Abstract
Objective: To construct and validate a nomogram model for predicting sepsis complicated by acute lung injury (ALI). Methods: The healthcare records of 193 sepsis patients hospitalized at The Affiliated Tai’an City Central Hospital of Qingdao University from January 2022 to December 2023 were retrospectively reviewed. Among these patients, 69 were in the ALI group and 124 in the non-ALI group. A nomogram prediction model was constructed using logistic regression analysis. Its predictive performance was evaluated through various measures, including the area under the curve (AUC), calibration curve, decision curve, sensitivity, specificity, accuracy, recall rate, and precision rate. Results: The predictive factors included the neutrophil/lymphocyte ratio (NLR), oxygenation index (PaO2/FiO2), tumor necrosis factor-α (TNF-α), and acute physiology and chronic health evaluation II (APACHE II). The nomogram training set achieved an AUC of 0.959 (95% CI: 0.924-0.995), an accuracy of 92.59%, a recall of 96.70%, and a precision of 92.63%. In the validation set, the AUC was 0.938 (95% CI: 0.880-0.996), with an accuracy of 89.66%, a recall of 93.94%, and a precision of 88.57%. The calibration curve demonstrated that the prediction results were consistent with the actual findings. The decision curve indicated that the model has clinical applicability. Conclusion: NLR, PaO2/FiO2, TNF-α, and APACHE II are closely associated with ALI in sepsis patients. A nomogram model based on these four variables shows strong predictive performance and may be used as a clinical decision-support tool to help physicians better identify high-risk groups.
Keywords: Sepsis, acute lung injury, nomogram, forecasting model
Introduction
Severe organ failure caused by an ineffective host response to an infection is known as sepsis. It is the leading cause of death in critical care units [1,2]. Acute lung injury (ALI), a common and severe complication of sepsis, occurs in 25% to 45% of sepsis cases, with a mortality rate ranging from 30% to 40%, with adverse patient prognosis [3,4]. The treatment of ALI is challenging, with limited efficacy from pharmacologic interventions. Current treatment strategies primarily include lung protective ventilation, strict fluid management, and extracorporeal membrane oxygenation [5]. Many researchers recommend early detection and management of sepsis-induced ALI to improve outcomes for sepsis patients. Studies have shown that various inflammatory mediators and clinical features are associated with the likelihood of developing ALI in sepsis [6,7].
The use of machine learning in the medical field has grown in popularity as big data analysis technology continues to advance. Analyzing large amounts of patient data from electronic medical records can lead to the development of predictive models that assist physicians in disease prediction and severity assessment. Nomogram models have become a mainstream method for predicting the occurrence and progression of diseases and have been applied in various fields [8-10]. However, there is a lack of research combining multiple predictive factors to develop a reliable nomogram for identifying sepsis patients at risk of developing ALI. Therefore, this research aims to examine the influencing factors of ALI in sepsis patients using multidimensional indicators and to construct a nomogram model, providing clinicians with an easy-to-use risk prediction tool.
Materials and methods
Research subjects
This study was approved by the Ethics Committee of The Affiliated Tai’an City Central Hospital of Qingdao University. A retrospective analysis was conducted on the medical records of 193 sepsis patients admitted to the Affiliated Tai’an City Central Hospital of Qingdao University between January 2022 and December 2023. The patients were divided into two groups based on the presence or absence of ALI: ALI (n = 69) and non-ALI (n = 124). All subjects were over 80 years old and had complete clinical data. Sepsis was defined using the SEPSIS-3 criteria [11], and ALI was diagnosed according to the Berlin Definition [12].
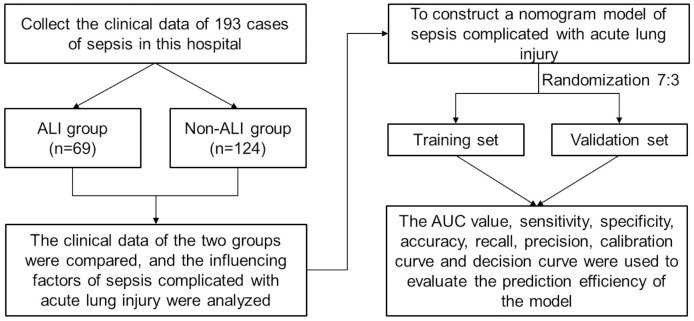
Exclusion criteria included patients who declined medical intervention or died within a day of admission, as well as those with hematological diseases, tumors, immunological disorders, or those on long-term treatment with immunosuppressants, corticosteroids, and other medications. The study was approved by the Ethics Committee of the Affiliated Tai’an City Central Hospital of Qingdao University, and all medical records were used and analyzed anonymously. The investigation’s flow chart is illustrated in Figure 1.
Figure 1.
Flow chart for this investigation. ALI: acute lung injury.
Data collection
Medical information was collected from the hospital’s electronic health records system, including age, gender, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), history of hypertension, history of coronary heart disease, history of diabetes, infection site, C-reactive protein (CRP), procalcitonin (PCT), neutrophil/lymphocyte ratio (NLR), oxygenation index (PaO2/FiO2), tumor necrosis factor-α (TNF-α), acute physiology and chronic health evaluation II (APACHE II) score, and sequential organ failure assessment (SOFA) score.
Upon admission, peripheral venous blood was collected from patients for the assessment of routine blood indices, CRP, PCT, and TNF-α. Routine blood tests were conducted using an automated hematology analyzer (XN-1000, Sysmex, Japan). The serum levels of CRP (CRP assay kit, Roche, Switzerland), PCT (PCT CLIA kit, Mindray, China), and TNF-α (TNF-α ELISA kit, Proteintech, China) were determined.
Statistical methods
SPSS Statistics 23.0 software was used for statistical analysis. Quantitative data conforming to a normal distribution were expressed as mean ± standard deviation, and differences between groups were compared using the t-test. Quantitative data with a skewed distribution were expressed as median and interquartile range, and differences between groups were compared using the rank-sum test. Counted data were expressed as percentages, and differences between groups were compared using the chi-square test. Logistic regression was employed to analyze the influencing factors of sepsis complicated with lung injury. R 4.2.3 software was used to construct the model, dividing the data into training and validation sets in a 7:3 ratio using the sample function. The nomogram model was constructed using the ‘rms’ package based on the training set data. The ROC curve, calibration curve, and decision curve were plotted, and the area under the curve (AUC), sensitivity, specificity, accuracy, recall rate, and precision rate were calculated. The model’s predictive efficiency was evaluated and verified using the validation set. A P-value of <0.05 was considered significant.
Results
Comparison of clinical data
The study included 193 sepsis patients, 69 of whom had concurrent ALI, resulting in an ALI incidence rate of 35.75%. The ALI group showed higher rates of lung infection, PCT, NLR, TNF-α, APACHE II score, and SOFA score compared to the non-ALI group (all P<0.05). Additionally, the ALI group had significantly lower PaO2/FiO2 values than the non-ALI group (P<0.05). Detailed information can be found in Table 1.
Table 1.
Comparison of medical records from patients
| Factor | Non-ALI group (n = 124) | ALI group (n = 69) | Statistic value | P value |
|---|---|---|---|---|
| Sex | 0.127a | 0.722 | ||
| Female | 56 (45.16) | 33 (47.83) | ||
| Male | 68 (54.84) | 36 (52.17) | ||
| Hypertension | 0.072a | 0.789 | ||
| No | 60 (48.39) | 32 (46.38) | ||
| Yes | 64 (51.61) | 37 (53.62) | ||
| Coronary heart disease | 0.085a | 0.771 | ||
| No | 71 (57.26) | 41 (59.42) | ||
| Yes | 53 (42.74) | 28 (40.58) | ||
| Diabetes | 0.995a | 0.318 | ||
| No | 65 (52.42) | 31 (44.93) | ||
| Yes | 59 (47.58) | 38 (55.07) | ||
| Infection sites | 8.372a | 0.015 | ||
| Lung | 56 (45.16) | 46 (66.67) | ||
| Abdomen | 41 (33.07) | 15 (21.74) | ||
| Other places | 27 (21.77) | 8 (11.59) | ||
| Age, year | 57.06±8.11 | 58.55±7.42 | -1.257b | 0.210 |
| BMI, kg/m2 | 23.64±3.70 | 23.41±3.75 | 0.406b | 0.685 |
| SBP, mmHg | 125.22±28.63 | 129.42±29.48 | -0.967b | 0.335 |
| DBP, mmHg | 71.02±18.14 | 66.96±16.65 | 1.534b | 0.127 |
| CRP, mg/L | 63.85±11.21 | 66.30±10.89 | -1.466b | 0.144 |
| PCT, ng/mL | 11.69±4.99 | 13.17±4.77 | -2.008b | 0.046 |
| NLR | 4.22±0.84 | 5.54±1.33 | -8.360b | <0.001 |
| PaO2/FiO2 | 176.86±12.29 | 149.65±18.29 | 12.317b | <0.001 |
| TNF-α, μg/L | 16.27±3.93 | 21.59±4.95 | -8.191b | <0.001 |
| APACHE II, score | 16 (13, 20) | 21 (19, 25) | -6.164c | <0.001 |
| SOFA, score | 7 (6, 9) | 8 (7, 9) | -3.337c | 0.001 |
The statistical value of the chi-square test;
The statistical value of the t-test;
The statistical value of the non-parametric test;
ALI: acute lung injury; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; CRP: C-reactive protein; PCT: procalcitonin; NLR: neutrophil/lymphocyte ratio; PaO2/FiO2: oxygenation index; TNF-α: tumor necrosis factor-α; APACHE II: acute physiology and chronic health evaluation II; SOFA: sequential organ failure assessment.
Risk predictors of sepsis complicated with ALI
Multifactorial logistic regression analysis was conducted with the incidence of ALI as the dependent variable (no ALI = 0, with ALI = 1) and infection site (pulmonary infection = 0, abdominal infection = 1, other infections = 2), PCT (original values), NLR (original values), TNF-α (original values), PaO2/FiO2 (original values), APACHE II score (original values), and SOFA score (original values) as independent variables. The occurrence of ALI in sepsis patients was found to be influenced by NLR, PaO2/FiO2, TNF-α, and APACHE II score (all P<0.05), as shown in Table 2.
Table 2.
Risk predictors of ALI in patients with sepsis
| Variable | β | S.E. | Wald χ2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Infection site | -0.392 | 0.372 | 1.111 | 0.292 | 0.676 | 0.329-1.400 |
| PCT | 0.132 | 0.075 | 3.084 | 0.079 | 1.142 | 0.985-1.324 |
| NLR | 0.721 | 0.271 | 7.094 | 0.008 | 2.056 | 1.210-3.493 |
| TNF-α | 0.297 | 0.080 | 13.774 | <0.001 | 1.346 | 1.150-1.574 |
| PaO2/FiO2 | -0.113 | 0.026 | 18.990 | <0.001 | 0.893 | 0.849-0.940 |
| APACHE II | 0.188 | 0.060 | 9.952 | 0.002 | 1.207 | 1.074-1.356 |
| SOFA | 0.256 | 0.162 | 2.501 | 0.114 | 1.291 | 0.941-1.773 |
ALI: acute lung injury; BMI: body mass index; PaO2/FiO2: oxygenation index; TNF-α: tumor necrosis factor-α; APACHE II: acute physiology and chronic health evaluation II; SOFA: sequential organ failure assessment.
Construction of a nomogram model
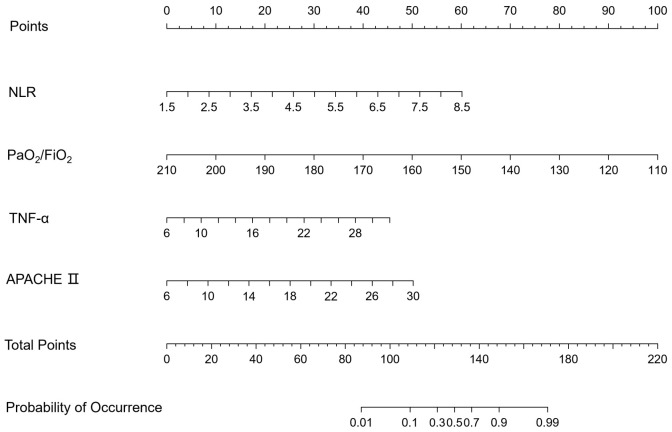
All samples were randomly divided into two datasets in a 7:3 ratio, with 135 individuals in the training set and 58 individuals in the validation set, ensuring an even distribution of samples in both datasets (Table 3). Multifactorial logistic regression analysis identified important variables potentially predicting the development of ALI in sepsis patients. The regression equation is as follows: Y = 2.104 + 0.721 * NLR + 0.297 * TNF-α - 0.113 * PaO2/FiO2 + 0.188 * APACHE II. Using the regression coefficients of the variables from the logistic regression equation, a risk prediction nomogram model was created with R software, as shown in Figure 2.
Table 3.
Comparison of the validation and training sets
| Criterion | Training set (n = 135) | Validation set (n = 58) | t/χ2 | P |
|---|---|---|---|---|
| Complicated with ALI (Yes/No) | 44/91 | 25/33 | 1.951 | 0.162 |
| NLR | 4.64±1.19 | 4.80±1.27 | -0.851 | 0.396 |
| PaO2/FiO2 | 167.99±19.15 | 165.11±20.78 | 0.932 | 0.353 |
| TNF-α | 18.18±4.84 | 18.16±5.42 | 0.028 | 0.978 |
| APACHE II | 17.89±5.42 | 18.50±5.51 | -0.705 | 0.481 |
ALI: acute lung injury; NLR: neutrophil/lymphocyte ratio; PaO2/FiO2: oxygenation index; TNF-α: tumor necrosis factor-α; APACHE II: acute physiology and chronic health evaluation II.
Figure 2.
Nomogram model. NLR: neutrophil/lymphocyte ratio; PaO2/FiO2: oxygenation index; TNF-α: tumor necrosis factor-α; APACHE II: acute physiology and chronic health evaluation II.
Verification of the nomogram model
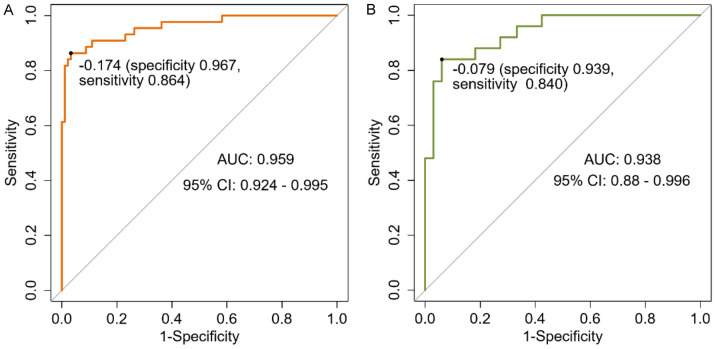
The area under the curve (AUC) for the training set was 0.959 (95% CI: 0.924-0.995), while the AUC for the validation set was 0.938 (95% CI: 0.880-0.996), indicating the nomogram’s high discriminative capacity (Figure 3). Table 4 presents the confusion matrix for the training and validation sets. The training set achieved 92.59% accuracy, 96.70% recall, and 92.63% precision, while the validation set had 89.66% accuracy, 93.94% recall, and 88.57% precision.
Figure 3.
ROC curves. A: Training set; B: Validation set.
Table 4.
Confusion matrix
| Training set prediction classification | Total | Validation set prediction classification | Total | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| ALI | Non-ALI | ALI | Non-ALI | ||||
| Actual | ALI | 37 | 7 | 44 | 21 | 4 | 25 |
| Non-ALI | 3 | 88 | 91 | 2 | 31 | 33 | |
| Total | 40 | 95 | 23 | 35 | |||
ALI: acute lung injury.
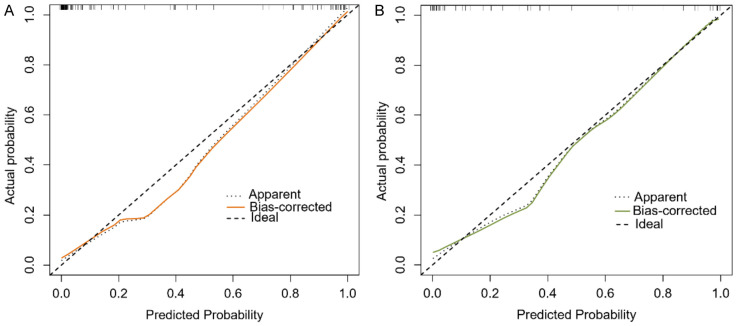
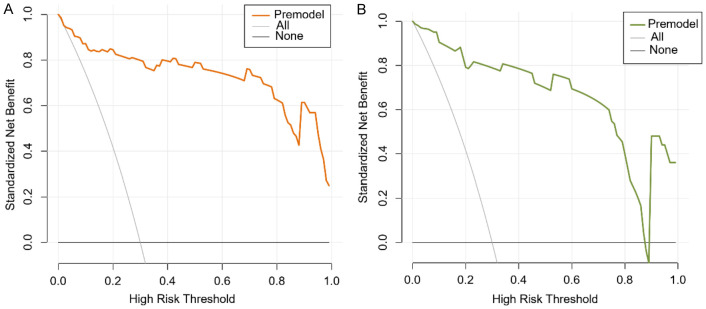
The calibration curve (Figure 4) demonstrated high agreement between predicted and actual outcomes. The decision curve (Figure 5) showed that for the training set, across a threshold range of 0.1 to 1.0, the predictive model’s net benefit was higher than the reference line. For the validation set, within a threshold range of 0.0 to 0.9, the predictive model’s net benefit was also higher than the reference line, indicating that the model had clinical utility.
Figure 4.
Calibration curves. A: Training set; B: Validation set.
Figure 5.
Decision curves. A: Training set; B: Validation set.
Discussion
The most prevalent serious condition in critical care facilities is sepsis. This condition can rapidly trigger severe inflammatory responses in the body, leading to multiple organ dysfunction and posing a significant threat to the patient’s life [13]. Acute lung injury (ALI) is a common and severe complication of sepsis, affecting up to 35.75% of patients in our study, which aligns with both domestic and international reports [14-16]. Moreover, the high mortality rate of ALI further underscores its threat to the prognosis of sepsis patients [17]. Therefore, effectively predicting the occurrence of ALI is crucial.
Our retrospective research identified NLR, PaO2/FiO2, TNF-α, and the APACHE II score as significant predictors of ALI in sepsis patients. The NLR reflects both the degree of current inflammation and the balance of the immune system. An elevated NLR typically indicates an exacerbation of the inflammatory response and a disruption of immune balance [18]. Huang et al. [19] highlighted the significant predictive value of NLR in the diagnosis and prognosis of pneumonia. Additionally, NLR has been reported as an independent risk factor for in-hospital mortality in patients with infective endocarditis, with its predictive value surpassing other indicators [20]. In our study, the ALI group exhibited higher NLR levels than the non-ALI group, and an increased NLR was identified as a risk factor for sepsis patients complicated with ALI.
Neutrophils are the first to respond to infection or inflammation sites during sepsis, playing a crucial role in clearing infections and resolving inflammation. Neutrophil extracellular traps represent a relatively new effector function of neutrophils, capable of killing pathogens by releasing DNA modified with histones and granular proteins. However, if the immune system’s overactivation is not effectively controlled, it may lead to lung injury and even trigger multiple organ failure or death [21-23].
The PaO2/FiO2 ratio is an important metric of lung oxygenation capability. Studies have indicated that the PaO2/FiO2 ratio is negatively associated with the severity of lung illnesses, meaning that the more severe the lung injury, the lower the PaO2/FiO2 ratio [24,25]. It is also a key measure for diagnosing acute respiratory distress syndrome (ARDS), with a ratio between 200-300 mmHg indicating mild ARDS [26]. This study found that the PaO2/FiO2 ratio in the ALI group was lower than that in the non-ALI group, consistent with previous research [27]. In sepsis patients, the large release of inflammatory mediators can damage vascular endothelial and alveolar epithelial cells, causing fluid and proteins to leak into the alveoli, resulting in alveolar fluid accumulation. This reduces lung oxygenation efficiency and increases the risk of lung dysfunction, leading to more severe respiratory problems [28].
Sepsis is characterized by a dysfunctional inflammatory and immunologic response, with TNF-α being a key inflammatory mediator. This study found that TNF-α is an independent predictor of ALI in sepsis patients. Kothari et al. [29] also found that as sepsis patients’ conditions worsen and ALI severity increases, TNF-α levels rise. TNF-α promotes inflammation by facilitating white blood cell passage through the endothelial cell wall and accelerating their arrival at the inflammatory site. It also increases neutrophil infiltration in lung tissue and alveoli, leading to ALI symptoms [30,31].
The APACHE II score is an objective scoring system for assessing chronic organ dysfunction and determining the need for resuscitation, holding high reference value [32]. Research has indicated that patients with inflammatory disorders have considerably higher APACHE II scores than healthy individuals, with the increase corresponding to the severity of the condition [33]. Bardají-Carrillo et al. [34] also found that ARDS and sepsis patients with higher APACHE II scores are at higher risk of death. In this research, the APACHE II score was a reliable indicator of ALI in sepsis patients, with the ALI group having substantially higher scores than the non-ALI group.
We created a nomogram using the four influential factors mentioned above. Scores were assigned based on the impact of each risk factor on ALI risk. The overall score was calculated by summing the individual scores, which was then converted to evaluate the individual risk likelihood of ALI in sepsis patients. In the validation set, the nomogram had an AUC of 0.938, an accuracy of 89.66%, a recall of 93.94%, and a precision of 88.57%, demonstrating good predictive performance. The calibration curve showed good agreement between the model’s predicted values and the actual outcomes. The decision curve validated the nomogram’s clinical utility. The nomogram simplifies the complex regression formula into an easy-to-understand visual tool, facilitating individualized patient assessment by clinicians.
However, our research has certain limitations. As a single-center retrospective investigation, we cannot eliminate selection bias. Moreover, the model has not been validated with external data, so the applicability of the results needs further confirmation. Future studies will require multicenter, large-sample, long-term prospective studies.
In conclusion, we created a nomogram model that integrates NLR, PaO2/FiO2, TNF-α, and the APACHE II score to accurately predict ALI in sepsis patients. The predictive model demonstrated good predictive ability and internal validation capability.
Disclosure of conflict of interest
None.
References
- 1.Anesi GL, Dress E, Chowdhury M, Wang W, Small DS, Delgado MK, Bayes B, Barreda FX, Halpern SD, Liu VX. Hospital strain and variation in sepsis ICU admission practices and associated outcomes. Crit Care Explor. 2023;5:e0858. doi: 10.1097/CCE.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oczkowski S, Alshamsi F, Belley-Cote E, Centofanti JE, Hylander Moller M, Nunnaly ME, Alhazzani W. Surviving sepsis campaign guidelines 2021: highlights for the practicing clinician. Pol Arch Intern Med. 2022;132:16290. doi: 10.20452/pamw.16290. [DOI] [PubMed] [Google Scholar]
- 3.Huang X, Zhang R, Fan G, Wu D, Lu H, Wang D, Deng W, Sun T, Xing L, Liu S, Wang S, Cai Y, Tian Y, Zhang Y, Xia J, Zhan Q CHARDSnet group. Incidence and outcomes of acute respiratory distress syndrome in intensive care units of mainland China: a multicentre prospective longitudinal study. Crit Care. 2020;24:515. doi: 10.1186/s13054-020-03112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398:622–637. doi: 10.1016/S0140-6736(21)00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowery NT, Terzian WTH, Nelson AC. Acute lung injury. Curr Probl Surg. 2020;57:100777. doi: 10.1016/j.cpsurg.2020.100777. [DOI] [PubMed] [Google Scholar]
- 6.Mayow AH, Ahmad F, Afzal MS, Khokhar MU, Rafique D, Vallamchetla SK, Palleti SK, Saleem F. A systematic review and meta-analysis of independent predictors for acute respiratory distress syndrome in patients presenting with sepsis. Cureus. 2023;15:e37055. doi: 10.7759/cureus.37055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y, Wang L, Yu S, Ma X, Li X. Risk factors for acute respiratory distress syndrome in sepsis patients: a retrospective study from a tertiary hospital in China. BMC Pulm Med. 2022;22:238. doi: 10.1186/s12890-022-02015-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y, Xu J, Shang X, Fang X, Gao C, Sun D, Yao L, Zhou T, Pan S, Zou X, Shu H, Yang X, Shang Y. Clinical characteristics and risk factors associated with ICU-acquired infections in sepsis: a retrospective cohort study. Front Cell Infect Microbiol. 2022;12:962470. doi: 10.3389/fcimb.2022.962470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li XD, Li MM. A novel nomogram to predict mortality in patients with stroke: a survival analysis based on the MIMIC-III clinical database. BMC Med Inform Decis Mak. 2022;22:92. doi: 10.1186/s12911-022-01836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Lu J, Song Z, Zhou Y, Liu T, Zhang D. From past to future: bibliometric analysis of global research productivity on nomogram (2000-2021) Front Public Health. 2022;10:997713. doi: 10.3389/fpubh.2022.997713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 13.Srzic I, Nesek Adam V, Tunjic Pejak D. Sepsis definition: what’s new in the treatment guidelines. Acta Clin Croat. 2022;61(Suppl 1):67–72. doi: 10.20471/acc.2022.61.s1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auriemma CL, Zhuo H, Delucchi K, Deiss T, Liu T, Jauregui A, Ke S, Vessel K, Lippi M, Seeley E, Kangelaris KN, Gomez A, Hendrickson C, Liu KD, Matthay MA, Ware LB, Calfee CS. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med. 2020;46:1222–1231. doi: 10.1007/s00134-020-06010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujishima S, Gando S, Saitoh D, Kushimoto S, Ogura H, Abe T, Shiraishi A, Mayumi T, Sasaki J, Kotani J, Takeyama N, Tsuruta R, Takuma K, Yamashita N, Shiraishi SI, Ikeda H, Shiino Y, Tarui T, Nakada TA, Hifumi T, Otomo Y, Okamoto K, Sakamoto Y, Hagiwara A, Masuno T, Ueyama M, Fujimi S, Yamakawa K, Umemura Y JAAM FORECAST ARDS Study Group. Demographics, treatments, and outcomes of acute respiratory distress syndrome: the focused outcomes research in emergency care in acute respiratory distress syndrome, sepsis, and trauma (FORECAST) study. Shock. 2020;53:544–549. doi: 10.1097/SHK.0000000000001416. [DOI] [PubMed] [Google Scholar]
- 16.Pan S, Zhang F, Ma X, Zhang Z. Clinical value of neutrophil/lymphocyte ratio in early prediction of the incidence of organ dysfunction and 28-day mortality in patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33:665–670. doi: 10.3760/cma.j.cn121430-20210325-00437. [DOI] [PubMed] [Google Scholar]
- 17.Wang DH, Jia HM, Zheng X, Xi XM, Zheng Y, Li WX. Attributable mortality of ARDS among critically ill patients with sepsis: a multicenter, retrospective cohort study. BMC Pulm Med. 2024;24:110. doi: 10.1186/s12890-024-02913-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Wang Y, Li W, Wang G. The association between the baseline and the change in neutrophil-to-lymphocyte ratio and short-term mortality in patients with acute respiratory distress syndrome. Front Med (Lausanne) 2021;8:636869. doi: 10.3389/fmed.2021.636869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Liu A, Liang L, Jiang J, Luo H, Deng W, Lin G, Wu M, Li T, Jiang Y. Diagnostic value of blood parameters for community-acquired pneumonia. Int Immunopharmacol. 2018;64:10–15. doi: 10.1016/j.intimp.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Meshaal MS, Nagi A, Eldamaty A, Elnaggar W, Gaber M, Rizk H. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as independent predictors of outcome in infective endocarditis (IE) Egypt Heart J. 2019;71:13. doi: 10.1186/s43044-019-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Payal N, Srivastava VK, Kaushik S, Saxena J, Jyoti A. Neutrophil extracellular traps and organ dysfunction in sepsis. Clin Chim Acta. 2021;523:152–162. doi: 10.1016/j.cca.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Nie S, Wang H, Liu Q, Tang Z, Tao W, Wang N. Prognostic value of neutrophils to lymphocytes and platelets ratio for 28-day mortality in patients with acute respiratory distress syndrome: a retrospective study. BMC Pulm Med. 2022;22:314. doi: 10.1186/s12890-022-02112-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Gao C, Li F, Yang L, Chen J, Guo S, He Y, Guo Q. Monocyte-to-lymphocyte ratio is associated with 28-day mortality in patients with acute respiratory distress syndrome: a retrospective study. J Intensive Care. 2021;9:49. doi: 10.1186/s40560-021-00564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feiner JR, Weiskopf RB. Evaluating pulmonary function: an assessment of PaO2/FIO2. Crit Care Med. 2017;45:e40–e48. doi: 10.1097/CCM.0000000000002017. [DOI] [PubMed] [Google Scholar]
- 25.Sartini S, Massobrio L, Cutuli O, Campodonico P, Bernini C, Sartini M, Cristina ML, Castellani L, Ceschi L, Spadaro M, Gratarola A, Barbera P. Role of SatO2, PaO2/FiO2 ratio and PaO2 to predict adverse outcome in COVID-19: a retrospective, cohort study. Int J Environ Res Public Health. 2021;18:11534. doi: 10.3390/ijerph182111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokra D. Acute lung injury - from pathophysiology to treatment. Physiol Res. 2020;69(Suppl 3):S353–S366. doi: 10.33549/physiolres.934602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seethala RR, Hou PC, Aisiku IP, Frendl G, Park PK, Mikkelsen ME, Chang SY, Gajic O, Sevransky J. Early risk factors and the role of fluid administration in developing acute respiratory distress syndrome in septic patients. Ann Intensive Care. 2017;7:11. doi: 10.1186/s13613-017-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Li D, Chen Y, Abudou H, Wang H, Cai J, Wang Y, Liu Z, Liu Y, Fan H. Classic signaling pathways in alveolar injury and repair involved in sepsis-induced ALI/ARDS: new research progress and prospect. Dis Markers. 2022;2022:6362344. doi: 10.1155/2022/6362344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kothari N, Bogra J, Abbas H, Kohli M, Malik A, Kothari D, Srivastava S, Singh PK. Tumor necrosis factor gene polymorphism results in high TNF level in sepsis and septic shock. Cytokine. 2013;61:676–681. doi: 10.1016/j.cyto.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Qiao X, Yin J, Zheng Z, Li L, Feng X. Endothelial cell dynamics in sepsis-induced acute lung injury and acute respiratory distress syndrome: pathogenesis and therapeutic implications. Cell Commun Signal. 2024;22:241. doi: 10.1186/s12964-024-01620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou S, Jie H, Han X, Wang J. The role of neutrophil extracellular traps in sepsis and sepsis-related acute lung injury. Int Immunopharmacol. 2023;124:110436. doi: 10.1016/j.intimp.2023.110436. [DOI] [PubMed] [Google Scholar]
- 32.Xu C, Zheng L, Jiang Y, Jin L. A prediction model for predicting the risk of acute respiratory distress syndrome in sepsis patients: a retrospective cohort study. BMC Pulm Med. 2023;23:78. doi: 10.1186/s12890-023-02365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quintairos A, Pilcher D, Salluh JIF. ICU scoring systems. Intensive Care Med. 2023;49:223–225. doi: 10.1007/s00134-022-06914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bardaji-Carrillo M, Martin-Fernandez M, Lopez-Herrero R, Priede-Vimbela JM, Heredia-Rodriguez M, Gomez-Sanchez E, Gomez-Pesquera E, Lorenzo-Lopez M, Jorge-Monjas P, Poves-Alvarez R, Villar J, Tamayo E. Post-operative sepsis-induced acute respiratory distress syndrome: risk factors for a life-threatening complication. Front Med (Lausanne) 2024;11:1338542. doi: 10.3389/fmed.2024.1338542. [DOI] [PMC free article] [PubMed] [Google Scholar]