Abstract
Rheumatoid arthritis (RA) is an autoimmune illness affecting joint articulations, leading to a disability state. Currently, there is no satisfying optimal therapy except for immunosuppressants, which have variable and bad effects after long-term use. Hence, researchers have attempted to develop other alternative, safer, and more effective natural treatment agents that are effective and without undesirable effects. The objective of this research is to assess the antiarthritic properties of navel orange peel ethanolic extract (NOPEE) and naringin (NAR) in experimentally induced RA in male Wistar rats. RA was induced via two successive subcutaneous injections of 0.1 mL complete Freund’s adjuvant (CFA) into a footpad of the right hind leg. The arthritic rats were orally treated with 100 mg/kg body weight (b.w.)/day of NOPEE or with 25 mg/kg b.w./day of NAR for 14 days. Results showed that treatment with NOPEE or NAR obviously counteracted the increased ankle joint circumference, inflammatory cell infiltration, pannus development, cartilage degradation, and synovial hyperplasia that developed in CFA-induced arthritic rats. Additionally, the elevation of serum rheumatoid factor (RF), prostaglandin E2 (PGE-2), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-17 (IL-17) were significantly declined in parallel to enhanced level of serum interleukin-4 (IL-4). Furthermore, NOPEE and NAR supplementation, reversed the negative oxidative effects of lipid peroxidation (LPO), nitric oxide (NO), as well as improved the antioxidant glutathione level (GSH), glutathione reductase (GR) and superoxide dismutase (SOD) activities. Overall, the anti-arthritic effects of NOPEE and NAR may be mediated through their modulatory effects on T helper (Th)1/Th2/Th17 cytokines, oxidative stress, and the antioxidant defense system.
Keywords: Rheumatoid arthritis, navel orange peel ethanolic extract, naringin, Wistar rats
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that causes stiffness, musculoskeletal discomfort, swelling, degradation of the joints and bones, with an increased risk of developing osteoporosis, and deformity that leads to progressive disability [1-4]. It affects between 0.3 and 1% of the population and is more common in women and in developed economies and countries [5]. The cytokine network’s imbalance is important in the pathophysiology of RA. The etiology of RA is based on the patient’s body reacting to autoantigens, such as citrullinated peptides, or a foreign peptide, such as a viral or bacterial peptide. The synovial membrane in joints is then penetrated by activated T and B lymphocytes and monocytes, which generate cytokines and encourage leukocyte migration as well as the expansion of synovial fbroblast- and macrophage-like cells [6]. In rheumatoid joints, a disproportion between proinflammatory and anti-inflammatory cytokines favors the induction of autoimmunity, chronic inflammation and thereby joint destruction [7-9]. The regulation of the two key proinflammatory cytokines in RA, tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), is of crucial importance in RA [5,10-12]. In arthritis and osteoclastogenesis pathology, the investigation of the roles of these cytokines and other cytokines, such as interleukin-17 (IL-17), interleukin-18 (IL-18), and receptor activator of nuclear factor κB (RANK) ligand, the receptor activator of nuclear factor-kappa B (NF-κB), will help us better understand the pathophysiology of chronic arthritis and may help to improve present treatments [13]. Moreover, interleukin-4 (IL-4) also interleukin-10 (IL-10) are cytokines that have anti-inflammatory properties and thought to be promising modulators in the treatment of RA [5,11,14]. Although prostaglandin E2 (PGE-2) is also produced in response to proinflammatory cytokines, IL-1β, and TNF-α, it was found to have both pro- and anti-inflammatory effects, dependent on the receptor subtype, cell population, activation environment, and tissue receptor gene expression [15-17]. As a result, cytokines are regarded as potentially powerful indicators of RA, with roles expected to expand in the future, and a thorough understanding of the cytokine balance in RA may aid both the diagnostic and therapeutic processes [9,18-20].
Adjuvant-induced arthritis (AIA) in rats [11,21] or mice [22,23] is a chronic inflammatory illness marked by synovial membrane invasion and joint destruction [2,24,25]. It is more like human RA clinically, pathologically, immunologically, and histologically [5,11,12,21]. Not only does AIA assist in the knowledge of RA etiology in people, but it also aids in the development of new RA therapeutics [21]. Therefore, this research used complete Freund’s adjuvant (CFA)-induced arthritis as an investigational animal model to assess the ameliorative effects of various tested agents.
Alternative, safer, and more effective natural product-based medications are required due to the side effects and toxicity of widely used anti-arthritic pharmaceuticals [11,26].
Citrus flavonoids, which make up a large group of flavonoids, have been shown to have anti-inflammatory properties [27,28] and have the ability to decline lipid peroxidation (LPO) and increase the antioxidant defence system’s potency [29]. The antioxidant properties of the peel (flavedo and albedo) and juice of some commercially grown citrus fruits (Rutaceae), including grapefruit (Citrus paradisi), lemon (Citrus limon), lime (Citrus aurantiifolia), and sweet orange (Citrus sinensis), were assessed in vitro and it was found that the volatile and polar fractions of citrus peels as well as crude and polar fraction of citrus fruit juices revealed their free radicals (FRs) scavenging capacity and LPO inhibiting efficiency [30]. Citrus flavonoids, and naringin (NAR), which is one of the important components, have been shown to have potent anti-inflammatory and antioxidant properties in metabolic disorders [27,29,31]. Moreover, the therapeutic effects and underlying mechanisms of NAR, a flavanone glycoside of naringenin, against RA were clarified using network pharmacology research and in vitro clinical confirmation. NAR has been shown to decrease the intensity of clinical symptoms and to reduce the generation of inflammatory mediators and proinflammatory cytokines both in vitro and in vivo [30].
However, the effects of NOPEE and NAR on RA have not been studied. As a result, the goal of this study is to evaluate the effect of NOPEE and NAR on arthritic rats and discover their likely action mechanisms.
Materials and methods
Experimental animals
Forty-healthy male Wistar rats (divided into 4 categories, each with ten animals) with an average weight of 120±10 gm were obtained from the Egyptian holding company for biological products and vaccines (VACSERA) Animal Facilities (Helwan Station, Cairo, Egypt) and utilized as a mammalian animal model in this experiment. The rats were overseen for about two weeks before starting experimental work to eradicate any infections. In the animal house, the animals were kept in polypropylene cages with well-aerated stainless-steel lids (Zoology Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt) at normal range of temperature (22±2°C) and relative humidity (55±5%). The rats were given an adequate amount of standard food pelleted diet and water ad libitum and weighed weekly during the experimental periods. All techniques follow the requirements of the Faculty of Science’s Experimental Animal Ethics Commission, Beni-Suef University, Egypt (Ethical Approval Number: BSU/FS/2015/5).
Chemicals
CFA, a heat-destroyed Mycobacterium tuberculosis mineral oil-based suspension, was purchased from Sigma Chemical Company (MO, USA). NAR was obtained from Sigma-Aldrich Chemie GmbH, Eschenstr. 582024 Taufkirchen, Germany. The rest of the chemicals are of analytical reagent grade.
Induction of RA
The male Wistar rats were given CFA to induce RA. For more extensive severity the disease, arthritis was induced by a double subcutaneous (s.c.) injection of 0.1 mL CFA into a footpad of a rat’s right hind leg for two days [32,33].
Preparation of NOPEE
The navel orange fruits were obtained from the local market. The fruits were peeled by hand and rinsed under running water until they were clean. After that, the peels were air-dried. An electric grinder was used to grind the dried peels. For 72 hours, the powder (500 g) was steeped in 100% ethanol. To remove the peel particles, the mixture was filtered through a Whatman No. 2 filter paper. To guarantee full extraction, the residue was extracted twice under the same conditions. A rotary evaporator was used to filter the extract and evaporate it to semisolid bulk. The extract was stored in dark jars in the refrigerator at 4°C until it was needed.
Dose preparation of NOPEE and NAR
NOPEE as well as NAR were given to arthritic rats via oral gavage at doses of 100 mg/kg b.w./day [34] and 25 mg/kg b.w./day [35] for 14 days. These doses were added and dissolved in a volume of 5 mL carboxymethylcellulose (CMC) and at a concentration of 1% w/v.
Animal grouping
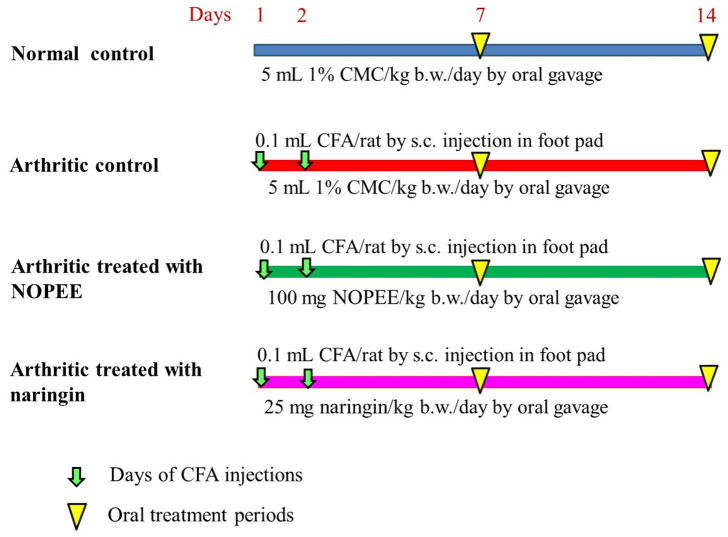
The animals were placed into 4 categories, each with ten animals (Figure 1): 1) Normal control group: Rats of this group were provided the corresponding volume of 1% CMC via oral gavage for 14 days. 2) Arthritic control group: The arthritic rats were given the corresponding volume of 1% CMC (5 mL/kg b.w./day) via oral gavage for 14 days. 3) Arthritic group treated with NOPEE: The rats of this group were treated daily with 100 mg/kg b.w. of NOPEE (dissolved in 5 mL 1% CMC) via oral gavage for 14 days. 4) Arthritic group treated with NAR: The rats were treated daily with 25 mg/kg b.w. of NAR (dissolved in 5 mL 1% CMC) via oral gavage for 14 days.
Figure 1.
Experimental design and animal grouping. CMC, carboxymethylcellulose; s.c., subcutaneous; kg. b.w., kilogram body weight; CFA, complete Freund’s Adjuvant; NOPEE, navel orange peel ethanolic extract; NAR, naringin.
By the end of experiment, the ankle circumference of the rats’ right hind leg was measured. Blood was drawn from the jugular vein after the rats were anaesthetized by inhaling ether to reduce pain, suffering and distress. The serum was separated by centrifugation at 3,000 rpm for 15 minutes after the blood had clotted. The pure, non-hemolyzed supernatant sera were rapidly collected, split into three separate portions for each animal, and stored at temperature 30°C until use. The animals were ethically euthanized. Each rat’s hind right ankle was detached and treated in 10% neutralized formalin buffer for histopathology. A half gram of liver was homogenized using a Teflon homogenizer (Glas-Col, Terre Haute, USA) in 5 mL cold ice, 0.9% NaCl for the determination of antioxidants and oxidative stress parameters. The homogenate was gained and saved in the freezer at -30°C.
Detection of markers related to inflammation and oxidative stress
Serum PGE-2, RF, IL-1β, TNF-α, IL-4 and IL-17 levels in the serum were assayed following the manufacturer’s instructions using kits of enzyme-linked immunosorbent assay (ELISA) technique bought from R&D Systems (Minneapolis, MN, USA). Moreover, the liver LPO and NO as well as reduced GSH levels were assayed according to manufacturer’s methods [36-38], respectively. The GR and SOD was measured dependent on the procedure of Goldberg & Spooner [39] as well as Marklund and Marklund [40].
Histopathological investigation
The articular bones in the right leg ankle were detached, cleaned in 0.9% NaCl, and preserved in 10% neutralized formalin buffer. The fixed ankles were decalcified for 2-3 weeks at 4°C with a 10% ethylenediamine tetra-acetic acid (EDTA) solution in distilled water with a pH of 7.4 and EDTA renewal every week [41]. The samples were regularly processed, implanted in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) in the Histopathology Department, Faculty of Veterinary Medicine, Beni-Suef University [42]. Histological lesions and alterations were studied in stained sections put on glass slides.
Statistical analysis
The statistics were examined using one-way and two-way analysis of variance (ANOVA) via PC-STAT (Version IA (C), University of Georgia, USA), followed by Least Significant Difference (LSD) analysis, to determine the major effects and compare the various groups [43]. The number of samples in each group is six (n=6). The general effect between groups is expressed by the F-probability for each marker. The mean and standard error were used to express the data. For LSD, statistically non-significant differences were defined as P>0.05, whereas statistically significant differences were defined as P<0.05 and P<0.01 respectively.
Results
Effect on paw circumference of the right hind leg
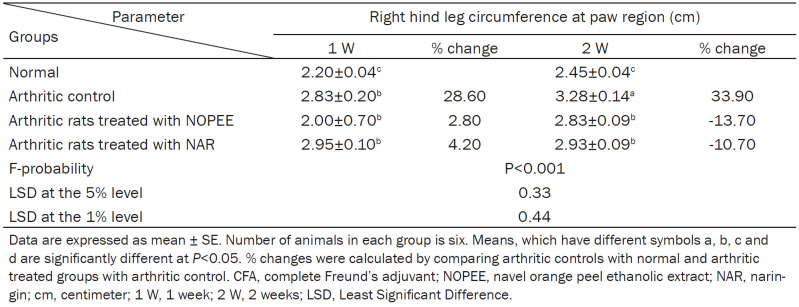
The circumference of the right hind leg in the paw region was used as an indicator of paw edema, it significantly (P<0.01; LSD) increased during the 1st and 2nd weeks with percentage changes of 28.60 and 33.90, respectively, comparing with the corresponding normal controls. The administration of arthritic rats with NOPEE and NAR induced a non-significant effect (P>0.05; LSD) during the 1st week and a highly significant effect (P<0.01; LSD) during the 2nd week (Table 1).
Table 1.
Effects of NOPEE and NAR on right hind leg circumference at the paw region in CFA-induced arthritic rats
Effect on serum RF and PGE-2 levels
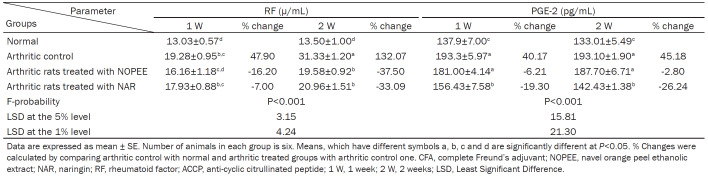
The serum RF level (P<0.01; LSD) increased significantly in arthritic rats during the 1st and 2nd weeks with percentage changes of 47.90 and 132.07, respectively, compared to the corresponding normal controls. The arthritic rats treated with NOPEE and NAR produced a non-significant action during the first week, although they developed a highly significant effect during the 2nd week (Table 2).
Table 2.
Effects of NOPEE and NAR on serum RF and PGE-2 levels in CFA-induced arthritic rats
The serum PGE-2 level was significantly (P<0.01; LSD) increased in CFA-induced arthritic rats during the 1st and 2nd weeks with percentage changes of 40.17 and 45.18, respectively, compared to the corresponding normal controls. The arthritic rats treated with NOPEE produced a non-significant (P>0.05; LSD) decrease in the PGE-2 level, while the administration of NAR induced a highly significant decrease (P<0.01; LSD).
Effect on the IL-1β, TNF-α, and IL-17 levels
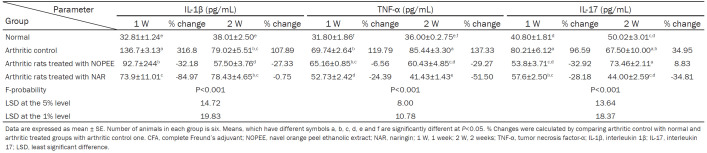
Shown in Table 3, there was a remarkably significant elevation (P<0.01; LSD) in the levels of serum IL-1β, TNF-α, and IL-17 in the CFA group during the 1st and 2nd weeks in comparison to control rats with recorded percentage changes of 316.80, 119.79, and 96.59 during the 1st week and 107.89, 137.33, and 34.95 during the 2nd week, respectively.
Table 3.
Effects of NOPEE and NAR on serum IL-1β, TNF-α and IL-17 levels in CFA-induced arthritic rats
The treatment of arthritic rats with NOPEE induced a highly significant reduction (P<0.01; LSD) in the higher serum IL-1β level during the 1st (-32.18%) and 2nd (-27.33%) weeks, while the arthritic group treated with NAR resulted in a significant enhancement (P<0.01; LSD) only during the 1st week (-84.97%). The raised serum TNF-α level in arthritic animals significantly improved due to treatment with NAR during the 1st week and because of treatments with both NOPEE and NAR during the 2nd week. On the other hand, the elevated serum IL-17 level in arthritic rats was significaltly ameliorated due to treatments with NOPEE and NAR during the 1st week as well as treatment with NAR only during the 2nd week (Table 3).
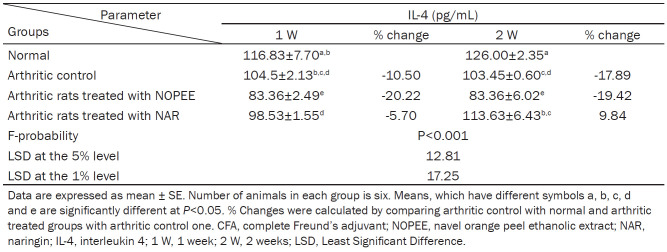
Effect on serum IL-4 level
The level of IL-4 in serum was remarkably reduced in CFA-rats during 1st and 2nd weeks with percentage changes of -10.50 and -17.89, respectively, compared to the equivalent values of the control group. However, the effect was significant (P<0.01; LSD) during the 2nd week and non-significant (P>0.01; LSD) during the 1st week after CFA injection. The arthritic rats treated with the NOPEE induced a highly significant decrease (P<0.01; LSD) during the 1st and 2nd weeks, while the treatment with NAR showed no significant effect (P>0.05; LSD) at both tested periods (Table 4). The IL-4 concentration increased detectably due to NAR treatment for 2 weeks with a recorded percentage change of 9.84 as compared with the control group (Table 4).
Table 4.
Effects of NOPEE and NAR on serum IL-4 level in CFA-induced arthritic rats
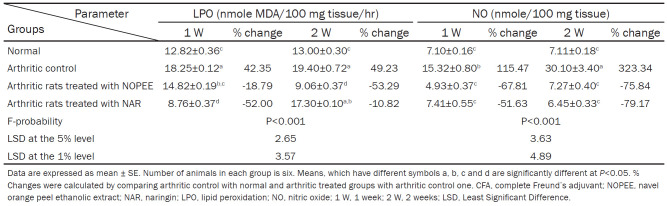
Effect on liver LPO and NO level
The levels of the LPO and NO were significantly increased (P<0.01; LSD) in the CFA group during the 1st and 2nd weeks compared with the control group with recorded percentage changes of 42.35 and 115.47 during the 1st week and 49.23 and 323.34 during the 2nd week, respectively, as compared with the respective values of the control group. The treatment of arthritic rats with NOPEE induced a highly significant effect (P<0.01; LSD) in the levels of LPO and NO during the 1st and 2nd weeks. The treatment of arthritic rats with NAR induced a highly significant effect (P<0.01; LSD) in the level of NO during the 1st and 2nd weeks and a significant effect (P<0.05; LSD) on LPO during the 1st week and a non-significant effect (P>0.05; LSD) during the 2nd week (Table 5).
Table 5.
Effects of NOPEE and NAR on liver LPO and NO level in CFA-induced arthritic rats
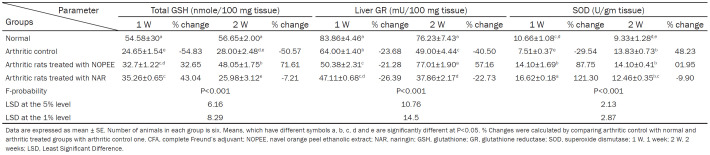
Effect on liver GSH content and GR and SOD activities
GSH content showed a highly significant (P<0.01; LSD) reduction in liver of CFA-induced arthritic rats during the 1st and 2nd weeks with recorded percentage changes of -54.83 and -50.57, respectively, compared to those of control rats’ group. The arthritic rats given NOPEE and NAR, had a significant (P<0.05; LSD) and highly significant (P<0.01; LSD) respectively, decrease in GSH content during the 1st week with recorded percentage changes of 32.65 and 43.04 as compared to control group. As the period extended to 2 weeks, the NOPEE induced a significant change in the lowered GSH content, while NAR treatment showed no significant effect (P>0.05; LSD).
The GR activity was highly significantly diminished in the liver of CFA-induced rats during the 1st and 2nd week with recorded percentage changes of -23.68 and -40.50, respectively, compared to the consistent control group. The treatment with NOPEE produced a significant decrease (P<0.05; LSD) during the 1st week, while it induced a significant increase (P<0.01; LSD) during the 2nd week. Conversely, the treatment with NAR induced a highly significant and a significant decrease in GR activity during the 1st and 2nd weeks with recorded percentage decreases of 26.39 and 22.73, respectively, compared to the arthritic control group.
The SOD activity was significantly declined (P<0.01; LSD) during the 1st week compared to the control group, while it improved significantly during the 2nd week. The treatment of rats with NOPEE and NAR triggered a significant increase (P<0.01; LSD) in SOD activity, while it produced a non-significant result during the 2nd week (Table 6).
Table 6.
Effects of NOEE and NAR on liver GSH content and GR and SOD activities in CFA-induced arthritic rats
In Tables 7 and 8, the data of one-way besides two-way ANOVA analysis were represented. One-way test of ANOVA for all detected parameters revealed that the overall effect among groups was very highly significant (P<0.001). A two-way test of ANOVA depicted that the efficacy of treatment-time interaction was significant on most of the tested parameters.
Table 7.
Analyses of variance of the effects of NOPEE and NAR on right hind leg circumference, RF, PGE-2, IL-1β, TNF-α, IL-17 and IL-4 in CFA-induced arthritic rats at various periods of time
| Source of variation | Right hind paw circumference | RF | PGE-2 | IL-1β | TNF-α | IL-17 | IL-4 | |
|---|---|---|---|---|---|---|---|---|
| One-way ANOVA | General effect | |||||||
| In between groups | P<0.001 | |||||||
| Two-way ANOVA | Normal-arthritic effect | |||||||
| Treatment | P>0.05 | |||||||
| Time | P>0.05 | |||||||
| Treatment-time | P>0.05 | P<0.001 | P<0.05 | P<0.001 | P<0.05 | P<0.05 | ||
| Arthritic-NOPEE effect | ||||||||
| Treatment | P>0.05 | P<0.05 | ||||||
| Time | P>0.05 | |||||||
| Treatment-time | P>0.05 | P<0.001 | P>0.05 | P<0.01 | P<0.05 | P>0.05 | ||
| Arthritis-NAR effect | ||||||||
| Treatment | P>0.05 | P>0.05 | P>0.05 | |||||
| Time | P>0.05 | P<0.05 | P>0.05 | |||||
| Treatment-time | P>0.05 | P<0.001 | P>0.05 | P<0.001 | P>0.05 | P<0.05 | ||
CFA, Complete Freund’s Adjuvant; NOPEE, navel orange peel ethanolic extract; NAR, naringin; RF, rheumatoid factor; ACCP, anti-cyclic citrullinated peptide; TNF-α, tumor necrosis factor-α; IL-1β, interleukin 1β; IL-17, interleukin 17; IL-4, interleukin 4; ANOVA, analysis of variance.
Table 8.
Analyses of variance of the effects of NOPEE and NAR on various oxidant and antioxidant markers in CFA-induced arthritic rats at various periods of time
| Source of variation | LPO | NO | GSH | GR | SOD | |
|---|---|---|---|---|---|---|
| One-way ANOVA | General effect | |||||
| In between groups | P<0.001 | |||||
| Two-way ANOVA | Normal-arthritic effect | |||||
| Treatment | P>0.05 | |||||
| Time | P>0.05 | |||||
| Treatment-time | P>0.05 | P<0.001 | P<0.05 | P>0.05 | P<0.001 | |
| Arthritic-NOPEE effect | ||||||
| Treatment | P>0.05 | |||||
| Time | P>0.05 | |||||
| Treatment-time | P<0.01 | P<0.001 | ||||
| Arthritis-NAR effect | ||||||
| Treatment | P>0.05 | |||||
| Time | P>0.05 | |||||
| Treatment-time | P<0.001 | P<0.01 | P>0.05 | P<0.001 | ||
CFA, complete Freund’s adjuvant; NOPEE, navel orange peel ethanolic extract; NAR, naringin; LPO, lipid peroxidation; NO, nitric oxide; GSH, glutathione; GR, glutathione reductase; SOD, superoxide dismutase; ANOVA, Analysis of variance.
Histopathological effects
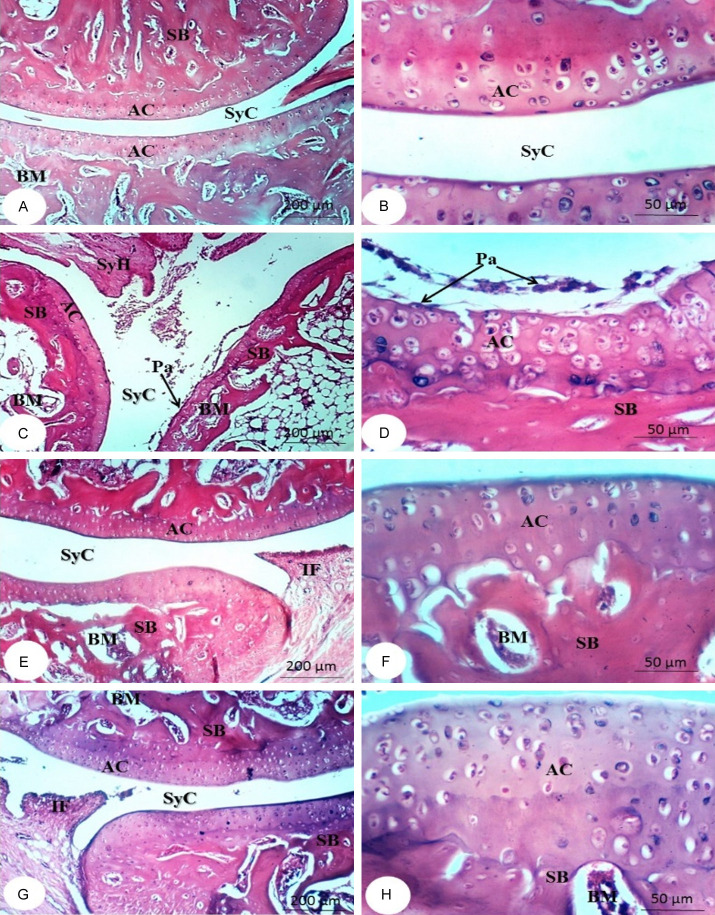
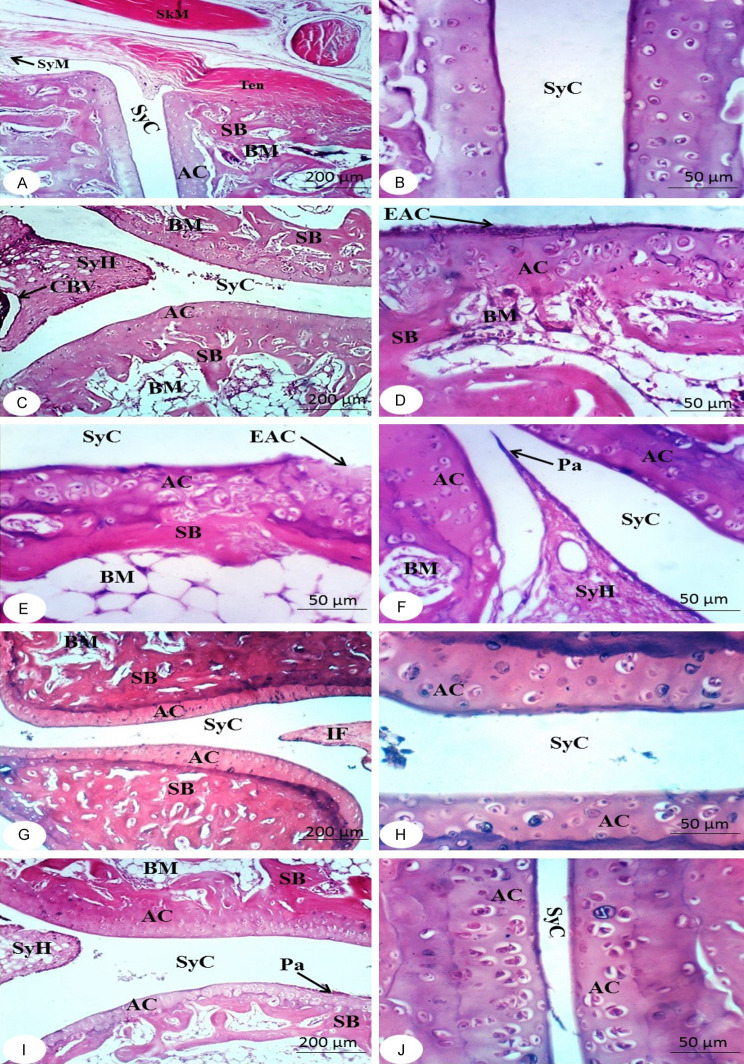
Figures 2 and 3 show the histological modifications to the right ankle joint in the tested groups. The bone surfaces in the synovium, the joint cavity between two movable bones, are enclosed by articular cartilage, which deficiencies a perichondrium. A two-part articular capsule surrounds and connects the ends of the two bones. The outer portion is a fibrous capsule that surrounds each bone’s articular cartilage and extends far beyond it. The inner part is called the synovial membrane. As a result, either the synovial membrane or articular cartilage lines the whole joint cavity. A thin sheet of connective tissue with many blood and lymphatic vessels makes up the synovial membrane. The surface facing the joint cavity is coated with epithelioid cells, which secrete hyaluronic acid and phagocytize debris. They are not epithelial because they do not rest on a basement membrane. The viscous synovial fluid, which lubricates the joints, is made up of hyaluronic acid and a dialysate of plasma from the blood vessels of the membrane (Figures 2A, 2B, 3A and 3B).
Figure 2.
Photomicrographs of sections in ankles of rats from normal group (A and B), arthritic control (C and D) and arthritic groups treated with NOPEE (E and F) and NAR (G and H) at the end of the 1st week showing articulating cartilage (AC), synovial cavity (SyC), sponge bone (SB), bone marrow (BM), skeletal muscle (SkM), synovial hyperplasia (SyH), pannus formation (Pa), and eroded articulating cartilage (EAC). CFA, complete Freund’s adjuvant; NOPEE, navel orange peel ethanolic extract; NAR, naringin.
Figure 3.
Photomicrographs of sections in ankles of rats from rats from normal group (A and B), arthritic control (C-F) and arthritic groups treated with NOPEE (G and H) and NAR (I and J) at the end of the 2nd week showing cartilage (AC), sponge bone (SB), bone marrow (BM), synovial cavity (SyC), synovial membrane (SyM), congested blood vessel (CBV), tendon (Ten), skeletal muscle (SkM), erosin in articular cartilage (EAC), synovial hyperplasia (SyH) and pannus formation (Pa). CFA, complete Freund’s adjuvant; NOPEE, navel orange peel ethanolic extract; NAR, naringin.
The characteristics of CFA rats’ joints are synovial hyperplasia, with excessive synovial growth resulting in pannus and cell exudation into the joint space. Erosion of bone and cartilage during the 1st week (Figure 2C and 2D) and 2nd week (Figure 3C-F) after CFA injection. CFA rats had a higher incidence of synovial hyperplasia, a huge influx of inflammatory cells, and an accumulation of many monomorphonuclear and polymorphonuclear cells in the joint area was observed compared with the normal group. In contrast, the NOPEE-treated arthritic rats showed a considerable decrease in joint damage and/or inflammation as indicated during the 1st week (Figure 2E and 2F) and 2nd week (Figure 3G and 3H). The synovial membrane in the joints became almost like normal synovium. The joint histology was almost identical with that of the normal group. On the other hand, less decrease in arthritis severity was detected in the rats treated with NAR at the 1st week (Figure 2G and 2H) and 2nd week (Figure 3I and 3J). Despite the absence of obvious joint injury, like bone and cartilage erosions, synovial hyperplasia and the invasion of inflammatory cells are present.
Discussion
RA is a progressive autoimmune disease that affects roughly 1% of people and its prevalence rises with age. It is primarily related to increased inflammatory responses within the synovial joints of articular joints like bone, ligaments, hyaline cartilage, tendons, and synovial membrane and affects mostly the foot, hand and wrist, but it can also affect large joints [44,45].
This study uses the Wistar rat CFA-induced arthritic model because it shares characteristics with human RA, including the invasion of inflammatory cells, synovial membrane proliferation, and joint damage [46]. Most researchers agree that inhibiting arthritis induced in rats via CFA is one of the best test methods to identify drugs that have anti-arthritic action since it strongly matches human arthritis [21,47,48].
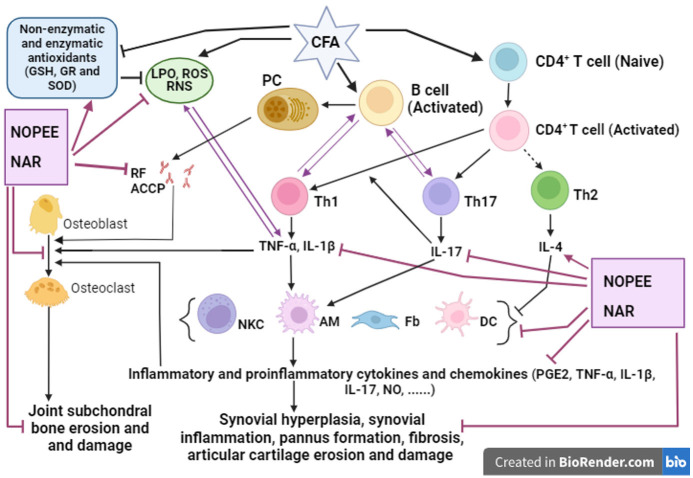
In our study, the increased paw circumference of arthritic rats of the right leg was significantly decreased during the 2nd week because of treating them with NOPEE and NAR. The decrease in the right leg circumference due to treatment with NOPEE and NAR reflects the reduction in the rate of swelling, that could be attributed to the reduction in edema, diminution of inflammation, and a decrease in synovial tissue hyperplasia as described by the histology data of the present study and stated also by other previous studies [11,49]. Such macroscopic investigation was done through microscopic and histologic examination of the joint sections. The CFA rats showed an enormous inflammatory response, synovial hyperplasia, and an aggregation of many monomorphonuclear and polymorphonuclear cells in the joint space compared with the control group. Conversely, rats treated with NOPEE demonstrated a clear reduction in joint damage or inflammation as indicated during the 1st and 2nd weeks. Moreover, the arthritic animals treated with NAR showed no signs of harm or damage to the joint although the inflammation and synovium proliferation were somewhat pronounced. Therefore, the preceding findings illustrated the suppressed arthritis-related paw edema and inflammatory responses in both treatments. RF is the main circulating immunoglobulin G antibody and an important serum test for the diagnosis of RA [50,51]. B lymphocytes are the source of RF and anticitrullinated protein antibodies (ACCP), which support the development of immunological complexes in the joint (Figure 4) [5,6]. Moreover, they respond and produce cytokines and chemokines which promote angiogenesis, leukocyte infiltration, and synovial hyperplasia [52].
Figure 4.
Schematic diagram showing the immunomodulatory effect of NOPEE and NAR in CFA-induced arthritis. Activation, →; inhibition, ┬; CFA, Complete Freund’s Adjuvant; NOPEE, navel orange peel ethanolic extract; NAR, naringin; PC, plasma cell; ROS, reactive oxygen species; LPO, lipid peroxidation; RNS, reactive nitrogen species; RF, rheumatoid factor; ACCP, anti-cyclic citrullinated peptide; CD, Cluster Differentiation; Th, T helper cell; NKC, natural killer cells; AM, activated macrophages; DC, dendritic cells; Fb, fibroblast; PGE2, prostaglandin E2; TNF-α, tumor necrosis factor-α; IL, interleukin; NO, nitric oxide; GSH, glutathione; GR, glutathione reductase; SOD, superoxide dismutase.
In RA, TNF-α is the major mediator of the inflammatory response [53]. TNF-α is released by macrophages during the erosive process of cartilage and bone, and it causes the creation of other cytokines like IL-1β [54]. Additionally, IL-17 is able to promote inflammation and it is very important in the etiology of RA by triggering a number of proinflammatory mediators, such as chemokines, cytokines, macrophages, monocytes, chondrocytes and other mediators of the breakdown of bone and cartilage in synovial fibroblasts (Figure 4) [55]. On the other hand, the anti-inflammatory mediator, IL-4, is a cytokine synthesized by CD4+ Th2 lymphocytes, which is a characteristic for this subset of lymphocytes. In addition, IL-4 is also secreted by mast cells and basophils [56,57]. In the present study, the CFA-induced arthritic rats displayed a significant increase in the serum proinflammatory cytokines IL-1β, TNF-α and IL-17, while it showed an obvious decrease in the anti-inflammatory cytokine IL-4 in parallel with the increase of serum RF after 1 and 2 weeks of CFA administration. Overall, in CFA-induced arthritis, there is a Th1 and Th17 dominant response (Figure 4).
The PGE-2 pathway is active in the rheumatoid joint, which also exhibits high amounts of this mediator in the synovial fluid and robust expression of its synthesis-related enzymes in the synovium like cyclooxygenase (COX)-1 and 2 as well as microsomal PGE-2 synthase-1 (mPGES-1) [58-61]. PGE-2 promotes the growth of auto-aggressive T helper 17 (Th17) cells, which maintains inflammatory pathways [62,63]. This is confirmed by the results of the present study, which indicated that the elevation in the serum PGE-2 level in arthritic rats was concomitant with the increase in the serum IL-17 level during the 1st and 2nd weeks.
In the present study, the increased serum RF was considerably decreased by treatment with NOPEE and NAR. The decrease in the RF level by treatment with NOPEE and NAR indicated a decrease in the inflammatory cells. This finding is consistent with other research [64] which revealed that many members of the Citrus genus are famous for their therapeutic, physiological, and pharmacological properties, which include antibacterial, antioxidant, and anti-inflammatory actions [65]. In the same way, it was indicated that flavonoids are strong anti-inflammatory compounds [27,66].
To research the potential anti-inflammatory mechanisms of action of NOPEE and NAR in the present study, the serum concentrations of RF, PGE-2, TNF-α, IL-1β, IL-17, and IL-4 were determined. The serum RF, PGE-2 (acute inflammatory cytokines), TNF-α, IL-1β, and IL-17 (proinflammatory cytokines) were pointedly elevated in the arthritic control rats. The anti-inflammatory IL-4 serum level was depleted. These changes in the cytokines ensure that in experimental CFA-induced arthritis, a predominance of Th1 and Th17 on Th2 can be observed (Figure 1). Numerous earlier authors have supported this finding [11,44,67]. Unbalanced Th1/Th2/Th17 lymphocyte cytokine production undoubtedly contributes significantly to the development of RA. The Th2 cytokine, IL-4, stimulates antibody-mediated immune responses while the Th17 cytokine, IL-17, is principally in charge of eliminating extracellular as well fungal pathogens [7,68-70].
In the current study, the NOPEE treatment caused a significant drop in the high serum level of RF, IL-1β, and TNF-α levels during the 1st and 2nd weeks while IL-17 levels changed during the 1st week. In addition, the IL-4 concentration clearly decreased during the 1st week of NOPEE treatment. These effects could be explained by the lowered levels of proinflammatory markers [IL-6, monocyte chemotactic protein 1 (MCP-1), IFN-γ, and TNF-α], and higher levels of adiponectin and IL-10 in the plasma or liver as indicated by [71].
NAR therapy led to reduction in the increased serum RF, PGE-2, IL-1β, TNF-α, and IL-17 levels, although it caused a non-significant change in the decreased serum IL-4 level in arthritic rats. Thus, this flavonoid may induce anti-inflammatory activities by decreasing the proinflammatory and inflammatory cytokines. These results are consistent with the study of Jain and Parmar [72] that suggested that the anti-inflammatory effects could be attributed to the increase in adiponectin and IL-10 in addition to the decrease in the proinflammatory cytokines in the plasma and liver.
The cause of RA is mainly joint inflammation initiated by oxidative stress [73]. Oxidative stress that is produced within an inflamed joint can produce connective tissue destruction and autoimmune phenomena in rheumatoid synovitis [74]. Moreover, oxidative stress also has a vital role in the pathology of autoimmune diseases by breaking down the immunological tolerance, enhancing the inflammation, and inducing apoptotic cell death [75].
Reactive oxygen species (ROS) and LPO generation that is stimulated suggests that an imbalance between ROS production and clearance occurs, resulting in oxidative stress [76] that in turn contributes to tissue damage [77]. Cell membrane polyunsaturated fatty lipids undergo oxidation, which results in the production of lipid peroxyl radicals. These radicals then promote oxidation and cause cell membrane damage [78]. Additionally, elevated NO production is important to the pathophysiology of RA (Figure 4). Treatment for chronic autoimmune illnesses may involve a novel therapeutic strategy called NOS inhibition [79,80].
In the current study, the LPO and NO level significantly increased in the liver of CFA-administered rats compared with the normal group [7]. The therapy of arthritic rats with NOPEE significantly improved the elevated LPO during the 1st and 2nd weeks, while the treatment with NAR produces a significant decrease only during the 1st week. Both NOPEE and NAR made a significant reduction in the high NO in arthritic rats at both tested periods. Similarly, other reports revealed that NAR has antioxidant, metal chelating, and FR scavenging properties and offers some protection against mutagenesis and LPO [81,82] and largely, citrus and its flavonoids have antioxidant properties [83].
Tripeptide γ-glutamylcysteinylglycine or GSH is a significant non-enzymatic modulator of intracellular redox homeostasis and is present at millimolar concentrations in all cell types. GSH is an important FR scavenging compound, which catalyzes the conversion of ROS into a less reactive radical. Moreover, in a process known as glutathionylation, GSH can bind to proteins covalently and function as an enzyme cofactor for a variety of defense-related enzymes. Thus, glutathione can actively intercept free radicals (FRs) or serve as a substrate for GPx and GST when lipid hydroperoxides, hydrogen peroxides, and electrophilic chemicals are detoxified [84]. Furthermore, GSH is a crucial tripeptide and an antioxidant that is not enzymatically produced inside of cells, which participates in the removal of FRs, such as superoxide anions, H2O2, and alkoxy radicals, and the management of membrane protein thiols and serves as a substrate for GPx and GR [85]. Similarly, GSH, an amino acid derivative with a sulfhydryl group, is a highly unique compound with several crucial functions, including acting as a sulfhydryl buffer to protect cells from oxidative damage. It also has the capacity to prevent oxidative stress, preserve the usual reduced state within the cell, and act as a peroxide scavenger to make up for catalase’s diminished role in antioxidant defense [86]. Thus, glutathione plays a fundamental role in combating oxidative stress [87].
In the present findings, a decrease in the content of GSH and GR activity in the liver of arthritic rats was observed. As a result, these decreased GSH levels may be related to the use of GSH with GPx and GST as its substrates [88]. However, treating the arthritic rats with NOPEE significantly increased their liver GSH content during the 1st and 2nd weeks of CFA injection, though the treatment with NAR significantly increased liver GSH content only during the 1st week. These results agreed with the reports of Jagetia and Reddy [72] who stated that NAR as a flavonoid can increase the GSH level and also, Abdel Moneim [89] reported that the citrus peel has a scavenging ability in rats after exposure to oxidative stress.
In the present study, the liver GR activity of the arthritic rats was significantly decreased as a result of treatment with NOPEE and NAR for one week. As the period continued to two weeks, GR activity significantly increased as a result of treatment with NOPEE, while it decreased as a result of NAR treatment. Such discrepancy in the effect of NOPEE and NAR may be due to the suggestion that peel extracts are extremely complicated combinations of many components with varying activity [90,91].
SOD is crucial to the development and treatment of RA since the extracellular SOD serves as the main catalytic antioxidant in the joint fluid [92]. In the present study, the lowered SOD activity in the arthritic rats during the 1st week was greatly enhanced because of NOPEE and NAR treatment which produced a remarkable but a non-significant effect during the 2nd week. These findings demonstrated in a straightforward manner the efficiency of NOPEE and NAR as anti-arthritic and anti-inflammatory medications in preventing the onset and spread of RA.
TNF-α and ROS commonly influence each other in a positive feedback loop [93] as shown in Figure 4. Accordingly, the therapeutic inhibition of TNF-α signaling efficiently inhibits ROS production and reduces joint inflammation in RA [94]. Therefore, it can be suggested that the link between TNF-α and ROS production may have an important role in the development of CFA-induced RA and inhibition of TNF-α and ROS production have crucial role in the therapy of the disease (Figure 4).
Conclusion
The NOPEE as well as NAR exhibit strong anti-inflammatory and antioxidant effects in rat RA induced with CFA. Both treatments effectively inhibit the damaging actions in the articular ankle joints. Thus, these therapeutic agents could be applied in the treatment of RA. However, further studies are needed to determine their negative effects on human health.
Disclosure of conflict of interest
None.
References
- 1.Chu J, Wang X, Bi H, Li L, Ren M, Wang J. Dihydromyricetin relieves rheumatoid arthritis symptoms and suppresses expression of pro-inflammatory cytokines via the activation of Nrf2 pathway in rheumatoid arthritis model. Int Immunopharmacol. 2018;59:174–180. doi: 10.1016/j.intimp.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Kim MS, Lee SH, Kim JH, Kim OK, Yu AR, Baik HH. Anti-inflammatory effects of step electrical stimulation on complete Freund’s adjuvant (CFA) induced rheumatoid arthritis rats. J Nanosci Nanotechnol. 2019;19:6546–6553. doi: 10.1166/jnn.2019.17077. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R, Singh S, Saksena AK, Pal R, Jaiswal R, Kumar R. Effect of boswellia serrata extract on acute inflammatory parameters and tumor necrosis factor-α in complete Freund’s adjuvant-induced animal model of rheumatoid arthritis. Int J Appl Basic Med Res. 2019;9:100–106. doi: 10.4103/ijabmr.IJABMR_248_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandão PL, de Albuquerque Filho ST, dos Santos JG, Junior MP, Lessa GP, Pascoal DB, da Cruz CM. Estudo comparativo das doenças inflamatórias articulares espondilite anquilosante e artrite reumatóide. Brazilian Applied Science Review. 2020;4:2258–2268. [Google Scholar]
- 5.Ahmed EA, Ahmed OM, Fahim HI, Mahdi EA, Ali TM, Elesawy BH, Ashour MB. Combinatory effects of bone marrow-derived mesenchymal stem cells and indomethacin on adjuvant-induced arthritis in Wistar rats: roles of IL-1β, IL-4, Nrf-2, and oxidative stress. Evid Based Complement Alternat Med. 2021;2021:8899143. doi: 10.1155/2021/8899143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mucke J, Krusche M, Burmester GR. A broad look into the future of rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2022;14 doi: 10.1177/1759720X221076211. 1759720X221076211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed OM, Ashour MB, Fahim HI, Ahmed NA. The role of Th1/Th2/Th17 cytokines and antioxidant defense system in mediating the effects of lemon and grapefruit peel hydroethanolic extracts on adjuvant-induced arthritis in rats. J Appl Pharm Sci. 2018;8:069–081. [Google Scholar]
- 8.Berthelot JM, Le Goff B, Maugars Y. Bone marrow mesenchymal stem cells in rheumatoid arthritis, spondyloarthritis, and ankylosing spondylitis: problems rather than solutions? Arthritis Res Ther. 2019;21:239. doi: 10.1186/s13075-019-2014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dissanayake K, Jayasinghe C, Wanigasekara P, Sominanda A. Potential applicability of cytokines as biomarkers of disease activity in rheumatoid arthritis: enzyme-linked immunosorbent spot assay-based evaluation of TNF-α, IL-1β, IL-10 and IL-17A. PLoS One. 2021;16:e0246111. doi: 10.1371/journal.pone.0246111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed OM, EL-Abd SF, El Mahdi EA, Abdou EA. Curcumin ameliorative efficacy on type 1 diabetes mellitus coexisted with rheumatoid arthritis in Wistar rats. Merit Res J Med Med Sci. 2015;3:256–270. [Google Scholar]
- 12.Ahmed EA, Ahmed OM, Fahim HI, Ali TM, Elesawy BH, Ashour MB. Potency of bone marrow-derived mesenchymal stem cells and indomethacin in complete Freund’s adjuvant-induced arthritic rats: roles of TNF-α, IL-10, iNOS, MMP-9, and TGF-β1. Stem Cells Int. 2021;2021:6665601. doi: 10.1155/2021/6665601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi Y, Tanaka H, Nakai K, Kitami S, Murakami F, Morita T, Tanabe N, Kawato T, Maeno M. RANKL induces IL-18 binding protein expression in RAW264.7 cells. J Hard Tissue Biol. 2016;25:173–180. [Google Scholar]
- 14.Ahmed OM, Ashour MB, Fahim HI, Ahmed NA. Citrus limon and paradisi fruit peel hydroethanolic extracts prevent the progress of complete Freund’s adjuvant-induced arthritis in male Wistar rats. Adv Anim Vet Sci. 2018;6:443–455. [Google Scholar]
- 15.Akaogi J, Nozaki T, Satoh M, Yamada H. Role of PGE2 and EP receptors in the pathogenesis of rheumatoid arthritis and as a novel therapeutic strategy. Endocr Metab Immune Disord Drug Targets. 2006;6:383–394. doi: 10.2174/187153006779025711. [DOI] [PubMed] [Google Scholar]
- 16.Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fattahi MJ, Mirshafiey A. Prostaglandins and rheumatoid arthritis. Arthritis. 2012;2012:239310. doi: 10.1155/2012/239310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Astry B, Harberts E, Moudgil KD. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res. 2011;31:927–40. doi: 10.1089/jir.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatesha SH, Dudics S, Acharya B, Moudgil KD. Cytokine-modulating strategies and newer cytokine targets for arthritis therapy. Int J Mol Sci. 2014;16:887–906. doi: 10.3390/ijms16010887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra SI, Gioia D, Childress S, Barnet B, Webster RL. Adherence to medication regimens among low-income patients with multiple comorbid chronic conditions. Health Soc Work. 2011;36:249–58. doi: 10.1093/hsw/36.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi W, Lin C, Fan K, Chen Z, Liu L, Feng X, Zhang H, Shao Y, Fang H, Zhao C, Zhang R, Cai D. Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice. Chem Biol Interact. 2019;306:19–28. doi: 10.1016/j.cbi.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Omorogbe O, Ajayi AM, Ben-Azu B, Oghwere EE, Adebesin A, Aderibigbe AO, Okubena O, Umukoro S. Jobelyn® attenuates inflammatory responses and neurobehavioural deficits associated with complete Freund-adjuvant-induced arthritis in mice. Biomed Pharmacother. 2018;98:585–593. doi: 10.1016/j.biopha.2017.12.098. [DOI] [PubMed] [Google Scholar]
- 24.Mei WY, Yu MJ, Yao S, Wang KL, Yao RS. Anti-inflammatory effects of a small molecule gastrin-releasing peptide receptor antagonist on adjuvant-induced rheumatoid arthritis in rats. Chem Pharm Bull (Tokyo) 2018;66:410–415. doi: 10.1248/cpb.c17-00887. [DOI] [PubMed] [Google Scholar]
- 25.Kocyigit A, Guler EM, Kaleli S. Anti-inflammatory and antioxidative properties of honey bee venom on Freund’s complete adjuvant-induced arthritis model in rats. Toxicon. 2019;161:4–11. doi: 10.1016/j.toxicon.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Nasuti C, Bordoni L, Fedeli D, Gabbianelli R. Effect of nigella sativa oil in a rat model of adjuvant-induced arthritis. 2019;17:16. doi: 10.3390/antiox8090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed OM, AbouZid SF, Ahmed NA, Zaky MY, Liu H. An up-to-date review on citrus flavonoids: chemistry and benefits in health and diseases. Curr Pharm Des. 2021;27:513–530. doi: 10.2174/1381612826666201127122313. [DOI] [PubMed] [Google Scholar]
- 28.Benavente-García O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 29.Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5:404–417. doi: 10.3945/an.113.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guimarães R, Barros L, Barreira JC, Sousa MJ, Carvalho AM, Ferreira IC. Targeting excessive free radicals with peels and juices of citrus fruits: grapefruit, lemon, lime and orange. Food Chem Toxicol. 2010;48:99–106. doi: 10.1016/j.fct.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Niu D, Ren EF, Li J, Zeng XA, Li SL. Effects of pulsed electric field-assisted treatment on the extraction, antioxidant activity and structure of naringin. Sep Purif Technol. 2021;265:118480. [Google Scholar]
- 32.Ahmed OM, Soliman HA, Mahmoud B, Gheryany RR. Ulva lactuca hydroethanolic extract suppresses experimental arthritis via its anti-inflammatory and antioxidant activities. Beni Suef Univ J Basic Appl Sci. 2017;6:394–408. [Google Scholar]
- 33.Shaaban HH, Hozayen WG, Khaliefa AK, El-Kenawy AE, Ali TM, Ahmed OM. Diosmin and trolox have anti-arthritic, anti-inflammatory and antioxidant potencies in complete Freund’s adjuvant-induced arthritic male Wistar rats: roles of NF-κB, iNOS, Nrf2 and MMPs. Antioxidants (Basel) 2022;11:1721. doi: 10.3390/antiox11091721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed OM, Hassan MA, Abdel-Twab SM, Abdel Azeem MN. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed Pharmacother. 2017;94:197–205. doi: 10.1016/j.biopha.2017.07.094. [DOI] [PubMed] [Google Scholar]
- 35.Chtourou Y, Aouey B, Aroui S, Kebieche M, Fetoui H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem Biol Interact. 2016;243:1–9. doi: 10.1016/j.cbi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Preuss HG, Jarrell ST, Scheckenbach R, Lieberman S, Anderson RA. Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr. 1998;17:116–123. doi: 10.1080/07315724.1998.10718736. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery HAC, Dymock JF, Thom NS. The rapid colorimetric determination of organic acids and their salts in sewage-sludge liquor. Analyst. 1962;87:949–955. [Google Scholar]
- 38.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 39.Goldberg DM, Spooner RJ. Glutathione reductase. In: Bergmeyer HU, Bergmeyer J, GraBI M, editors. Methods of Enzymatic Analysis. 3rd edition. Verlag Chemie, Weinheim: Scientific Research Publishing; 1983. pp. 258–265. [Google Scholar]
- 40.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 41.Alers JC, Krijtenburg PJ, Vissers KJ, van Dekken H. Effect of bone decalcification procedures on DNA in situ hybridization and comparative genomic hybridization. EDTA is highly preferable to a routinely used acid decalcifier. J Histochem Cytochem. 1999;47:703–710. doi: 10.1177/002215549904700512. [DOI] [PubMed] [Google Scholar]
- 42.Bancroft JD, Stevens A, Turner DR. Theory and Practice of Histological Techniques. 4th edition. Churchill Livingstone, London, Toronto: Scientific Research Publishing; 1996. [Google Scholar]
- 43.Roa M, Blane K, Zonneberg M. PC-STAT one-way analysis of variance. In: Roa M, Blane K, Zonneberg M, editors. The University of Georgia. Version 1 A (C) copyright. USA: University of Georgia; 1985. [Google Scholar]
- 44.Mohamed MA, Mahmoud MF, Rezk AM. Effect of pentoxifylline and pioglitazone on rheumatoid arthritis induced experimentally in rats. Menoufia Med J. 2014;27:766. [Google Scholar]
- 45.Shams S, Martinez JM, Dawson JRD, Flores J, Gabriel M, Garcia G, Guevara A, Murray K, Pacifici N, Vargas MV, Voelker T, Hell JW, Ashouri JF. The therapeutic landscape of rheumatoid arthritis: current state and future directions. Front Pharmacol. 2021;12:680043. doi: 10.3389/fphar.2021.680043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Kakkar R, Wang J. In vivo and in vitro approach to anti-arthritic and anti-inflammatory effect of crocetin by alteration of nuclear factor-E2-related factor 2/hem oxygenase (HO)-1 and NF-κB expression. Front Pharmacol. 2018;9:1341. doi: 10.3389/fphar.2018.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamani Gandomani M, Forouzandeh Malati E. Evaluation of protective efficacy of avicennia marina (forssk.) vierh leaves against complete Freund’s adjuvant-induced arthritis in Wistar. Iran J Pharm Res. 2014;13:945–51. [PMC free article] [PubMed] [Google Scholar]
- 48.Prakash A. Impact of sewage sludge on pea (Pisum sativum) in saline usar soil. Indian J Sci Res. 2020;10:21–6. [Google Scholar]
- 49.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devi SM, Balachandar V, Sasikala K, Manikantan P, Arun M, Kumar SS, Sudha S. Elevated rheumatoid factor (RF) from peripheral blood of patients with rheumatoid arthritis (RA) has altered chromosomes in Coimbatore population, South India. J Clin Med Res. 2010;2:167–174. [Google Scholar]
- 51.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 52.Silverman GJ, Carson DA. Roles of B cells in rheumatoid arthritis. Arthritis Res Ther. 2003;5(Suppl 4):S1–6. doi: 10.1186/ar1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vervoordeldonk MJ, Tak PP. Cytokines in rheumatoid arthritis. Curr Rheumatol Rep. 2002;4:208–217. doi: 10.1007/s11926-002-0067-0. [DOI] [PubMed] [Google Scholar]
- 54.Dai SM, Shan ZZ, Xu H, Nishioka K. Cellular targets of interleukin-18 in rheumatoid arthritis. Ann Rheum Dis. 2007;66:1411–8. doi: 10.1136/ard.2006.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miossec P. Interleukin-17 in fashion, at last: ten years after its description, its cellular source has been identified. Arthritis Rheum. 2007;56:2111–2115. doi: 10.1002/art.22733. [DOI] [PubMed] [Google Scholar]
- 56.Serre K, Bénézech C, Desanti G, Bobat S, Toellner KM, Bird R, Chan S, Kastner P, Cunningham AF, Maclennan IC, Mohr E. Helios is associated with CD4 T cells differentiating to T helper 2 and follicular helper T cells in vivo independently of Foxp3 expression. PLoS One. 2011;6:e20731. doi: 10.1371/journal.pone.0020731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Partsch G, Wagner E, Leeb BF, Bröll H, Dunky A, Smolen JS. T cell derived cytokines in psoriatic arthritis synovial fluids. Ann Rheum Dis. 1998;57:691–693. doi: 10.1136/ard.57.11.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, Koki A, Tripp CS. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002;46:1789–1803. doi: 10.1002/art.10356. [DOI] [PubMed] [Google Scholar]
- 59.Chizzolini C, Chicheportiche R, Alvarez M, De Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El Mansouri FE, Chabane N, Zayed N, Kapoor M, Benderdour M, Martel-Pelletier J, Pelletier JP, Duval N, Fahmi H. Contribution of H3K4 methylation by SET-1A to interleukin-1-induced cyclooxygenase 2 and inducible nitric oxide synthase expression in human osteoarthritis chondrocytes. Arthritis Rheum. 2011;63:168–179. doi: 10.1002/art.27762. [DOI] [PubMed] [Google Scholar]
- 61.Korotkova M, Westman M, Gheorghe KR, Af Klint E, Trollmo C, Ulfgren AK, Klareskog L, Jakobsson PJ. Effects of antirheumatic treatments on the prostaglandin E2 biosynthetic pathway. Arthritis Rheum. 2005;52:3439–3447. doi: 10.1002/art.21390. [DOI] [PubMed] [Google Scholar]
- 62.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 63.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 64.Ladaniya MS. Citrus Fruit: Biology, Technology and Evaluation. Atlanta, USA: Elsevier Inc.; 2008. pp. 1–10. [Google Scholar]
- 65.García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537–52. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 66.Vezza T, Rodríguez-Nogales A, Algieri F, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Flavonoids in inflammatory bowel disease: a review. Nutrients. 2016;8:211. doi: 10.3390/nu8040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chizzolini C, Dayer JM, Miossec P. Cytokines in chronic rheumatic diseases: is everything lack of homeostatic balance? Arthritis Res Ther. 2009;11:246. doi: 10.1186/ar2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berner B, Akça D, Jung T, Muller GA, Reuss-Borst MA. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol. 2000;27:1128–1135. [PubMed] [Google Scholar]
- 69.Amirshahrokhi K, Dehpour AR, Hadjati J, Sotoudeh M, Ghazi-Khansari M. Methadone ameliorates multiple-low-dose streptozotocin-induced type 1 diabetes in mice. Toxicol Appl Pharmacol. 2008;232:119–124. doi: 10.1016/j.taap.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed OM. Anti-hyperglycemic, immunomodulatory and anti-oxidant efficacy of vasoactive intestinal peptide in streptozotocin-induced diabetic mice. Int J Zool Res. 2009;5:42–61. [Google Scholar]
- 71.Park HJ, Jung UJ, Cho SJ, Jung HK, Shim S, Choi MS. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid-regulating enzymes in db/db mice. J Nutr Biochem. 2013;24:419–427. doi: 10.1016/j.jnutbio.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 72.Jain M, Parmar HS. Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm Res. 2011;60:483–491. doi: 10.1007/s00011-010-0295-0. [DOI] [PubMed] [Google Scholar]
- 73.Desai PB, Manjunath S, Kadi S, Chetana K, Vanishree J. Oxidative stress and enzymatic antioxidant status in rheumatoid arthritis: a case control study. Eur Rev Med Pharmacol Sci. 2010;14:959–967. [PubMed] [Google Scholar]
- 74.Vasanthi P, Nalini G, Rajasekhar G. Status of oxidative stress in rheumatoid arthritis. Int J Rheum Dis. 2009;12:29–33. doi: 10.1111/j.1756-185X.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- 75.El-barbary AM, Abdel-Khalek MA, Elsalawy AM, Hazaa SM. Assessment of lipid peroxidation and antioxidant status in rheumatoid arthritis and osteoarthritis patients. Egypt Rheumatol. 2011;33:179–185. [Google Scholar]
- 76.Biller JD, Takahashi LS. Oxidative stress and fish immune system: phagocytosis and leukocyte respiratory burst activity. An Acad Bras Cienc. 2018;90:3403–3414. doi: 10.1590/0001-3765201820170730. [DOI] [PubMed] [Google Scholar]
- 77.Mateen S, Moin S, Khan AQ, Zafar A, Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One. 2016;11:e0152925. doi: 10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hitchon CA, El-Gabalawy HS. Oxidation in rheumatoid arthritis. Arthritis Res Ther. 2004;6:265–278. doi: 10.1186/ar1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagy G, Koncz A, Telarico T, Fernandez D, Érsek B, Buzás E, Perl A. Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2010;12:210. doi: 10.1186/ar3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fresneda Alarcon M, McLaren Z, Wright HL. Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: same foe different M.O. Front Immunol. 2021;12:649693. doi: 10.3389/fimmu.2021.649693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amudha K, Pari L. Beneficial role of naringin, a flavanoid on nickel induced nephrotoxicity in rats. Chem Biol Interact. 2011;193:57–64. doi: 10.1016/j.cbi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Castro-Vazquez L, Alañón ME, Rodríguez-Robledo V, Pérez-Coello MS, Hermosín-Gutierrez I, Díaz-Maroto MC, Jordán J, Galindo MF, Arroyo-Jiménez Mdel M. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (citrus paradisi macf.) Oxid Med Cell Longev. 2016;2016:8915729. doi: 10.1155/2016/8915729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Couto N, Wood J, Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med. 2016;95:27–42. doi: 10.1016/j.freeradbiomed.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 85.Naik SR, Thakare VN, Patil SR. Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property. Exp Toxicol Pathol. 2011;63:419–431. doi: 10.1016/j.etp.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 86.Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH. Glutathione. Arabidopsis Book. 2011;9:e0142. doi: 10.1199/tab.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwon DH, Cha HJ, Lee H, Hong SH, Park C, Park SH, Kim GY, Kim S, Kim HS, Hwang HJ, Choi YH. Protective effect of glutathione against oxidative stress-induced cytotoxicity in RAW 264.7 macrophages through activating the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway. Antioxidants (Basel) 2019;8:82. doi: 10.3390/antiox8040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Umamaheswari S, Prathiba D, Jeyabalan S, Reddy U. Antioxidant potential of a polyherbal formulation (Diabet) on alloxan induced oxidative stress in rats. Drug Invention Today. 2009;1:46–49. [Google Scholar]
- 89.Abdel Moneim AE. Citrus peel extract attenuates acute cyanide poisoning-induced seizures and oxidative stress in rats. CNS Neurol Disord Drug Targets. 2014;13:638–646. doi: 10.2174/1871527312666131206095142. [DOI] [PubMed] [Google Scholar]
- 90.Mensor LL, Menezes FS, Leitão GG, Reis AS, Dos Santos TC, Coube CS, Leitão SG. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–30. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 91.Hou WC, Lin RD, Cheng KT, Hung YT, Cho CH, Chen CH, Hwang SY, Lee MH. Free radical-scavenging activity of Taiwanese native plants. Phytomedicine. 2003;10:170–175. doi: 10.1078/094471103321659898. [DOI] [PubMed] [Google Scholar]
- 92.Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16:515–521. doi: 10.1016/j.joca.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Blaser H, Dostert C, Mak TW, Brenner D. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–261. doi: 10.1016/j.tcb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Kennedy A, Ng CT, Chang TC, Biniecka M, O’Sullivan JN, Heffernan E, Fearon U, Veale DJ. Tumor necrosis factor blocking therapy alters joint inflammation and hypoxia. Arthritis Rheum. 2011;63:923–932. doi: 10.1002/art.30221. [DOI] [PubMed] [Google Scholar]