Abstract
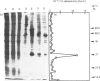
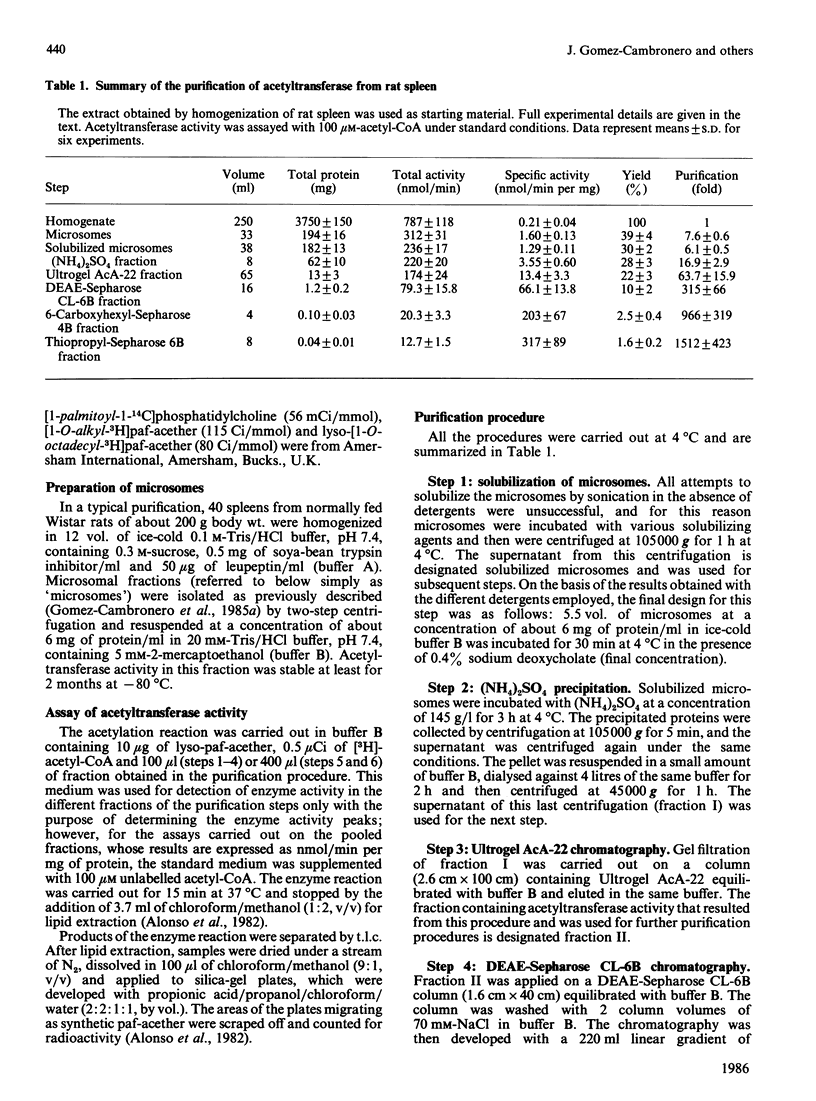
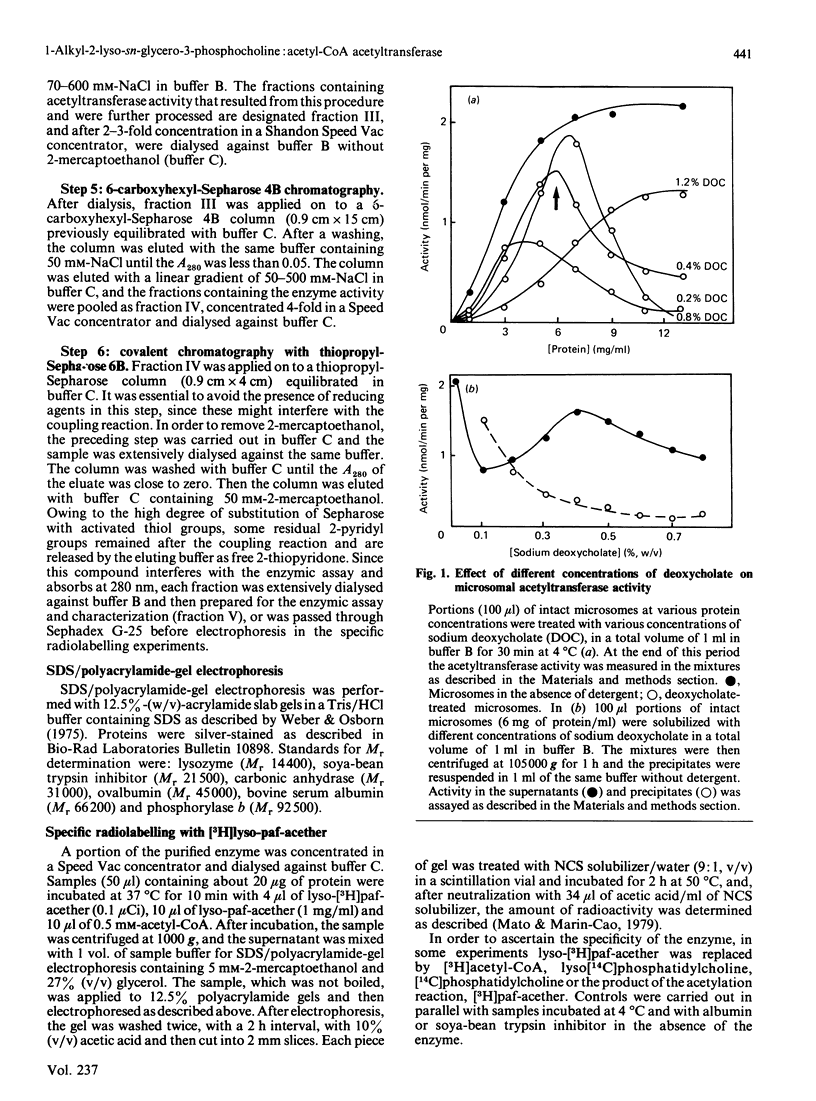
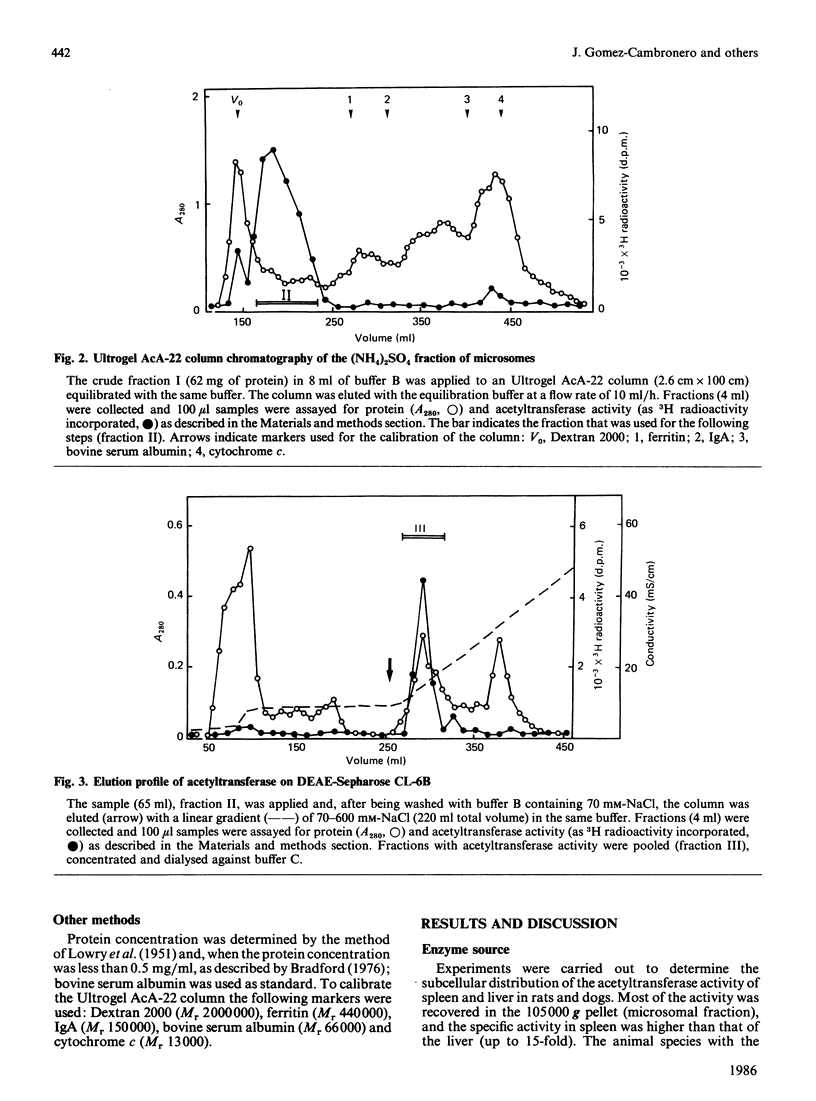
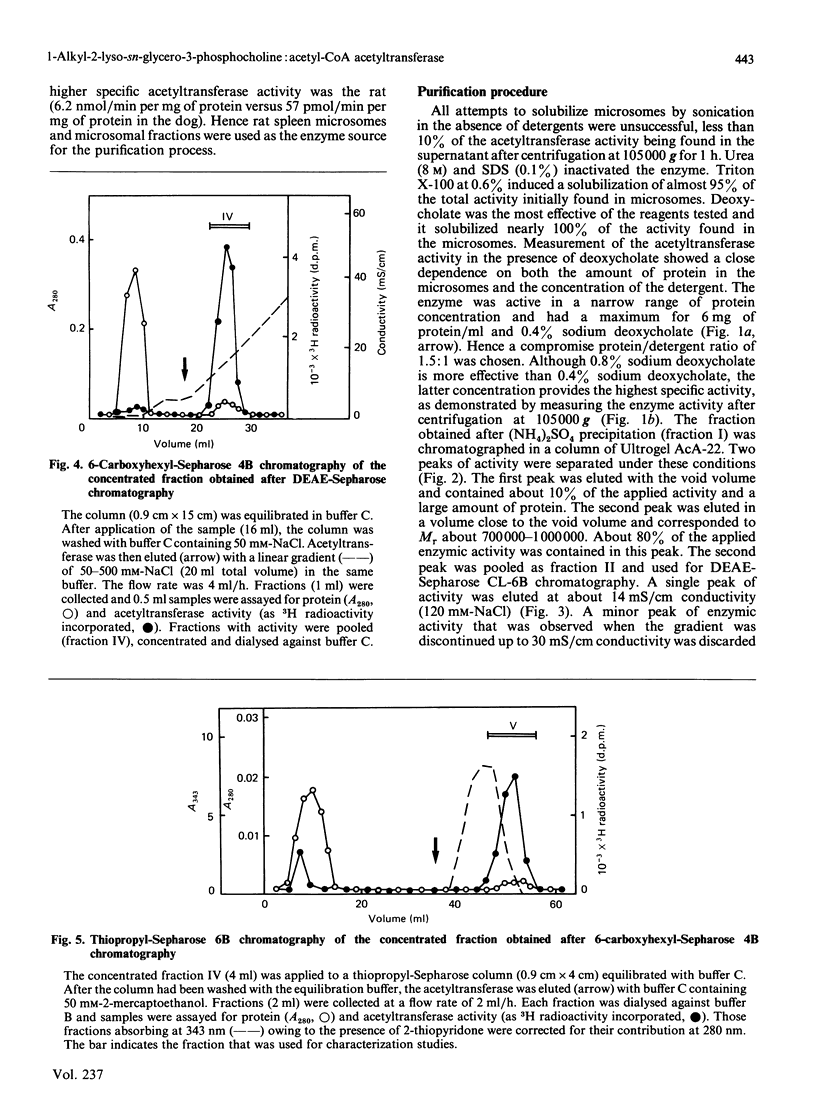
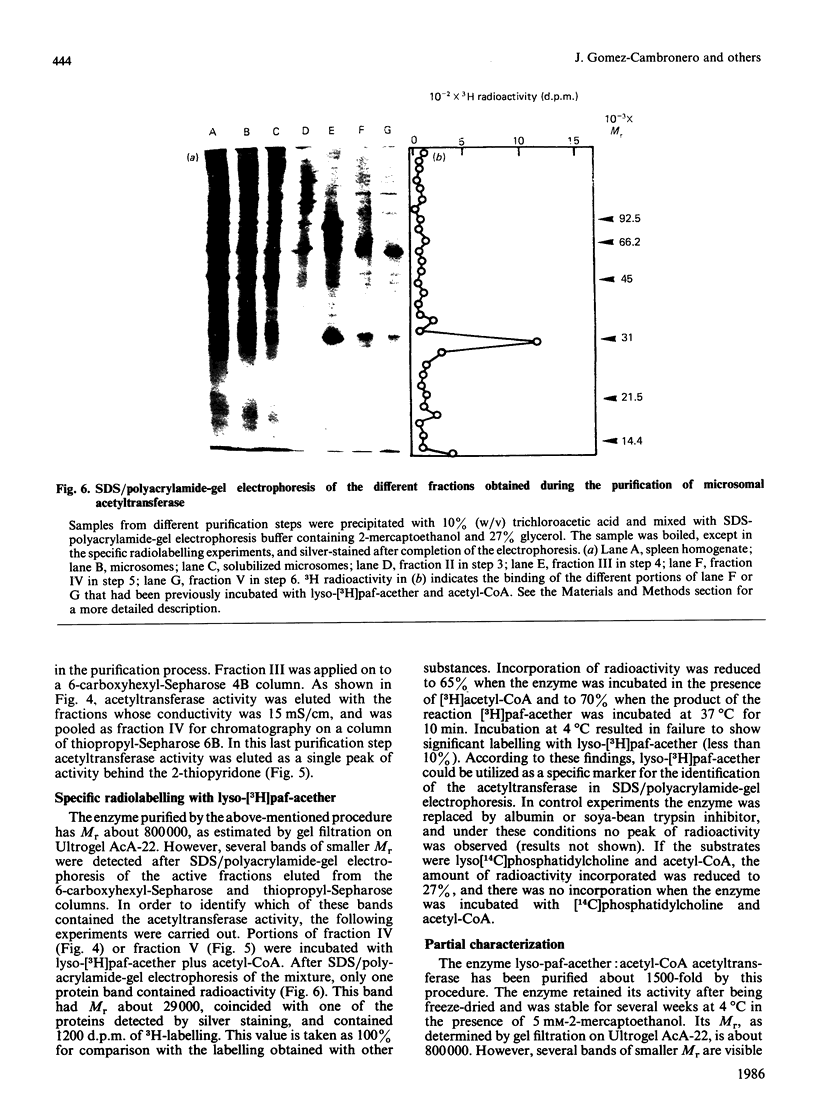
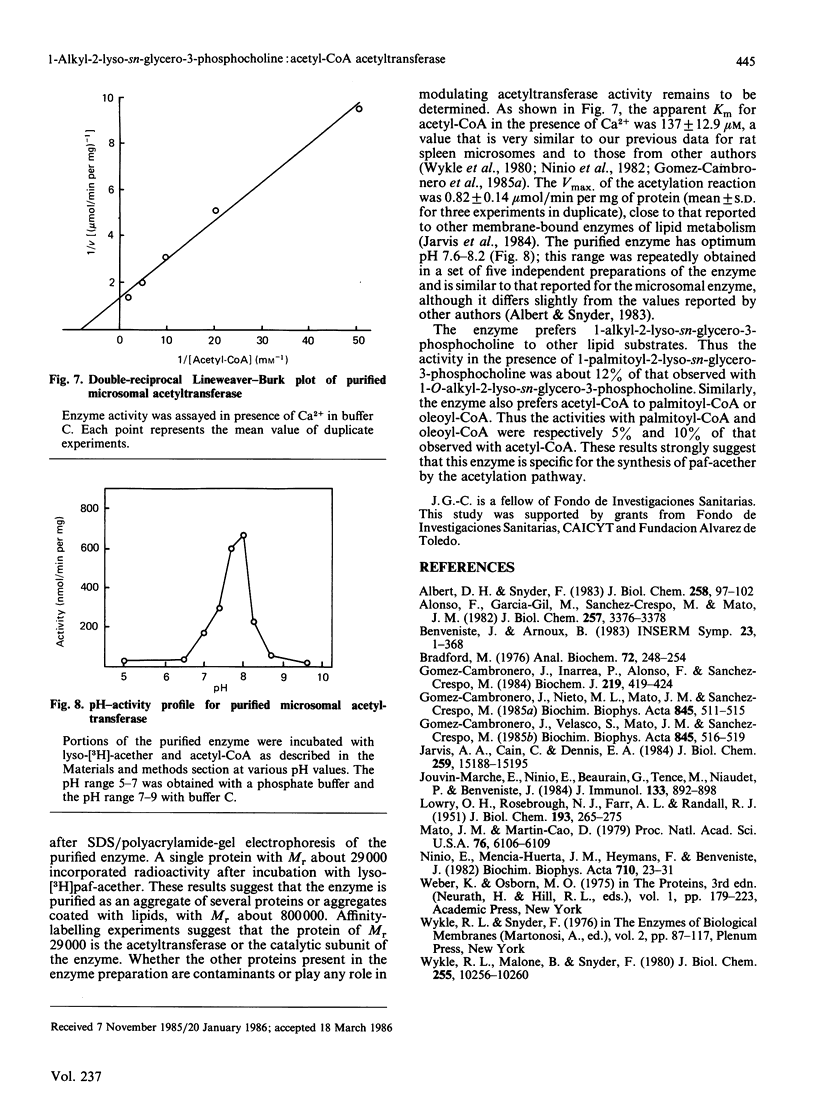
The enzyme 1-O-alkyl-2-lyso-sn-glycero-3-phosphocholine: acetyl-CoA acetyltransferase (EC 2.3.1.67) was purified from rat spleen approx. 1500-fold in 1.6% yield. The specific activity of the purified enzyme was 0.317 +/- 0.089 mumol/min per mg of protein (mean +/- S.D., n = 6). The Km for the substrate acetyl-CoA was 137 +/- 13 microM and the pH optimum was about 8. Incubation of the purified enzyme was 1-O-[3H]octadecyl-2-lyso-sn-glycero-3-phosphocholine followed by electrophoresis resulted in the incorporation of radioactivity into a protein of Mr 29,000. The enzyme was most active towards 1-O-alkyl-2-lyso-sn-glycero-3-phosphocholine as substrate, 1-palmitoyl-2-lyso-glycero-3-phosphocholine being a poor substrate. In addition, the enzyme preferred acetyl-CoA to palmitoyl-CoA or oleoyl-CoA as substrate.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. H., Snyder F. Biosynthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) from 1-alkyl-2-acyl-sn-glycero-3-phosphocholine by rat alveolar macrophages. Phospholipase A2 and acetyltransferase activities during phagocytosis and ionophore stimulation. J Biol Chem. 1983 Jan 10;258(1):97–102. [PubMed] [Google Scholar]
- Alonso F., Gil M. G., Sánchez-Crespo M., Mato J. M. Activation of 1-alkyl-2-lysoglycero-3-phosphocholine. Acetyl-CoA transferase during phagocytosis in human polymorphonuclear leukocytes. J Biol Chem. 1982 Apr 10;257(7):3376–3378. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gómez-Cambronero J., Iñarrea P., Alonso F., Sánchez Crespo M. The role of calcium ions in the process of acetyltransferase activation during the formation of platelet-activating factor (PAF-acether). Biochem J. 1984 Apr 15;219(2):419–424. doi: 10.1042/bj2190419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Cambronero J., Nieto M. L., Mato J. M., Sánchez-Crespo M. Modulation of lyso-platelet-activating factor: acetyl-CoA acetyltransferase from rat splenic microsomes. The role of calcium ions. Biochim Biophys Acta. 1985 Jun 30;845(3):511–515. doi: 10.1016/0167-4889(85)90218-6. [DOI] [PubMed] [Google Scholar]
- Gómez-Cambronero J., Velasco S., Mato J. M., Sánchez-Crespo M. Modulation of lyso-platelet activating factor: acetyl-CoA acetyltransferase from rat splenic microsomes. The role of cyclic AMP-dependent protein kinase. Biochim Biophys Acta. 1985 Jun 30;845(3):516–519. doi: 10.1016/0167-4889(85)90219-8. [DOI] [PubMed] [Google Scholar]
- Jarvis A. A., Cain C., Dennis E. A. Purification and characterization of a lysophospholipase from human amnionic membranes. J Biol Chem. 1984 Dec 25;259(24):15188–15195. [PubMed] [Google Scholar]
- Jouvin-Marche E., Ninio E., Beaurain G., Tence M., Niaudet P., Benveniste J. Biosynthesis of Paf-acether (platelet-activating factor). VII. Precursors of Paf-acether and acetyl-transferase activity in human leukocytes. J Immunol. 1984 Aug;133(2):892–898. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mato J. M., Marín-Cao D. Protein and phospholipid methylation during chemotaxis in Dictyostelium discoideum and its relationship to calcium movements. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6106–6109. doi: 10.1073/pnas.76.12.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio E., Mencia-Huerta J. M., Heymans F., Benveniste J. Biosynthesis of platelet-activating factor. I. Evidence for an acetyl-transferase activity in murine macrophages. Biochim Biophys Acta. 1982 Jan 15;710(1):23–31. doi: 10.1016/0005-2760(82)90185-0. [DOI] [PubMed] [Google Scholar]
- Wykle R. L., Malone B., Snyder F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J Biol Chem. 1980 Nov 10;255(21):10256–10260. [PubMed] [Google Scholar]