Abstract
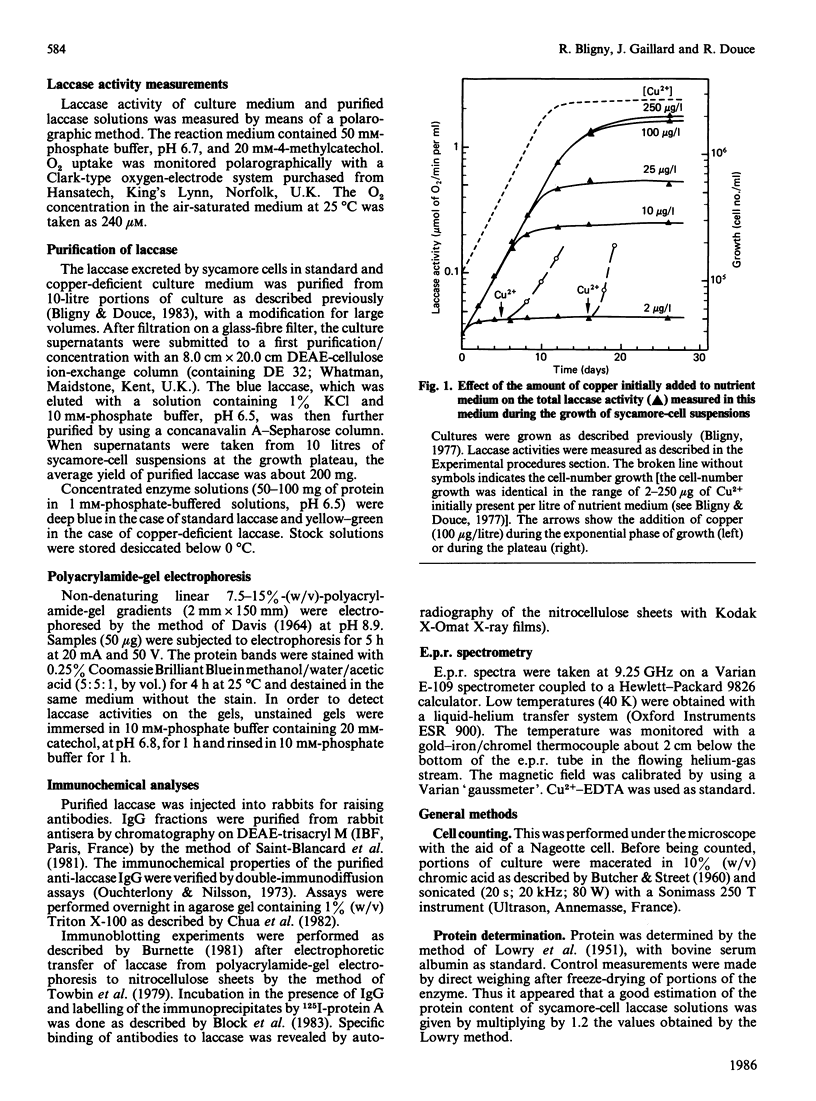
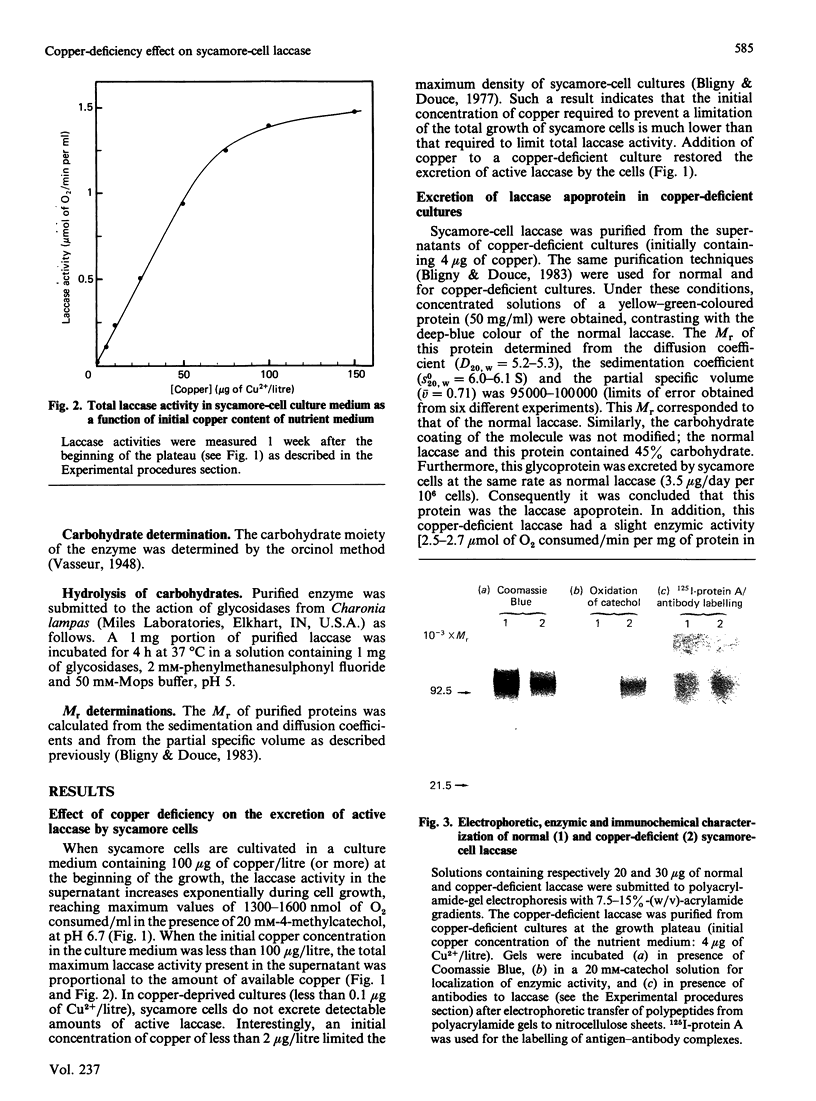
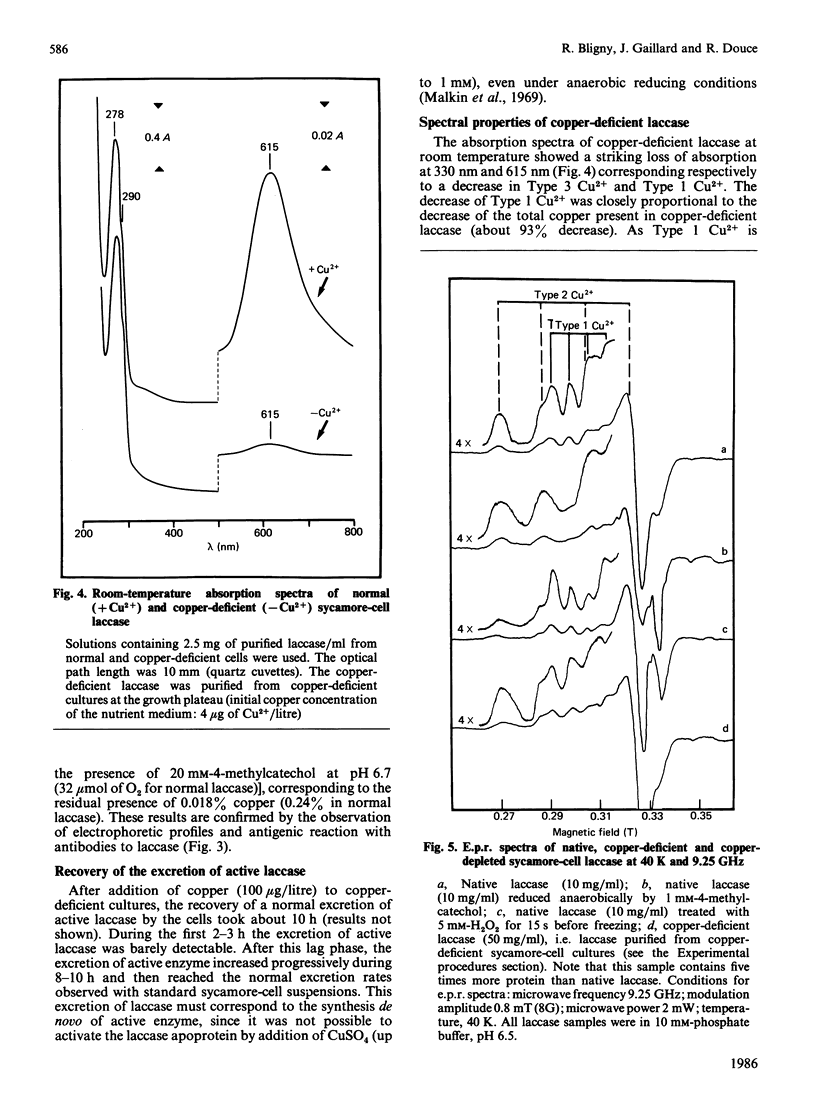
Copper-deprived sycamore (Acer pseudoplatanus) cells do not excrete molecules of active laccase in their culture medium. In the range of 2-100 micrograms of copper initially present per litre of nutrient solution, the total laccase activity measured in the cell suspensions at the end of the exponential phase of growth was closely proportional to the amount of added copper. However, copper-deprived cells excreted the laccase apoprotein (laccase without copper) at the same rate as copper-supplied cells excreted the active, copper-containing, laccase. When the culture medium was initially supplied with limiting amounts of copper, the active laccase was excreted until all copper molecules were metabolized. Thereafter, the laccase apoprotein was excreted. Consequently, at the end of the exponential phase of growth, the cell supernatants contained a mixture of apoprotein and copper-containing laccase. After purification and concentration, this mixture of copper-containing laccase (blue) and laccase apoprotein (slightly yellow) showed a yellow-green colour. Under copper-limiting culture conditions an equivalent decrease of Type 1, Type 2 and Type 3 Cu2+ was observed. Addition of copper to copper-deficient enzyme solutions does not result in a recovery of the enzyme activity. However, when added to copper-deficient sycamore-cell suspensions, copper induced a recovery of the excretion of active enzyme, at a normal rate, within about 10 h. The first molecules of active laccase were excreted after 3-4 h.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bligny R., Douce R. Excretion of laccase by sycamore (Acer pseudoplatanus L.) cells. Purification and properties of the enzyme. Biochem J. 1983 Feb 1;209(2):489–496. doi: 10.1042/bj2090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligny R., Douce R. Mitochondria of Isolated Plant Cells (Acer pseudoplatanus L.): II. Copper Deficiency Effects on Cytochrome C Oxidase and Oxygen Uptake. Plant Physiol. 1977 Nov;60(5):675–679. doi: 10.1104/pp.60.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligny R. Growth of Suspension-cultured Acer pseudoplatanus L. Cells in Automatic Culture Units of Large Volume. Plant Physiol. 1977 Mar;59(3):502–505. doi: 10.1104/pp.59.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. A., Dorne A. J., Joyard J., Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. I. Electrophoretic and immunochemical analyses. J Biol Chem. 1983 Nov 10;258(21):13273–13280. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Frank P., Farver O., Pecht I. The Type 3 copper site is intact but labile in Type 2-depleted laccase. J Biol Chem. 1983 Sep 25;258(18):11112–11117. [PubMed] [Google Scholar]
- LAMPORT D. T. CELL SUSPENSION CULTURES OF HIGHER PLANTS: ISOLATION AND GROWTH ENERGETICS. Exp Cell Res. 1964 Jan;33:195–206. doi: 10.1016/s0014-4827(64)81026-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leguay J. J., Guern J. Quantitative Effects of 2,4-Dichlorophenoxyacetic Acid on Growth of Suspension-cultured Acer pseudoplatanus Cells. Plant Physiol. 1975 Sep;56(3):356–359. doi: 10.1104/pp.56.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G., Vänngård T. The reversible removal of one specific copper(II) from fungal laccase. Eur J Biochem. 1969 Jan;7(2):253–259. doi: 10.1111/j.1432-1033.1969.tb19600.x. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Hartmann H. J., Desideri A., Weser U., Rotilio G. Yeast copper-thionein can reconstitute the Japanese-lacquer-tree (Rhus vernicifera) laccase from the Type 2-copper-depleted enzyme via a direct copper(I)-transfer mechanism. Biochem J. 1983 May 1;211(2):515–517. doi: 10.1042/bj2110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill P., Fielden E. M., Avigliano L., Marcozzi G., Ballini A., Agrò F. Pulse-radiolysis studies on the interaction of one-electron reduced species with blue oxidases. Reduction of type-2-copper-depleted ascorbate oxidase. Biochem J. 1984 Aug 15;222(1):65–70. doi: 10.1042/bj2220065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhammar B., Oda Y. Spectroscopic and catalytic properties of Rhus vernicifera laccase depleted in type 2 copper. J Inorg Biochem. 1979 Oct;11(2):115–127. doi: 10.1016/s0162-0134(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Saint-Blancard J., Fourcart J., Limonne F., Girot P., Boschetti E. Nouveaux échangeurs d'ions Trisacryl: intérêt et application au fractionnement des protéines du plasma humain. Ann Pharm Fr. 1981;39(5):403–409. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]



