Abstract
The hepatitis C virus (HCV) envelope proteins, E1 and E2, form noncovalent heterodimers and are leading candidate antigens for a vaccine against HCV. Studies in mammalian cell expression systems have focused primarily on E2 and its folding, whereas knowledge of E1 folding remains fragmentary. We used a cell-free in vitro translation system to study E1 folding and asked whether the flanking proteins, Core and E2, influence this process. We translated the polyprotein precursor, in which the Core is N-terminal to E1, and E2 is C-terminal, and found that when the core protein was present, oxidation of E1 was a slow, E2-independent process. The half-time for E1 oxidation was about 5 h in the presence or absence of E2. In contrast with previous reports, analysis of three constructs of different lengths revealed that the E2 glycoprotein undergoes slow oxidation as well. Unfolded or partially folded E1 bound to the endoplasmic reticulum chaperones calnexin and (with lower efficiency) calreticulin, whereas no binding to BiP/GRP78 or GRP94 could be detected. Release from calnexin and calreticulin was used to assess formation of mature E1. When E1 was expressed in the absence of Core and E2, its oxidation was impaired. We conclude that E1 folding is a process that is affected not only by E2, as previously shown, but also by the Core. The folding of viral proteins can thus depend on complex interactions between neighboring proteins within the polyprotein precursor.
Most proteins that enter the secretory pathway are targeted to the endoplasmic reticulum (ER) by signal sequences (59). These are usually located in the N terminus and are cotranslationally removed by the signal peptidase (38, 58). The signal sequences of the hepatitis C virus (HCV) glycoproteins, E1 and E2, when cleaved still remain part of the mature proteins Core and E1, which precede them in the viral polyprotein sequence (29). Given the organization of Core–E1–E2 in HCV, it is possible that the signal sequences contribute to proper folding prior to cleavage. A correlation between the rate of signal sequence cleavage and posttranslational protein folding has recently been observed for the gp160 glycoprotein of human immunodeficiency virus type 1 (33).
HCV, the major cause of chronic hepatitis, is an enveloped positive-stranded RNA virus belonging to the Flaviviridae (29). The viral genome contains a single open reading frame of approximately 9.5 kb that codes for a precursor which is proteolytically processed into at least 10 polypeptides (15, 54). The N-terminal segment contains the putative structural proteins, including a cytoplasmic 21-kDa capsid protein (Core) and two membrane-anchored glycoproteins, E1 and E2, which are synthesized in the ER (15, 27, 49). Immediately C-terminal from E2 is a 7-kDa peptide (p7) and a 24-kDa protein of unknown function (NS2) (34). The signal peptidase in the host ER membrane is responsible for all cleavages in the Core–E1–E2-p7–NS2 segment (27, 34, 41, 49).
No tissue culture system that allows reproducible culture of HCV is available. However, subgenomic replicons of nonstructural proteins have recently been expressed in cultured cells (2, 36). Given this limitation, all studies on viral structural proteins and their functions rely on recombinant-DNA technology. Although the detailed structure and composition of HCV are unknown, it is assumed that the viral genome is contained within a capsid surrounded by a lipid envelope containing the E1 and E2 glycoproteins. The formation of noncovalently linked heterodimers of E1 and E2 has been described (10, 32, 39, 49). Previous studies have also shown that the folding rates of the two glycoproteins are significantly different, although dimerization requires 1 to 3 h in a variety of cell lines (10, 11). E2 was found to reach its oxidized conformation in less than 5 min without detectable intermediates, whereas E1 needed at least 1 h to complete its folding (10, 11). Folding of E1 required coexpression of almost full length E2 (40).
In living cells, viral glycoproteins are translocated cotranslationally across the ER membrane. In this compartment they find an oxidized environment required for formation of inter- and/or intrachain disulfide bonds, and they are offered assistance in their maturation process by a number of membrane-bound and luminal folding factors (8, 60). Two lectin-like chaperones, calnexin and calreticulin, and the ERp57 oxidoreductase directly aid in the folding of cysteine-rich glycoproteins (24, 26). Substrates for calnexin and calreticulin are generated both cotranslationally, by removal of the two outermost glucoses from the core oligosaccharides by the sequential activities of glucosidases I and II (16, 20), and posttranslationally, by the action of UDP-glucose:glycoprotein glucosyltransferase (GT), which re-forms monoglucosylated N-linked oligosaccharides on incompletely folded glycoproteins (46). The slow removal of mannose from the core oligosaccharides is thought to provide a timing mechanism for protein maturation, since inhibition of ER α-mannosidase I blocks degradation of misfolded proteins (14). These factors are components of the ER quality control system, which functions to ensure that newly synthesized proteins reach their native structures and that misfolded proteins do not exit the ER (13).
It has been shown that an in vitro translation system, comprising reticulocyte lysate and rough ER-derived microsomal membranes, contains all the components necessary for this ER quality control (3, 19, 20, 23, 30, 31, 37, 48, 53). In this cell-free system, formation of native disulfide bonds on newly synthesized proteins is achieved when exogenous oxidized glutathione (GSSG) is present (31, 37, 53). Therefore, for HCV, which lacks robust cellular culture systems, the in vitro translation and translocation approach provides a viable alternative for protein folding studies.
The HCV E2 protein has been the subject of many studies, since it is an obvious candidate for inclusion in an HCV vaccine. E2 has been shown to bind the cellular receptor CD81 (47). HCV patients frequently express antibodies to E2 (7), and antibodies that disrupt CD81 binding correlate with protection (50). Moreover, recent studies have demonstrated that an HCV vaccine containing E1/E2 heterodimers, while not preventing acute HCV infection, prevents the development of chronic infection in chimpanzees (M. Houghton and S. Abrignani, unpublished data). Thus, a more complete understanding of E1 folding and assembly of E1/E2 heterodimers should aid in the larger-scale preparation of an HCV vaccine.
We sought to investigate the folding of HCV structural proteins, focusing on E1, using a cell-free expression system. We started by designing conditions that would allow folding of the Core–E1–E2–p7–NS2 region of the HCV genome, spanning 1,026 amino acids (aa), and proceeded to analyze shorter constructs.
MATERIALS AND METHODS
Reagents.
Rabbit reticulocyte lysate, canine pancreas microsomal membranes, and an amino acid mixture minus methionine and cysteine were purchased from Promega (Madison, Wis.). Pro-mix l-[35S]Methionine and l-[35S]Cysteine in vitro cell labeling mix and protein A-Sepharose were obtained from Amersham Pharmacia Biotech (Little Chalfont, Buckinghamshire, United Kingdom). For calnexin immunoprecipitation, a rabbit antiserum was used (20). Anti-BiP/Grp78 and anti-calreticulin rabbit polyclonal antiserum were provided by StressGen Biotechnologies Corp. (Victoria, British Columbia, Canada). Anti-GRP94 is a commercial mouse monoclonal antibody (Affinity Bioreagents, Inc., Golden, Colo.). The E1/E2 antibody Ch-L559 is a purified immunoglobulin G (IgG) fraction from the serum of a chimpanzee immunized with purified E1 and E2 and protected against experimental HCV infection (4). Mouse monoclonal E1 (linear) antibody 3D5/C3 was raised against an E1/E2 heterodimer purified from HeLa cells and selected in an enzyme-linked immunosorbent assay against purified E1 (M. Houghton, unpublished data). Castanospermine and deoxymannojirimicin were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). The protease inhibitor cocktail Complete (EDTA free), 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), HEPES, endoglycosidase H, and RNase inhibitor were obtained from Roche Molecular Biochemicals (Manheim, Germany). All other reagents were purchased from Sigma Biochemicals (St. Louis, Mo.).
Plasmids and RNA synthesis.
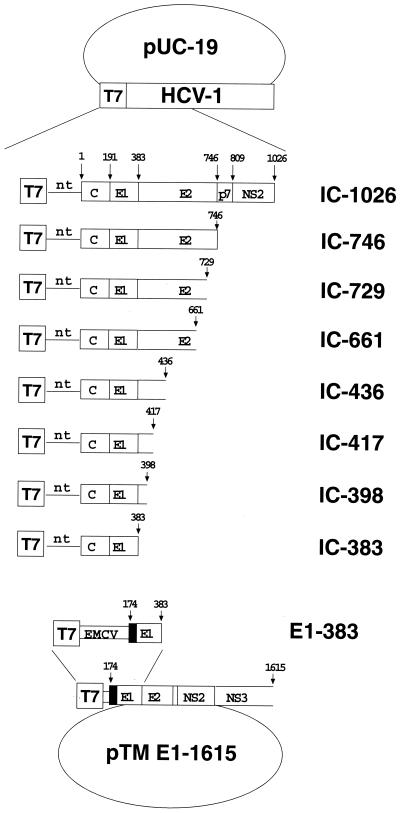
The full-length consensus sequence of HCV type 1 (HCV-1) was cloned into the HindIII-XbaI site of pUC19 (Invitrogen), resulting in the pUC-HCV-1PC clone (A. J. Weiner, unpublished data). DNA templates for RNA synthesis were generated by amplification of this recombinant plasmid with the appropriate pair of primers. All amplified DNA fragments from plasmid pUC-HCV-1PC (referred to as IC in primer and construct designations [see Fig. 1]) included the T7 promoter of the pUC vector at their 5′ ends by using the forward primer 5′-TCACGACGTTGTAAAACGACGGCC-3′. The following reverse primers, each containing a stop codon (underlined) immediately downstream of the indicated amino acid position (the number following IC-) in the polypeptide, were used: IC-383 (5′-TCCCCCGGTGACGTGGGTTTACGC-3′), IC-398 (5′-GAGGAGGCTAACTCATCCAGACACAGT-3′), IC-417 (5′-GAGGTGCCAACTTCAGTTGGTGTTGAT-3′), IC-436 (5′-AAGCCCTGCCAATCAGCCGGTGTTGAG-3′), IC-661 (5′-CAGCAGTAACGGGCTCTACTCGGA-3′), IC-729 (5′-AAGCAGGAGCAGACTTACGCGTCTGCA-3′), IC-746 (5′-GGTTCTCCAATTACGCTTCCGCTTGGG-3′), and IC-1026 (5′-GATGGGTTACAGCAACCTCCAACC-3′). All HCV polypeptides translated from mRNAs of the IC series start from amino acid number 1.
FIG. 1.
Recombinant plasmids and DNA templates. Plasmids pUC19.HCV1 and pTM E1–1615 were used as templates for DNA amplification and subsequent RNA synthesis. Amplified products are diagrammed, their designations are given on the right. IC, plasmid pUC-HCV-1PC. Numbers in designations correspond to the last amino acid of the HCV polypeptide (HCV-1 strain) included in the construct and followed by a stop codon. nt, 341-bp nontranslated region of HCV; EMCV, internal ribosomal entry site sequence carried by the pTM1 vector (45). Oligonucleotides used for the amplification procedure are described in Materials and Methods. RNA was synthesized by using a T7 polymerase kit.
To generate pTME1-1615, the HCV genomic region coding for E1 (including the N-terminal ER targeting sequence corresponding to the C-terminal sequence of the core protein), E2, p7, NS2, and truncated NS3, corresponding to amino acid positions 174 to 1615 of the polypeptide, was cloned into the pTM1 vector (45). Since we used the NcoI/SpeI sites on pTM1, a silent mutation was introduced on pUC.HCV1 (Ser363 TCC→TCG) to destroy the internal NcoI site on the HCV genome. The silent mutated HCV sequence was amplified with the forward primer 5′-CCTTCCTGCCATGGCTTTCTCTATCTTC-3′ and the reverse primer 5′-TGTTGGCCCTCTAGAAGCTTACTTGAGGCG-3′. The resulting DNA was digested with NcoI/XbaI (underlined) and cloned into the NcoI/SpeI sites of pTM1. Between the starting methionine and the first amino acid of the core protein's C terminus (phenylalanine 174), a GCT codon coding for alanine was introduced for cloning. The absence of additional mutations in the HCV region was verified by DNA sequencing. The DNA template for E1-383 mRNA synthesis (coding for aa 174 to 383 of the HCV polypeptide) was generated by amplifying the pTME1-1615 plasmid with the forward primer 5′-GACAGTTCTTTCCAGACATTGTTG-3′ and the reverse primer IC-383 (see above).
All amplification reactions were performed in a 50-μl reaction mixture by using Pwo DNA polymerase (Roche Molecular Biochemicals) up to 3,000 bases or PCR SuperMIX High Fidelity (Gibco-BRL) according to the manufacturer's instructions. The amplified DNA was then purified with a QIAquick PCR purification kit (Qiagen, Hilden, Germany), and 5 μl was used as a template for T7 polymerase RNA synthesis by MEGAscript (Ambion, Austin, Tex.) according to the manufacturer's instructions. Finally, RNA was precipitated, resuspended in 100 to 200 μl of RNase-free water to reach an approximate concentration of 1 μg/μl, and stored at −80°C in aliquots.
In vitro translation, translocation, and folding of HCV proteins.
35S-labeled HCV proteins were translated and translocated into dog pancreas microsomes using the following mixture (final volume, 100 μl): 40 to 60 μl of reticulocyte lysate, 5 μl of microsomes, 10 to 20 μl of 35S metabolic labeling mixture (10 to 15 mCi/mmol) (Pro-mix), 5 μl of an amino acid mixture minus methionine and cysteine, 5 μl of an RNase inhibitor, and 1 μl of mRNA (about 1 μg/μl). Except for the experiment for which results are shown in Fig. 2, where GSSG concentrations are specified, synthesis and posttranslational incubation were performed under oxidizing conditions with a final GSSG concentration of 10 mM. Optimal conditions for synthesis and posttranslation incubation were established for each batch of pancreatic microsomal membranes and reticulocyte lysate by titrating the amount of reticulocyte lysate in combination with the following reagents: potassium acetate (0 to 150 mM), dithiothreitol [DTT] (0 to 2 mM), and MgCl2 (0 to 2.5 mM). All experiments were performed at 30°C. Samples were incubated for 2 h, and posttranslational folding was initiated by adding cycloheximide (CHX) to a final concentration of 0.5 mM. Aliquots were removed at various time points, alkylated by adding 20 mM N-ethylmaleimide (NEM) to block free sulfhydryls, and subjected to immunoprecipitation followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. For long posttranslational incubation, the same aliquots of 20 mM HEPES (pH 7.2), 100 mM potassium acetate, 10 mM GSSG, and 6 mM tetracaine were added to the mixture 5 to 6 h after CHX addition.
FIG. 2.
Protein synthesis of IC-1026 mRNA under different oxidizing conditions and with endoglycosidase H digestion. Each sample was incubated for 2 h at 30°C in a 20-μl translation mixture to which RNase-free water (lanes 1 and 12) or a solution of GSSG (lanes 2 to 6 and 13 to 17), or diamide (lanes 7 to 11 and 18 to 22) was added to reach the final concentrations given above the gel. After synthesis and membrane solubilization in HBS buffer containing 2% CHAPS, proteins were alkylated with 20 mM NEM for 5 min on ice and subjected to immunoprecipitation with anti-E1/E2 Ch-L559 antibody. Samples were then resuspended in 40 μl of SDS loading buffer, and proteins were eluted from beads by heating at 95°C for 5 min. (a) Two 10-μl aliquots were used for SDS–7.5% PAGE analysis under both nonreducing (NR) and reducing (Red) conditions. For the reduced samples, 1 μl of 1 M DTT was added before reheating at 95°C for an additional 3 min and loading. Labeled species were visualized by autoradiography and phosphorimager analysis. The autoradiogram shown was obtained with a short exposure time to better emphasize the difference in expression level relative to the increasing amount of oxidizing agents. The positions of molecular size markers are indicated. (b) Aliquots (10 μl) of samples 1 to 6 from panel a were subjected to endoglycosidase H (Endo-H) digestion as described in Materials and Methods. Proteins were then separated on an SDS–12.5% PAGE gel under reducing conditions. The positions of molecular size markers are indicated.
Immunoprecipitation.
A 10- to 20-μl volume of the in vitro reaction mixture per antibody was used for immunoprecipitation. Samples were solubilized in ice-cold 2% CHAPS in HBS (20 mM HEPES–150 mM NaCl) including protease inhibitor cocktail (Complete, EDTA free; Roche Molecular Biochemicals) and 1 mM phenylmethylsulfonyl fluoride. For BiP/GRP78 immunoprecipitation, 20 U of Apyrase (Sigma Biochemicals, St. Louis, Mo.) was added to the lysis buffer to deplete the ATP pool. Lysates were precleared by addition of 20 μl of protein A-Sepharose (Amersham Pharmacia Biotech) in lysis buffer and end-over-end rotation for 1 to 3 h at 4°C. Beads were gently pelleted by centrifugation, and supernatants were transferred to new tubes. An additional 20 μl of protein A-Sepharose and the appropriate antibody were added, and samples were subjected to overnight end-over-end rotation at 4°C. Immunocomplexes were recovered by centrifugation at 2.500 × g, washed three times with 0.5% CHAPS in HBS, and resuspended in 40 μl of SDS sample buffer (50 mM Tris-HCl [pH 6.8], 3% SDS, 10% glycerol, 0.05% bromophenol blue). Proteins were eluted from beads by heating at 95°C for 5 min and were then divided into 10-μl aliquots. One aliquot was loaded directly for nonreducing analysis, a second aliquot was supplemented with 100 mM (final concentration) DTT for reducing SDS-PAGE, and if required, a third aliquot was used for endoglycosidase H digestion. Proteins were then separated on SDS-PAGE gels (7.5 or 10% polyacrylamide), which were stained with Coomassie brilliant blue to verify equal protein loading. Following neutralization and a wash with salicylic acid (0.125 M salicylic acid in 30% methanol) for 15 min, gels were dried and submitted to autoradiography (XAR-5 films; Kodak Co., Rochester, N.Y.) and/or densitometric analysis with a digital gel scanner (Molecular Dynamics).
Endoglycosidase H digestion.
Endoglycosidase H digestion was performed using a 10-μl aliquot of the immunoprecipitates equilibrated in SDS sample buffer. Each sample was diluted 50 times in 100 mM sodium acetate (pH 5.8) and 100 mM β-mercaptoethanol to adjust the pH and to dilute the SDS to about 0.05 M. Samples were heated at 95°C for 3 min and incubated overnight at 37°C in the presence of 10 U of endoglycosidase H and protease inhibitors. Proteins were then precipitated with trichloroacetic acid, washed, dried, and resuspended in SDS loading buffer containing 100 mM DTT for SDS-PAGE.
RESULTS
In vitro expression of HCV glycoproteins is sensitive to oxidizing conditions.
HCV genome translation and translocation in a cell-free system using a standard reticulocyte lysate in the presence of canine pancreatic microsomal membranes has been previously used to identify the cleaved products from the polyprotein precursor (27, 52). Our aim was to extend this system to study the folding of the HCV structural proteins E1 and E2 by the procedure described for the folding of influenza virus hemagglutinin (22).
The different DNA templates from which mRNAs were transcribed and translated in vitro are shown in Fig. 1. We first established whether the translation, translocation, and maturation of E1 and E2 were influenced by redox conditions. Instead of using the standard reticulocyte translation protocol, designed for optimal translation efficiency but not for disulfide bond formation, we translated IC-1026 mRNA in the presence of different concentrations of two oxidants: GSSG and diamide. This region of the HCV-1 genome spans the initial 3,419 bases and contains 341 nontranslated bases and a unique open reading frame coding for 5 proteins: Core, E1, E2, p7, and NS2 (29). After translation HCV glycoproteins were immunoprecipitated with anti-E1/E2 antibody (Ch-L559) (4) and separated on SDS-PAGE gels under nonreducing or reducing conditions (Fig. 2a). Equal fractions of total synthesized E1 and E2 are immunoprecipitated by the antibody (data not shown). In addition to E1 and E2, expression of Core and NS2 from this construct was verified by specific antibodies (data not shown).
The 383-residue sequence of E2 includes 11 potential glycosylation sites and 20 cysteine residues. The apparent molecular size in SDS-PAGE of the fully glycosylated product has been reported to be 68 to 74 kDa (29). E2 is synthesized as a precursor with the p7 protein at its C-terminal end; this precursor is slowly cleaved by the action of the host signal peptidase (34). The E2-p7 precursor should have an apparent molecular size of 75 to 81 kDa. As shown in Fig. 2a (lanes 1 to 6), the uncleaved and cleaved products could be detected only when the GSSG concentration was 7.5 mM or higher. Furthermore, the intensities of the bands increased with higher oxidant concentrations. At 5 mM GSSG and below, only a small amount of E2 could be detected (Fig. 2a, lanes 1 to 3). The results do not allow discrimination between the following possibilities: (i) E2-p7 synthesis is impaired under reducing conditions, (ii) the protein is rapidly degraded after synthesis, or (iii) E2-p7 acquires a misfolded conformation that is not recognized by the antibody.
E1 is a 192-aa protein with 8 cysteines and, in the HCV-1 strain, 4 core-glycosylated asparagines out of the 5 potentially core-glycosylated asparagines included in the consensus sequences. The molecular size of the protein is about 21 kDa, and the apparent molecular size of the glysosylated product ranges from 31 to 35 kDa (29). The intensity of the E1 band increased with increasing concentration of GSSG (Fig. 2a). In addition, its mobility in SDS-PAGE shifted when the oxidizing agent was present during synthesis (Fig. 2a, lanes 1 to 6). The difference in migration was due to a real change in molecular size and not to disulfide bond formation, since the mobility pattern was identical for samples separated on SDS-PAGE gels under reducing conditions (lanes12 to 17). To determine whether the mobility shift was due to the sugar moiety of E1, we performed endoglycosidase H digestion on samples 1 to 6 and separated the products on SDS-PAGE gels. Endoglycosidase H removes all sugar residues from ER glycoproteins except for the innermost N-linked N-acetylglucosamine. Endoglycosidase H treatment of E1 generated bands with the same mobility irrespective of the oxidizing conditions used to synthesize them (Fig. 2b). Thus, the difference in the apparent molecular size was most likely due to the differential trimming of the sugar moiety of the molecules. This result led us to conclude that synthesis under reducing conditions generates an instantaneously trimmed E1 and provided a strong indication that a proteolytic degradation pathway is favored, as seen in other systems (14).
The oxidant GSSG, a natural component of the ER lumen, has been used to induce oxidative protein folding in in vitro systems (31, 37, 53). To test whether the protection of E1 from fast sugar trimming was due to GSSG specifically or to the oxidizing conditions in general, we used the more-potent, membrane-permeating sulfhydryl oxidizing agent diamide. Addition of increasing concentrations of diamide in the translation reaction produced an analogous covalent mobility shift in the migration of E1 (Fig. 2a, lanes 7 to 11 and 18 to 22). Moreover, at 5 mM diamide, a smeared E1 band appeared, indicating that diamide induced heterogeneous disulfide bonds. By contrast, E2 was poorly visible on the gel when diamide was used as an oxidative agent during synthesis, suggesting that E2 is more sensitive to strong oxidizing agents (Fig. 2a, lanes 7 to 11 and 18 to 22). This result is consistent with a previous report showing that different oxidants are not equivalent in promoting proper disulfide bond formation (37). These findings established that a GSSG concentration of 10 mM in our system provided the optimal compromise between HCV protein synthesis and redox potential required for native sulfhydryl oxidation. We used these oxidizing conditions for all further studies.
Oxidizing conditions allow in vitro folding of HCV glycoproteins.
We next determined whether in vitro synthesis under oxidizing conditions induced a conformational change in HCV glycoproteins through the generation of intramolecular disulfide bonds. IC-1026 mRNA was translated for 2 h in the presence of 10 mM GSSG before protein synthesis was arrested by addition of 0.5 mM CHX (time zero). After prolongation of posttranslational incubation for an additional 2, 4, or 6 h, E1 and E2 oxidation was assessed by comparative analysis of these proteins by both nonreducing and reducing SDS-PAGE. This procedure is used routinely to monitor the formation of correct intrachain disulfide bonds, since the oxidized form of a protein with its intact disulfide bonds generally migrates faster than its reduced counterpart. (3, 37, 53). While S-S bonds are resistant to detergent, reduction of samples before SDS-PAGE analysis cancels all mobility differences, since only linear forms are present in the gel.
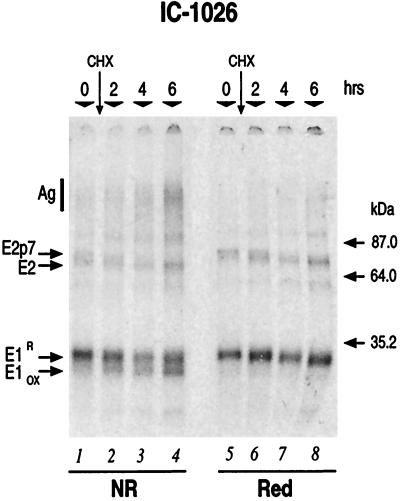
Oxidizing conditions promoted folding of both HCV glycoproteins in the cell-free system (Fig. 3). At time zero a single E1 band, migrating with an apparent molecular size of 33 to 34 kDa, was predominant in both nonreducing and reducing gels (Fig. 3, lanes 1 and 5). Therefore, after the 2-h “pulse” period (time zero), almost all E1 was found in a linear form, which we refer to as reduced E1 (E1R). During posttranslational incubation, a second, faster-migrating form of E1 appeared with increasing intensity over time and only in the nonreducing gel (Fig. 3, lanes 2 to 4). We refer to this band as oxidized E1 (E1OX). The increased mobility of E1OX was not due to a difference in molecular size, because after reduction, only a single species of E1 was detected, and its mobility was equivalent to that of E1R (Fig. 3, lanes 6 to 8). Thus, the faster-migrating form (E1OX) represented an E1 conformer with more-compact structure due to intramolecular disulfide bonds. As no heterogeneous products were visible, we concluded that the conditions used for this analysis promoted correct oxidation of E1. Extending the posttranslational incubation time led to a slight increase in the electrophoretic mobility of E1R (Fig. 3, lanes 5 to 8). This effect almost disappeared in the presence of deoxymannojirimicin, an ER α-mannosidase inhibitor, indicating that this shift was mainly due to slow removal of mannose residues (data not shown).
FIG. 3.
Folding of IC-1026. IC-1026 mRNA was translated in a 100-μl volume including 10 mM GSSG. Incubation was performed at 30°C for 2 h (time zero) before addition of 0.5 mM (final concentration) CHX. Posttranslation incubation was then prolonged for an additional 6 h. At the times indicated above the gel, 20-μl aliquots were withdrawn, treated with NEM, solubilized, and subjected to anti-E1/E2 immunoprecipitation with Ch-L559 antibody as described in Materials and Methods. Immunoprecipitates were resuspended in SDS loading buffers and removed from beads by heating at 95°C for 5 min. Proteins were then separated on SDS–10% PAGE gels under both nonreducing (NR) (lanes 1 to 4) and reducing (Red) (lanes 5 to 8) conditions. The positions of molecular size markers are indicated. Ag, high-molecular-weight aggregates. The position of E2 was identified by in vitro translation of E2-746 mRNA (not shown).
In the upper part of the gel, time-dependent oxidation of E2 could be observed (Fig. 3). The kinetics of disulfide bond formation was difficult to follow in this experiment because there was a conformational mobility shift during the entire posttranslational incubation time (Fig. 3; compare samples under nonreducing and reducing conditions). This is most likely due to the simultaneous actions of E2 oxidation and cleavage of p7. During the initial 2 h of posttranslational incubation, E2 underwent oxidation (Fig. 3; compare lanes 2 and 6) even though it did not seem to be cleaved from the E2p7 precursor. Cleavage of p7 from the full-length E2 appeared to be complete only after 4 h (Fig. 3, lane 7). A broad band, indicating heterogeneously oxidized products, appeared immediately after synthesis (Fig. 3, time zero, lane 1). After reduction it was resolved as E2p7 (lane 5). Finally, after 2 to 6 h of posttranslational incubation, E2 showed a mobility shift that clearly indicated the presence of an oxidized E2 species (Fig. 3; compare lanes 3 and 4 with lanes 7 and 8). This implies that oxidation of E2 starts while it is still linked to p7 and that the process proceeds more slowly than previously reported (10, 11).
On the gel shown in Fig. 3 we also performed a densitometric analysis of the relative radioactivity incorporated in the aggregate (Ag), E2p7/E2, and E1 fractions, respectively (Table 1). E1 incorporated a larger fraction of radioactivity and appeared to be more efficiently expressed than the downstream E2. The high-molecular-weight fraction (Ag) showed a time-dependent increase that confirmed the tendency of HCV proteins to aggregate. The aggregates were only partially (60%) reduced by DTT, showing that this fraction includes not only interchain SS-linked molecules but also SDS-insoluble species, which are probably hydrophobically associated. Interestingly, the E2p7/E2 fraction exhibited a drastic time-dependent reduction in radiaoctive signal under nonreducing conditions, but not under reducing conditions. This indicated that the two HCV glycoproteins tend to form different high-molecular-weight aggregates when expressed in the context of Core-NS2. E2 preferentially aggregated through homologous interchain SS-linked species, leaving only a small percentage for the generation of the properly oxidized species. Conversely, E1 favored the formation of hydrophobic, insoluble aggregates.
TABLE 1.
Densitometric analysis of Ag, E2-p7/E2, and E1 fractions
| Fraction | % of radioactivity incorporated at the indicated timea
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Nonreduced samples
|
Reduced samples
|
|||||||
| 0 h | 2 h | 4 h | 6 h | 0 h | 2 h | 4 h | 6 h | |
| Ag | 11.49 | 12.82 | 19.72 | 26.34 | 5.25 | 5.42 | 7.27 | 10.76 |
| E2-p7/E2 | 17.79 | 19.26 | 15.57 | 11.93 | 23.39 | 22.26 | 22.87 | 25.30 |
| E1 | 70.72 | 67.93 | 64.71 | 61.73 | 71.36 | 72.32 | 69.86 | 63.95 |
Number of hours of posttranslation incubation.
IC-746 and IC-661 folding analysis.
Previous reports showed that E1 folding depends on cotranslation of full-length E2 (40) or truncated E2 lacking the C-terminal 85 aa (9). Under these conditions the oxidation of E2 was seen to be very rapid, without detectable intermediates. We tested the analogous constructs in our cell-free system. The two mRNAs IC-746 and IC-661, encoding the core protein, E1, and the full-length or truncated (minus 85 aa) E2, respectively, were subjected to folding analysis under the same conditions as our previous experiments. To obtain an optimal separation for each of the proteins, immunoprecipitated E1 and E2 were resolved under nonreducing or reducing conditions on separate SDS-PAGE gels with 10 or 7.5% polyacrylamide, respectively (Fig. 4).
FIG. 4.
Folding of IC-746 and IC-661. Each mRNA was translated in a 100-μl mixture under oxidizing conditions (10 mM GSSG). Translation and posttranslation incubation were performed at 30°C. The time course and immunoprecipitations were performed as described in the legend to Fig. 3. Proteins were resolved by SDS-PAGE (7.5% [a] or 10% [b] polyacrylamide). Gels were stained, neutralized, dried, and subjected to autoradiography. NR, nonreducing conditions of electrophoresis (lanes 1 to 4). For analysis under reducing conditions (Red), 100 mM (final concentration) DTT was added to samples before loading (lanes 5 to 8).
Analysis of E2-746 folding clearly showed time-dependent formation of oxidized species, although a smeared area appeared instead of discrete bands (Fig. 4a, upper panel). This phenomenon indicates the presence of heterogeneously disulfide bonded E2 conformers, including off-pathway structures. Folding analysis of E2-661 (Fig. 4a, bottom panel) showed that this species generated a sharper band that underwent a time-dependent increase in an SDS-PAGE mobility shift (compare lanes 2 to 4 with lanes 6 to 8). As observed for E2p7 (Fig. 3), the truncated soluble E2-661 generated nonheterogeneous conformational species. Taken together, these observations indicate that E2, in vitro, is able to acquire a more compact structure, which required at least 4 h of posttranslational incubation for completion. Although in this study we did not pursue a deeper analysis of E2 folding (see Discussion), our data show that complete oxidation of E2 seems to be a slow process in our system.
Posttranslational incubation of E1 from both IC-746 and IC-661 led to the time-dependent formation of E1ox (Fig. 4b). These data are consistent with previous reports (40) and confirm that folding analysis of HCV proteins is possible in vitro.
E1 enters a folding pathway in the absence of E2.
We next addressed two major questions. (i) What is the minimum length of E2 required for proper oxidation and folding of E1? (ii) Is the oxidized E1 species correctly folded or, at least, a folding intermediate?
To establish the minimal length of E2 needed to obtain oxidized E1, we translated truncated mRNAs containing a stop codon at amino acid position 730, 437, 418, 399, or 384. The 384 construct codes for the full-length E1 in the complete absence of E2, while the other mRNAs code for longer portions of E2. Addressing our second question was less straightforward, given that no anti-E1 conformational antibodies are available. Therefore, we devised a strategy to estimate the proportion of E1OX lacking linear epitopes. To this end we used two different antibodies: (i) Ch-L559, which is a purified antiE1/E2 chimpanzee antiserum (4) expected to recognize a variety of E1 conformations and (ii) 3D5/C3, a monoclonal antibody which recognizes only the linear form of E1 (Houghton, unpublished). Decreased binding to monoclonal antibody 3D5/C3 indicated conformational changes in E1OX, and the ratio of E1OX binding to 3D5/C3 and Ch-L559 was used to quantitate these changes.
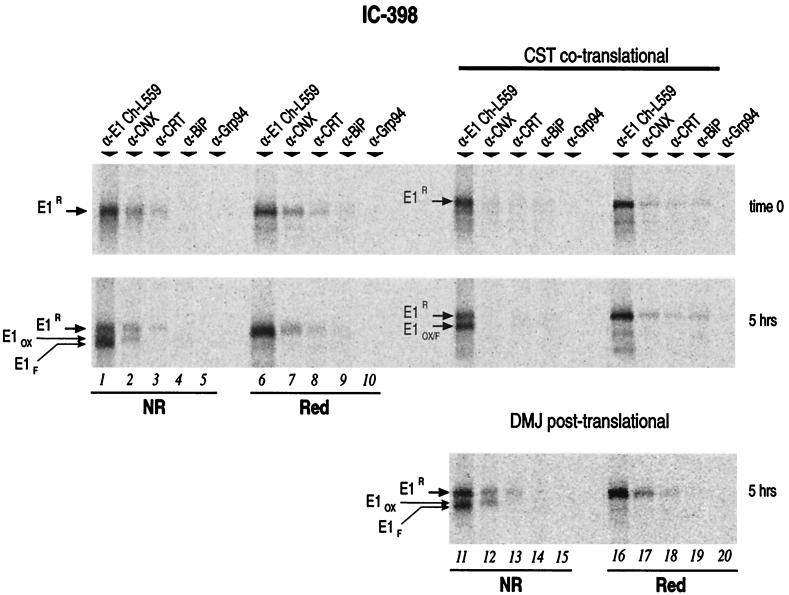
We translated the mRNAs in our in vitro system for 2 h (time zero), and the posttranslational incubation was extended to 20 h. Aliquots were taken after 2, 5, 7, and 20 h, at which point the lysates were split in two and subjected to immunoprecipitation with the antibodies. Results from SDS-PAGE analysis (Fig. 5a) indicated that E1 does not require downstream sequences for its own folding. Analysis of the Ch-L559 anti-E1/E2 immunoprecipitates at time zero of posttranslational incubation revealed an additional, faster-migrating band in the IC-383 construct (Fig. 5a, top panel, lanes 1 and 6 [E13g]). This species was identified as an E1 protein with 3 instead of 4 N-linked glycans based on the facts that (i) endoglycosidase H treatment of the immunoprecipitated sample generated a 21-kDa single band and (ii) the amount of E13g, as well as those of other underglycosylated forms, increased when E1 from different constructs was synthesized in a reducing environment, whereas under oxidizing conditions these forms were apparent only when E1 was expressed without downstream sequences (reference 9 and data not shown). The amount of this underglycosylated form, however, decreased during the incubation time (Fig. 5a; compare lanes 6 in all the left panels, from top to bottom). Conversely, the amount of E1OX relative to E1R increased over time in the presence of the oxidizing agent (Fig. 5a, lanes 1 to 5). As expected for a disulfide-bonded conformational protein, it disappeared when samples were reduced before gel loading (lanes 6 to 10).
FIG. 5.
E1 folding dependence by E2 sequences. IC-383, IC-398, IC-417, IC-436, and IC-729 mRNAs were translated separately in 150-μl mixtures under oxidizing conditions. Translation and posttranslation incubation were performed at 27°C. The time course and immunoprecipitations were performed as indicated in the legend to Fig. 3 using the anti-E1/E2 antibody Ch-L559 (left panels, lanes 1 to 10) or the anti-E1 mouse monoclonal antibody 3D5/C3 (right panels, lanes 11 to 20). After the 20-μl aliquot was withdrawn at 5 h, the same aliquots of 20 mM HEPES (pH 7.2), 100 mM potassium acetate, 10 mM GSSG, and 6 mM tetracaine were added to samples and incubation was prolonged for an additional 2 and 15 h. (a) Immunoprecipitates were analyzed by SDS–10% PAGE under nonreducing (NR) and reducing (Red) conditions as described in Materials and Methods. E13g, E1 containing three N-linked core glycosylated units (see the text for details). (b) Plot of the autoradiograms of E1OX, EIR, and E13g analyzed by densitometric analysis. The sum of the radioactivity incorporated by E1OX, EIR, and E13g was considered to equal 100%. Values are expressed as percentages of E1OX radioactivity at the corresponding time of incubation. E13g values under reducing conditions were considered to be background (panel a, lanes 6 to 10) and were used to normalize E1OX values. Folding efficiency was calculated as the intensity of E1OX relative to that of the E1 bands of the corresponding gel under reducing conditions (for example, E1OX in lane 1 versus E1R plus E13g in lane 6). Using this parameter, the DTT-sensitive fraction of the high-molecular-weight aggregates was included in the analysis.
The kinetics of E1OX formation for all the constructs analyzed appeared similar whether the protein was translated in the presence or absence of downstream sequences (Fig. 5). We performed a densitometric analysis of the gels shown in the left panels of Fig. 5. For each construct we calculated the radioactivity incorporated into the E1OX bands relative to the sum of the radioactivity incorporated by E1R and E13g (considered as background). The calculated values were plotted against the corresponding posttranslational incubation time (Fig. 5b). Each point represents the fraction of synthesized E1 that achieved an oxidized conformation. For nearly all constructs the half-time of oxidation (t1/2) (i.e., the time required for equal distribution of radioactivity between E1R and E1OX) was around 5 h, with only IC-383 giving slightly higher values (or faster kinetics) at early stages. Furthermore, the similar folding rate of E1 in all constructs was assessed also by calculating their folding efficiencies. After a 20-h incubation, these values were about 82 and 79% for IC-383 and IC-729, respectively (Fig. 5).
The right side of Fig. 5a (lanes 11 to 20) shows the SDS-PAGE analysis of the immunoprecipitates obtained using the antibody that recognizes E1 containing linear epitopes (3D5/C3). E1OX was not recognized by 3D5/C3, while both E1R and E13g were. Therefore E1OX likely represents a conformer with a more compact structure. Taken together, we conclude that E1 does not require the presence of E2 in order to enter a productive folding pathway.
E1OX contains a folding intermediate(s).
To establish whether E1OX is a folding intermediate or a fully folded molecule, we tested its interaction with ER chaperones. The ER quality control system ensures that newly synthesized proteins interact with resident chaperones through the entire folding and assembly process and are released when they reach a mature conformation (13). The folding of viral glycoproteins is assisted primarily by the ER-resident chaperones calnexin, calreticulin, BiP/Grp78, and Grp94 (42).
To minimize the interference of the E13g product in our analysis, we translated in vitro the IC-398 construct instead of the IC-383. After translation, samples were incubated for an additional 5 h to allow posttranslational folding. In parallel, castanospermine was added cotranslationally to a second sample. This molecule blocks glucosidases I and II (12), thereby preventing the removal of any of the three glucoses in the core glycans and, consequently, association of calnexin and calreticulin. Deoxymannojirimycin, an inhibitor of the ER mannosidases (12), was also used in these experiments but was added posttranslationally to block mannose trimming, which we have shown to be irrelevant during E1 synthesis (Fig. 3). Samples were then immunoprecipitated with an anti E1/E2 Ch-L559, anti-calnexin, anti-calreticulin, anti-BiP/Grp78, or anti-GRP94 antibody and analyzed on SDS–10% PAGE gels.
At the end of the synthesis (time zero), linear E1 (E1R) is predominant (Fig. 6, top panel, lane 1), and this form is associated with calnexin and calreticulin but not with BiP/Grp78 or GRP94 (top panel, lanes 2 to 5). After 5 h of posttranslational incubation the anti-E1 antibody resolved the reduced and oxidized E1 (center panel, lane 1), but calnexin association was limited to a more slowly migrating fraction of E1OX (center panel, lane 2). From this result we conclude that E1OX consisted of two closely migrating components, a slower-migrating calnexin-bound form (E1OX) and a faster-migrating calnexin-released species that represented the fully folded E1 (E1F). Posttranslational folding in the presence of deoxymannojirimycin diminished the difference in migration between E1OX and E1F (bottom panel). Therefore, the observed mobility shift is caused not only by mannose trimming but also by an even more compact conformation. As already described for other systems (19), prevention of calnexin and calreticulin association by inhibition of glucose trimming did not impair E1 folding but rather accelerated this process (Fig. 6, center panel, lanes 11 to 20). Incorporation of radioactivity by E1OX increased from 53.5% in the absence of inhibitor to 64.5% in its presence (center panel; compare lanes 1 and 11). This demonstrates that E1 coexpressed with core protein was oxidized and likely acquired a mature conformation even in the absence of chaperone association. Figure 6 also shows that calreticulin transiently associated with reduced E1 and not the oxidized form (lane 3), while BiP/Grp78 association was limited to the aggregate fraction of E1, as it is slightly visible in the reduced samples (lane 8). It was not possible to detect any Grp94 association in this study (Fig. 6, lanes 5, 10,15, and 20).
FIG. 6.
E1 folding, expressed as IC-398 mRNA, is not impaired by sugar trimming inhibitors. IC-398 mRNA was translated in two different mixtures under oxidizing conditions. A 150-μl mixture was translated as specified previously, while 0.5 mM castanospermine (CST) was added cotranslationally to a 100-μl mixture. After 2 h at 30°C, CHX was added to both samples, and 50-μl aliquots were used for analysis at time zero. At this time, a second 50-μl aliquot of sample incubated in the absence of CST was withdrawn and 0.5 mM deoxymannojirimycin was added to it. Samples were then incubated for an additional 5 h before proceeding to membrane lysis and immunoprecipitation as described in Materials and Methods. Proteins were then separated by SDS–10% PAGE under nonreducing (NR) or reducing (Red) conditions and subjected to autoradiography. The area of E1 migration is magnified for better resolution. Antibodies used for immunoprecipitations are specified above the gel. Ch-L559 anti-E1/E2 has been described previously. α-CNX, α-CRT, and α-BiP are rabbit polyclonal antibodies that recognize calnexin, calreticulin, and BiP, respectively. α-Grp94 is a mouse monoclonal antibody that recognizes GRP94. E1F, folded E1.
The E1 folding pathway is impaired in the absence of other HCV proteins.
Our results indicated that E1 folding is a slow, E2-independent process, but we did not address whether the core protein plays any role in this process. To answer this question, we analyzed the folding of E1-383 (without the Core) under the same conditions described previously (Fig. 6). The results clearly demonstrated a dramatic change in the E1 folding pathway (Fig. 7).
FIG. 7.
E1 folding, expressed as E1–383 mRNA, is severely impaired. Expression and analysis of E1–383 mRNA were performed exactly as described in the legend to Fig. 6. E13g, E1 containing three N-linked core glycosylated units. Immunoprecipitation with the anti-Grp94 antibody is not shown in the figure.
Immediately after synthesis, (time zero) the linear form of E1, as well as the underglycosylated species, was associated with calnexin and calreticulin but, again, not with BiP/Grp78 (Fig. 7, top panel, lanes 1 to 4). However, after 5 h of posttranslational incubation, formation of oxidized E1 was dramatically reduced (about 22% of total E1 was oxidized) and the E1F species was barely detectable (center panel, lanes 1 and 2). The effect was even more dramatic when calnexin and calreticulin association was prevented by adding castanospermine. In this case almost no E1OX was generated (center panel, lane 9). Analysis of the E1 species recovered after 5 h of posttranslational incubation in the presence of the mannose trimming inhibitor deoxymannojirimycin (bottom panel, lane 9) showed that the amount of total E1 recovered by immunoprecipitation was 3 times higher, although the percentage of E1OX did not increase (23.7%). Furthermore, there was no visible difference in mobility between the calnexin-bound and the calnexin-released form, indicating that the formation of E1F under these conditions is unlikely. We conclude that inhibition of ER mannosidase action protects E1 from degradation but does not affect the folding pathway. Thus, E1-383 folding under these conditions generates mostly incorrectly oxidized forms that are normally targeted to degradation.
DISCUSSION
Most previous studies on HCV folding have focused on E2 and have relied on powerful viral vectors to overexpress this protein in cells. Our goal was to study primarily the HCV E1 protein, and we chose an in vitro strategy to gain insight into its folding process. The key limitation of this system, which relies on rabbit extracts and dog-derived microsomal membranes, is the potential lack of human-specific factors necessary for correct folding and assembly of HCV structural proteins. However, in vivo studies on expression, processing, localization, and folding of these proteins using human, monkey, pig, mouse, hamster, and insect cells have all yielded similar results (10, 28, 32, 35, 39, 40, 49, 57). Therefore, the insights that can be gained from our system far outweigh this theoretical disadvantage. Specifically, we show that (i) E1 can fold properly in the absence of E2, but only if the core protein is present, (ii) in the absence of both the core protein and E2, E1 generates unstable, heterogeneously oxidized species targeted to degradation by mannose trimming, (iii) E1 associates in the ER with calnexin and calreticulin, and (iv) E2 oxidation kinetics are slower than previously reported.
We have used an anti-E1 antibody recognizing a linear epitope to demonstrate that E1 undergoes proper oxidation, and we have analyzed chaperone association to reveal the fully folded form. Our results show that the E1 folding pathway is almost identical when E1 is coexpressed as part of the N-terminal 1,026 aa as well as core-E1 and all other intermediate sequential truncations. The ER-resident chaperone calnexin appears to play the major role in assisting E1 folding. When E1 was coexpressed with the core protein, E1 folding was accelerated by the presence of castanospermine, an inhibitor of calnexin association. This result is consistent with current models, since molecular chaperones are known to increase folding efficiency by preventing incorrect interactions but do not give directions for specific folding pathways (18). On the other hand, expression of E1 in the absence of other HCV proteins generates oxidized intermediates stably associated with calnexin. Misfolding pathways are thus predominant under these conditions, and castanospermine inhibition of calnexin association accelerates degradation. In fact, the posttranslational addition of deoxymannojirimycin, an inhibitor of α-mannosidase activities, protected this molecule from degradation without increasing the amount of the oxidized species. Mannose removal by the ER α-mannosidase I is a prerequisite for misfolded glycoprotein degradation, which is blocked by inhibiting such activity (6, 14). Conversely, when the core protein was coexpressed, the amount of oxidized and folded E1 was not affected by deoxymannojirimycin treatment, suggesting that under these conditions degradation is prevented by the formation of a correct tertiary structure.
In general, spike glycoproteins present on the surfaces of enveloped viruses are locked into a metastable conformation that is usually obtained through proteolytic processing of a precursor(s) (1, 25, 55). This processing has never been observed for HCV structural proteins. A second mechanism which can lock a conformation into an active state with a level of free energy higher than the thermodynamic minimum has been described for the α-lytic protease (56). In this case, the pro-region brings the protease into a metastable conformation, acting as a catalyst by stabilizing the folding transition state (56). Taken together with those of others, our data strongly suggest that either the core protein or E2 has the potential to direct E1 through a productive folding pathway, thus playing a role analogous to the pro-region of the α-lytic protease. In this context, E1OX generated from either IC-398 or E1-383 represents products of separate folding pathways: the first species has a kinetically trapped functional conformation, while the second has a dead-end conformation at its thermodynamic minimum that is not released from calnexin. This model can also explain previous reports that chaperone overexpression did not lead to any increase in the level of correctly folded E1-E2 complexes (5).
In our model, the core protein plays an important role in E1 folding. It will be important to define which region of the Core is involved. The core protein has been reported to be ER associated and to interact with the ER membrane through its internal hydrophobic domain (27, 43, 51). Furthermore, direct Core-E1 interactions through the carboxy-terminal regions of both proteins have been reported (35).
Our finding that E1 undergoes proper folding in the absence of E2 contrasts with previous data obtained using a recombinant vaccinia virus system to infect BHK-21 cells (40). These authors did not detect any oxidized E1 when expressing the Core-E1 region (40). Since our data do not support an absolute requirement of E2 for proper E1 folding, it is probable that the different expression systems are responsible for this discrepancy. More recently, use of a vaccinia virus system for infection in HepG2 cells has shown that oxidized E1 is generated very inefficiently in the absence of other HCV proteins (9). This observation is in agreement with our data, since we also found that E1 folding is severely impaired in the absence of the core protein.
E1 association with ER chaperones appears to fit with classical models of glycoprotein folding in which assistance is provided by the two lectin-like chaperones calnexin and calreticulin, and possibly by the ERp57 oxidoreductase (26). In E1 from the HCV-1 strain, only 4 out of the 5 glycosylation acceptor sequences (N-X-S/T) are used (data not shown). Three are located in the first 43 residues (amino acid positions 197, 209, and 234 of the polypeptide). We have shown that calnexin binds both the reduced and oxidized E1 species, whereas calreticulin appears to associate transiently with reduced E1 only. Contrary to what has been reported (5), we have been unable to detect any binding of E1 to BiP/Grp78. Our data are consistent with the cotranslational calnexin and/or calreticulin chaperone selection by nascent glycoproteins with N-linked glycans located within the first 50 NH2-terminal residues (42). Proteins that fulfill this criterion usually fold independently of BiP/Grp78. Although the two lectin-like chaperones possess identical oligosaccharide specificity (Glc1Man7–9NacGlc2), it has been shown that they differ partially in substrate specificity (21). As a soluble protein, calreticulin preferentially binds to residues easily accessible from the ER lumen, while membrane-proximal carbohydrates seem to be captured more efficiently by the membrane-bound calnexin. Our hypothesis is that E1 includes a lumen-oriented domain that folds rapidly, while the rest of the molecule remains close to the membrane and folds more slowly.
We found that under oxidizing conditions the Core-NS2 polypeptide generates an E2-p7 precursor that is further processed to full-length E2 within 2 to 4 h after synthesis in vitro. Intrachain disufide bond formation appears to start immediately after synthesis, as revealed by a mobility shift in nonreducing SDS-PAGE. Due to the overlapping migration of E2p7 precursor with some E2 oxidized forms, it is difficult to determine exactly how much time is necessary for complete oxidation. The same analysis conducted on the IC-746 and IC-661 products showed that cysteine oxidation was still occurring 4 to 6 h after blocking of protein synthesis. Previous studies using high-expression viral vectors (vaccinia virus) to study E2 folding in cells have reported it to be a very rapid process (less than 5 min) (11). Our data suggest that the rate of E2 folding is much slower (hours) and comparable to that of E1 in this low-expression system. These large differences cannot be explained by the fact that protein folding is 2 to 3 times slower in in vitro systems than in in vivo systems (20).
The use of recombinant proteins derived from infectious agents has begun to replace the more traditional approach to vaccine design, with great success (17). For HCV the use of a recombinant protein vaccine is an absolute requirement due to (i) the lack of a robust culture system for the virus and (ii) potential safety problems of an attenuated or killed HCV vaccine because the virus gives rise to chronic infection. A leading candidate vaccine for HCV consists of a recombinant E1/E2 heterodimer which is effective at preventing chronic infection in experimentally infected chimpanzees (Houghton and Abrignani, unpublished). Large-scale preparation of this recombinant vaccine is impaired by a number of obstacles; one of the most important is our incomplete knowledge of the expression, folding, and assembly of the E1/E2 heterodimer. Studies of the folding of HCV structural proteins have so far been carried out using overexpression systems driven mainly by vaccinia virus vectors (10, 32, 40, 49). Although this vector has been used successfully to transiently express high levels of foreign proteins in cells, the fate of such products may be altered by the contemporary presence of large amounts of vaccinia virus proteins, including factors that interfere with host metabolism (44). High expression levels of HCV proteins in cells have been reported to generate extensive formation of high-molecular-weight aggregates and cellular toxicity (15, 43). Using low-expression systems, such as in vitro translation and translocation, we found results differing from those reported previously using the more-standard high-expression systems. The differences in folding requirements for E1 and E2 in the two systems should provide clues to the natural folding and assembly of the E1/E2 heterodimer and how this might be optimized for vaccine production. Thus, further study of E1 and E2 folding, comparing both in vivo and in vitro systems, are warranted.
ACKNOWLEDGMENTS
We thank Maurizio Molinari, Ivo Lorentz, and Bruno Martoglio for fruitful discussions and generous gifts of reagents. We are grateful to Susanna Campagnoli for technical assistance and to Giorgio Corsi for artwork. We also thank Nicholas Valiante for critical review of the manuscript.
This work was partially funded by the Italian Ministry of University and Scientific and Technological Research (MURST 229605-1371/576).
REFERENCES
- 1.Baker D, Agard D A. Influenza hemagglutinin: kinetic control of protein function. Structure. 1994;2:907–910. doi: 10.1016/s0969-2126(94)00091-3. [DOI] [PubMed] [Google Scholar]
- 2.Blight K J, Kolykhalov A A, Rice C M. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1975. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 3.Bulleid N J, Freedman R B. Defective cotranslational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature. 1988;335:649–651. doi: 10.1038/335649a0. [DOI] [PubMed] [Google Scholar]
- 4.Choo Q L, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choukhi A, Ung S, Wychowski C, Dubuisson J. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J Virol. 1998;72:3851–3858. doi: 10.1128/jvi.72.5.3851-3858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung D H, Ohashi K, Watanabe M, Miyasaka N, Hirosawa S. Mannose trimming targets mutant alpha(2)-plasmin inhibitor for degradation by the proteasome. J Biol Chem. 2000;275:4981–4987. doi: 10.1074/jbc.275.7.4981. [DOI] [PubMed] [Google Scholar]
- 7.Depraetere S, Van Kerschaever E, Van Vlierberghe H, Elewaut A, Brouwer J T, Niesters H G, Schalm S W, Maertens G, Leroux-Roels G. Long term response to interferon treatment in chronic hepatitis C patients is associated with a significant reduction in anti-E1 envelope antibody titers. J Med Virol. 2000;60:126–132. [PubMed] [Google Scholar]
- 8.Doms R W, Lamb R A, Rose J K, Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993;193:545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- 9.Dubuisson J, Duvet S, Meunier J C, Op De Beeck A, Cacan R, Wychowski C, Cocquerel L. Glycosylation of the hepatitis C virus envelope protein E1 is dependent on the presence of a downstream sequence on the viral polyprotein. J Biol Chem. 2000;275:30605–30609. doi: 10.1074/jbc.M004326200. [DOI] [PubMed] [Google Scholar]
- 10.Dubuisson J, Hsu H H, Cheung R C, Greenberg H B, Russell D G, Rice C M. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J Virol. 1994;68:6147–6160. doi: 10.1128/jvi.68.10.6147-6160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubuisson J, Rice C M. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J Virol. 1996;70:778–786. doi: 10.1128/jvi.70.2.778-786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbein A D. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991;5:3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- 13.Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–1888. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 14.Frigerio L, Lord J M. Glycoprotein degradation: do sugars hold the key? Curr Biol. 2000;10:R674–R677. doi: 10.1016/s0960-9822(00)00680-1. [DOI] [PubMed] [Google Scholar]
- 15.Grakoui A, Wychowski C, Lin C, Feinstone S M, Rice C M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993;67:1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond C, Braakman I, Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci USA. 1994;91:913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansson M, Nygren P A, Stahl S. Design and production of recombinant subunit vaccines. Biotechnol Appl Biochem. 2000;32:95–107. doi: 10.1042/ba20000034. [DOI] [PubMed] [Google Scholar]
- 18.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 19.Hebert D N, Foellmer B, Helenius A. Calnexin and calreticulin promote folding, delay oligomerization and suppress degradation of influenza hemagglutinin in microsomes. EMBO J. 1996;15:2961–2968. [PMC free article] [PubMed] [Google Scholar]
- 20.Hebert D N, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- 21.Hebert D N, Zhang J X, Chen W, Foellmer B, Helenius A. The number and location of glycans on influenza hemagglutinin determine folding and association with calnexin and calreticulin. J Cell Biol. 1997;139:613–623. doi: 10.1083/jcb.139.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hebert D N, Zhang J X, Helenius A. Protein folding and maturation in a cell-free system. Biochem Cell Biol. 1998;76:867–873. doi: 10.1139/bcb-76-5-867. [DOI] [PubMed] [Google Scholar]
- 23.Hedley M L, Urban R G, Strominger J L. Assembly and peptide binding of major histocompatibility complex class II heterodimers in an in vitro translation system. Proc Natl Acad Sci USA. 1994;91:10479–10483. doi: 10.1073/pnas.91.22.10479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helenius A, Trombetta E S, Hebert D N, Simons J F. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997;7:193–200. doi: 10.1016/S0962-8924(97)01032-5. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez L D, Hoffman L R, Wolfsberg T G, White J M. Virus-cell and cell-cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 26.High S, Lecomte F J, Russell S J, Abell B M, Oliver J D. Glycoprotein folding in the endoplasmic reticulum: a tale of three chaperones? FEBS Lett. 2000;476:38–41. doi: 10.1016/s0014-5793(00)01666-5. [DOI] [PubMed] [Google Scholar]
- 27.Hijikata M, Kato N, Ootsuyama Y, Nakagawa M, Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci USA. 1991;88:5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hijikata M, Mizushima H, Akagi T, Mori S, Kakiuchi N, Kato N, Tanaka T, Kimura K, Shimotohno K. Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus. J Virol. 1993;67:4665–4675. doi: 10.1128/jvi.67.8.4665-4675.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houghton M. Hepatitis C viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1035–1058. .. [Google Scholar]
- 30.Huppa J B, Ploegh H L. In vitro translation and assembly of a complete T cell receptor-CD3 complex. J Exp Med. 1997;186:393–403. doi: 10.1084/jem.186.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaderbhai M A, Austen B M. Studies on the formation of intrachain disulphide bonds in newly biosynthesised bovine prolactin. Role of protein-disulphide isomerase. Eur J Biochem. 1985;153:167–178. doi: 10.1111/j.1432-1033.1985.tb09283.x. [DOI] [PubMed] [Google Scholar]
- 32.Lanford R E, Notvall L, Chavez D, White R, Frenzel G, Simonsen C, Kim J. Analysis of hepatitis C virus capsid, E1, and E2/NS1 proteins expressed in insect cells. Virology. 1993;197:225–235. doi: 10.1006/viro.1993.1583. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Luo L, Thomas D Y, Kang C Y. The HIV-1 Env protein signal sequence retards its cleavage and down-regulates the glycoprotein folding. Virology. 2000;272:417–428. doi: 10.1006/viro.2000.0357. [DOI] [PubMed] [Google Scholar]
- 34.Lin C, Lindenbach B D, Pragai B M, McCourt D W, Rice C M. Processing in the hepatitis C virus E2–NS2 region: identification of p7 and two distinct E2-specific products with different C termini. J Virol. 1994;68:5063–5073. doi: 10.1128/jvi.68.8.5063-5073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo S Y, Selby M J, Ou J H. Interaction between hepatitis C virus core protein and E1 envelope protein. J Virol. 1996;70:5177–5182. doi: 10.1128/jvi.70.8.5177-5182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 37.Marquardt T, Hebert D N, Helenius A. Post-translational folding of influenza hemagglutinin in isolated endoplasmic reticulum-derived microsomes. J Biol Chem. 1993;268:19618–19625. [PubMed] [Google Scholar]
- 38.Martoglio B, Dobberstein B. Signal sequences: more than just greasy peptides. Trends Cell Biol. 1998;8:410–415. doi: 10.1016/s0962-8924(98)01360-9. [DOI] [PubMed] [Google Scholar]
- 39.Matsuura Y, Suzuki T, Suzuki R, Sato M, Aizaki H, Saito I, Miyamura T. Processing of E1 and E2 glycoproteins of hepatitis C virus expressed in mammalian and insect cells. Virology. 1994;205:141–150. doi: 10.1006/viro.1994.1629. [DOI] [PubMed] [Google Scholar]
- 40.Michalak J P, Wychowski C, Choukhi A, Meunier J C, Ung S, Rice C M, Dubuisson J. Characterization of truncated forms of hepatitis C virus glycoproteins. J Gen Virol. 1997;78:2299–2306. doi: 10.1099/0022-1317-78-9-2299. [DOI] [PubMed] [Google Scholar]
- 41.Mizushima H, Hijikata M, Tanji Y, Kimura K, Shimotohno K. Analysis of N-terminal processing of hepatitis C virus nonstructural protein 2. J Virol. 1994;68:2731–2734. doi: 10.1128/jvi.68.4.2731-2734.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molinari M, Helenius A. Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science. 2000;288:331–333. doi: 10.1126/science.288.5464.331. [DOI] [PubMed] [Google Scholar]
- 43.Moradpour D, Kary P, Rice C M, Blum H E. Continuous human cell lines inducibly expressing hepatitis C virus structural and nonstructural proteins. Hepatology. 1998;28:192–201. doi: 10.1002/hep.510280125. [DOI] [PubMed] [Google Scholar]
- 44.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2637–2671. [Google Scholar]
- 45.Moss B, Elroy-Stein O, Mizukami T, Alexander W A, Fuerst T R. Product review. New mammalian expression vectors. Nature. 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 46.Parodi A J. Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation. Biochem J. 2000;348:1–13. [PMC free article] [PubMed] [Google Scholar]
- 47.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner A J, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 48.Qu D, Teckman J H, Omura S, Perlmutter D H. Degradation of a mutant secretory protein, α1-antitrypsin Z, in the endoplasmic reticulum requires proteasome activity. J Biol Chem. 1996;271:22791–22795. doi: 10.1074/jbc.271.37.22791. [DOI] [PubMed] [Google Scholar]
- 49.Ralston R, Thudium K, Berger K, Kuo C, Gervase B, Hall J, Selby M, Kuo G, Houghton M, Choo Q L. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia viruses. J Virol. 1993;67:6753–6761. doi: 10.1128/jvi.67.11.6753-6761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosa D, Campagnoli S, Moretto C, Guenzi E, Cousens L, Chin M, Dong C, Weiner A J, Lau J Y, Choo Q L, Chien D, Pileri P, Houghton M, Abrignani S. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93:1759–1763. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santolini E, Migliaccio G, La Monica N. Biosynthesis and biochemical properties of the hepatitis C virus core protein. J Virol. 1994;68:3631–3641. doi: 10.1128/jvi.68.6.3631-3641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santolini E, Pacini L, Fipaldini C, Migliaccio G, Monica N. The NS2 protein of hepatitis C virus is a transmembrane polypeptide. J Virol. 1995;69:7461–7471. doi: 10.1128/jvi.69.12.7461-7471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheele G, Jacoby R. Conformational changes associated with proteolytic processing of presecretory proteins allow glutathione-catalyzed formation of native disulfide bonds. J Biol Chem. 1982;257:12277–12282. [PubMed] [Google Scholar]
- 54.Selby M J, Choo Q L, Berger K, Kuo G, Glazer E, Eckart M, Lee C, Chien D, Kuo C, Houghton M. Expression, identification and subcellular localization of the proteins encoded by the hepatitis C viral genome. J Gen Virol. 1993;74:1103–1113. doi: 10.1099/0022-1317-74-6-1103. [DOI] [PubMed] [Google Scholar]
- 55.Skehel J J, Wiley D C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 56.Sohl J L, Jaswal S S, Agard D A. Unfolded conformations of alpha-lytic protease are more stable than its native state. Nature. 1998;395:817–819. doi: 10.1038/27470. [DOI] [PubMed] [Google Scholar]
- 57.Spaete R R, Alexander D, Rugroden M E, Choo Q L, Berger K, Crawford K, Kuo C, Leng S, Lee C, Ralston R, et al. Characterization of the hepatitis C virus E2/NS1 gene product expressed in mammalian cells. Virology. 1992;188:819–830. doi: 10.1016/0042-6822(92)90537-y. [DOI] [PubMed] [Google Scholar]
- 58.Stroud R M, Walter P. Signal sequence recognition and protein targeting. Curr Opin Struct Biol. 1999;9:754–759. doi: 10.1016/s0959-440x(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 59.Walter P, Johnson A E. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- 60.Zapun A, Jakob C A, Thomas D Y, Bergeron J J. Protein folding in a specialized compartment: the endoplasmic reticulum. Structure Fold Des. 1999;7:R173–R182. doi: 10.1016/s0969-2126(99)80112-9. [DOI] [PubMed] [Google Scholar]