Abstract
Oxidative stress and lipid peroxide levels in the brain increase with aging. The carotenoids lutein and zeaxanthin have potent antioxidant properties and the ability to improve cognitive function. However, their effects on neuronal damage via lipid peroxidation remain unknown. Therefore, we aimed to elucidate the effects of these carotenoids on neuronal damage induced by accumulated peroxidized lipids. We developed an oxidative stress model of lipid peroxidation-induced neuronal damage using differentiated neuronal cells derived from human neuroblastoma SH-SY5Y cells in vitro. Combining rotenone and RSL3 increased mitochondrial oxidative stress and lipid reactive oxygen species (ROS), which resulted in enhanced neuronal damage. Lutein and zeaxanthin were added to the cells for 1 week, and these carotenoids suppressed mitochondrial oxidative stress and lipid peroxidation in differentiated neuronal cells and mitigated neuronal damage. Further investigation is required to clarify the underlying pathways in detail.
Keywords: Lutein, Zeaxanthin, Lipid peroxidation, Neuron
Graphical abstract
Highlights
-
•
We developed a model of neuronal damage caused by lipid peroxidation in vitro.
-
•
Lutein and zeaxanthin block mitochondrial oxidative stress and lipid peroxidation.
-
•
Lutein and zeaxanthin mitigate neuronal damage.
1. Introduction
Aging is progressing in countries around the world, highlighting the necessity of preventing cognitive decline. Lipids account for more than half of the brain's dry weight [1,2]. In particular, the brain features a high content of polyunsaturated fatty acids (PUFAs) [3,4], and it is a metabolically active tissue with high oxygen consumption rates that generate significant amounts of reactive oxygen species (ROS) [5]. Oxidative stress in the brain increases with age [6], and increased ROS levels lead to the peroxidation of lipids, resulting in neuronal cell damage [[6], [7], [8]]. Accumulated peroxidized lipids could cause ferroptotic cell damage [9]. Thus, inhibiting lipid peroxidation might help prevent the decline of cognitive function in the central nervous system [10].
A multitude of studies have explored the impact of various food components on cognitive health. Sustaining cognitive function is largely dependent on a well-balanced diet [11]. Carotenoids are found in green and yellow vegetables and have powerful antioxidant properties. Of the 16 types of carotenoids found in the human brain, lutein and zeaxanthin are abundant [[12], [13], [14]] and exert numerous antioxidant effects [15]. Continuous intake leads to lutein and zeaxanthin accumulation in the brain [16], and epidemiological findings have associated higher intake or blood concentrations of lutein and zeaxanthin with decreased cognitive decline [[17], [18], [19]]. Intervention trials have also found improved cognitive function with continuous intake of lutein and zeaxanthin [20,21]. Furthermore, lutein and zeaxanthin inhibit the death of neuronal cells in vitro [[22], [23], [24]] and lipid peroxidation in other types of cells [25]. The accumulation of peroxidized lipids in the brain correlates with the amount of lutein [26]. However, the effects of lutein and zeaxanthin on lipid peroxidation-mediated neuronal cell damage have not been proven.
Therefore, in this study, we aimed to clarify the effects of lutein and zeaxanthin on neuronal cell damage induced by accumulated lipid peroxidation.
2. Materials and methods
2.1. SH-SY5Y cells
The Ethics Committee at Suntory Holdings approved all experiments conducted in this study (experimental code 20046, approved on July 31, 2019). Human neuroblastoma SH-SY5Y cells (ECACC) were cultured in DMEM/F12 (Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10 % fetal bovine serum (FBS; Merck, Darmstadt, Germany) and antibiotics (Nacalai Tesque Inc., Kyoto, Japan) at 37 °C under a 5 % CO2 atmosphere. The cells were passaged before reaching confluence.
2.2. Differentiation of SH-SY5Y
Neuronal cells were differentiated using SH-SY5Y cells as previously described, with some modifications [[27], [28], [29]]. For neuronal differentiation, the cells were incubated in DMEM/F12 (Thermo Fisher Scientific Inc.) containing 5 % FBS and 2 μM retinoic acid (Cayman Chemical, Ann Arbor, MI, USA) for 7 days in plates coated with a Corning® Matrigel® Matrix (Corning Inc., Corning, NY, USA). The culture medium was changed 7 days later to neurobasal medium containing 2 % B-27 (Thermo Fisher Scientific Inc.), 50 ng/mL BDNF (Merck), 2 mM db-cAMP (Merck), 20 mM KCl (Nacalai Tesque Inc.), GlutaMAX (Thermo Fisher Scientific Inc.), and antibiotics (Nacalai Tesque Inc.) and incubated for another 7 days. On day 14, the neuronal cells were analyzed. The culture medium was changed twice a week.
2.3. Compound treatment in cultured cells
Lutein and zeaxanthin (both from Extrasynthese S.A., Genay, France) were dissolved in dimethyl sulfoxide (DMSO; Nacalai Tesque Inc.) and mixed with medium to a final concentration of 0.1 % (v/v). Lutein (1–2 μM) and zeaxanthin (0.2–0.4 μM) were added at the indicated concentrations. We combined lutein and zeaxanthin in a 5:1 ratio, as this proportion has been shown to improve cognitive function in clinical studies [20,21]. The effects of these compounds on cell proliferation were analyzed using Cell Proliferation Kit I (Merck) and EdU Cell Proliferation Kit (Thermo Fisher Scientific Inc.). Rotenone (Merck), RSL3 (Selleck Chemicals, Houston, TX, USA), Z-VAD-FMK (Selleck Chemicals), and ferrostatin-1 (Selleck Chemicals) were also dissolved in DMSO and mixed with the medium to a final concentration of 0.1 % (v/v) on day 14. We added rotenone (0.05–1 μM), RSL3 (0.25–1 μM), Z-VAD-FMK (0.5–1 μM), and ferrostatin-1 (5 μM) at the indicated concentrations.
2.4. Immunocytochemistry (ICC)
The cells were fixed in 4 % paraformaldehyde (PFA) (Nacalai Tesque Inc.) for 10 min at room temperature (20–25 °C), permeabilized in 0.1 % Triton X-100 (Nacalai Tesque Inc.) for 10 min, and then incubated with 5 % goat serum (Abcam Plc., Cambridge, UK) for 1 h. The cells were incubated overnight with primary antibody (TUJ1, Abcam) at 4 °C, followed by incubation with secondary anti-mouse Alexa Fluor 488 (Thermo Fisher Scientific Inc.) for 1 h at room temperature and DAPI (Thermo Fisher Scientific Inc.) for 10 min. Cell images were acquired and evaluated using a BZ-X800 microscope and a BZ-X analyzer, respectively (both from Keyence, Osaka, Japan).
2.5. ROS measurement
Intracellular mitochondrial ROS levels were measured using MitoSOX (Thermo Fisher Scientific Inc.) as described by the manufacturer. The cells were washed with PBS (Nacalai Tesque Inc.), then incubated with neurobasal medium containing MitoSOX and Hoechst 33342 (Thermo Fisher Scientific Inc.) for 10 min at 37 °C, under a 5 % CO2 atmosphere. The cells were washed with PBS; subsequently, fluorescence was measured in Hank's balanced salt solution (HBSS; Thermo Fisher Scientific Inc.) using Infinite (TECAN, Männedorf, Switzerland). The excitation/emission wavelengths were 510/580 nm for MitoSOX and 350/460 nm for Hoechst 33342. Fluorescence intensity was measured sequentially. The MitoSOX intensity was normalized to that of Hoechst 33342. Lipid ROS levels were measured using the same method with BODIPY™ 581/591 C11 as a fluorescent probe (Thermo Fisher Scientific Inc.) [30]. Excitation and emission wavelengths were 480/520 nm.
2.6. Lactate dehydrogenase (LDH) assay
Culture supernatants were centrifuged at 400×g for 10 min, and then PBS supplemented with 0.1 % Triton X-100 was added to the cells, which were then centrifuged at 2000×g for 15 min. Thereafter, the absorbance of the culture supernatant and cell lysate was measured at 490 nm using a Cytotoxicity LDH Assay Kit (FujiFilm, Tokyo, Japan). The amount of LDH in the culture supernatant was calculated as a ratio (%) of the total amount of LDH in the culture supernatant and the cell lysate.
2.7. Statistical analysis
Data are expressed as means ± standard error (SE). We analyzed the results using Dunnett tests or Tukey multiple comparison tests to determine the statistical significance of the data compared with the control. All data were analyzed using IBM SPSS Statistics for Windows version 26 (IBM Corp., Armonk, NY, USA) and Prism 9 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Overview of SH-SY5Y cell culture system
We differentiated SH-SY5Y cells using a two-step method with retinoic acid and neurobasal medium as previously described, with some modifications [[27], [28], [29]]. Schematic diagram of the culture system is shown in Fig. 1A. Immunostaining confirmed the expression of neuronal marker proteins and the morphology of the neurons with numerous neurites (Fig. 1B). Lutein and zeaxanthin added to the medium from day 7 onwards did not affect cell proliferation or differentiation (Fig. 1C–E).
Fig. 1.
Overview of SH-SY5Y cell culture system.
(A) Schematic diagram of culture conditions. Cells were incubated with lutein and zeaxanthin from day 7. (B) Representative immunostained image of differentiated neurons on day 14. Scale bar: 100 μm. (C and D) Effects of lutein and zeaxanthin on cell proliferation on day 14 analyzed using MTT (C) and EdU (D) assays. Means ± SE, n = 3 (C), n = 5–8 (D). (E) Effects of lutein and zeaxanthin on differentiation on day 14 analyzed using immunostaining. Scale bar: 200 μm.
SE, standard error.
3.2. Confirmation of a neuronal cell damage model with lipid peroxidation
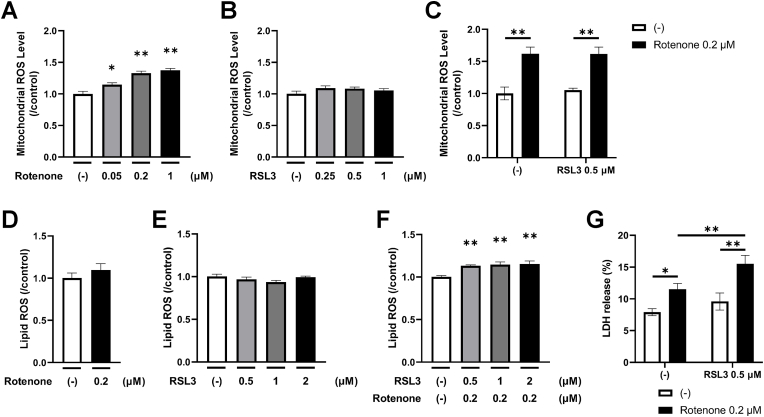
Next, we constructed a model of neuronal cell damage involving lipid peroxidation. Mitochondrial oxidative stress was induced by rotenone, which inhibits mitochondrial respiratory chain complex I. We also used RSL3, which inhibits glutathione peroxidase-4 (GPX4) activity, to accumulate lipid ROS derived from lipid peroxidation. When rotenone was combined with RSL3 at the described concentrations, rotenone dose-dependently increased mitochondrial ROS levels, independent of RSL3 (Fig. 2A‒C). Rotenone and RSL3 increased lipid ROS levels when applied together but not when either was added alone (Fig. 2D‒F). Rotenone and RSL3 together released more LDH into the medium compared with either alone (Fig. 2G). These findings suggest that the combination of rotenone and RSL3 at the described concentrations may induce neuronal cell damage through a mechanism involving lipid peroxidation. To better understand the mechanism of cell death induced by combined rotenone and RSL3 in this culture system, we evaluated LDH release in the presence of a caspase inhibitor. However, the caspase inhibitor did not inhibit neuronal cell damage (Fig. 3A). In contrast, ferrostatin-1, a well-known ferroptosis inhibitor, inhibited neuronal cell damage (Fig. 3B). These results suggest that neuronal cell death induced by combined rotenone and RSL3 treatment is not apoptotic but most likely ferroptotic [26], which is characterized by the accumulation of lipid peroxidation.
Fig. 2.
Modeling neuronal cell damage with lipid peroxidation using rotenone and RSL3.
(A–C) Cells were incubated with rotenone and RSL3 alone or in combination on day 14. After 24 h, mitochondrial ROS was analyzed using MitoSOX and relative values are described compared with no oxidative stress condition (control). Means ± SE, n = 8, Dunnett's test (A) or Tukey's multiple comparisons test (C). ∗p < 0.05, ∗∗p < 0.01.
(D–F) Cells were incubated with rotenone and RSL3 alone or in combination on day 14. After 24 h, lipid ROS was analyzed using BODIPY™ 581/591 C11 and are described compared with no oxidative stress condition (control). Mean ± SE, n = 8, Dunnett's test, ∗∗p < 0.01.
(G) Cells were incubated in medium containing rotenone combined with RSL3. After 24 h, the ratio of LDH release to the culture supernatant was analyzed. Means ± SE, n = 3, Tukey's multiple comparisons test, ∗p < 0.05, ∗∗p < 0.01.
LDH, lactate dehydrogenase; ROS, reactive oxygen species; SE, standard error.
Fig. 3.
Neuronal cell damage is ameliorated with ferroptosis inhibitor.
Compounds were added together to the medium at final concentration of 0.1 % v/v on day 14. Rotenone and RSL3 combined with Z-VAD-FMK (A) or ferrostatin-1 (B). After 48 h, ratios of LDH released into culture supernatant was analyzed. Means ± SE, n = 5, Dunnett's test, ∗∗p < 0.01.
LDH, lactate dehydrogenase; SE, standard error.
3.3. Effects of lutein and zeaxanthin on mitochondrial ROS, lipid ROS, and neuronal cell damage
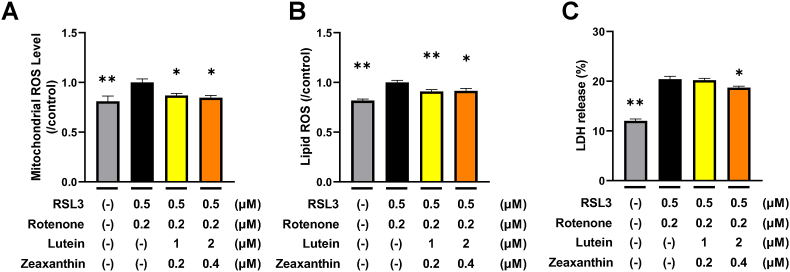
We incubated neuronal cells in the medium supplemented with lutein and zeaxanthin from day 7 to determine their effects. In the model of neuronal cell damage with lipid peroxidation, we found that mitochondrial ROS (Fig. 4A), lipid ROS (Fig. 4B), and LDH release (Fig. 4C) were all significantly reduced by lutein and zeaxanthin. These results indicated a decrease in oxidative stress and lipid peroxidation in the cells, ultimately inhibiting neuronal damage.
Fig. 4.
Effects of lutein and zeaxanthin on mitochondrial ROS, lipid ROS, and neuronal cell damage.
(A and B) Lutein and zeaxanthin were continuously added to the medium from day 7. Rotenone and RSL3 were added from day 14. Mitochondrial ROS (A) and lipid ROS (B) were measured 24 h later, and values relative to controls with oxidative stress were analyzed. Means ± SE, n = 7–8, Dunnett's test, ∗p < 0.05, ∗∗p < 0.01.
(C) Lutein and zeaxanthin were continuously added to medium from day 7. Rotenone and RSL3 were added from day 14. After 48 h, ratio of LDH released into culture supernatant was analyzed. Means ± SE, n = 9–10 (C), Dunnett's test, ∗p < 0.05, ∗∗p < 0.01.
LDH, lactate dehydrogenase; ROS, reactive oxygen species; SE, standard error.
4. Discussion
In this study, we developed an in vitro oxidative stress model of lipid peroxidation-induced neuronal damage using a combination of rotenone and RSL3. We demonstrated the inhibitory effect of lutein and zeaxanthin on oxidative stress, lipid peroxidation, and ferroptotic neuronal cell damage. The inhibition of neuronal cell damage through the antioxidant effects of lutein and zeaxanthin has been reported previously in studies using rodent-derived neuronal cells [23,31]. The novelty of our study lies in the use of human neuronal cells, and that demonstrating lutein and zeaxanthin can suppress ferroptotic neuronal cell damage with lipid peroxidation.
We constructed a model of induced lipid peroxide accumulation in vitro using rotenone combined with RSL3. Previous findings have shown that rotenone immediately induces lipid peroxidation in neuronal cells. However, lipid peroxides might not accumulate unless GPX4 activity, which degrades them, is inhibited [32]. By promoting the progression and accumulation of lipid peroxides, the combination of rotenone and RSL3 is a valuable approach to create a model for evaluating lipid peroxide accumulation in neuronal cells. An increase in oxidative stress and ferroptosis sensitivity associated with aging has been reported [33], and this model might reproduce some of the age-related alterations in vitro.
We quantified lipid ROS levels using BODIPY™ 581/591 C11 to evaluate lipid peroxidation. The lipid ROS assessed using this fluorescent probe can determine the total, but not specific, peroxidized intracellular unsaturated fatty acids (UFAs). Neuronal cells contain several types of UFAs with double bonds, including arachidonic acid and docosahexaenoic acid [34]. Therefore, lipid ROS evaluated using BODIPY™ 581/591 C11 might be derived from the double bonds of these UFAs, suggesting that lutein and zeaxanthin inhibit their peroxidation. Evaluation of variations in oxidative metabolites generated from UFAs remains a topic for future investigation.
In this study, continuous addition of lutein and zeaxanthin to neuronal cells from day 7 resulted in the suppression of oxidative stress and lipid ROS, along with the inhibition of ferroptotic neuronal cell damage. Lutein was added at the concentration of 1–2 μM, and zeaxanthin at 0.2–0.4 μM. Compared to the concentrations of lutein and zeaxanthin in human blood [21], these levels are within physiologically plausible concentrations. The inhibition of lipid peroxidation and neuronal cell damage by lutein and zeaxanthin may represent a mechanism for maintaining cognitive function through the dietary intake of these carotenoids [20,21].
This study has some limitations. Neuronal differentiation of SH-SY5Y cells was induced using an established protocol. However, despite having the morphology of mature neurons, the differentiated cells formed a heterogeneous group of cells that partially proliferated [28]. Such heterogeneity among the cells might have affected our results. Despite their important role in neural lipid metabolism, glial cells were absent from our culture system [[35], [36]]. Furthermore, we confirmed that the neuronal cell death induced by rotenone and RSL3 combination was ferroptotic using cell death inhibitors; however, we have not verified other characteristics of ferroptosis [37], and further investigation is required regarding the type of cell death. Lutein and zeaxanthin exert antioxidant effects through two distinct mechanisms. One involves the oxidation of these carotenoids, while another one is relies on changes in the expression of antioxidant enzymes, including HO1 and NQO1 [15]. Therefore, further investigation is required to clarify these pathways in details.
5. Conclusion
Lutein and zeaxanthin reduced oxidative stress, lipid peroxidation, and ferroptotic cell damage in neuronal cells differentiated from human neuroblastoma SH-SY5Y. These effects should be further verified in human neurons co-cultured with glial cells to reproduce physiological conditions more accurately. In addition, further investigation is required to clarify the underlying mechanisms of protection from neuronal damage.
Funding
This work was supported by Suntory Wellness Ltd. This study received no external funding.
CRediT authorship contribution statement
Satoshi Morita: Writing – review and editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Sueyasu Toshiaki: Writing – review and editing, Conceptualization. Hisanori Tokuda: Writing – review and editing, Conceptualization. Yoshihisa Kaneda: Writing – review and editing, Conceptualization, Project administration. Takayuki Izumo: Writing – review and editing, Project administration. Yoshihiro Nakao: Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Satoshi Morita reports a relationship with Suntory Wellness Ltd. That includes: employment.
Sueyasu Toshiaki reports a relationship with Suntory Wellness Ltd. That includes: employment.
Hisanori Tokuda reports a relationship with Suntory Wellness Ltd. That includes: employment.
Yoshihisa Kaneda reports a relationship with Suntory Wellness Ltd. That includes: employment.
Takayuki Izumo reports a relationship with Suntory Wellness Ltd. That includes: employment.
Yoshihiro Nakao reports a relationship with Suntory Wellness Ltd. That includes: employment.
Acknowledgements
We thank all our coworkers for their support. Part of the figure was created using images from Servier Medical Arts. Servier Medical Art is licensed under the Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Data availability
Data will be made available on request.
References
- 1.Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 1968;9:570–579. doi: 10.1016/S0022-2275(20)42702-6. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien J.S., Sampson E.L. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J. Lipid Res. 1965;6:537–544. doi: 10.1016/S0022-2275(20)39619-X. [DOI] [PubMed] [Google Scholar]
- 3.McNamara R.K., Liu Y., Jandacek R., Rider T., Tso P. The aging human orbitofrontal cortex: decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot. Essent. Fatty Acids. 2008;78:293–304. doi: 10.1016/j.plefa.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carver J.D., Benford V.J., Han B., Cantor A.B. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res. Bull. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 5.Clarke D.D., Sokolof L. In: Basic Neurochem. Mol. Cell. Med. Asp. sixth ed. Siegel G.J., Agranoff B., Albers R.W., Fisher S.K., Uhler M.D., editors. Lippincott-Raven; 1999. Circulation and energy metabolism in the brain; pp. 637–669. [Google Scholar]
- 6.Cabré R., Naudí A., Dominguez-Gonzalez M., Ayala V., Jové M., Mota-Martorell N., Piñol-Ripoll G., Gil-Villar M.P., Rué M., Portero-Otín M., Ferrer I., Pamplona R. Sixty years old is the breakpoint of human frontal cortex aging. Free Radic. Biol. Med. 2017;103:14–22. doi: 10.1016/j.freeradbiomed.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Domínguez-González M., Puigpinós M., Jové M., Naudi A., Portero-Otín M., Pamplona R., Ferrer I. Regional vulnerability to lipoxidative damage and inflammation in normal human brain aging. Exp. Gerontol. 2018;111:218–228. doi: 10.1016/j.exger.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Chia L.S., Thompson J.E., Moscarello M.A. Changes in lipid phase behaviour in human myelin during maturation and aging: involvement of lipid peroxidation. FEBS Lett. 1983;157:155–158. doi: 10.1016/0014-5793(83)81136-3. [DOI] [PubMed] [Google Scholar]
- 9.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiko T., Nakagawa K., Tsuduki T., Suzuki T., Arai H., Miyazawa T. Significance of lutein in red blood cells of Alzheimer's disease patients. J. Alzheim. Dis. 2012;28:593–600. doi: 10.3233/JAD-2011-111493. [DOI] [PubMed] [Google Scholar]
- 11.Otsuka R., Nishita Y., Tange C., Tomida M., Kato Y., Nakamoto M., Imai T., Ando F., Shimokata H. Dietary diversity decreases the risk of cognitive decline among Japanese older adults. Geriatr. Gerontol. Int. 2017;17:937–944. doi: 10.1111/ggi.12817. [DOI] [PubMed] [Google Scholar]
- 12.Manochkumar J., Doss C.G.P., El-Seedi H.R., Efferth T., Ramamoorthy S. The neuroprotective potential of carotenoids in vitro and in vivo. Phytomedicine. 2021;91 doi: 10.1016/j.phymed.2021.153676. [DOI] [PubMed] [Google Scholar]
- 13.Maoka T. Carotenoids as natural functional pigments. J. Nat. Med. 2020;74:1–16. doi: 10.1007/s11418-019-01364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craft N.E., Haitema T.B., Garnett K.M., Fitch K.A., Dorey C.K. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J. Nutr. Health Aging. 2004;8:156–162. https://pubmed.ncbi.nlm.nih.gov/15129301/ [PubMed] [Google Scholar]
- 15.Ahn Y.J., Kim H. Lutein as a modulator of oxidative stress-mediated inflammatory diseases. Antioxidants. 2021;10:1448. doi: 10.3390/antiox10091448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon S., Li Q., Rubakhin S.S., V Sweedler J., Smith J.W., Neuringer M., Kuchan M., Erdman J.W. 13C-lutein is differentially distributed in tissues of an adult female rhesus macaque following a single oral administration: a pilot study. Nutr. Res. 2019;61:102–108. doi: 10.1016/j.nutres.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan C., Chen H., Wang Y., Schneider J.A., Willett W.C., Morris M.C. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: a community-based cohort of older adults. Am. J. Clin. Nutr. 2021;113:200–208. doi: 10.1093/ajcn/nqaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan C., Fondell E., Ascherio A., Okereke O.I., Grodstein F., Hofman A., Willett W.C. Long-term intake of dietary carotenoids is positively associated with late-life subjective cognitive function in a prospective study in US women. J. Nutr. 2020;150:1871–1879. doi: 10.1093/jn/nxaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feeney J., O'Leary N., Moran R., O'Halloran A.M., Nolan J.M., Beatty S., Young I.S., Kenny R.A. Plasma lutein and zeaxanthin are associated with better cognitive function across multiple domains in a large population-based sample of older adults: findings from the Irish longitudinal study on aging, Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 2017;72:1431–1436. doi: 10.1093/gerona/glw330. [DOI] [PubMed] [Google Scholar]
- 20.Renzi-Hammond L.M., Bovier E.R., Fletcher L.M., Miller L.S., Mewborn C.M., Lindbergh C.A., Baxter J.H., Hammond B.R. Effects of a lutein and zeaxanthin intervention on cognitive function: a randomized, double-masked, placebo-controlled trial of younger healthy adults. Nutrients. 2017;9:1246. doi: 10.3390/nu9111246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sueyasu T., Yasumoto K., Tokuda H., Kaneda Y., Obata H., Rogi T., Izumo T., Kondo S., Saito J., Tsukiura T., Nakai M. Effects of long-chain polyunsaturated fatty acids in combination with lutein and zeaxanthin on episodic memory in healthy older adults. Nutrients. 2023;15:2825. doi: 10.3390/nu15132825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y., Zhang X., Lian F., Yang J., Xu X. Combination of lutein and DHA alleviate H2O2 induced cytotoxicity in PC12 cells by regulating the MAPK pathway. J. Nutr. Sci. Vitaminol. 2021;67:234–242. doi: 10.3177/jnsv.67.234. [DOI] [PubMed] [Google Scholar]
- 23.Singhrang N., Tocharus C., Thummayot S., Sutheerawattananonda M., Tocharus J. Protective effects of silk lutein extract from Bombyx mori cocoons on β-amyloid peptide-induced apoptosis in PC12 cells. Biomed. Pharmacother. 2018;103:582–587. doi: 10.1016/j.biopha.2018.04.045. [DOI] [PubMed] [Google Scholar]
- 24.Zeni A.L.B., Camargo A., Dalmagro A.P. Lutein prevents corticosterone-induced depressive-like behavior in mice with the involvement of antioxidant and neuroprotective activities. Pharmacol. Biochem. Behav. 2019;179:63–72. doi: 10.1016/j.pbb.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Leung H.H., Galano J.M., Crauste C., Durand T., Lee J.C.Y. Combination of lutein and zeaxanthin, and DHA regulated polyunsaturated fatty acid oxidation in H2O2-stressed retinal cells. Neurochem. Res. 2020;45:1007–1019. doi: 10.1007/s11064-020-02994-4. [DOI] [PubMed] [Google Scholar]
- 26.Mohn E.S., Erdman J.W., Kuchan M.J., Neuringer M., Johnson E.J. Lutein accumulates in subcellular membranes of brain regions in adult rhesus macaques: relationship to DHA oxidation products. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gimenez‐Cassina A., Lim F., Diaz‐Nido J. Differentiation of a human neuroblastoma into neuron-like cells increases their susceptibility to transduction by herpesviral vectors. J. Neurosci. Res. 2006;84:755–767. doi: 10.1002/jnr.20976. [DOI] [PubMed] [Google Scholar]
- 28.Pezzini F., Bettinetti L., Di Leva F., Bianchi M., Zoratti E., Carrozzo R., Santorelli F.M., Delledonne M., Lalowski M., Simonati A. Transcriptomic profiling discloses molecular and cellular events related to neuronal differentiation in SH-SY5Y neuroblastoma cells. Cell. Mol. Neurobiol. 2017;37:665–682. doi: 10.1007/s10571-016-0403-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shipley M.M., Mangold C.A., Szpara M.L. Differentiation of the SH-SY5Y human neuroblastoma cell line. J. Vis. Exp. 2016;108 doi: 10.3791/53193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummen G.P.C., van Liebergen L.C.M., den Kamp J.A.F.O., Post J.A. C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002;33:473–490. doi: 10.1016/s0891-5849(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 31.Muangnoi C., Phumsuay R., Jongjitphisut N., Waikasikorn P., Sangsawat M., Rashatasakhon P., Paraoan L., Rojsitthisak P. Protective effects of a lutein ester prodrug, lutein diglutaric acid, against H2O2-induced oxidative stress in human retinal pigment epithelial cells. Int. J. Mol. Sci. 2021;22:4722. doi: 10.3390/ijms22094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong Z.-B., Kim H., El Touny L., Simeonov A., Gerhold D. LUHMES dopaminergic neurons are uniquely susceptible to ferroptosis. Neurotox. Res. 2022;40:1526–1536. doi: 10.1007/s12640-022-00538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren J.X., Sun X., Yan X.L., Guo Z.N., Yang Y. Ferroptosis in neurological diseases. Front. Cell. Neurosci. 2020;14:218. doi: 10.3389/fncel.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kan I., Melamed E., Offen D., Green P. Docosahexaenoic acid and arachidonic acid are fundamental supplements for the induction of neuronal differentiation. J. Lipid Res. 2007;48:513–517. doi: 10.1194/jlr.C600022-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.van Deijk A.F., Camargo N., Timmerman J., Heistek T., Brouwers J.F., Mogavero F., Mansvelder H.D., Smit A.B., Verheijen M.H.G. Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia. 2017;65:670–682. doi: 10.1002/glia.23120. [DOI] [PubMed] [Google Scholar]
- 36.Ioannou M.S., Jackson J., Sheu S.-H., Chang C.-L., V Weigel A., Liu H., Pasolli H.A., Xu C.S., Pang S., Matthies D., Hess H.F., Lippincott-Schwartz J., Liu Z. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell. 2019;177:1522–1535.e14. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Ji Y., Zheng K., Li S., Ren C., Shen Y., Tian L., Zhu H., Zhou Z., Jiang Y. Insight into the potential role of ferroptosis in neurodegenerative diseases. Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.1005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.