were contrary in KIRC, OV, PCPG, STAD, and THCA. Specifically, the combined analysis of GEPIA2.0 and Kaplan–Meier plotter tools showed that low EBNA1BP2 expression was significantly associated with longer OS in BLCA, LIHC, and SARC. These findings were primarily derived from the TCGA database. Additionally, we further assessed the OS for EBNA1BP2 using the PrognoScan website, which mainly extracted data from the GEO database. The results demonstrated that EBNA1BP2 expression was significantly associated with bladder, brain, and lung cancers, and surprisingly, low EBNA1BP2 expression was also related to better OS (Supplementary Figure S4A-C). These findings suggest that EBNA1BP2 may serve as a potential prognostic marker for a wide variety of cancer types.
Genetic alteration landscape analysis
The EBNA1BP2 gene mutation has been shown to affect cellular functions [20]. To analyze the mutation status of the EBNA1BP2 gene in various types of tumors, we utilized the cBioPortal platform. The results revealed that in ovarian cancer (OV), the primary type of alteration was "amplification," with the highest frequency of EBNA1BP2 alteration (> 5%). UCEC had the highest incidence of "mutation," with a frequency of approximately 2% of cases (Fig. 4A). Figure 4B illustrates other mutations and their locations within the EBNA1BP2 gene. We observed that missense mutations were the most common type of mutation in EBNA1BP2. Among these, the G291 = /K291N/X291_splice mutation was the most frequently mutated region of the EBNA1BP2 protein. Figure 4C displays the gene site visualized in the 3D structure of the EBNA1BP2 protein. Furthermore, we analyzed the potential association between EBNA1BP2 gene alterations and pan-cancer survival prognosis. However, we found that alterations in the EBNA1BP2 gene did not significantly impact patient prognosis (Supplementary Figure S5). Further verification may require additional patient clinical data in the future.
Fig. 4.
EBNA1BP2 gene mutation in different cancers. The altered frequencies of different mutation types (A) and mutation sites (B) in pan-cancer were shown using cBioPortal. C The 3D protein structure of EBNA1BP2 was shown. cBioPortal, The cBio Cancer Genomics Portal
Methylation level analysis
Abnormal methylation of gene promoter regions is frequently implicated as a contributing factor in carcinogenesis [21]. To investigate the methylation levels of the EBNA1BP2 gene in tumor and normal tissues, we utilized the UALCAN tool. The results demonstrated that the promoter methylation of the EBNA1BP2 gene was significantly higher in normal tissues compared to tumor tissues. However, in KIRC and KIRP, the methylation of the EBNA1BP2 promoter was significantly increased (Fig. 5; Supplementary Figure S6). These findings suggest that altered promoter methylation may play a role in the transcriptional expression of EBNA1BP2.
Fig. 5.
Comparison of differences in promoter methylation levels of EBNA1BP2 in cancer. EBNA1BP2 methylation values of normal tissues and primary tumor tissues were analyzed by UALCAN tool
Immune infiltration analysis data
Studies have demonstrated that immune cell infiltration is closely associated with various tumor behaviors, including tumor occurrence and development [22, 23]. Therefore, we utilized multiple algorithms such as TIMER, EPIC, QUANTISEQ, XCELL, MCPCOUNTER, CIBERSORT, CIBERSORT-ABS, and TIDE to investigate the correlation between EBNA1BP2 expression and immune cell infiltration in pan-cancer. In LUSC, we observed an inverse correlation between EBNA1BP2 expression and B-cell infiltration (Fig. 6A). Additionally, in BRCA, STAD, TGCT, and THCA, there was an inverse correlation between cancer-associated fibroblast infiltration and EBNA1BP2 expression (Fig. 6B). In UVM, EBNA1BP2 expression was positively correlated with CD8+ T cell infiltration (Fig. 6C). Furthermore, we found that EBNA1BP2 expression was positively correlated with neutrophils in KIRC and negatively correlated with monocytes in THCA (Supplementary Figure S7; Supplementary Figure S8A-E). These findings suggest that EBNA1BP2 may have potential as a tumor immune-related biomarker in the future.
Fig. 6.
The correlation between EBNA1BP2 expression level and immune cells in tumors. A–C TIMER2.0 database was used to analyze the relationship between EBNA1BP2 expression and immune infiltration of B cells (A), cancer-associated fibroblasts (B) and T cells CD8+ (C). TIMER, EPIC, TIDE, QUANTISEQ, CIBERSORT, CIBERSORT-ABS, XCELL, MCPCOUNTER and other algorithms are used for analysis. Red for positive correlation (0–1) and blue negative correlation (− 1 to 0). A correlation of p < 0.05 was considered statistically significant. A cross indicates that the correlation is not significant. TIMER2.0: Tumor Immune Estimation Resource, version 2
Single cell sequencing data analysis
To further validate the potential functions of the candidate genes at the level of the single cell, we used the CancerSEA tool to investigate the correlation of EBNA1BP2 gene expression with the function of cancer cells in pan-cancer. EBNA1BP2 expression was positively correlated with angiogenesis, differentiation, and inflammation in retinoblastoma (RB). Conversely, it was inversely correlated with cell cycle, DNA damage response, and DNA repair response. In uveal melanoma (UM), EBNA1BP2 expression showed negative correlations with almost all tumor biological behaviors, including apoptosis, DNA repair response, invasion, and metastasis (Fig. 7A). Furthermore, Fig. 7B shows significant correlations between EBNA1BP2 expression and angiogenesis differentiation and DNA repair in RB, DNA repair and DNA damage in UM, and quiescence in LUAD. And then, EBNA1BP2 single-cell expression profile of RB, UM and LUAD by T-SNE plots is also shown in Fig. 7C.
Fig. 7.
EBNA1BP2 gene expression at the single-cell level. A, B Using CancerSEA tools analysis EBNA1BP2 expression and tumor in the relationship between different functional status. C The single-cell expression profile of EBNA1BP2 in RB, UM and LUAD was shown by T-SNE plot. *p < 0.05; **p < 0.01; ***p < 0.001. RB: retinoblastoma; UM: uveal melanoma; LUAD: lung adenocarcinoma
Gene enrichment analysis
Finally, we combined all of the tumor expression data from the TCGA from the GEPIA2.0 tool to obtain the top 100 genes associated with the expression of EBNA1BP2 (Supplementary Table S1). Subsequently, functional enrichment analysis was performed to evaluate these potential mechanisms. Using the BioGRID network tool, we identified 11 molecules that interact with EBNA1BP2 (Fig. 8A). Notably, EBNA1BP2 expression showed a high correlation with Homo sapiens peptidylprolyl isomerase H (PPIH), diphthamide biosynthesis 2 (DPH2), Homo sapiens Y-box in most cancers binding protein 1 (YBX1), Homo sapiens MRT4 homolog, ribosome maturation factor (MRTO4), Homo sapiens mediator complex subunit 8 (MED8), and Homo sapiens cell division cycle 20 (CDC20) (Fig. 8B, C). Furthermore, GO and KEGG enrichment analysis revealed that the genes associated with EBNA1BP2 expression were primarily related to the nucleoplasm and RNA binding pathways (Fig. 8D). These findings suggest that EBNA1BP2 may play a role in tumorigenesis and development through these pathways.
Fig. 8.
Functional enrichment and pathway analysis of EBNA1BP2-related genes. A EBNA1BP2 related gene is available by the BioGRID website and 11 proteins have been shown. B The top six genes most related to EBNA1BP2 were obtained using GEPIA2.0, which were PPIH, DPH2, YBX1, MRTO4, MED8 and CDC20. p-value < 0.001. C Heat map results confirmed EBNA1BP2 gene expression and six (PPIH DPH2, YBX1, MRTO4, MED8 and CDC20) in pan-carcinoma were positively correlated. D GO and KEGG enrichment analysis of EBNA1BP2-related genes. BioGRID: Biological General Repository for Interaction Datasets; PPIH: Homo sapiens peptidylprolyl isomerase H; DPH2: diphthamide biosynthesis 2; YBX1: Homo sapiens Y-box in most cancers binding protein 1; MRTO4: Homo sapiens MRT4 homolog, ribosome maturation factor; MED8: Homo sapiens mediator complex subunit 8; CDC20: Homo sapiens cell division cycle 20; GO: Gene Ontology; KEGG: Kyoto Encyclopedia of Genes and Genomes
The expression of genes in cancer and normal tissue
In addition, we detected the expression of EBNA1BP2 in clinical samples of LUAD and normal lung tissue using RT-PCR method. The results showed that the expression of the EBNA1BP2 was upregulated in lung cancer tissues compared to normal tissues (Supplementary Figure S9).
Discussion
Traditional research suggests that EBNA1BP2 primarily functions in EBV latent infections [24]. However, its expression and specific functions in malignant tumors are still not well understood. In this study, we conducted a comprehensive analysis of the EBNA1BP2 gene in 33 different types of tumors. Our findings revealed a significant upregulation of EBNA1BP2 expression in various tumor tissues, including BLCA, BRCA, CESC, COAD, ESCA, and HNSC. Survival analysis demonstrated that low expression of EBNA1BP2 was associated with better survival outcomes in certain tumors such as ACC, BLCA, LGG, LIHC, and others. These findings suggest that EBNA1BP2 has the potential to serve as a biomarker for predicting the prognosis of cancer patients. However, further studies are needed to validate the prognostic value of EBNA1BP2 in different types of cancer.
EBV is the first human tumorigenic virus identified to establish lifelong asymptomatic persistence and is associated with a wide range of diseases, including benign disease, lymphoid malignancies, and epithelial cancers. Through various EBV coding genes, EBV can establish latent infection in host cells, which contributes to the development of related diseases. Among these genes, EBNA1 is the only virus-encoded gene expressed in all EBV-associated tumors [25, 26]. Previous studies have shown that EBNA1BP2 interacts with EBNA1, leading us to hypothesize that EBNA1BP2 is also closely involved in tumorigenesis [27]. Furthermore, previous research has demonstrated that EBNA1BP2 is expressed ubiquitously in human tissues and is highly expressed in Burkitt's lymphoma, melanoma, lung cancer, and colorectal cancer [28]. Another study shows that EBNA1BP2 is overexpressed in most tumors, but not in kidney tumors [29]. In our study, we not only found that EBNA1BP2 has a similar expression pattern in lung cancer and colorectal cancer based on public databases, but also observed differential expression of EBNA1BP2 in various tumors compared to normal tissues, such as BRCA, KIRC, LIHC, and others. These findings strongly indicate that the expression of EBNA1BP2 plays a crucial role in tumor incidence and development.
EBNA1BP2 can also serve as a prognostic predictor for some malignant tumors. Studies have shown that EBNA1BP2 is associated with a poorer prognosis in high-grade bladder cancer [30]. According to the TCGA database in GEPIA2.0, low expression level of EBNA1BP2 was significantly correlated with the increase of overall survival in ACC, BLCA, LGG, LIHC, MESO, SARC and UCS. Similarly, EBNA1BP2 low expression also showed statistically significant differences with increased disease-free survival in ACC, HNSC, KIRP, LGG, PRAD, SARC and UCS. We also obtained from Kaplan–Meier plotter data that low expression of EBNA1BP2 in BLCA, LIHC, LUAD, SARC, and UCEC was associated with overall survival improvement. Overall, the combined analysis of GEPIA2.0 and Kaplan–Meier plotter data showed that low EBNA1BP2 expression was significantly associated with longer overall survival in BLCA, LIHC, and SARC. In addition to TCGA database, we also used PrognoScan based on GEO database to obtain the prognostic relationship of EBNA1BP2 expression in tumors. The results showed that EBNA1BP2 expression was significantly associated with bladder, brain, and lung cancer, and we also found that low EBNA1BP2 expression was significantly associated with increased OS.
Immune cell infiltration plays a crucial role in the malignant behavior of tumors and the immune microenvironment [31]. Li et al. discovered that the proportion of plasma cells, CD4 activated memory T cells, and follicular helper T cells increased as the expression of EBNA1BP2 elevated in COVID-19 samples [32]. Another study demonstrated that the expression of EBNA1BP2 was positively correlated with mast cells and negatively correlated with B cells in vitiligo [33]. These studies indirectly suggest a relationship between EBNA1BP2 expression and lymphocyte infiltration, but there is a lack of research on tumor cells. In our study, we observed a positive correlation between EBNA1BP2 expression and infiltration of B cells, cancer-associated fibroblasts, and CD8+ T cells in several tumor types. Our results indicated the underlying effect of EBNA1BP2 in tumor immunity and it may be an effective target for immunotherapy and provide new hope for treating cancer patients in the clinic.
EBNA1BP2 is a highly conserved protein in eukaryotes and is predicted to form coiled-coil interactions and it is contributed to the biogenesis of human ribosomes because it was identified in the nucleolus of HeLa cells [34, 35]. Studies suggest that EBNA1BP2 is a novel binding partner of c-Myc, which regulates nucleolar c-Myc function, cell proliferation, and tumorigenesis through positive feedback [9, 36]. However, the specific function of EBNA1BP2 in tumorigenesis remains unclear. Previous research has reported that EBNA1BP2 interacts with nucleophosmin‐anaplastic lymphoma kinase (NPM-ALK) in the nucleolus and promotes the proliferation of anaplastic large‐cell lymphoma (ALCL) cells. Knockdown of EBNA1BP2 has been shown to activate the tumor suppressor p53 [10]. Additionally, EBNA1BP2 has been found to destabilize p53 by inhibiting its interaction with HAUSP/USP7, a deubiquitination enzyme for p53 [37, 38]. However, the detailed roles and underlying mechanisms of EBNA1BP2 in human tumors require further exploration. In this study, we performed gene enrichment analysis and found that the genes expressed by EBNA1BP2 were mainly associated with nucleoplasmic and RNA binding pathways. We also investigated the correlation between EBNA1BP2 gene expression, methylation levels, and mutation frequency in pan-cancer. These results will provide a foundation for understanding the role and molecular mechanisms of EBNA1BP2 in malignant tumors.
In conclusion, our comprehensive bioinformatics analysis using various tools and databases explored the expression level, clinical prognosis, genetic mutations, methylation levels, immune cell infiltration, and pathway mechanisms of EBNA1BP2 in pan-cancers. Our findings suggest that EBNA1BP2 may serve as a potential novel biomarker associated with the prognosis of cancer patients. Furthermore, this study lays the foundation for further investigation into the mechanisms underlying the occurrence, development, and treatment of pan-cancer involving EBNA1BP2.
Limitation
Despite our integration of information from multiple website tool, this research remain has limitations. Firstly, this study relies on bioinformatics analysis and lacks validation of expression and prognosis using clinical samples from various cancer types. Second, we lack in vitro/in vivo experiments to further verify the mechanism of EBNA1BP2.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
All authors are grateful for the GEO and TCGA databases to providing public data and all the bioinformatics tools used to analyze data.
Author contributions
LS, and YJ took part in article conception and design. QX, KX and XX acquired the data. XZ and JS analyzed and interpreted the data. LS, and YJ wrote the manuscript. LL and XZ supervised the whole research. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by National Natural Science Foundation of China (No. 82302640), the Guangzhou Science and Technology Plan Project (Grant Nos. 202002030404 & 2023A04J1129), the Foundation of Guangdong Second Provincial General Hospital (Grant No. 3DA2021015), Doctoral Workstation Foundation of Guangdong Second Provincial General Hospital (Grant No. 2021BSGZ018), the science foundation of Guangdong Second Provincial General Hospital (Grant No. TJGC-2021007), Guangdong Medical Scientific Research (Grant No. B2023038), and Natural Science Foundation of Guangdong Province (Grant No. 2021A1515012329).
Data availability
Publicly datasets were analyzed in this study can be found in online. This date can be found in the article and supplementary material.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li-Yue Sun, Yu-Ying Jiang and Xin-Xin Zeng contributed equally to this work.
Contributor Information
Lei Liang, Email: leiliang@jnu.edu.cn.
Xu-Hui Zhang, Email: 9158863@qq.com.
References
- 1.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45:1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrell PJ. Epstein-Barr virus and cancer. Annu Rev Pathol. 2019;14:29–53. 10.1146/annurev-pathmechdis-012418-013023. [DOI] [PubMed] [Google Scholar]
- 5.Huber MD, Dworet JH, Shire K, Frappier L, McAlear MA. The budding yeast homolog of the human EBNA1-binding protein 2 (Ebp2p) is an essential nucleolar protein required for pre-rRNA processing. J Biol Chem. 2000;275(37):28764–73. 10.1074/jbc.M000594200. [DOI] [PubMed] [Google Scholar]
- 6.Tsujii R, Miyoshi K, Tsuno A, et al. Ebp2p, yeast homologue of a human protein that interacts with Epstein-Barr virus nuclear antigen 1, is required for pre-rRNA processing and ribosomal subunit assembly. Genes Cells. 2000;5(7):543–53. 10.1046/j.1365-2443.2000.00346.x. [DOI] [PubMed] [Google Scholar]
- 7.Liao P, Wang W, Shen M, et al. A positive feedback loop between EBP2 and c-Myc regulates rDNA transcription, cell proliferation, and tumorigenesis. Cell Death Dis. 2014;5(1): e1032. 10.1038/cddis.2013.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchihara Y, Tago K, Tamura H, Funakoshi-Tago M. EBP2, a novel NPM-ALK-interacting protein in the nucleolus, contributes to the proliferation of ALCL cells by regulating tumor suppressor p53. Mol Oncol. 2021;15(1):167–94. 10.1002/1878-0261.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–14. 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–102. 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandrashekar DS, Karthikeyan SK, Korla PK, et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27. 10.1016/j.neo.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–58. 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Luo D, Zhong N, et al. GPC2 is a potential diagnostic, immunological, and prognostic biomarker in pan-cancer. Front Immunol. 2022;13: 857308. 10.3389/fimmu.2022.85730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei Y, Yu T, Li C, et al. Expression of CAMK1 and its association with clinicopathologic characteristics in pancreatic cancer. J Cell Mol Med. 2021;25(2):1198–206. 10.1111/jcmm.16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–4. 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Luo G, Yan Y, Peng J. A pan-cancer analysis of copper homeostasis-related gene lipoyltransferase 1: its potential biological functions and prognosis values. Front Genet. 2022;13:1038174. 10.3389/fgene.2022.1038174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan H, Yan M, Zhang G, et al. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 2019;47(D1):D900–8. 10.1093/nar/gky939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oughtred R, Rust J, Chang C, et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021;30(1):187–200. 10.1002/pro.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587–92. 10.1093/nar/gkac963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okano A, Wan K, Kanda K, Yabuki Y, Funato K, Mizuta K. SMY2 and SYH1 suppress defects in ribosome biogenesis caused by ebp2 mutations. Biosci Biotechnol Biochem. 2015;79(9):1481–3. 10.1080/09168451.2015.1031077. [DOI] [PubMed] [Google Scholar]
- 21.Chen XY, Zhang J, Zhu JS. The role of m6A RNA methylation in human cancer. Mol Cancer. 2019;18(1):103. 10.1186/s12943-019-1033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren L, Yi J, Yang Y, et al. Systematic pan-cancer analysis identifies APOC1 as an immunological biomarker which regulates macrophage polarization and promotes tumor metastasis. Pharmacol Res. 2022;183: 106376. 10.1016/j.phrs.2022.106376. [DOI] [PubMed] [Google Scholar]
- 23.Dieci MV, Miglietta F, Guarneri V. Immune infiltrates in breast cancer: recent updates and clinical implications. Cells. 2021;10(2):223. 10.3390/cells10020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong Y, Meehan MT, Burrows SR, Doolan DL, Miles JJ. Estimating the global burden of Epstein-Barr virus-related cancers. J Cancer Res Clin Oncol. 2022;148(1):31–46. 10.1007/s00432-021-03824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu H, Robertson ES. Epstein-Barr virus history and pathogenesis. Viruses. 2023;15(3):714. 10.3390/v15030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odumade OA, Hogquist KA, Balfour HH Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev. 2011;24(1):193–209. 10.1128/CMR.00044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shire K, Ceccarelli DF, Avolio-Hunter TM, Frappier L. EBP2, a human protein that interacts with sequences of the Epstein-Barr virus nuclear antigen 1 important for plasmid maintenance. J Virol. 1999;73(4):2587–95. 10.1128/JVI.73.4.2587-2595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henning D, Valdez BC. Expression of p40/Epstein-Barr virus nuclear antigen 1 binding protein 2. Biochem Biophys Res Commun. 2001;283(2):430–6. 10.1006/bbrc.2001.4780. [DOI] [PubMed] [Google Scholar]
- 29.Pilarsky C, Wenzig M, Specht T, Saeger HD, Grützmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6(6):744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang ZG, Shi ZD, Dong JJ, Chen YA, Cao MY, Li YT, Ma WM, Hao L, Pang K, Zhou JH, Zhang WD, Dong Y, Han CH. Novel potential urinary biomarkers for effective diagnosis and prognostic evaluation of high-grade bladder cancer. Transl Cancer Res. 2023;12(8):1992–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng K, Cai N, Zhu J, Yang X, Liang H, Zhang W. Tumor-associated macrophages in liver cancer: from mechanisms to therapy. Cancer Commun. 2022;42(11):1112–40. 10.1002/cac2.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L, Li GS, Li JD, et al. Identification of the susceptibility genes for COVID-19 in lung adenocarcinoma with global data and biological computation methods. Comput Struct Biotechnol J. 2021;19:6229–39. 10.1016/j.csbj.2021.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao H, Dong Y, Xiao L, Liang X, Zheng J. Identification of key gene contributing to vitiligo by immune infiltration. Int J Clin Exp Pathol. 2022;15(4):157–67. [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen JS, Lyon CE, Fox AH, et al. Directed proteomic analysis of the human nucleolus. Curr Biol. 2002;12(1):1–11. 10.1016/s0960-9822(01)00650-9. [DOI] [PubMed] [Google Scholar]
- 35.Scherl A, Couté Y, Déon C, et al. Functional proteomic analysis of human nucleolus. Mol Biol Cell. 2002;13(11):4100–9. 10.1091/mbc.e02-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee MC, Hsieh CH, Wei SC, et al. Ectopic EBP2 expression enhances cyclin E1 expression and induces chromosome instability in HEK293 stable clones. BMB Rep. 2008;41(10):716–21. 10.5483/bmbrep.2008.41.10.716. [DOI] [PubMed] [Google Scholar]
- 37.Saridakis V, Sheng Y, Sarkari F, et al. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein-Barr nuclear antigen 1 implications for EBV-mediated immortalization. Mol Cell. 2005;18(1):25–36. 10.1016/j.molcel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Machida YJ, Chen Y, Machida Y, Malhotra A, Sarkar S, Dutta A. Targeted comparative RNA interference analysis reveals differential requirement of genes essential for cell proliferation. Mol Biol Cell. 2006;17(11):4837–45. 10.1091/mbc.e06-04-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly datasets were analyzed in this study can be found in online. This date can be found in the article and supplementary material.