Abstract
As the global prevalence of chronic liver disease continues to rise, the need to determine which patients will develop end-stage liver disease and require liver transplantation is increasingly important. However, current prognostic models perform sub-optimally. We aim to determine microRNA profiles associated with clinical decompensation and mortality/transplantation within 1 year. We examined microRNA expression profiles in plasma samples from patients across the spectrum of cirrhosis (n = 154), acute liver failure (ALF) (n = 22), sepsis (n = 20) and healthy controls (HC) (n = 20). We demonstrated that a microRNA-based model (miR-24 and -27a) associated with systemic inflammation differentiated decompensated cirrhosis states from compensated cirrhosis and HC (AUC 0.77 (95% CI 0.69–0.85)). 6 patients within the compensated cirrhosis group decompensated the subsequent year and their exclusion improved model performance (AUC 0.81 (95% CI 0.71–0.89)). miR-191 (associated with liver injury) predicted risk of mortality across the cohort when acutely decompensated and acute-on-chronic-liver failure patients were included. When they were excluded miR-24 (associated with systemic inflammation) predicted risk of mortality. Our findings demonstrate that microRNA associated with systemic inflammation and liver injury predict adverse outcomes in cirrhosis. miR-24 and -191 require further investigation as prognostic biomarkers and therapeutic targets for patients with liver disease.
Subject terms: Biomarkers, Hepatology
Introduction
Morbidity and mortality from chronic liver disease (CLD) has become an international health crisis. CLD significantly impacts people of working age with alcohol-related liver disease (ARLD) the predominant etiology of patients who develop decompensated cirrhosis within Europe1,2. The transition through the development of cirrhosis to clinically significant portal hypertension and subsequent decompensation is associated with increased morbidity and mortality3. The PREDICT study demonstrated that following acute decompensation (AD), patients followed three distinct clinical pathways, including the development of acute-on-chronic liver failure (ACLF), with each pathway having a significantly different risk of mortality4. Whilst there are effective treatments for specific etiologies of liver disease, the only effective treatment for end-stage cirrhosis remains liver transplantation (LT). Accurate prediction of progression through this pathway to poor prognostic outcomes remains elusive.
There has been extensive work undertaken to determine novel biomarkers which predict which patients with cirrhosis are at risk of clinical decompensation. However, to this point, none have outperformed well-established models which include standard clinical and laboratory data and perform most optimally over longer-term follow-up5,6. Following decompensation, conventional prognostic models focus on clinical and biochemical markers of liver failure to predict mortality7–9. With the development of classifications for ACLF and AD, novel models which integrate conventional markers of liver and non-liver organ failure, inflammation and patient age have been established which improve prognostication within these specific subgroups of cirrhosis10,11. However, these prognostic models again perform optimally later in the patient’s clinical course, limiting their clinical value. There is a need for novel biomarkers that predict decompensation and mortality in patients with cirrhosis earlier in their clinical course.
MicroRNA (miRNA) have long been heralded as potential biomarkers across a range of clinical conditions including CLD12–16. However, there has been limited translation into routine clinical practice. This likely reflects a lack of standardized methodology in quantifying miRNA expression as well as the complex pathways that govern miRNA expression. We have previously demonstrated that miRNA expression is time dependent in acute liver failure (ALF)17. A previous study exploring miRNA expression in patients with cirrhosis following an acute decompensation event showed that grouping patients with similar characteristics likely improves miRNA performance as prognostic biomarkers12. A standardized methodological approach examining miRNA expression in carefully defined cohorts at set time points may potentially improve the performance of miRNA as clinically tractable biomarkers.
We have previously described a distinct hepatic miRNA signature associated with successful native liver regeneration following auxiliary LT (ALT)18. This miRNA signature is present in the circulation of patients who recover from ALF and cirrhotic patients who clinically recompensate following direct-acting antiviral therapy (DAA) for hepatitis C (HCV)19. We have integrated this regeneration-linked miRNA signature within a prognostic model for ALF which outperformed conventional models as opposed to microRNA associated with cell-death and response to cellular injury which did not17. We hypothesized that circulating miRNA may have utility in predicting clinical decompensation and prognosis in patients with cirrhosis. We investigated whether miRNA-based models can identify decompensated cirrhosis states and those at risk of clinical decompensation. In addition, we investigated whether these miRNA have the potential to be developed as biomarkers to predict 1 year risk of mortality/transplantation for patients with cirrhosis.
Results
Plasma miRNA expression across the spectrum of cirrhosis, ALF, sepsis and HC
Minimal hemolysis was observed across samples (8/216) and there was a stable expression of quality control markers for RNA isolation, RT and PCR efficiency (Supplementary Figs. 1, 2). No samples were excluded from further analysis. All samples expressed the normalizing miRNA (miR-23a, -26a and -103) therefore all samples were included in the study.
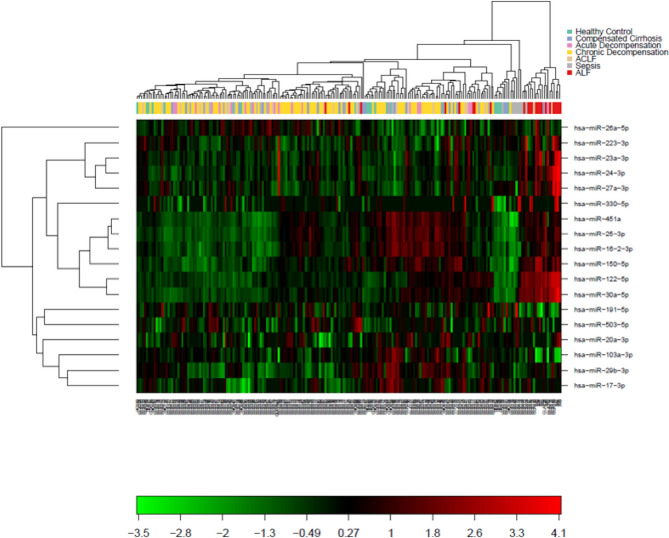
Across clinical groups, 18 miRNA were expressed in greater than a third of samples and these were used to perform two-way unsupervised hierarchical clustering (Fig. 1). Whilst the ALF and sepsis clinical cohorts clustered based on miRNA expression, the cirrhosis groups (ACLF/AD/cCLD/dCLD) and HCs did not.
Fig. 1.
Heatmap and 2-way hierarchical clustering of miRNA expression across all patient groups. Clustering was performed on all samples including all miRNA expressed in more than a third of samples.
miRNA expression profiles of patients with acute presentations of liver disease and sepsis
Clinical, demographic and laboratory data from patients within ACLF, AD, ALF and sepsis cohorts are shown in Supplementary Table 3. There were several differences between groups; namely patient age (ALF patients youngest and sepsis patients oldest), laboratory biomarkers of infection and antibiotic use (highest in sepsis group although notably 19/20 of the ACLF cohort (11.76% positive bacterial cultures within 72 h) and 15/20 of the AD cohort (5.56% positive bacterial cultures within 72 h) were being treated for infection), biomarkers of liver injury/failure and MELD score (highest in the ALF group) and need for organ support (lowest in the AD group).
There was significant variation in the expression of the following miRNA; miR-122, -150, -16-2, -191, -24, -25, -26a, -30a and -451a (Supplementary Table 4A). Following conversion of less prevalently expressed miRNA into categorical variables, miR-503 expression was demonstrated to vary significantly across clinical groups (Supplementary Table 4B).
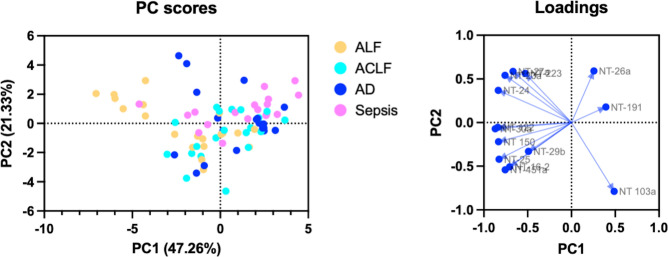
Principle component analysis was utilized to identify clustering of prevalently expressed miRNA to understand expression profiles in AD and ACLF states compared with ALF and sepsis states (Fig. 2). Patients with ALF and sepsis had distinct, divergent expression profiles. Patients with ACLF associated more with the sepsis cohort rather than ALF based on miRNA expression.
Fig. 2.
Principle component analysis of prevalently expressed miRNA in patients with ACLF, AD, ALF and sepsis. Presented with loadings.
miRNA expression profiles of patients across the cirrhosis spectrum
Comparisons of clinical, demographic and laboratory data from patients with ACLF, AD, cCLD, dCLD and HC are shown in Supplementary Table 5. HC individuals were younger than patients within the cirrhosis groups. Active alcohol consumption was highest amongst patients with acute presentations of cirrhosis (ACLF/AD). Markers of systemic inflammation and infection (white cell counts (WCC) and CRP), hepatic synthetic failure (bilirubin, INR, ascites and ammonia), hepatic inflammation (aspartate aminotransferase (AST)) and renal impairment (creatinine) were highest in the ACLF cohort.
Significant variations in expression across patient groups were demonstrated across a number of miRNA. Notably, acute presentations of cirrhosis had opposing expression profiles to the HC and cCLD cohorts, with the most significant being for miR-122, -24 and -30a (Supplementary Table 6A and Supplementary Fig. 3). However, there were opposing expression profiles of certain miRNA across the decompensated CLD groups including; miR-223 (dCLD and ACLF), -23a (dCLD and ACLF/AD), -26a (ACLF and AD) and -103a (AD and ACLF/dCLD) (Supplementary Table 6A and Supplementary Fig. 3). There was significant variation in the detection of miR-503 across the cohorts with the lowest rate of detection in the AD cohort (Supplementary Table 6B).
Determining miRNA associated with decompensated CLD states
Decompensated CLD states (ACLF/AD/dCLD) were grouped together and miRNA expression profiles were compared to cCLD/HC cohort (Table 1A + B). Significant differences in miRNA expression between groups were observed for miR-122, -223, -24, -27a, -29b, -30a, -330 and -663. The best performing prevalently expressed miRNA at discriminating patients with decompensated CLD states from those without was miR-27a (AUC 0.67 (95% CI 0.58–0.76)). Conversion of miRNA into categorical variables did not identify further miRNA which discriminated between patient with decompensated states and those without.
Table 1.
miRNA expression in decompensated cirrhosis states and HC/cCLD.
| miRNA | N | Decompensated (n = 134) | N | cCLD/HC (n = 40) | p value | AUC |
|---|---|---|---|---|---|---|
| (A) | ||||||
| miR-103a | 134 | 298.72 (2.36) | 40 | 298.39 (2.07) | 0.43 | |
| miR-122 | 134 | 278.29 (12.02) | 40 | 272.35 (15.73) | 0.01* | 0.62 (0.51–0.73) |
| miR-150 | 134 | 280.95 (7.97) | 40 | 280.56 (9.15) | 0.79 | |
| miR-16-2 | 134 | 269.62 (9.15) | 39 | 269.15 (9.51) | 0.78 | |
| miR-17 | 113 | 243.87 (11.45) | 36 | 246.23 (13.34) | 0.30 | |
| miR-191 | 131 | 295.54 (1.10) | 39 | 295.37 (1.14) | 0.40 | |
| miR-200b | 19 | 236.38 (23.35) | 5 | 224.04 (25.23) | 0.31 | |
| miR-20a | 101 | 247.91 (6.86) | 35 | 249.25 (4.83) | 0.29 | |
| miR-223 | 134 | 310.35 (1.78) | 40 | 311.09 (1.58) | 0.02* | 0.63 (0.53–0.73) |
| miR-23a | 134 | 302.94 (1.55) | 40 | 302.90 (1.86) | 0.90 | |
| miR-24 | 134 | 302.60 (1.80) | 40 | 301.89 (1.90) | 0.03* | 0.62 (0.52–0.73) |
| miR-25 | 134 | 299.73 (6.30) | 40 | 297.51 (6.85) | 0.06 | |
| miR-26a | 134 | 296.91 (1.68) | 40 | 297.28 (1.76) | 0.22 | |
| miR-27a | 134 | 294.65 (2.08) | 40 | 295.69 (1.81) | 0.005* | 0.67 (0.58–0.76) |
| miR-29b | 134 | 261.77 (8.68) | 40 | 266.01 (6.86) | 0.005* | 0.65 (0.56–0.74) |
| miR-30a | 134 | 272.43 (8.27) | 40 | 268.23 (11.14) | 0.01* | 0.63 (0.52–0.74) |
| miR-330 | 45 | 226.72 (12.69) | 19 | 216.51 (14.51) | 0.006* | 0.72 (0.58–0.87) |
| miR-451a | 134 | 321.18 (6.02) | 40 | 321.16 (6.02) | 0.99 | |
| miR-503 | 102 | 251.84 (7.58) | 35 | 249.04 (7.09) | 0.06 | |
| miR-663 | 9 | 231.85 (19.60) | 3 | 192.46 (35.28) | 0.03* | 0.85 (0.62–1.00) |
| miRNA | Decompensated (n = 134) | Compensated (n = 40) | p value | |||
|---|---|---|---|---|---|---|
| (B) | ||||||
| miR-149 | 2 (1.49%) | 0 (0.00%) | > 0.9999 | |||
| miR-17 | 113 (84.33%) | 36 (90%) | 0.45 | |||
| miR-200b | 19 (14.18%) | 5 (12.50%) | > 0.9999 | |||
| miR-20a | 101 (75.4%) | 35 (87.50%) | 0.13 | |||
| miR-330 | 45 (33.58%) | 19 (47.5%) | 0.14 | |||
| miR-503 | 102 (76.12%) | 35 (87.50%) | 0.19 | |||
| miR-663 | 9 (6.72%) | 3 (7.50%) | > 0.9999 | |||
| miRNA | β value | OR | p value | |||
|---|---|---|---|---|---|---|
| (C) | ||||||
| miR-24 | 0.6491 | 1.91 (1.45–2.62) | < 0.0001* | |||
| miR-27a | − 0.6201 | 0.54 (0.41–0.69) | < 0.0001* | |||
(A) Results of t tests between patients with decompensated CLD states and HC/cCLD. Results given as mean (SD). If p < 0.05, AUC was calculated. (B) For miRNA detected in < 85% of samples, reanalysis was performed after converting results to either detected (1) or undetected (0) using Fisher’s exact test. Results given as number (%). (C) miRNA model utilized to distinguish decompensated CLD (n = 174, AUC 0.77 (95% CI 0.69-0.85, p < 0.0001*); pseudo r2 = 0.1849, HL stat 8.95 (p = 0.35). Statistical significance set after correction for false discovery with the Benjamini-Hochberg procedure (p < 0.0344) and designated by *.
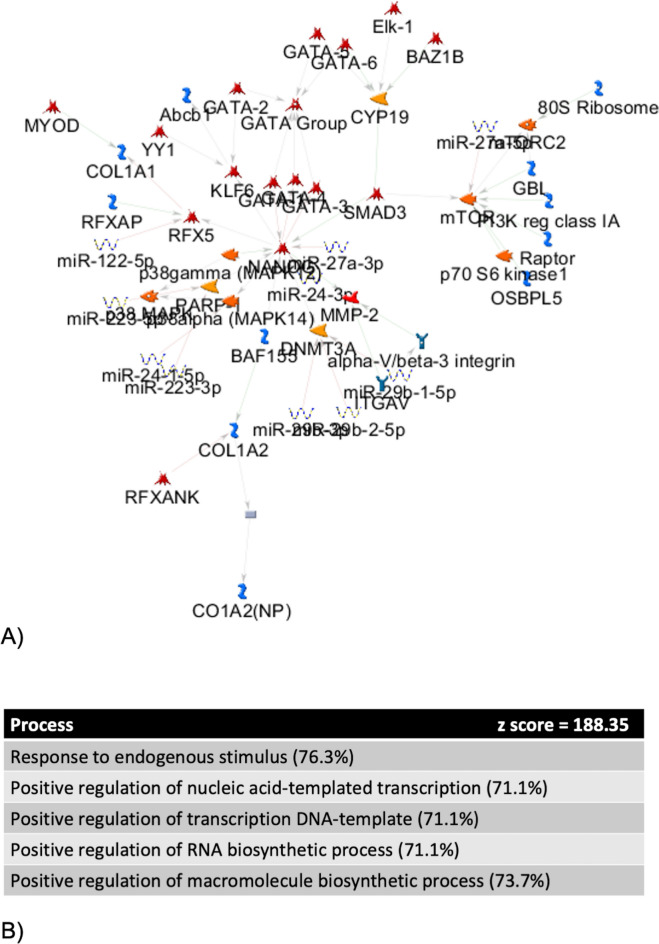
MetaCore™ pathway analysis was utilized to identify biological pathways regulated by the miRNA which discriminated patients with decompensated CLD states from those without (miR-122, -223, -24, -27a, -29b and -30a). This demonstrated that these miRNA regulate the response to endogenous stimuli, nucleic acid transcription, RNA and macromolecule biosynthesis (Fig. 3), which are key processes in the response to systemic inflammation. Within the network were genes implicated with progression of liver fibrosis/cirrhosis (MMP-2, GATA family, COL120–22). NANOG, a gene with key roles in promoting stem cell pluripotency and suppressing apoptosis23–25, was central to the network and upregulated.
Fig. 3.
MetaCore™ pathway analysis of prevalently expressed miRNA differentiating decompensated cirrhosis states from cCLD and HC. (A) Network of gene and miRNA expression from MetaCore™ pathway analysis of miRNA which discriminated between compensated and decompensated states. Thick cyan lines indicate fragments of canonical pathways. Up-regulated genes are marked with red circles. Down-regulated genes are marked with blue circles. ‘Checkerboard’ color indicates mixed expression for the gene between files or between multiple tags of the same gene. (B) Processes associated with miRNA from this signature.
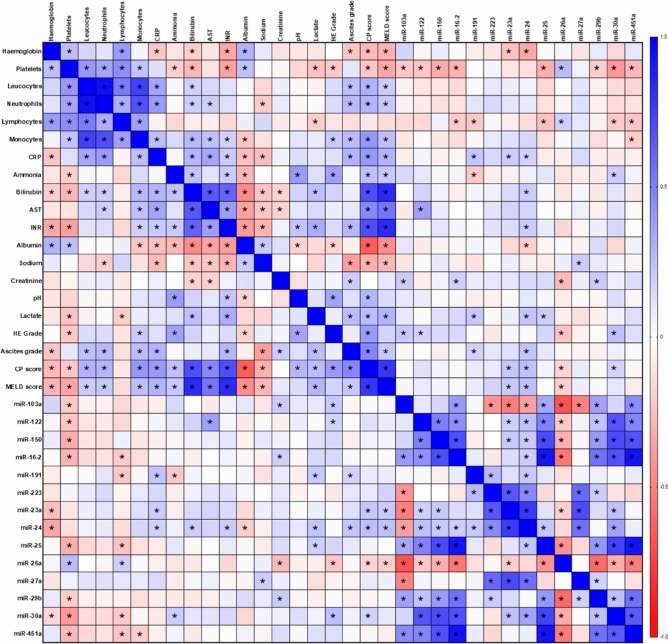
A Spearman’s correlation matrix was utilized to further understand relationships between miRNA and clinical, laboratory and prognostic model data across the cirrhosis cohorts (Fig. 4) with the aim to investigate the relationship between these miRNA and systemic inflammation. Significant correlations with CP and MELD scores were seen with miR-23a (rs = 0.21 (95% CI 0.05–0.36), p = 0.01*), -24 (rs = 0.25 (95% CI 0.09–0.40), p = 0.002*) and -26a (rs = − 0.19 (95% CI − 0.34 to − 0.02), p = 0.02*). miR-24 was also demonstrated to significantly correlate with markers of inflammation (CRP, rs = 0.18 (95% CI 0.01–0.33), p = 0.03*), portal hypertension (ascites grade, rs = 0.23 (95% CI 0.07–0.38), p = 0.004*) and liver failure (bilirubin (rs = 0.19 (95% CI 0.03–0.35), p = 0.02*), INR (rs = 0.20 (95% CI 0.04–0.35), p = 0.01*), albumin (rs = − 0.19 (95% CI − 0.34 to − 0.03), p = 0.02*) and lactate (rs = 0.23 (95% CI 0.05–0.39), p = 0.008*)). miR-26a demonstrated significant correlations with markers of portal hypertension (platelet count (rs = 0.27 (95% CI 0.11–0.41), p = 0.001*) and HE (rs = − 0.23 (95% CI − 0.38 to − 0.07), p = 0.004*) and inflammation (lymphocyte count (rs = 0.19 (95% CI 0.03–0.34), p = 0.02*)). miR-23a demonstrated significant correlation with CRP (rs = 0.20 (95% CI 0.04–0.36), p = 0.01*). miR-30a demonstrated significant correlations with CP score (rs = 0.17 (95% CI 0.01–0.33), p = 0.04*), inflammation (lymphocyte count (rs = − 0.21 (95% CI − 0.36 to − 0.05), p = 0.009*)) and markers of portal hypertension (platelet count (rs = − 0.40 (95% CI − 0.53 to − 0.26), p < 0.0001*) and ammonia (rs = 0.20 (95% CI 0.02–0.37), p = 0.02*). miR-191 demonstrated significant correlation with markers of inflammation (CRP (rs = 0.23 (95% CI 0.07–0.38), p = 0.005*, lymphocyte (rs = − 0.22 (95% CI − 0.37 to − 0.05), p = 0.008*) and liver failure (lactate (rs = 0.22 (95% CI 0.04–0.38), p = 0.01*, ammonia (rs = − 0.21 (95% CI − 0.37 to − 0.03), p = 0.02*, and ascites grade (rs = 0.19 (95% 0.01–0.33 CI), p = 0.03*).
Fig. 4.
Spearman’s rank correlation matrix of miRNA expression with clinical and laboratory variables. Statistical significance set after correction for false discovery with the Benjamini–Hochberg procedure (p < 0.0344) and designated by *.
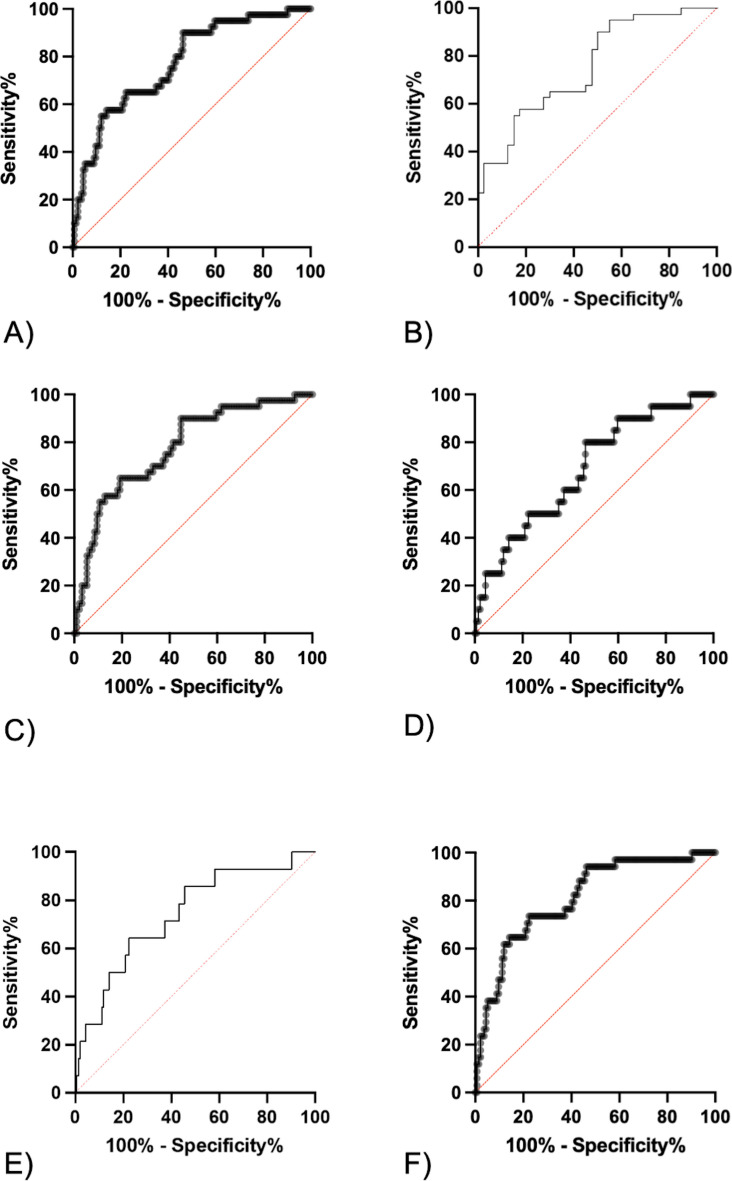
Multiple logistic regression was utilized to generate a microRNA-based model to discriminate patients with decompensated cirrhosis states from those without (Table 1C, Fig. 5A). This included miR-24 and -27a and generated an AUC of 0.77 (95%CI 0.69–0.85, p < 0.0001*). Given the heterogeneity of the cohorts, sensitivity analyses were performed to ensure that performance was maintained across patient groups. Excluding acute (ACLF/AD; AUC 0.78 (95% CI 0.69–0.86)) and chronic (dCLD; AUC 0.76 (95% CI 0.65–0.86) decompensation patient groups did not impact on model performance (Fig. 5B + C). However, excluding the HC cohort did reduce the model performance (AUC 0.69 (95% CI 0.56–0.81) (Fig. 5D). Further interrogation of the cCLD cohort led to the discovery that 6 patients decompensated within the subsequent year (3 patients with new HE and 3 patients with new ascites) (Table 2). Exclusion of these patients with HC individuals improved sensitivity analysis (AUC 0.74 (95% CI 0.60–0.88)) (Fig. 5E). Excluding these patients from the entire cohort improved overall model performance (AUC 0.81 (95% CI 0.73–0.89)) (Fig. 5F).
Fig. 5.
Model discriminating patients with decompensated cirrhosis from those without. (A) Original model (n = 174,AUC 0.77 (95% CI 0.69–0.85, p < 0.0001*). (B) Excluding patients with dCLD (n = 80, AUC 0.76 (95% CI 0.65–0.86, p < 0.0001*). (C) Excluding patients with ACLF and AD (n = 134, AUC 0.78 (95% CI 0.69–0.86, p < 0.0001*). (D) Excluding healthy controls (n = 154, AUC 0.69 (95% CI 0.56–0.81, p = 0.008*). (E) Excluding HC and the 6 cCLD patients who decompensated the year after sampling (n = 148, AUC 0.74 (95% CI 0.60–0.88, p < 0.0001*). (F) Excluding the 6 cCLD patients who decompensated the year after sampling (n = 168, AUC 0.81 (95% CI 0.73–0.89, p < 0.0001*). Statistical significance set after correction for false discovery with the Benjamini–Hochberg procedure (p < 0.0344) and designated by *.
Table 2.
Clinical characteristics of the 6 cCLD patients who decompensated the year after sampling.
| Variable | Number (%) or median (IQR) |
|---|---|
| Age | 54 (43–64) |
| Sex (male) | 4 (66.67%) |
| ARLD | 3 (50%) |
| Active alcohol consumption | 1 (16.67%) |
| WCC (× 109/l) | 3.68 (1.95–5.32) |
| Hb (g/dl) | 111.50 (107.75–148.25) |
| Platelets (× 109/l) | 71.00 (49.00–181.75) |
| INR | 1.23 (1.10–1.36) |
| CRP (mg/L) | 3.25 (1.75–11.38) |
| Bilirubin (mg/dl) | 1.29 (0.85–1.46) |
| AST (U/L) | 43.50 (29.00–71.00) |
| Albumin (g/L) | 37.50 (30.75–43.25) |
| Na (mmol/L) | 140 (136.250142) |
| Creatinine (mg/dl) | 0.70 (0.61–0.95) |
| Ammonia (µmol/L) | 77.50 (31.25–125.50) |
| Any ascites | 0 (0%) |
| Any HE | 1 (16.67%) |
| MELD score | 10.00 (8.00–11.25) |
| Child Pugh score | 5.50 (5.00–6.00) |
Evaluating performance of miRNA in predicting 1 year risk of mortality and/or transplantation in patients with cirrhosis
Across the entire cirrhotic cohort (ACLF/AD/cCLD/dCLD), 46 patients underwent LT (ACLF = 1/20, AD = 1/20. cCLD = 2/20, dCLD = 42/94) and 28 patients died (ACLF = 10/20, AD = 4/20, cCLD = 0/20. dCLD = 14/94) within 1 year of sampling. When mortality and LT were used as a composite end-point, only miR-330 discriminated clinical outcomes and did not remain significant when adjusted for both MELD and CP scores (Table 3A + B). Competing risk analysis was then performed to look for associations of microRNA expression with LT. No microRNA was able to discriminate outcome in this cohort (Table 3C). Performance of MELD and CP scores to discriminate patients who underwent LT was also poor (Supplementary Table 7).
Table 3.
One year risk of mortality/LT and competing risk analysis for 1 year risk of LT.
| microRNA | N | HR (95% CI) | C-statistic (95% CI) |
|---|---|---|---|
| (A) | |||
| miR-103a | 154 | 1.03 (0.94–1.15) | |
| miR-122 | 154 | 0.99 (0.98–1.01) | |
| D/ND miR-149 | 154 | 1.02 (0.06–4.61) | |
| miR-150 | 154 | 1.00 (0.98–1.03) | |
| miR-16-2 | 153 | 1.02 (0.996–1.05) | |
| miR-17 | 129 | 1.01 (0.99–1.03) | |
| D/ND miR-17 | 154 | 0.63 (0.37–1.14) | |
| miR-191 | 151 | 1.21 (0.99–1.47) | |
| D/ND miR-200b | 154 | 1.68 (0.87–3.03) | |
| miR-20a | 116 | 0.81 (0.50–1.37) | |
| D/ND miR-20a | 154 | 1.24 (0.73–2.02) | |
| miR-223 | 154 | 1.05 (0.93–1.19) | |
| miR-23a | 154 | 1.04 (0.91–1.17) | |
| miR-24 | 154 | 1.08 (0.97–1.20) | |
| miR-25 | 154 | 1.03 (0.999–1.07) | |
| miR-26a | 154 | 0.90 (0.79–1.03) | |
| miR-27a | 154 | 0.98 (0.88–1.09) | |
| miR-29b | 154 | 0.99 (0.97–1.02) | |
| miR-30a | 154 | 1.01 (0.99–1.04) | |
| miR-330 | 54 | 1.05 (1.01–1.09) | 0.67 (0.54–0.80) |
| D/ND miR-330 | 154 | 0.59 (0.34–0.96) | 0.56 (0.51–0.61) |
| miR-451 | 154 | 1.03 (0.99–1.07) | |
| miR-503 | 117 | 1.01 (0.97–1.05) | |
| D/ND miR-503 | 154 | 0.84 (0.51–1.43) | |
| D/ND miR-663 | 154 | 0.81 (0.25–1.97) | |
| microRNA | aHR (95% CI) | ||
|---|---|---|---|
| (B) | |||
| D/ND miR-330 with MELD | 0.66 (0.38–1.09) | ||
| D/ND miR-330 with CP | 0.71 (0.41–1.17) | ||
| microRNA | N | SHR (95% CI) | |
|---|---|---|---|
| (C) | |||
| miR-103a | 154 | 1.03 (0.93–1.15) | |
| miR-122 | 154 | 0.99 (0.97–1.01) | |
| D/ND miR-149 | 154 | 1.94 (0.29–12.99) | |
| miR-150 | 154 | 0.98 (0.95–1.01) | |
| miR-16-2 | 153 | 1.02 (0.99–1.05) | |
| miR-17 | 129 | 1.01 (0.98–1.04) | |
| D/ND miR-17 | 154 | 0.77 (0.38–1.58) | |
| miR-191 | 151 | 1.00 (0.79–1.28) | |
| D/ND miR-200b | 154 | 1.59 (0.73–3.47) | |
| miR-20a | 116 | 0.97 (0.93–1.02) | |
| D/ND miR-20a | 154 | 0.57 (0.31–1.03) | |
| miR-223 | 154 | 0.94 (0.81–1.09) | |
| miR-23a | 154 | 0.92 (0.79–1.07) | |
| miR-24 | 154 | 0.98 (0.85–1.13) | |
| miR-25 | 154 | 1.02 (0.98–1.07) | |
| miR-26a | 154 | 1.03 (0.88–1.20) | |
| miR-27a | 154 | 0.93 (0.83–1.05) | |
| miR-29b | 154 | 0.98 (0.94–1.01) | |
| miR-30a | 154 | 1.00 (0.97–1.03) | |
| miR-330 | 54 | 1.02 (0.98–1.06) | |
| D/ND miR-330 | 154 | 0.75 (0.41–1.39) | |
| miR-451 | 154 | 1.02 (0.97–1.07) | |
| miR-503 | 117 | 1.02 (0.97–1.08) | |
| D/ND miR-503 | 154 | 0.75 (0.40–1.43) | |
| D/ND miR-663 | 154 | 1.02 (0.31–3.32) | |
(A) One year risk of mortality/LT and association with microRNA expression. For microRNA with significant HR, C-statistic is calculated. (B) D/ND miR-330 adjusted for MELD (MELD score performance n = 151, C-statistic = 0.69 (95% CI 0.63-0.75)) and CP (CP score performance n = 151, C-statistic = 0.69 (95% CI 0.63-0.75)). (C) Competing risk analysis for 1 year risk of LT. D/ND = microRNA converted into categorical variable and marked as detected = 1 or not detected = 0.
Competing risk analysis was then used to look for associations of microRNA expression and patient mortality (Table 4). On univariable analyses, miR-150 (SHR 1.04 (95% CI 1.00–1.08)) miR-191 (SHR 1.52 (95% CI 1.12–2.05), miR-24 (SHR 1.20 (95% CI 1.08–1.35)) and miR 26a (SHR 0.79 (95% CI 0.63–0.99)) predicted 1 year risk of mortality (Table 4A). On multivariable analyses, only miR-191 remained significant when adjusted for MELD (aSHR 1.85 (95% CI 1.32–2.60)) and CP scores (SHR 1.63 (95% CI 1.19–2.24)) (Table 4B + C). The analysis was then repeated in patients with a diagnosis of ARLD, patients with ascites and the dCLD cohort alone. miR-191 was the only microRNA to predict 1 year mortality which remained significant when adjusted for both MELD and CP scores in the ARLD and ascites cohort (Table 4D + E). However, in the dCLD cohort, miR-191 did not predict risk of mortality at 1 year on univariable analyses. miR-24 did predict risk of mortality at 1 year for patients with dCLD and remained significant when adjusted for MELD (aSHR 1.40 (95% CI 1.04–1.90) and CP scores (aSHR 1.36 (95% CI 1.01–1.82)) (Table 4D + E).
Table 4.
Evaluation of microRNA performance in predicting 1 year risk of mortality.
| microRNA | N | SHR (95% CI) |
|---|---|---|
| (A) | ||
| miR-103a | 154 | 1.00 (0.82–1.21) |
| miR-122 | 154 | 1.01 (0.98–1.03) |
| miR-150 | 154 | 1.04 (1.00–1.08) |
| miR-16-2 | 153 | 1.02 (0.98–1.07) |
| miR-17 | 129 | 1.01 (0.97–1.05) |
| D/ND miR-17 | 154 | 0.56 (0.24–1.30) |
| miR-191 | 151 | 1.52 (1.12–2.05) |
| D/ND miR-200b | 154 | 1.21 (0.40–3.64) |
| miR-20a | 116 | 1.03 (0.95–1.12) |
| D/ND miR-20a | 154 | 1.58 (0.61–4.13) |
| miR-223 | 154 | 1.24 (0.999–1.54) |
| miR-23a | 154 | 1.20 (1.04–1.40) |
| miR-24 | 154 | 1.20 (1.08–1.35) |
| miR-25 | 154 | 1.04 (0.99–1.10) |
| miR-26a | 154 | 0.79 (0.63–0.99) |
| miR-27a | 154 | 1.08 (0.88–1.33) |
| miR-29b | 154 | 1.03 (0.98–1.08) |
| miR-30a | 154 | 1.02 (0.99–1.06) |
| miR-330 | 54 | 1.08 (0.995–1.17) |
| D/ND miR-330 | 154 | 0.48 (0.19–1.19) |
| miR-451 | 154 | 1.04 (0.98–1.11) |
| miR-503 | 117 | 0.99 (0.94–1.04) |
| D/ND miR-503 | 154 | 0.99 (0.43–2.27) |
| D/ND miR-663 | 154 | 0.53 (0.06–4.10) |
| microRNA | MELD aSHR (95% CI) | |
|---|---|---|
| (B) | ||
| miR-150 | 1.03 (0.98–1.08) | |
| miR-191 | 1.85 (1.32–2.60) | |
| miR-23a | 1.08 (0.87–1.33) | |
| miR-24 | 1.10 (0.93–1.30) | |
| miR-26a | 0.84 (0.66–1.07) | |
| microRNA | CP aHR (95% CI) | |
|---|---|---|
| (C) | ||
| miR-150 | 1.02 (0.98–1.07) | |
| miR-191 | 1.63 (1.19–2.24) | |
| miR-23a | 1.08 (0.92–1.28) | |
| miR-24 | 1.06 (0.92–1.21) | |
| miR-26a | 0.83 (0.66–1.05) | |
| microRNA | MELD aSHR (95% CI) | |
|---|---|---|
| (D) | ||
| miR-191 (ARLD) | 1.88 (1.30–2.72) | |
| miR-191 (Ascites) | 1.91 (1.37–2.65) | |
| miR-16-2 (dCLD) | 1.08 (1.01–1.15) | |
| miR-24 (dCLD) | 1.40 (1.04–1.90) | |
| microRNA | CP aSHR (95% CI) | |
|---|---|---|
| (E) | ||
| miR-191 (ARLD) | 1.66 (1.13–2.43) | |
| miR-191 (Ascites) | 1.59 (1.16–2.19) | |
| miR-24 (dCLD) | 1.36 (1.01–1.82) | |
(A) Competing risk analysis for 1 year risk of mortality. (B) MELD adjustment of prevalently expressed microRNA which discriminated 1 year risk of mortality. Performance of MELD adjusted miR-191; n = 148, Wald Chi2 = 26.31, p < 0.001*. (C) CP adjustment of prevalently expressed microRNA which discriminated 1 year risk of mortality. Performance of CP adjusted miR-191; n = 148, Wald Chi2 = 53.69, p < 0.001*. (D) microRNA which discriminated outcome on sensitivity analyses and remained significant following adjustment for MELD. (E) microRNA which discriminated outcome on sensitivity analyses and remained significant following adjustment for CP. D/ND = microRNA converted into categorical variable and marked as detected = 1 or not detected = 0. See Supplementary Tables 8–10 for model performance.
Discussion
To our knowledge, this is the largest study of miRNA expression across the full clinical spectrum of liver failure syndromes. We have demonstrated that miRNA associated with systemic inflammation are more prevalently expressed in patients with decompensated cirrhosis states compared to compensated cirrhosis and HC individuals. These microRNA may be present in patients with compensated cirrhosis prior to clinical decompensation and may predict risk of mortality in dCLD patients. We also demonstrate that miR-191 expression predicts risk of mortality within 1 year across the spectrum of cirrhosis when patients with ACLF and AD are included but does not when they are removed.
We have previously demonstrated the prognostic utility of miR-191 in ALF17. Whilst we generated a regeneration-linked microRNA model at the early time point and a cell-death related microRNA model at the late time point, it was notable that both miR-191 and -149 were included in both models. We previously have performed MetaCore™ pathway analysis to understand the processes that miR-191 and -149 were implicated in. This demonstrated that they were associated with responses to cellular injury. In this study we demonstrate that miR-191 correlates with markers of inflammation and liver failure. The association it has with inflammation has been demonstrated in other settings such as ulcerative colitis26. However, the ability of miR-191 to identify patients with liver disease was demonstrated in patients with alcohol use disorders where elevated levels differentiated patients with cirrhosis from those without27). It was notable that within our analysis that miR-191 did not differentiate between decompensated states and compensated/HC and that it was not able to predict risk of mortality at 1 year in the dCLD cohort on its own. We would hypothesize that miR-191 represents an acute marker of hepatocyte injury which is implicated in risk of mortality in acute presentations of liver disease (ACLF, AD and ALF). Further exploration is required to validate these findings and explore the role of it as a therapeutic target in these clinical scenarios.
Systemic inflammation is believed to be the major driver of progression from compensated to decompensated cirrhosis28,29. Here we show that there is a common, shared miRNA signature that defines decompensated cirrhosis states which is associated with critical epigenetic responses to systemic inflammation. Furthermore, we demonstrate that these miRNA correlate with conventional clinical and laboratory surrogate markers of systemic inflammation, portal hypertension and hepatic synthetic failure. A model containing miR-24 and -27a from this system inflammation signature discriminated between decompensated and compensated states. Both of these miRNAs have been demonstrated to have key roles in the regulation of macrophage and monocyte activation which supports this model being associated with systemic inflammation30–32. Sensitivity analyses of the miRNA model discriminating between decompensated and compensated states demonstrated minimal change in model performance when excluding patients dCLD patients or ACLF/AD patients, indicating that the miRNA within this model may reflect systemic inflammation related to underlying liver disease. This is supported by our systemic inflammation network including genes (MMP-2, GATA family, COL1) implicated in the progression of liver fibrosis/cirrhosis. However, excluding patients with cCLD who subsequently decompensate improves overall model performance. Furthermore, a subgroup analysis including only patients from the dCLD cohort demonstrated that miR-24 predicted 1 year risk of mortality and remained significant when adjusted for both MELD and CP score. These results would support the hypothesis that the onset of systemic inflammation may predate clinical decompensation and worsening systemic inflammation is associated with increased risk of adverse clinical outcomes in chronically decompensated patients. There are evolving data which suggest that carvedilol and rifaximin may have protective roles in the prevention of decompensation and mortality in cirrhosis33–36. There has also recently been characterization of ‘non-acute’ decompensation which is a progressive disorder without acute events associated with significant mortality37. These data combined with our findings may suggest that there is a therapeutic window to prevent the progression of cirrhosis through a compensated state to decompensation and mortality. These miRNA therefore may not only represent potential novel prognostic biomarkers, but also potential therapeutic targets for patients with cirrhosis. Subject to validation, they may be useful tools in the preventing decompensation and mortality, which remain a significant area of unmet clinical need.
We have demonstrated that miRNA expression in ACLF shares more similarity to sepsis than ALF. The importance of bacterial infection has been well described in ACLF and 95% of the ACLF cohort were being treated for infection (although only 11.76% of patients had positive cultures for bacterial infection)38. We have previously demonstrated the importance of regeneration-linked miRNA in determining outcome in ALF17–19. The difference in miRNA expression profile demonstrated in this study between patients with ACLF and patients with ALF implies reduced prognostic utility of regeneration-linked miRNA in cirrhosis compared to ALF. However, reversal of the underlying disease process that led to decompensation is likely to be relevant prior to recovery of underlying liver function and clinical recompensation. We have previously demonstrated that following DAA therapy for HCV, clinical recompensation is predicted by regeneration-linked miRNA19. Interleukin-22Fc therapy has also been demonstrated to ameliorate bacterial infection and subsequently upregulate regenerative pathways in an ACLF animal model39. Here, we have demonstrated that the detection of miR-150, which was previously utilized within our regeneration-linked ALF prognostic model17, is associated with 1 year risk of mortality but does not remain significant when adjusted for MELD or CP score. We also demonstrate that NANOG, a gene with a key role in processes which govern successful regeneration23–25, is upregulated and central within our systemic inflammation network. It remains unclear whether liver regeneration plays a significant role in determining clinical outcomes in all cirrhosis patients following control of the underlying cause of decompensation.
It is notable that the inclusion of patients undergoing LT within 1 year significantly impacted the analysis of the results with 1 year mortality/LT as a composite endpoint. LT in the UK is offered to patients with cirrhosis with a greater than 10% risk of liver related mortality at 1 year which is assessed by the United Kingdom model for End-stage Liver Disease (UKELD score)40. As such, whilst patients listed for LT have a higher risk of mortality, this does not mean there is an absolute correlation with the patient cohort that dies within 1 year. This is likely reflected by the suboptimal performance of MELD and CP scores in predicting LT within 1 year in our cohort. As such, competing risk analysis is required to evaluate the impact of this if used as a composite endpoint.
The limitations of this study include the retrospective design. However, samples and data were collected prospectively and investigators were blinded to outcome data when performing miRNA analysis. This study was performed in a single high-volume LT center in a large city in the United Kingdom and outcome, disease etiology and severity data likely reflects this. Future multicentered prospective work should be conducted at specialist and non-specialist hepatology centers across a broad geographical area. Formal mechanistic work is required to investigate the role of these miRNA in; the development of liver cirrhosis and portal hypertension, response to pharmacotherapy in cirrhosis, and to understand if modulation of miRNA expression can impact on patient clinical trajectory through different cirrhosis states. This may allow for the development of miRNA-based therapeutics to manage cirrhosis. Finally, the findings described in this study require validation in an external cohort. An adequately powered prospective study, with sufficient longitudinal follow up to allow for detailed time-to-event analyses as well as multiple sampling time points, is also required to evaluate the performance of these miRNA in predicting clinical decompensation amongst patients with cCLD. Understanding the relationship these microRNA have with the development of infection is essential and conventional tests may not adequately detect infection in cirrhotic patients. The field of molecular diagnostics for infection is rapidly evolving and these novel technologies could be utilized to better understand this association41 However, this remains a large-scale translational study which included patients from across the spectrum of cirrhosis as well as a septic non-CLD, ALF and HC cohorts. The miRNA panel utilized was based on our previous study in dCLD19 and we ensured quality control for each step of miRNA analysis. Therefore, our findings are robust and warrant further investigation.
In conclusion, we identify a common miRNA signature associated with systemic inflammation which discriminates patients with decompensated cirrhosis from those without. This signature may have utility in identifying patients with compensated cirrhosis at the highest risk of clinical decompensation as well as predicting mortality in patients with non-acute decompensation. We demonstrated that miR-191 predicts mortality across the cirrhotic cohort when patients with AD and ACLF are included but not when they are excluded. miR-191 was previously included in both our cell-death and regeneration linked prognostic models in ALF and is associated with hepatocyte injury. This may suggest that miR-191 represents a prognostic biomarker associated with poor clinical outcomes across acute presentations of liver disease. Biomarkers associated with systemic inflammation and hepatocyte injury may improve prognostication of patients with cirrhosis.
Materials and methods
Study design
This study was designed as a cohort study of prospectively collected data and biological samples from patients enrolled in the ‘Gut-liver axis in liver disease and liver transplantation’ (GLA) and ‘Immunometabolism in sepsis, systemic inflammation and liver failure syndromes’ (IMET) studies based at King’s College Hospital (United Kingdom) between 2014 and 2021. Patients were categorized into the following clinical groups; ACLF, AD, decompensated cirrhosis (dCLD), compensated cirrhosis (cCLD), HC, ALF and sepsis (definitions/inclusion criteria in supplementary Table 1). Patients were excluded if; they were younger than 18 years old, if they had an underlying diagnosis of malignancy (liver or non-liver in origin), if they had previously undergone any organ transplantation, if they had an active viraemia secondary to hepatitis B or C, if they were taking immunosuppression or if they had been previously sampled. Patients with acute presentations (ACLF, AD, ALF and sepsis) were all sampled within 72 h of admission. Non-acute presentations (cCLD, dCLD and HC) were sampled in outpatient or in elective clinical settings prior to any elective clinical intervention.
Consecutively enrolled patients fulfilling inclusion and exclusion criteria from the GLA study were included as follows; 19 ACLF, 20 AD, 94 dCLD, 20 cCLD, 20 HC, 14 ALF and 20 sepsis patients. Patients from the IMET study were randomly selected to ensure that each clinical group had a minimum of 20 patients and included 1 ACLF and 8 ALF patients. Total patient number was 216 which included; 20 ACLF, 20 AD, 94 dCLD, 20 cCLD, 20 HC, 22 ALF and 20 sepsis patients. Patients were further grouped by; decompensated states (ACLF, AD and dCLD, n = 134) and compensated/healthy states (cCLD and HC, n = 40). One year mortality/LT was used as a composite endpoint for patients with cirrhosis (ACLF, AD, cCLD and dCLD) with competing risk analysis subsequently performed to understand associations with LT and death separately. Sensitivity analysis was performed for patients with cirrhosis 1 year risk of mortality for; patients with dCLD, patients with alcohol related liver disease (ARLD) and patients with ascites.
This study was approved by the London East Ethics Committee ethics committee (12/LO/1417 and 19/NW/0750) and the King’s College Hospital Research and Innovation Committee, and has been conducted according to the principles expressed in the 1975 Declaration of Helsinki. Patients provided written informed consent; if patients were unable to provide informed consent due to incapacity, written informed assent was provided by the patients next of kin. Reporting of the analysis of this study complies to the STROBE Guidelines for reporting of cohort studies42. All authors had access to the study data and reviewed and approved the final manuscript.
Clinical data
Demographic, clinical and laboratory data were recorded prospectively simultaneous to blood sampling as part of enrolment to GLA and IMET studies. Data assessed in this study include; laboratory (full blood count, c-reactive protein (CRP), electrolytes, creatinine, liver function tests, albumin, INR, ammonia, lactate and arterial pH), ascites grade (as per Child–Pugh (CP) score7,8), hepatic encephalopathy (HE) grade (as per West Haven criteria43) and requirement for organ support (mechanical ventilation, vasopressors and renal replacement therapy). Infection was characterized by treatment with antibiotics and a positive culture within 72 h of sampling. Prognostic scores including CP score7,8 and MELD score9 were calculated from these data.
miRNA analysis
RNA extraction
Plasma samples were thawed on ice and centrifuged at 3000 × g for 5 min in a 4 °C microcentrifuge. An aliquot of 200µL per sample was transferred to a FluidX tube and 60µL of lysis solution biofluids containing 1µg carrier-RNA and RNA spike-in temperature mixture were added. Each sample was then mixed for 1 min and incubated for 7 min at room temperature. Following this, 20µL of protein precipitation solution biofluids were added to each sample. Total RNA was extracted from plasma using the miRCURY™ RNA isolation kit—biofluids (QIAGEN, Hilden, Germany) using according to the manufacturer’s protocol (high-throughput bead based protocol v.1) into an automated 96 well format44. The purified total RNA was eluted to a final volume of 50µL.
miRNA real-time quantitative PCR
20µL of RNA was reverse transcribed using the miRCURY™ LNA RT kit (QIAGEN, Hilden Germany). Complementary DNA (cDNA) was diluted 50 × and assayed in 10µL polymerase-chain reactions (PCR) according to the manufacturer’s protocol44. 21 miRNAs were selected for analysis based on our previous study findings17–19, review of the literature for miRNA associated with outcome in CLD12,16 and to allow for normalization (see supplementary Table 2). Also included within this panel were; an RNA spike in (UniSp4) to ensure RNA isolation efficiency, a cDNA control marker (UniSp6) to assess reverse transcription (RT) and a DNA spike-in (UniSp3) to assess PCR efficiency. Each miRNA was assayed once by quantitative PCR (qPCR) on the miRNA Ready-to-Use PCR Custom Panel (Qiagen, Hilden Germany) using the miRCURY™ LNA SYBR Green master mix. Negative controls excluding the template from the RT reaction were performed and profiled in comparison to the samples. Amplification was performed in a LightCycler® 480 Real-Time PCR System (Roche, Basel, Switzerland) in 384 well plates. Amplification curves were analyzed using the Roche LC software v 1.5 (www.lifescience.roche.com/global/en/products/product-category/lightcycler.html, Roche, Basel, Switzerland), both for determination of quantification cycle (Cq) (by the second derivative method) and melting curve analysis.
Assessment of hemolysis
Two miRNAs were used to perform this: miRNA-451 which is expressed in red blood cells, and miRNA-23a which is relatively stable in serum and plasma but not affected by hemolysis45. The ratio between these two miRNAs correlates with the degree of hemolysis. In our experience, samples with ratios above seven will have an increased risk of being affected by hemolysis. Samples with lower ratios are generally not affected by hemolysis. These values refer to human samples and may vary with species and diseases studied.
miRNA data analysis
The amplification efficiency was calculated using algorithms similar to the LinReg software. All assays were inspected for distinct melting curves the Tm was checked to be within known specifications for the assay. To be deemed as detected, assays must be three Cq less than the negative control and have a Cq less than 37. Assays were deemed to be not detected within a sample if these criteria were not met.
Normalized Cq (dCq) values were generated subtracting the Cq of the assay of interest from the mean of the Cq of the normalizing miRNA developed from our previous work (miR-23a, -26a, -103)17. A positive dCq reflects a more abundantly expressed miRNA whereas a negative dCq reflects a less abundantly expressed miRNA. Samples where any of the normalizing miRNA (miR-23a, -26a and -103) were not detected were excluded from further analysis.
Statistical analysis
Comparisons were made between; acute presentation clinical groups (ACLF/AD/ALF/sepsis), decompensated (ACLF/AD/dCLD) and compensated/healthy clinical states, and between cirrhosis 1 year survivors and 1 year mortality/LT (ACLD/AD/cCLD/dCLD). Continuous demographic, clinical and laboratory variables were analyzed for normality using the D’Agostino and Pearson tests. Normally distributed data were analyzed with t tests (2 groups) or ANOVA (3 + groups) with results reported as mean (standard deviation (SD)). Non-normally distributed data were analyzed using Mann Whitney U tests (2 groups) or Kruskall-Wallis test (3 + groups) with results reported as median (interquartile range (IQR)). Categorical data were analyzed by Fisher’s exact tests (2 groups) or Chi-square test (3 + groups) and results reported as number (%).
Individual dCq values for each miRNA in each sample were utilized to demonstrate miRNA expression. These underwent a natural logarithmic (ln) transformation in a formula developed for this analysis to transform to a normal distribution. Given that dCq values were negative (demonstrating less abundant expression within a sample), 20 was added to the dCq value prior to ln transformation. 20 was chosen to ensure that no dCq values remained negative and all were greater than one. As the majority of dCq values were closer to the mean of the controls than 20 prior to the ln transformation leading to small differences being potentially significance, this value was subsequently multiplied by 100 to increase the standard deviation. Therefore the formula for transformation was 100 × ln(20 + dCq of miRNA of interest).
Two-way unsupervised hierarchical clustering of miRNA expression of samples from across the entire study was performed. miRNA were excluded from this analysis if not present in greater than a third of samples. Spearman’s rank correlation was used to correlate miRNA data with clinical/laboratory variables. When comparing miRNA data in decompensated to compensated/healthy clinical states, receiver-operating characteristic curve analysis was performed if a p value < 0.05 was achieved. This was reported as area under the curve (AUC) (95% confidence interval (CI)). When understanding the association of microRNA with 1 year risk of mortality/LT, univariable Cox regression was utilized with a C-Statistic (95% CI) generated if a significant result was achieved. To increase the utility of less prevalently expressed miRNA, those detected in less than 85% of samples were treated as categorical variables (detected (D) = 1, non-detected (ND) = 0) and analyzed as described above.
Prevalently expressed miRNA (expressed in > 85% of samples) data were scaled and principle component analysis was used to evaluate expression across acute presentations (ACLF/AD/ALF/sepsis). Scaling of prevalently expressed miRNA data (> 85% of samples) was performed using the following equation prior to principle component analysis;
Multiple logistic regression was used to develop miRNA-based models to predict decompensated cirrhosis clinical states. Multivariable Cox regression was utilized to understand the association of microRNA with 1 year mortality/LT following adjustment for liver disease severity as per CP and MELD score. Competing risk analysis was subsequently undertaken to specifically understand the risks of LT or death. miRNA were converted into categorical data if not present in more than 85% of samples. If a significant result was achieved with a microRNA expressed in less than 50% of samples, this was not included in multivariable analyses.
For the decompensated cirrhosis state model, variables with a p value of < 0.2 were included in each model at each time point and backwards elimination was performed. An r2 threshold with other variables within the model was set at < 0.50 to reduce co-linearity. Goodness of fit was assessed using Hosmer Lemeshow (HL) test and pseudo r2 values for multiple logistic regression analyses. Goodness of fit was assessed using log-likelihood ratios for multivariable Cox regression analyses. Results for multiple logistic regression analyses were recorded as adjusted odds ratios (aOR) with 95% CI and p value s. For Cox regression analyses, data were recorded as hazard ratio (HR) with 95% CI. Results from competing risk regression (based on Fine and Gray’s proportional subhazards model) were presented as subhazard ratio (SHR) with 95% CI with Walds Chi2 results. Sensitivity analyses were performed to assess performance of the decompensated cirrhosis model by simultaneously removing AD/ACLF, dCLD and HC patients. Sensitivity analyses were also performed to assess performance of microRNA in discriminating 1 year outcome in patients with ARLD, ascites and dCLD only. Complete case analysis was used, excluding individuals with missing data.
MetaCore™ pathway analysis V6.29 (www.portal.genego.com, GeneGo Inc, Michigan, USA) was used to identify biological processes associated with miRNA associated with decompensated cirrhosis states. The Analyze Networks algorithm was used to demonstrate biological processes associated with miRNA signatures developed. This uses a library of greater than 24,000 human genes in networks to predict pathways likely to be associated with inputted data46. The algorithm generates and ranks biological networks based on:- 1) the relative enrichment of the network with the uploaded data (miRNA) and 2) the relative saturation of these networks with canonical pathways. This generates a Z score based on the statistical likelihood that these processes are associated with the given data (the higher the Z score, the greater likelihood of relevance). Following this, results were excluded if they did not include all of the given miRNA of interest and the processes remaining with the highest Z score were reported.
Correction for multiple comparisons was performed using the Benjamini–Hochberg procedure with a false discovery rate (FDR) set at 0.1047. Competing risk analysis was performed using Stata V17.0 (www.stata.com, StataCorp LLC, Texas, USA). All other analyses were performed using Prism V10.2.3 (www.graphpad.com/features, GraphPad, San Diego, USA).
Supplementary Information
Acknowledgements
The authors are grateful to all the patient participants and healthy volunteers for agreeing to take part and participating in this study, and to the clinical and liver research teams at King’s College Hospital for facilitating recruitment and collecting metadata and biological samples.
Author contributions
Study supervision and guarantor of manuscript: V.R.A. Study concept: O.D.T., V.C.P., S.S., M.J.W.M., V.R.A. Study design: O.D.T., V.C.P., S.S., M.S., F.M.T., M.M., S.R., S.M., A.Z., E.C., K.M., A.P., M.A.H., K.A., M.J.W.M., V.R.A. Cohorting of patients and developing clinical data: O.D.T., V.C.P., M.S., M.M., D.J., F.M.T., S.R., S.M., A.Z., E.C. microRNA analysis and statistical analysis: O.D.T., S.S., M.J.W.M. Drafting of the manuscript: O.D.T., V.C.P., S.S., M.J.W.M., V.R.A. Critical revision of the manuscript: O.D.T., V.C.P., S.S., M.S., M.M., D.J., F.M.T., S.R., S.M., A.Z., E.C., K.M., A.P., M.A.H., K.A., M.J.W.M., V.R.A.
Funding
This study was supported by the Roche Organ Transplant Research Foundation (Grant No. 1-3336) and the National Institute for Health and Care Research Guy’s and St Thomas’ Biomedical Research Centre (Grant No. RE12566). The sponsors did not influence any aspect of the study or composition of this manuscript.
Data availability
Data available upon reasonable request by contacting the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Oliver D. Tavabie, Vishal C. Patel and Siamak Salehi.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72416-w.
References
- 1.Pimpin, L. et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol.69(3), 718–735 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Karlsen, T. H. et al. The EASL-Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet399(10319), 61–116 (2022). [DOI] [PubMed] [Google Scholar]
- 3.de Franchis, R., Bosch, J., Garcia-Tsao, G., Reiberger, T. & Ripoll, C. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol.76(4), 959–974 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trebicka, J. et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol.73(4), 842–854 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Åberg, F. et al. Development and validation of a model to predict incident chronic liver disease in the general population: The CLivD score. J. Hepatol.77, 302–311 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Innes, H. et al. Performance of routine risk scores for predicting cirrhosis-related morbidity in the community. J. Hepatol.77, 365–376 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Child, C. G. & Turcotte, J. G. Surgery and portal hypertension. Major Probl. Clin. Surg.1, 1–85 (1964). [PubMed] [Google Scholar]
- 8.Pugh, R. N., Murray-Lyon, I. M., Dawson, J. L., Pietroni, M. C. & Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg.60(8), 646–649 (1973). [DOI] [PubMed] [Google Scholar]
- 9.Kamath, P. S. et al. A model to predict survival in patients with end-stage liver disease. Hepatology33(2), 464–470 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Jalan, R. et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol.61(5), 1038–1047 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Jalan, R. et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J. Hepatol.62(4), 831–840 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Cisilotto, J. et al. MicroRNA profiles in serum samples from Acute-On-Chronic Liver Failure patients and miR-25-3p as a potential biomarker for survival prediction. Sci. Rep.10(1), 100 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benz, F., Roy, S., Trautwein, C., Roderburg, C. & Luedde, T. Circulating MicroRNAs as biomarkers for sepsis. Int. J. Mol. Sci.17(1), 78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortez-Dias, N. et al. Circulating miR-122-5p/miR-133b ratio is a specific early prognostic biomarker in acute myocardial infarction. Circ. J.80(10), 2183–2191 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Elhendawy, M. et al. MicroRNA signature in hepatocellular carcinoma patients: identification of potential markers. Mol. Biol. Rep.47, 4945–4953 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Blaya, D. et al. Profiling circulating microRNAs in patients with cirrhosis and acute-on-chronic liver failure. JHEP Rep.3(2), 100233 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavabie, O. D. et al. A novel microRNA-based prognostic model outperforms standard prognostic models in patients with acetaminophen-induced acute liver failure. J. Hepatol.75, 424–434 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salehi, S. et al. Human liver regeneration is characterized by the coordinated expression of distinct microRNA governing cell cycle fate. Am. J. Transpl.13(5), 1282–1295 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Salehi, S. et al. Serum MicroRNA signatures in recovery from acute and chronic liver injury and selection for liver transplantation. Liver Transpl.26(6), 811–822 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Takahara, T. et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology26(6), 1521–1529 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Winkler, M. et al. Endothelial GATA4 controls liver fibrosis and regeneration by preventing a pathogenic switch in angiocrine signaling. J. Hepatol.74(2), 380–393 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Bourbonnais, E. et al. Liver fibrosis protects mice from acute hepatocellular injury. Gastroenterology142(1), 130–9.e4 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Najafzadeh, B. et al. The oncogenic potential of NANOG: An important cancer induction mediator. J. Cell Physiol.236(4), 2443–2458 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Chen, T., Du, J. & Lu, G. Cell growth arrest and apoptosis induced by Oct4 or Nanog knockdown in mouse embryonic stem cells: A possible role of Trp53. Mol. Biol. Rep.39(2), 1855–1861 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Jeter, C. R. et al. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells27(5), 993–1005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu, K. Q. et al. Long non-coding RNA ANRIL regulates inflammatory factor expression in ulcerative colitis via the miR-191-5p/SATB1 axis. Inflammation47(2), 513–529 (2024). [DOI] [PubMed] [Google Scholar]
- 27.Shihana, F. et al. MicroRNAs signature panel identifies heavy drinkers with alcohol-associated cirrhosis from heavy drinkers without liver injury. Biology12(10), 1314 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arroyo, V. et al. The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J. Hepatol.74(3), 670–685 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Costa, D. et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J. Hepatol.74(4), 819–828 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Naqvi, A. R., Fordham, J. B. & Nares, S. miR-24, miR-30b, and miR-142-3p regulate phagocytosis in myeloid inflammatory cells. J. Immunol.194(4), 1916–1927 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha, B., Momen-Heravi, F., Kodys, K. & Szabo, G. MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals Naive monocytes to differentiate into M2 macrophages. J. Biol. Chem.291(1), 149–159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gierlikowski, W. & Gierlikowska, B. MicroRNAs as regulators of phagocytosis. Cells11(9), 1380 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villanueva, C. et al. Carvedilol reduces the risk of decompensation and mortality in patients with compensated cirrhosis in a competing-risk meta-analysis. J. Hepatol.77, 1014–1025 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Patel, V. C. et al. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J. Hepatol.76(2), 332–342 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Villanueva, C. et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): A randomised, double-blind, placebo-controlled, multicentre trial. Lancet393(10181), 1597–1608 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Salehi, S. et al. Rifaximin reduces the incidence of spontaneous bacterial peritonitis, variceal bleeding and all-cause admissions in patients on the liver transplant waiting list. Aliment Pharmacol. Ther.50(4), 435–441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tonon, M. et al. A new clinical and prognostic characterization of the patterns of decompensation of cirrhosis. J. Hepatol.80(4), 603–609 (2024). [DOI] [PubMed] [Google Scholar]
- 38.Moreau, R. et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology144(7), 1426–1437 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Xiang, X. et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J Hepatol72(4), 736–745 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barber, K. et al. Elective liver transplant list mortality: Development of a United Kingdom end-stage liver disease score. Transplantation92(4), 469–476 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Piano, S., Bunchorntavakul, C., Marciano, S. & Reddy, K. R. Infections in cirrhosis. Lancet Gastroenterol. Hepatol.10.1016/S2468-1253(24)00078-5 (2024). [DOI] [PubMed] [Google Scholar]
- 42.von Elm, E. et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bmj335(7624), 806–808 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conn, H. O. et al. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology72(4 Pt 1), 573–583 (1977). [PubMed] [Google Scholar]
- 44.QIAGEN. Qiagen Kit Handbooks. 2020. Accessed online 22/07/2020: https://www.qiagen.com/gb/service-and-support/learning-hub/search-resources/#filters=%7BF321478C-FDDA-437F-BE0B-87001D9936D3%7D.
- 45.Blondal, T. et al. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods59(1), S1-6 (2013). [DOI] [PubMed] [Google Scholar]
- 46.GeneGo. MetaCore. 2020. Accessed 28 Jun 2020.
- 47.Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc.57, 289–300 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon reasonable request by contacting the corresponding author.