Abstract
Objectives: To analyze the risk factors for peri-implantitis (PI) in patients with periodontitis after dental implantation and to establish a prediction model. Methods: A retrospective analysis was conducted using clinical data from 208 patients with periodontitis who required implant restoration due to tooth loss from various causes. These patients, meeting the indications for dental implantation, were treated at the Third People’s Hospital of Shenzhen from January 2019 to December 2023. The dataset was divided into training and validation sets in a 7:3 ratio. Logistic regression was used to identify risk factors for PI in these patients. Significant variables from the regression analysis were incorporated into the prediction model. The model’s accuracy was evaluated using Receiver Operating Characteristic (ROC) and calibration curves. A decision curve was also drawn to assess the clinical utility of the model. The model’s performance was evaluated using the Area Under the Curve (AUC), accuracy, sensitivity, and specificity. Results: Among the 208 patients, 68 developed PI, resulting in an incidence rate of 32.69%. Independent risk factors for PI included smoking history, diabetes, irregular periodontal treatment, high alveolar bone resorption, and a high plaque index score (all P < 0.05). Based on these risk factors, a logistic regression model was constructed to predict the occurrence of PI. The AUC of the logistic regression model was 0.911 for the training set and 0.823 for the validation set. The calibration curve indicated that the predicted probabilities closely matched the actual probabilities. The decision curve showed that the threshold probabilities for the training and validation sets were 0.1 to 0.85 and 0.1 to 0.81, respectively, suggesting that the net benefit was maximized within these ranges. Conclusion: Smoking history, diabetes, irregular periodontal treatment, high alveolar bone resorption, and a high plaque index score are significant risk factors for PI in patients with periodontitis. The logistic regression model constructed from these factors effectively predicts the probability of PI, providing a valuable reference for the prevention and management of PI.
Keywords: Periodontitis, postoperative, peri-implantitis, risk factors, prediction model
Introduction
Oral diseases have always significantly impacted human health. With improvements in diet and oral health awareness, dental implant restoration has become the preferred choice for patients with missing or defective teeth due to its stability, low foreign body sensation, and non-damaging effects on adjacent teeth. However, implant failure still occurs in clinical practice, with peri-implantitis (PI) being the most common cause [1]. PI is a frequent biological complication of dental implant restoration, beginning as peri-implant mucositis and progressing to PI. Clinical manifestations include soft tissue inflammation, peri-implant bone resorption, and even implant loosening and loss [2,3]. The soft and hard tissue destruction around the implant caused by PI results from mixed anaerobic bacterial infections, similar to periodontitis [4]. Additionally, periodontal pathogens in periodontitis patients can colonize around the implant, increasing the likelihood and severity of PI in these patients [5]. Preventing and treating PI is considered a major global health challenge. Therefore, predicting the probability of PI in patients with a history of periodontitis is crucial for enhancing doctor-patient communication and improving the success rate of implant restorations.
Currently, there is no effective treatment for PI, and the therapeutic effects are controversial. Most studies on PI in post-implantation periodontitis patients focus on risk factor analysis [6], and no effective risk prediction model has been developed. Logistic regression models are simple, convenient, and widely used for individual clinical outcome predictions [7-9]. This study aims to analyze the independent risk factors for PI in patients with a history of periodontitis and establish a logistic regression prediction model to provide a reference for predicting, preventing, and early treatment of PI in these patients.
Materials and methods
Research subjects
This study was approved by the Ethics Committee of the Third People’s Hospital of Shenzhen. A retrospective analysis was conducted on 208 periodontitis patients who required implant restoration due to tooth loss from various causes and who met the dental implantation criteria at the Third People’s Hospital of Shenzhen from January 2019 to December 2023. All patients successfully underwent dental implantation.
Inclusion criteria: (1) all subjects met the diagnostic criteria for periodontitis [10]; (2) all patients were ≥ 18 years old with complete baseline information, impact examination and other related data; (3) all patients were treated with periodontal treatment before operation, ensuring a stable periodontal condition; (4) all patients met oral implant indications.
Exclusion criteria: (1) patients with other oral diseases, such as leukoplakia, or herpes; (2) patients with diabetes, osteoporosis or severe systemic diseases that may affect periodontal disease; (3) pregnant or lactating women, or patients unable to complete regular follow-up; (4) patients taking bisphosphonates and warfarin.
Diagnostic criteria for periodontitis: Periodontitis sites [11]: gingival index > 0, probing depth > 3 mm and clinical attachment loss ≥ 3 mm. Criteria [12]: No or mild periodontitis: maximum attachment loss of 0-2 mm. Moderate periodontitis: maximum attachment loss of 3-4 mm. Severe periodontitis: maximum attachment loss of ≥ 5 mm.
Diagnostic criteria for PI [13]: Based on the consensus report of the 2017 International Symposium on New Periodontal Classification [19]: For patients with baseline data: presence of bleeding or pus on probing, increased probing depth compared to baseline, and bone resorption at the alveolar crest level one year after implant restoration. For patients without baseline data: probing depth ≥ 6 mm and/or bone resorption around the implant ≥ 3 mm, accompanied by bleeding or empyema.
Methods
Data collection
Medical records of all enrolled subjects were collected using the hospital medical record system, including: gender, age, smoking history (continuous or cumulative smoking for more than 6 months ≥ 1 cigarette/d), drinking history (drinking ≥ 1 time a week in the past year), diabetes, hypertension, osteoarthropathy, regular periodontal treatment, alveolar bone resorption degree, implant system, implant position, implant method, implant diameter, implant length, peri-implant mucosal thickness, bone increment, load time, keratinized gingival width, and dental plaque index.
Grouping methods
Patients were grouped based on the diagnostic criteria for PI: PI group (n = 68): Patients with peripheral inflammation, characterized by bleeding on probing and pus in the implant pocket, with marginal bone loss ≥ 2 mm as shown by imaging [14]. NPI group (n = 140): Patients without peripheral inflammation.
Clinical validation
From January to April 2024, 42 periodontitis patients requiring implant restoration due to tooth loss and meeting dental implantation criteria were collected. All patients successfully completed dental implantation.
Data preprocessing
To eliminate interactions between different factors, data centralization and standardization were performed. In this study, 70% of the patients with PI were randomly selected as the training set (n = 145), and 30% were used as the validation set (n = 63).
Statistical analysis
Statistical analysis was conducted using SPSS 26 and R 4.2.1 software. Measurement data were expressed as mean ± SD, and t-tests were performed. Count data were recorded as rates (%) and tested using the χ2 test. Logistic regression was used to analyze the risk factors for PI in patients with periodontitis after operation, and significant variables from the regression analysis were included in the prediction model. The model’s discrimination and calibration were evaluated using the Receiver Operating Characteristic (ROC) curve and calibration curve. The decision curve and confusion matrix were calculated in the validation set to assess the clinical utility of the PI risk prediction model. The model’s predictive value was evaluated based on the Area Under the Curve (AUC), accuracy, sensitivity, and specificity, and the logistic regression model’s predictive ability was analyzed.
A p-value of < 0.05 was considered statistically significant.
Results
Comparison of clinical data between the PI and NPI groups
A total of 208 patients with periodontitis were included in this study. Among them, 68 patients developed PI after implantation, resulting in an incidence rate of 32.69%. Significant differences were observed in smoking history, diabetes, regular periodontal treatment, alveolar bone resorption degree, and dental plaque index between the PI group and the NPI group (all P < 0.05). Other aspects were similar (all P > 0.05). See Table 1.
Table 1.
Comparison of clinical data analysis between the PI and NPI groups [n (%), (Mean ± SD)]
| Features | Classification | PI group (n = 68) | NPI group (n = 140) | t/χ2 | P |
|---|---|---|---|---|---|
| Male | 39 (57.35) | 74 (52.86) | 0.373 | 0.541 | |
| Age (year) | 53.20±7.28 | 53.17±6.54 | 0.034 | 0.973 | |
| Smoking history | 45 (66.18) | 33 (23.57) | 35.447 | < 0.001 | |
| Drinking history | 35 (51.47) | 56 (40.0) | 2.447 | 0.118 | |
| Diabetes | 44 (64.71) | 35 (25.0) | 30.633 | < 0.001 | |
| Hypertension | 36 (52.94) | 65 (46.43) | 0.777 | 0.378 | |
| Osteoarthrosis | 34 (50.0) | 63 (45.0) | 0.460 | 0.498 | |
| Periodic periodontal treatment | 28 (41.18) | 32 (22.86) | 26.159 | < 0.001 | |
| Alveolar bone resorption degree | Level 1 | 17 (25.00) | 64 (45.71) | 19.440 | < 0.001 |
| Level 2 | 21 (30.88) | 53 (37.86) | |||
| Level 3 | 30 (44.12) | 23 (16.43) | |||
| Implant system | Ankylos | 32 (47.06) | 73 (52.14) | 0.473 | 0.491 |
| Nobel | 36 (52.94) | 67 (47.86) | |||
| Dental implant position | Anterior teeth | 16 (23.53) | 31 (22.14) | 0.765 | 0.682 |
| Premolars | 29 (42.65) | 53 (37.86) | |||
| Molars | 23 (33.82) | 56 (40.0) | |||
| Embedding method | Submersible | 42 (61.76) | 78 (55.71) | 0.686 | 0.407 |
| Non-submerged | 26 (38.24) | 62 (44.29) | |||
| Plant diameter | < 3.5 mm | 18 (26.47) | 35 (25.0) | 0.915 | 0.633 |
| 3.6-5 mm | 31 (45.59) | 73 (52.14) | |||
| > 5 mm | 19 (27.94) | 32 (22.86) | |||
| Planting length | < 8 mm | 20 (29.41) | 40 (28.57) | 1.626 | 0.443 |
| 10-13 mm | 21 (30.88) | 33 (23.57) | |||
| > 13 mm | 27 (39.71) | 67 (47.86) | |||
| Mucosal thickness around the implant < 2 mm | Yes | 38 (55.88) | 79 (56.43) | 0.006 | 0.941 |
| No | 30 (44.12) | 61 (43.57) | |||
| Bone augmentation surgery | 18 (26.47) | 29 (20.71) | 0.867 | 0.352 | |
| Loading time | 1-3 years | 42 (61.76) | 93 (66.43) | 0.437 | 0.509 |
| 4-5 years | 26 (38.24) | 47 (33.57) | |||
| Keratinized gingival width | < 2 mm | 29 (42.65) | 45 (32.14) | 2.203 | 0.138 |
| ≥ 2 mm | 39 (57.35) | 95 (67.86) | |||
| Dental plaque index | 0 point | 17 (25.00) | 51 (36.43) | 8.038 | 0.045 |
| 1 point | 13 (19.12) | 41 (29.29) | |||
| 2 points | 16 (23.53) | 25 (17.86) | |||
| 3 points | 22 (32.35) | 23 (16.42) |
Note: PI is peri-implantitis; NPI is non-peri-implantitis.
Comparison of data in the training set and validation set
The differences between PI and NPI in both the training set and the validation set were statistically significant (P < 0.05). See Table 2.
Table 2.
Data analysis of training set and validation set [n (%), (Mean ± SD)]
| Features | Classification | Training set (n = 145) | Validation set (n = 63) | t/χ2 | P |
|---|---|---|---|---|---|
| Classification | |||||
| PI | 40 (27.59) | 28 (44.44) | 5.672 | 0.017 | |
| NPI | 105 (72.41) | 35 (55.56) | |||
| Male | 76 (52.41) | 37 (58.73) | 0.706 | 0.401 | |
| Age (year) | 54.33±6.75 | 54.28±7.16 | 0.048 | 0.562 | |
| Smoking history | 56 (38.62) | 22 (34.92) | 0.257 | 0.613 | |
| Drinking history | 55 (37.93) | 33 (52.38) | 3.757 | 0.053 | |
| Diabetes | 54 (37.24) | 32 (50.79) | 3.326 | 0.068 | |
| Hypertension | 70 (48.28) | 29 (46.03) | 0.089 | 0.766 | |
| Osteoarthrosis | 67 (46.21) | 30 (47.62) | 0.035 | 0.851 | |
| Periodic periodontal treatment | 50 (34.48) | 25 (39.68) | 0.515 | 0.473 | |
| Alveolar bone resorption degree | Level 1 | 51 (35.17) | 20 (31.75) | 0.835 | 0.659 |
| Level 2 | 46 (31.73) | 18 (28.57) | |||
| Level 3 | 48 (33.10) | 25 (39.68) | |||
| Implant system | Ankylos | 74 (51.03) | 32 (50.79) | 0.001 | 0.975 |
| Nobel | 71 (48.97) | 31 (49.21) | |||
| Dental implant position | Anterior teeth | 42 (28.97) | 15 (23.81) | 1.877 | 0.391 |
| Premolars | 53 (36.55) | 20 (31.75) | |||
| Molars | 50 (34.48) | 28 (44.44) | |||
| Embedding method | Submersible | 81 (55.86) | 29 (46.03) | 1.703 | 0.192 |
| Non-submerged | 64 (44.14) | 34 (53.97) | |||
| Plant diameter | < 3.5 mm | 45 (31.03) | 19 (30.16) | 0.265 | 0.876 |
| 3.6-5 mm | 59 (40.69) | 24 (38.09) | |||
| > 5 mm | 41 (28.28) | 20 (31.75) | |||
| Planting length | < 8 mm | 42 (28.97) | 18 (28.57) | 0.133 | 0.935 |
| 10-13 mm | 50 (34.48) | 24 (38.09) | |||
| > 13 mm | 43 (29.65) | 21 (33.34) | |||
| Mucosal thickness around the implant < 2 mm | Yes | 86 (59.31) | 30 (47.62) | 2.434 | 0.119 |
| No | 59 (40.69) | 33 (52.38) | |||
| Bone augmentation surgery | 43 (29.66) | 24 (38.09) | 1.433 | 0.231 | |
| Loading time | 1-3 years | 91 (62.76) | 33 (52.38) | 1.965 | 0.161 |
| 4-5 years | 54 (37.24) | 30 (47.62) | |||
| Keratinized gingival width | < 2 mm | 54 (37.24) | 28 (44.44) | 0.954 | 0.329 |
| ≥ 2 mm | 91 (62.76) | 35 (5.56) | |||
| Dental plaque index | 0 point | 49 (33.79) | 18 (28.57) | 3.835 | 0.280 |
| 1 point | 33 (22.76) | 21 (33.34) | |||
| 2 points | 28 (19.31) | 14 (22.22) | |||
| 3 points | 35 (24.14) | 10 (15.87) |
Regression analysis
The statistically significant indicators from the clinical data analysis were included as independent variables in the multivariate logistic regression analysis, with the occurrence of PI in patients with periodontitis as the dependent variable (occurrence = 1, non-occurrence = 0). The relevant variables were assigned as follows: smoking history (yes = 1, no = 0), diabetes (yes = 1, no = 0), regular periodontal treatment (yes = 1, no = 0), alveolar bone resorption degree, and dental plaque index were included as actual values. The results indicated that smoking history, diabetes, lack of regular periodontal treatment, alveolar bone resorption degree, and dental plaque index were risk factors for PI in patients with periodontitis after dental implantation (P < 0.05). See Table 3.
Table 3.
Regression analysis
| Risk Factor | B | S.E. | Wald value | OR value | 95% CI | P value |
|---|---|---|---|---|---|---|
| Smoking history | 1.598 | 0.399 | 16.048 | 4.944 | 2.262-10.805 | < 0.001 |
| Diabetes | 1.440 | 0.401 | 12.877 | 4.222 | 1.922-9.270 | < 0.001 |
| Regular periodontal treatment | -1.891 | 0.413 | 20.954 | 0.151 | 0.067-0.339 | < 0.001 |
| Alveolar bone resorption degree | 0.928 | 0.251 | 13.698 | 2.529 | 1.547-4.133 | < 0.001 |
| Dental plaque index | 0.449 | 0.170 | 6.598 | 1.566 | 1.122-2.187 | 0.008 |
| Constant | -3.388 | 0.659 | 26.442 | 0.034 | - | - |
Model construction
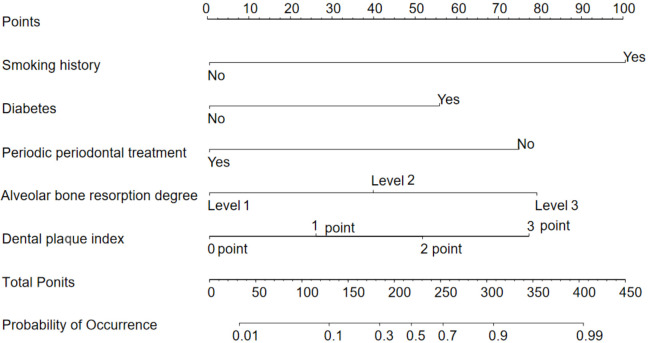
Based on the results of the multivariate logistic regression analysis, the model was constructed using smoking history, diabetes, regular periodontal treatment, alveolar bone resorption, and plaque index as variables. The model formula is as follows: Logistic = -3.388 + 1.598 × smoking history + 1.440 × diabetes - 1.891 × regular periodontal treatment + 0.928 × alveolar bone resorption degree + 0.449 × dental plaque index. The nomogram model is shown in Figure 1.
Figure 1.
Nomogram model.
ROC curve analysis of the prediction model
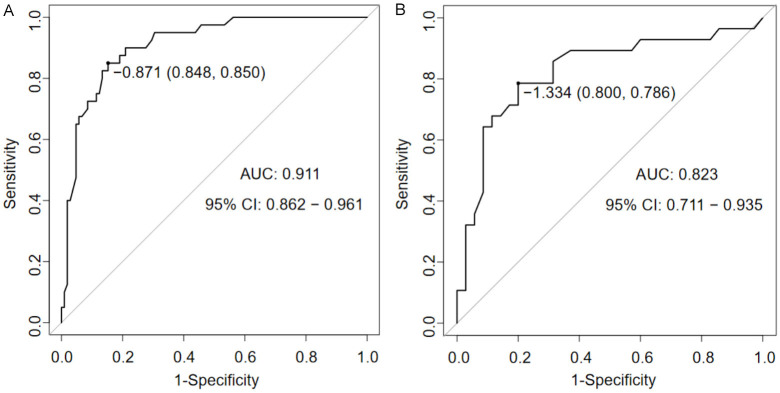
To further evaluate the predictive effectiveness of the logistic regression model for PI in periodontitis patients after dental implantation, ROC curves for both the training and validation sets were plotted. The AUC for the training set was 0.911 (Figure 2A), and the AUC for the validation set was 0.823 (Figure 2B), indicating that the logistic regression model has a good predictive rate.
Figure 2.
ROC curve of the logistic regression model (A is the training set, B is the validation set).
Calibration curve analysis of prediction model
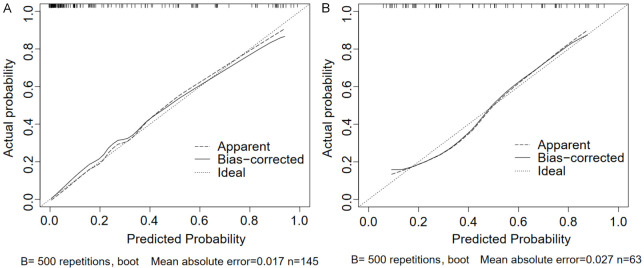
The calibration curves showed that the prediction curves for both the training and validation sets were close to the ideal curves (Figure 3A and 3B), indicating that the model’s predicted probabilities are consistent with the actual probabilities. The Hosmer-Lemeshow test result was χ2 = 10.671, P = 0.221 (P > 0.05), suggesting that the model was well fitted.
Figure 3.
Calibration curve of the logistic regression model (A is the training set, B is the validation set).
Decision curve analysis of the prediction model
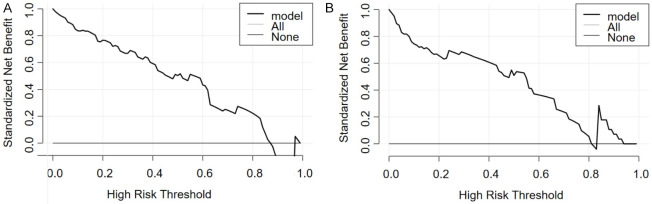
The decision curve analysis showed that the threshold probabilities for the training set and validation set were 0.1-0.85 (Figure 4A) and 0.1-0.81 (Figure 4B), respectively, suggesting that patients derived the greatest net benefit within these ranges.
Figure 4.
Decision curve of the logistic regression model (A is the training set, B is the validation set).
Clinical validation
To verify the clinical value of the logistic regression model, this study prospectively included 42 periodontitis patients. The results showed that the specificity, sensitivity, and accuracy of the clinical validation were 0.792, 0.722, and 0.762, respectively (Table 4).
Table 4.
Clinical validation evaluation
| Prediction results | Gold standard | Footing | |
|---|---|---|---|
|
| |||
| Occur | No occur | ||
| Occur | 13 | 5 | 18 |
| No occur | 5 | 19 | 24 |
| Footing | 18 | 24 | 42 |
Discussion
Periodontitis is a chronic inflammatory disease affecting the supporting tissues around natural teeth, characterized by soft tissue inflammation and alveolar bone resorption, and it is the main cause of tooth loss in adults [15]. With advancements in implant materials and technology and the improvement of China’s economic level, more patients with periodontitis opt for dental implants to restore missing teeth. However, periodontitis pathogens can colonize around the implant, significantly increasing the probability of PI. The mechanism of PI is complex, and if not controlled promptly and effectively, it can lead to implant loosening, aggravated bone resorption, and reduced implantation success rates [16,17]. Additionally, the lack of effective treatments for PI highlights the importance of analyzing PI risk factors in periodontitis patients, conducting preoperative evaluations, and reducing PI hazards to ensure long-term implant retention.
Current studies suggest that the factors influencing PI after implant surgery in patients with periodontitis are similar to those of implant failure, including smoking history, alveolar bone resorption, and periodontal conditions [18,19]. The occurrence of PI is also affected by factors such as the severity of periodontal disease, postoperative infection, and treatment response. To comprehensively identify the risk factors for PI in patients with periodontitis, this research combined findings from relevant reports on PI risk factors both domestically and internationally and analyzed multiple independent risk factors in the study subjects.
The results indicated that smoking history, diabetes, lack of regular periodontal treatment, high alveolar bone resorption degree, and high dental plaque index were independent risk factors for PI in patients with periodontitis. The consensus is that smoking adversely affects oral health. Long-term smoking increases the detection of inflammatory factors in gingival crevicular fluid [20,21], inhibits peri-implant osseointegration, and impedes postoperative healing. Compared to non-smokers, patients with periodontitis who smoke have a significantly higher risk and severity of PI [22].
Diabetes significantly increases the risk of PI in periodontitis patients due to the long-term abnormal metabolism of hyperglycemia. Chronic high blood glucose levels lead to microvascular disease and hypoxia in periodontal tissues, accelerating the reproduction of pathogenic bacteria and increasing the risk of bacterial infection, thus elevating the risk of PI after dental implantation in periodontitis patients [23,24].
Basic periodontal treatment has been shown to significantly improve the health of peri-implant tissues, reduce the incidence of PI, and promote the long-term retention of implants [25]. In this study, the increased risk of PI in periodontitis patients without regular periodontal treatment may be related to plaque accumulation around the implant. Therefore, it is crucial for periodontitis patients to undergo regular periodontal treatment to prevent PI.
Plaque is an aggregate formed by bacterial populations adhering to an extracellular matrix. When an implant is exposed to the oral environment, bacteria can easily accumulate on its surface, forming plaque, which induces and exacerbates PI. The plaque index is a key indicator of oral health. Ferreira et al. [26] found that patients with a plaque index ≥ 2 had a higher probability of PI (OR = 14.3). Wada et al. [27] studied the incidence of peri-implant diseases in a Japanese population with a loading time of three years or more and found that the plaque index was not only associated with peri-implant mucositis but also proved to be a risk factor for PI. In this research, the plaque index was also identified as a risk factor for PI in patients with periodontitis, consistent with previous findings. The occurrence of PI takes time, and the plaque index can change over time. Therefore, oral health management plays a crucial role in plaque control.
Moreover, the attenuation of alveolar bone mass caused by periodontitis is a key factor affecting the early stability of implants. Insufficient periodontal bone mass leads to a low implant-bone bonding rate and increases the risk of PI [28]. Severe bone resorption can cause mechanical stress on periodontal soft and hard tissues, inducing a local inflammatory response. When dental plaque accumulates, bone resorption can interact with bacterial factors to accelerate the development of PI, further aggravating bone resorption [29]. The patients included in this research all experienced tooth loss due to periodontitis. The degree of alveolar bone resorption varied individually, with patients exhibiting higher degrees of resorption at greater risk for PI. Studies indicate that patients with severe periodontitis have more pronounced alveolar bone resorption, deeper periodontal pockets, and more significant inflammation. Even if bacterial infections are controlled, the aggravation of bone resorption leads to the loss of periodontal tissue, indicating that the preoperative state of alveolar bone resorption significantly impacts postoperative PI [30].
Therefore, in clinical practice, evaluating alveolar bone resorption is essential, and intervention measures should be taken if necessary to reduce the mechanical load on implants. For periodontitis patients with extensive alveolar bone resorption, appropriate implants and surgical methods should be selected based on the extent and severity of bone defects assessed by imaging. Additionally, the literature has identified some gene polymorphisms that may be related to PI [31]. However, current research on the relationship between gene polymorphisms of inflammation and bone metabolism-related proteins and PI is insufficient, suggesting a potential focus for future research.
Logistic regression models can screen factors affecting the occurrence of periodontitis and evaluate the relative risk for patients [32]. In recent years, many studies have used logistic regression models to predict the risk of oral diseases effectively. These models have been used to predict the risk of root caries [33], the benign and malignant nature of oral parotid tumors [34], and the risk of PI in diabetic patients [35]. To predict the probability of PI in patients with periodontitis, this study established a logistic regression prediction model based on selected risk factors.
The results demonstrated that the logistic regression model had good predictive value for PI risk after dental implantation in patients with periodontitis. The model’s accuracy and discrimination were further evaluated using ROC and calibration curves. The results indicated that the prediction curves for both the training set and the validation set fitted well with the ideal curve, signifying good predictive value. The AUC of the ROC curve for the training set and validation set were 0.911 and 0.823, respectively, highlighting the model’s robust predictive ability. These findings suggest that the logistic regression model constructed in this research can be an effective tool for clinically predicting PI in patients with periodontitis. By identifying and mitigating relevant risk factors, the model can help reduce PI incidence, lower healthcare costs, improve implantation success rates, and enhance patient satisfaction.
This study may be limited by differences in sample size and feature selection between studies, leading to potential deviations in the model’s absolute prediction efficiency parameters. These differences may stem from variations in baseline characteristics such as age and smoking history among different populations, as well as subjective judgments during feature screening. Future research should involve more high-quality datasets to validate and establish reliable prediction models to guide clinical decision-making.
In summary, smoking history, diabetes, failure to perform regular periodontal treatment, high alveolar bone resorption, and high dental plaque index scores are all significant risk factors for PI in patients with periodontitis. Targeted interventions for these risk factors and the use of a stable and accurate prediction model can effectively reduce the incidence of PI and improve treatment outcomes. Furthermore, compared to traditional statistical methods, machine learning algorithms have shown higher accuracy in prediction results, offering potential for individualized PI risk prediction in periodontitis patients and warranting clinical promotion.
Disclosure of conflict of interest
None.
References
- 1.Liu M, Xu Z, Li H. Effect of orthodontic combined with implant repair on aesthetic effect and gingival crevicular fluid factor in patients with dentition defect and periodontitis. Biomed Res Int. 2022;2022:8065313. doi: 10.1155/2022/8065313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Güney Z, Karacaoglu F, Barıs E, Gezer KG, Akkaya MM. The relationship of peri-implant soft tissue wound healing with implant cover screw design: cross-sectional study. Clin Implant Dent Relat Res. 2024;26:299–308. doi: 10.1111/cid.13272. [DOI] [PubMed] [Google Scholar]
- 3.Renvert S, Persson GR, Pirih FQ, Camargo PM. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Periodontol. 2018;89(Suppl 1):S304–S312. doi: 10.1002/JPER.17-0588. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Meng HX. Research progress in microbiological characteristics of peri-implant disease. Zhonghua Kou Qiang Yi Xue Za Zhi. 2017;52:773–776. doi: 10.3760/cma.j.issn.1002-0098.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Roccuzzo A, Imber JC, Marruganti C, Salvi GE, Ramieri G, Roccuzzo M. Clinical outcomes of dental implants in patients with and without history of periodontitis: a 20-year prospective study. J Clin Periodontol. 2022;49:1346–1356. doi: 10.1111/jcpe.13716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravidà A, Siqueira R, Di Gianfilippo R, Kaur G, Giannobile A, Galindo-Moreno P, Wang CW, Wang HL. Prognostic factors associated with implant loss, disease progression or favorable outcomes after peri-implantitis surgical therapy. Clin Implant Dent Relat Res. 2022;24:222–232. doi: 10.1111/cid.13074. [DOI] [PubMed] [Google Scholar]
- 7.Pereira D, Machado V, Botelho J, Proença L, Rua J, Lemos C, Mendes JJ, Delgado AS. Impact of malocclusion, tooth loss and oral hygiene habits on quality of life in orthodontic patients: a cross-sectional study. Int J Environ Res Public Health. 2021;18:7145. doi: 10.3390/ijerph18137145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Tantawi M, Folayan MO, Oginni O, Adeniyi AA, Mapayi B, Yassin R, Chukwumah NM, Sam-Agudu NA. Association between mental health, caries experience and gingival health of adolescents in sub-urban Nigeria. BMC Oral Health. 2021;21:223. doi: 10.1186/s12903-021-01589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen GQ, Duan Y, Wang JF, Lian Y, Yin XL. Serum α-Klotho associated with oral health among a nationally representative sample of US adults. Front Endocrinol (Lausanne) 2022;13:970575. doi: 10.3389/fendo.2022.970575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brito LF, Taboza ZA, Silveira VR, Teixeira AK, Rego RO. Diagnostic accuracy of severe periodontitis case definitions: comparison of the CDC/AAP, EFP/AAP, and CPI criteria. J Periodontol. 2022;93:867–876. doi: 10.1002/JPER.21-0365. [DOI] [PubMed] [Google Scholar]
- 11.Casado PL, Pereira MC, Duarte ME, Granjeiro JM. History of chronic periodontitis is a high risk indicator for peri-implant disease. Braz Dent J. 2013;24:136–41. doi: 10.1590/0103-6440201302006. [DOI] [PubMed] [Google Scholar]
- 12.Timmerman MF, van der Weijden GA. Risk factors for periodontitis. Int J Dent Hyg. 2006;4:2–7. doi: 10.1111/j.1601-5037.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 13.Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, Camargo PM, Chen S, Cochran D, Derks J, Figuero E, Hämmerle CHF, Heitz-Mayfield LJA, Huynh-Ba G, Iacono V, Koo KT, Lambert F, McCauley L, Quirynen M, Renvert S, Salvi GE, Schwarz F, Tarnow D, Tomasi C, Wang HL, Zitzmann N. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S313–S318. doi: 10.1002/JPER.17-0739. [DOI] [PubMed] [Google Scholar]
- 14.Pan YH, Lin HK, Lin JC, Hsu YS, Wu YF, Salamanca E, Chang WJ. Evaluation of the peri-implant bone level around platform-switched dental implants: a retrospective 3-year radiographic study. Int J Environ Res Public Health. 2019;16:2570. doi: 10.3390/ijerph16142570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muramatsu R, Sato T, Hamamura K, Miyazawa K, Takeguchi A, Tabuchi M, Togari A, Goto S. Guanabenz inhibits alveolar bone resorption in a rat model of periodontitis. J Pharmacol Sci. 2021;147:294–304. doi: 10.1016/j.jphs.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Roccuzzo M, Mirra D, Pittoni D, Ramieri G, Roccuzzo A. Reconstructive treatment of peri-implantitis infrabony defects of various configurations: 5-year survival and success. Clin Oral Implants Res. 2021;32:1209–1217. doi: 10.1111/clr.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrivastava PK, Mahmood A, Datta S, Sengar P, Sybil D. Tetracycline impregnated bone grafts in the management of peri-implantitis and guided bone regeneration around dental implants: a systematic review. Saudi Dent J. 2022;34:689–698. doi: 10.1016/j.sdentj.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhakeem M, Kanounisabet N, Nowzari H, Aslroosta H, Moslemi N. Risk indicators of long-term outcome of implant therapy in patients with a history of severe periodontitis or no history of periodontitis: a retrospective cohort study. Int J Dent Hyg. 2023;21:227–237. doi: 10.1111/idh.12587. [DOI] [PubMed] [Google Scholar]
- 19.Wada M, Mameno T, Onodera Y, Matsuda H, Daimon K, Ikebe K. Prevalence of peri-implant disease and risk indicators in a Japanese population with at least 3 years in function-a multicentre retrospective study. Clin Oral Implants Res. 2019;30:111–120. doi: 10.1111/clr.13397. [DOI] [PubMed] [Google Scholar]
- 20.Abduljabbar T, Akram Z, Vohra F, Warnakulasuriya S, Javed F. Assessment of interleukin-1β, interleukin-6, and tumor necrosis factor-Α levels in the peri-implant sulcular fluid among waterpipe (narghile) smokers and never-smokers with peri-implantitis. Clin Implant Dent Relat Res. 2018;20:144–150. doi: 10.1111/cid.12557. [DOI] [PubMed] [Google Scholar]
- 21.Amaral AL, da Costa Andrade PA, Lwaleed BA, Andrade SA. Impacts of smoking on oral health-what is the role of the dental team in smoking cessation? Evid Based Dent. 2023;24:186–187. doi: 10.1038/s41432-023-00930-3. [DOI] [PubMed] [Google Scholar]
- 22.Beklen A, Sali N, Yavuz MB. The impact of smoking on periodontal status and dental caries. Tob Induc Dis. 2022;20:72. doi: 10.18332/tid/152112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner J, Spille JH, Wiltfang J, Naujokat H. Systematic review on diabetes mellitus and dental implants: an update. Int J Implant Dent. 2022;8:1. doi: 10.1186/s40729-021-00399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serón C, Olivero P, Flores N, Cruzat B, Ahumada F, Gueyffier F, Marchant I. Diabetes, periodontitis, and cardiovascular disease: towards equity in diabetes care. Front Public Health. 2023;11:1270557. doi: 10.3389/fpubh.2023.1270557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CY, Chen Z, Pan WL, Wang HL. The effect of supportive care in preventing peri-implant diseases and implant loss: a systematic review and meta-analysis. Clin Oral Implants Res. 2019;30:714–724. doi: 10.1111/clr.13496. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira SD, Martins CC, Amaral SA, Vieira TR, Albuquerque BN, Cota LOM, Esteves Lima RP, Costa FO. Periodontitis as a risk factor for peri-implantitis: systematic review and meta-analysis of observational studies. J Dent. 2018;79:1–10. doi: 10.1016/j.jdent.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Wada M, Mameno T, Onodera Y, Matsuda H, Daimon K, Ikebe K. Prevalence of peri-implant disease and risk indicators in a Japanese population with at least 3 years in function-a multicentre retrospective study. Clin Oral Implants Res. 2019;30:111–120. doi: 10.1111/clr.13397. [DOI] [PubMed] [Google Scholar]
- 28.Zhou R, Shen L, Yang C, Wang L, Guo H, Yang P, Song A. Periodontitis may restrain the mandibular bone healing via disturbing osteogenic and osteoclastic balance. Inflammation. 2018;41:972–983. doi: 10.1007/s10753-018-0751-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Tong Z, Luo H, Qu Y, Gu X, Si M. Titanium particles in peri-implantitis: distribution, pathogenesis and prospects. Int J Oral Sci. 2023;15:49. doi: 10.1038/s41368-023-00256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vagia P, Papalou I, Burgy A, Tenenbaum H, Huck O, Davideau JL. Association between periodontitis treatment outcomes and peri-implantitis: a long-term retrospective cohort study. Clin Oral Implants Res. 2021;32:721–731. doi: 10.1111/clr.13741. [DOI] [PubMed] [Google Scholar]
- 31.Lafuente-Ibáñez-de-Mendoza I, Marichalar-Mendia X, Setién-Olarra A, García-de-la-Fuente AM, Martínez-Conde-Llamosas R, Aguirre-Urizar JM. Genetic polymorphisms of inflammatory and bone metabolism related proteins in a population with dental implants of the Basque Country. A case-control study. BMC Oral Health. 2024;24:659. doi: 10.1186/s12903-024-04319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altun E, Walther C, Borof K, Petersen E, Lieske B, Kasapoudis D, Jalilvand N, Beikler T, Jagemann B, Zyriax BC, Aarabi G. Association between dietary pattern and periodontitis-a cross-sectional study. Nutrients. 2021;13:4167. doi: 10.3390/nu13114167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fee PA, Cassie H, Clarkson JE, Hall AF, Ricketts D, Walsh T, Goulão B. Development of a root caries prediction model in a population of dental attenders. Caries Res. 2022;56:429–446. doi: 10.1159/000526797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Z, Guo J, Feng J, Huang Y, Xu H, Zhou Q. Value of T2-weighted-based radiomics model in distinguishing Warthin tumor from pleomorphic adenoma of the parotid. Eur Radiol. 2023;33:4453–4463. doi: 10.1007/s00330-022-09295-0. [DOI] [PubMed] [Google Scholar]
- 35.Su N, Teeuw WJ, Loos BG, Kosho MXF, van der Heijden GJMG. Development and validation of a screening model for diabetes mellitus in patients with periodontitis in dental settings. Clin Oral Investig. 2020;24:4089–4100. doi: 10.1007/s00784-020-03281-w. [DOI] [PMC free article] [PubMed] [Google Scholar]