Abstract
Objective: To identify risk factors of postoperative keloid scar recurrence in patients using logistic regression analysis. Methods: A retrospective analysis was conducted with the use of clinical data collected from 132 keloid scars patients undergoing keloidectomy under local anaesthesia between January 2020 and June 2023 at The First Affiliated Hospital of the WANNAN Medical College. The recurrence of keloid scars in the included patients was analyzed, and their clinical data were subjected to univariate analysis. Factors showing significant differences were included in the multivariate logistic regression analysis. A receiver operating characteristics (ROC) curve was generated based on the independent risk factors to explore the predictive performance of joint-factor prediction for postoperative recurrence of keloid scars, and a corresponding Nomogram was generated. Results: Out of the 132 patients, 38 experienced keloid scar recurrence, accounting for 28.79% of the total cases. Logistic regression analysis identified infection, family history of keloid scars, relatively large scar size and the absence of radiotherapy and local hormone therapy as independent risk factors influencing postoperative recurrence of keloid scars. The prediction for postoperative recurrence of keloid scars based on the joint independent risk factors yielded an area under the ROC curve of 0.889, with a sensitivity, a specificity, and an accuracy of 78.72%, 86.84%, and 81.06%, respectively. Conclusion: Infection, family history of keloid scars, relatively large scar size, and the absence of radiotherapy and local hormone treatment have been identified as independent risk factors for postoperative recurrence of keloid scars in patients.
Keywords: Logistics regression analysis, keloid scar, recurrence, risk factors, predictive efficacy
Introduction
Keloid scars, also referred to as hypertrophic scars, are characterized by the formation of excessive fibrous tissue during skin healing process [1]. These scars typically develop at the sites of trauma, surgical incisions, burns, or other skin injuries [2]. Scarring represents a pathological response involving morphological changes in the skin as a protective mechanism in response to various types of injuries [3]. Generally, it is believed that the advent of keloid scars is in association with individual constitution, i.e., the pathological and physiological changes in the normal skin wound healing process can be affected by various factors in human body, leading to the formation of keloid scars [4,5].
Keloid scars typically manifest in various colours, such as red, dark, or light, in comparison to the surrounding normal skin. This can cause discomfort and negatively affect patients’ confidence and social activities [6,7]. Relatively large and thick keloid scars can trigger tensive or restrictive sensation in the surrounding tissues, impacting the motion or flexibility of that body part in patients [8]. Moreover, keloid scars often manifest symptoms such as itchiness, stinging, and heightened sensitivity to touch, which can lead to significant discomfort and distress for patients, interfering with their daily activities and sleep patterns [9,10]. Currently, there are various clinical treatments for keloid scars, including surgery, medication injections, postoperative compression, application of isotopes, and laser therapy [11,12]. Research has shown that the recurrence rate of keloid scars is relatively high in patients undergoing simple surgical excision. However, the scars tend to recover faster when the surgery is combined with radiation therapy, regardless of the fact that there is still a chance of recurrence [13]. Therefore, how to improve the treatment effectiveness of keloid scars and reduce their recurrence rate have become one of the urgent issues in clinical practice. Furthermore, keloid scars are a common skin condition that severely affects the quality of life of patients. Studying the factors associated with keloid scar recurrence has important clinical significance for the prevention and management of patients with recurrent keloid scars. According to the information we have searched, there is currently no available analysis of risk factors of keloid scar recurrence, specifically after scar excision surgery under local anaesthesia.
In this paper, logistic regression analysis was carried out to explore related risk factors resulting in the recurrence of keloid scars, so as to provide a basis for the treatment and prevention of patients with recurrent keloid scars. The innovative points of the study are as follows: The study employed a combination of univariate analysis and logistic regression analysis to systematically evaluate multiple potential influencing factors for the recurrence of keloid scars. Furthermore, this research has presented more scientific evidence in comparison to prior studies.
Methods and data
Sample source and processing
With approvals from the Medical Ethics Committee of The First Affiliated Hospital of the WANNAN Medical College, this study conducted a retrospective analysis using clinical data collected from 150 patients with keloid scars who underwent keloidectomy under local anaesthesia at The First Affiliated Hospital of the WANNAN Medical College between January 2020 and June 2023.
Inclusion criteria: Patients were eligible if they met the diagnostic criteria for keloid scars, including the presence of skin lesions extending beyond original injury site and/or the scar lasting over 9 months without spontaneous regression, or confirmed by pathological examination; they were compliant; and they had detailed medical history, accurate specialized physical examination records, and follow-up records within 6 months after surgery. Exclusion criteria: Patients were excluded if they were complicated with hypertrophic scars and other syndromes associated with keloid scars (such as Rubinstein-Taybi syndrome); they had severe diseases or their physical conditions were so bad that it might affect their survival; they were unable to comply with treatments; they had plans of conceiving children, or were pregnant or breastfeeding.
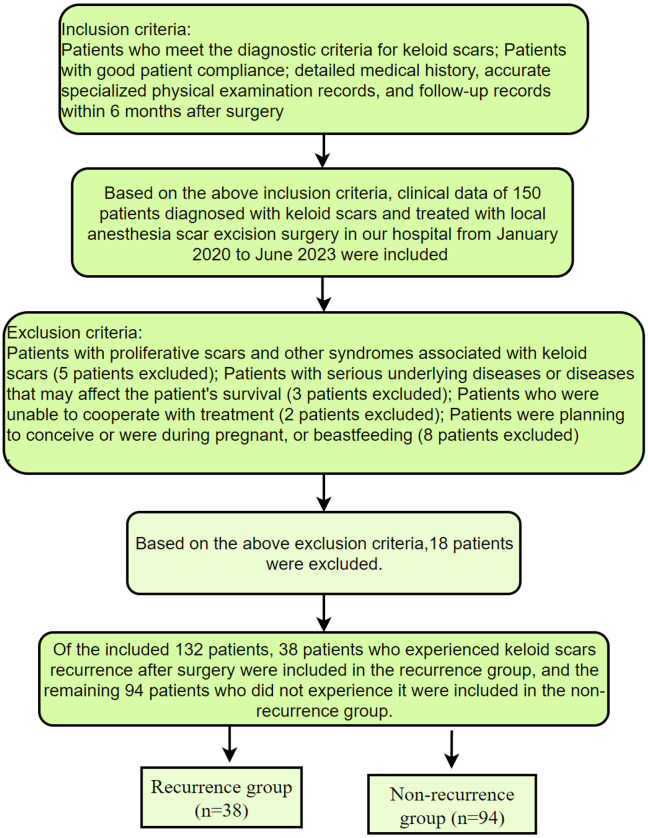
Based on the inclusion and exclusion criteria above, the study included a total of 132 patients with keloid scars undergoing keloidectomy under local anaesthesia. Among them, 38 patients were categorized into the recurrence group as they experienced postoperative recurrence of keloid scars, while the remaining 94 patients were categorized into the non-recurrence group. The screening and grouping processes are elaborated in Figure 1.
Figure 1.
Screening and grouping process.
Methods
By retrospectively studying the included patients’ medical and follow-up records, we collected information including their age, gender, distribution of keloid scars, size of the scars (maximum diameter), the presence of infection, family history of keloid scars, and histories of smoking, alcohol consumption and past treatments.
Outcomes of the included patients within 6 months after scar excision were categorized as cured, effective, ineffective, or recurrent [14]. Criteria for cured were that the scar tissue became softer, flatter, and in parallel with the normal skin surface without the presence of notable symptoms such as pain, itchiness, discomfort in the affected area, functional impairments or visible deformities. Criteria for effective included recovery observed in the scar tissue with extra thickness less than 2 mm when compared to the normal skin surface, alleviated symptoms such as pain, itchiness, or discomfort compared to before treatment, and notable improvements on both impaired functions and visible deformities. Criteria for ineffective encompassed slightly or not alleviated symptoms, and no changes in the size or texture of the keloid scars. Criteria for a recurrent included scar reappearance, continuance in scar growth, and no alleviations observed in symptoms such as itchiness or pain, and the presence of notable functional impairments and deformities. Univariate analysis was carried out with clinical data that exhibited significant differences between the two groups to identify risk factors for keloid scar recurrence in patients. Subsequently, multivariate logistic analysis was conducted for comparison of the identified risk factors that demonstrated significant differences between the two groups. Furthermore, the Receiver Operating Characteristics (ROC) curve was constructed using joint independent risk factors to assess their predictive performance for postoperative recurrence of keloid scars. This analysis aimed to explore the predictive ability of these factors in identifying patients at risk for postoperative recurrence of keloid scars. A corresponding Nomogram was generated on the website https://shiny.medsta.cn/coxpre1/ to visualize the risk factor predictive model. Graphs were generated using GraphPad Prism 7 for visual representation.
Statistical analyses
Statistical analysis in this study was conducted using SPSS 20.0 software. Counting data were presented by percentage and were compared between the two groups using chi-square test. Measurement data were presented by mean ± standard deviation (x±s) and were compared between groups with t-test. P<0.05 suggests a notable difference.
Results
Comparison of clinical baseline data between the two groups
The two groups did not differ notably in baseline data, including sex, age, BMI, etc. (P>0.05, Table 1).
Table 1.
Comparison of clinical baseline data between the two groups
| Recurrent group (n = 38) | Non-recurrent group (n = 94) | t/Χ2 | P value | |
|---|---|---|---|---|
| Sex | 2.557 | 0.110 | ||
| Male | 16 | 54 | ||
| Female | 22 | 40 | ||
| Age | 22.94±3.13 | 23.6±3.4 | 1.095 | 0.275 |
| BMI | 1.922 | 0.166 | ||
| ≥23 kg/m2 | 12 | 42 | ||
| <23 kg/m2 | 26 | 52 | ||
| Scar site | 4.011 | 0.135 | ||
| neck | 10 | 31 | ||
| Arms and legs | 13 | 42 | ||
| Trunk | 15 | 21 | ||
| History of alcohol consumption | 0.886 | 0.347 | ||
| Yes | 11 | 20 | ||
| No | 27 | 74 | ||
| Diabetes | 1.068 | 0.301 | ||
| Yes | 5 | 7 | ||
| No | 33 | 87 | ||
| Hypertension | 1.038 | 0.308 | ||
| Yes | 6 | 9 | ||
| No | 32 | 85 | ||
| Place of residence | 1.474 | 0.225 | ||
| Rural area | 25 | 51 | ||
| Urban area | 13 | 43 |
Efficacy analysis results
According to analysis on the treatment outcomes of the included patients, 38 out of 132 patients experienced recurrence of keloid scars, accounting for a relatively large proportion (28.79%, Table 2).
Table 2.
Efficacy analysis results
| Efficacy | Included patients (n = 132) |
|---|---|
| Cured | 22 (16.67%) |
| Effective | 50 (37.87) |
| Ineffective | 22 (16.67%) |
| Recurrent | 38 (28.79%) |
Comparison of univariate analysis of postoperative keloid scar recurrence risk factors between the two groups
Notable differences were found in risk factors including the presence of infection, family history of keloid scars, large scar size, history of smoking, the absence of radiotherapy and local hormone therapy between the two groups (P<0.05), as indicated in Table 3.
Table 3.
Comparison of univariate analysis of postoperative keloid scar recurrence risk factors between the two groups
| Recurrent group (n = 38) | Non-recurrent group (n = 94) | Χ2 | P value | |
|---|---|---|---|---|
| Sex | 2.557 | 0.110 | ||
| Male | 16 | 54 | ||
| Female | 22 | 40 | ||
| Age | 22.94±3.13 | 23.6±3.4 | 1.095 | 0.275 |
| Infection | 23.861 | <0.0001 | ||
| Yes | 25 | 20 | ||
| No | 13 | 74 | ||
| Family history of keloid scars | 7.431 | 0.006 | ||
| Yes | 12 | 11 | ||
| No | 26 | 83 | ||
| Scar size | 11.641 | 0.001 | ||
| ≥2 cm | 27 | 36 | ||
| <2 cm | 11 | 58 | ||
| Scar site | 4.011 | 0.135 | ||
| neck | 10 | 31 | ||
| Arms and legs | 13 | 42 | ||
| Trunk | 15 | 21 | ||
| Smoking history | 4.730 | 0.030 | ||
| Yes | 18 | 26 | ||
| No | 20 | 68 | ||
| History of alcohol consumption | 0.886 | 0.347 | ||
| Yes | 11 | 20 | ||
| No | 27 | 74 | ||
| Radiotherapy | 8.130 | 0.004 | ||
| Yes | 13 | 59 | ||
| No | 25 | 37 | ||
| Local hormone therapy | 21.911 | <0.0001 | ||
| Yes | 8 | 62 | ||
| No | 30 | 32 |
Comparison of multivariate logistic regression analysis of postoperative keloid scar recurrence risk factors between the two groups
Variables that exhibited significant differences between the two groups in the univariate analysis were assigned with individual values according to Table 4. The results of the multivariable logistic regression analysis employing the recurrence of keloid scars as the dependent variable, with 1 standing for the recurrence of keloid scars and 0 for otherwise, suggested that the presence of infection, family history of keloid scars, relatively large scar size, the absence of radiotherapy and local hormone treatment were all independent risk factors for keloid scar recurrence following surgical excision, as presented in Table 5.
Table 4.
Assignment of factor values
| Factors | Assignment |
|---|---|
| Infection | Yes = 1, No = 0. |
| Family history of keloid scars | Yes = 1, No = 0. |
| Scar size | ≥2 cm = 1, <2 cm = 0. |
| History of smoking | Yes = 1, No = 0. |
| Radiotherapy | No = 1, Yes = 0. |
| Local hormone therapy | No = 1, Yes = 0. |
| Recurrence of keloid | Yes = 1, No = 0. |
Table 5.
Comparison of multivariate logistics regression analysis of postoperative keloid scar recurrence risk factors between the two groups
| Factors | B | S.E. | Wals | Df | Sig. | Exp (B) | 95% C.I. for EXP (B) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower limit | Upper limit | |||||||
| Infection | 1.421 | 0.508 | 7.812 | 1 | 0.005 | 4.141 | 1.529 | 11.215 |
| Family history of keloid scars | 1.803 | 0.660 | 7.467 | 1 | 0.006 | 6.070 | 1.665 | 22.128 |
| Scar size | 1.438 | 0.534 | 7.257 | 1 | 0.007 | 4.214 | 1.480 | 11.999 |
| History of smoking | 0.809 | 0.539 | 2.251 | 1 | 0.134 | 2.245 | 0.781 | 6.457 |
| Radiotherapy | 1.013 | 0.521 | 3.785 | 1 | 0.052 | 2.753 | 0.993 | 7.636 |
| Local hormone therapy | 2.215 | 0.588 | 14.209 | 1 | <0.001 | 9.161 | 2.896 | 28.980 |
Predictive efficacy of joint independent risk factors on predicting postoperative recurrence of keloid scars in patients
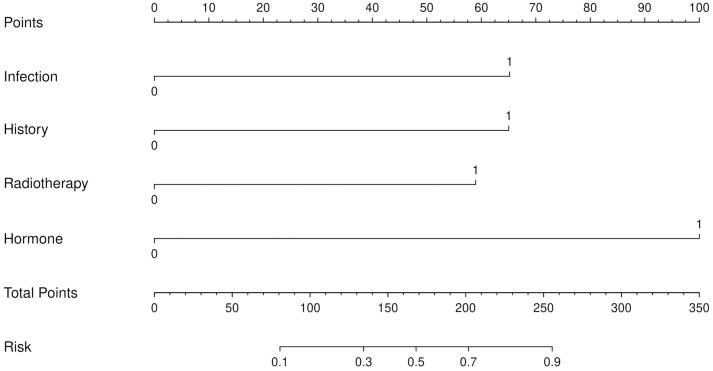
A ROC curve was plotted to evaluate the predictive efficacy of independent risk factors including the presence of infection, family history of keloid scars, large scar size, the absence of radiotherapy and local hormone treatment on recurrence of keloid scars after surgical excision. The results showed that the area under the curve (AUC) for evaluating the predictive performance was 0.889, with a sensitivity of 78.72%, a specificity of 86.84%, and an accuracy of 81.06% (Figure 2). Based on the logistic regression analysis results, we also constructed a Nomogram prediction model incorporating five independent factors to predict the risks of postoperative recurrence of keloid scars in patients (Figure 3).
Figure 2.
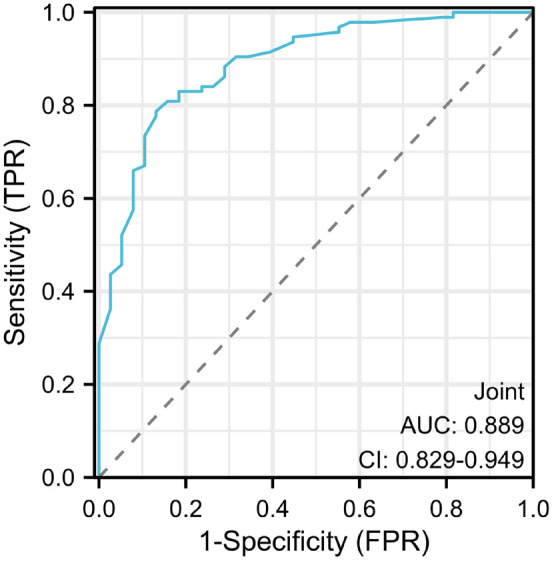
Predictive performance of joint independent risk factors for keloid scar recurrence after surgical excision.
Figure 3.
The Nomogram for predicting keloid scar recurrence after surgical excision.
Discussion
In this study, it was found that the recurrence of keloid scars after surgical excision was in close association with factors including scar infection, family history of keloid scars, large scar size, history of smoking, the absence of radiotherapy and local hormone treatment. Once a scar is observed with infection, it would normally be accompanied with symptoms such as redness, swelling, pain, fluid discharge, and fever. These symptoms disrupt the normal healing process, leading to the formation of abnormal scar tissues, prolonged healing time, and an increased risk of developing keloids [15]. Hence, in order to prevent infections in the scar, it is essential for postoperative patients to strictly follow their doctor’s instructions and adhere to proper wound care and hygiene practices. This includes keeping the surgical wound dry, ensuring wound cleanliness through regular cleaning, and timely changing of dressings as recommended by medical professionals. If there are any signs of infection, redness, swelling, fluid discharge, or fever, etc., it is important to consult a doctor immediately and seek appropriate treatment. The formation of keloid scars is a complex physiological process influenced by various factors, including genetics, hereditary predisposition, and environmental factors [1,16]. An individual with a family history of keloid formation may face a higher risk of developing recurrent keloid scars after surgery. Genetic factors can lead to different individual responses to trauma or surgical stimuli, making these individuals more prone to the formation of abnormal scar tissues [15,17]. Furthermore, this study found that the size of keloid scars was one of the important factors resulting in the recurrence of keloid scars, with larger size associated with higher recurrence risks. This might be explained by the fact that large keloid scar size increases the tension during wound recovery process, making patients susceptible to the recurrence of keloid scars [18,19]. Therefore, extra attention should be paid to patients with large keloid scar areas to minimize their risks of keloid scar recurrence. Similar to this study, research by Liu also found that keloid scar shape, maximum lesion diameter, and infection were risk factors for local recurrence of keloid scars [16], providing further support for the results of this study. Smoking is also identified as a detrimental factor in keloid scar formation. Smoking can impair blood circulation, leading to inadequate oxygen and nutrient supply in the human body, which in turn affects the wound healing process [21]. Furthermore, smoking may induce inflammation and immune suppression, further compromising the wound healing process [22]. Radiotherapy and local hormone treatment can reduce the recurrence of keloid scars after surgical excision. The reason may be as follows: Radiotherapy reduces inflammation and fibrotic reaction, thus improving the wound healing process, reducing scar tissue formation, and potentially promoting tissue repair and remodelling, thereby lowering the recurrence risk of keloid scars [20,22,23]. Local hormone treatment, aiming at alleviating symptoms in the scar, is a commonly used approach for the management of keloid scars. Hormones can reduce inflammatory responses, inhibit cell proliferation, and suppress collagen synthesis, thereby reducing the formation of excessive scar tissues [24]. Local hormone treatment can also improve the appearance and texture of scars and reduce the recurrence of keloid scars. Therefore, the employment of hormone and radiation therapies serve as protective factors against keloid scar recurrence. This finding suggests that for patients without contraindications, the consideration of hormone therapy or radiation therapy can be recommended as an approach to reduce the recurrence rate of keloid scars following surgery.
The final findings of the study revealed that infection, family history of keloid scars, large scar size, the absence of radiotherapy and local hormone treatment collectively had a high AUC value (0.889) in predicting the recurrence of keloid scars after surgery. Additionally, these factors demonstrated a high sensitivity (78.72%), specificity (86.84%) and accuracy (81.06%). These findings also indicated that the combined application of these five factors had a high accuracy and discriminatory power in predicting the recurrence of keloid scars after surgery. According to the research results, the combined application of these five risk factors, infection, family history of keloid scars, large scar size, the absence of radiotherapy and local hormone therapy, are effective in predicting the postoperative recurrence of keloid scars. The findings in the study are of great importance in clinical practice. Firstly, these five factors can serve as basic evidence for assessing the risks of keloid recurrence at the individual level. Before performing scar removal surgery for patients, doctors can comprehensively evaluate these factors to predict and assess the risks of keloid scar recurrence [25]. For high-risk patients, doctors can adopt more active preventive and management measures, such as more frequent follow-ups, application of improved surgical techniques, and more intensive post-operative health care, to reduce the probability of keloid scar recurrence [26]. Secondly, these five factors can also be used for risk prediction and stratification at the population level. In clinical studies, researchers can divide participants into high, medium, and low-risk groups based on these factors, which can better identify the impact of different risk factors on treatment outcomes and provide a basis for individualized diagnosis and treatment [27]. Meanwhile, more targeted preventive interventions can be carried out for high-risk groups to improve the overall success rate of treatment. Furthermore, these five factors can also play an important role in clinical decision-making. Doctors can customize treatment plans for patients by taking into consideration the five risk factors. For example, more aggressive surgical approaches combined with other comprehensive post-operative treatments are options for patients with high risks to reduce their chance in keloid scar recurrence. Similarly, patients with low risks can consider undergoing conservative surgical approaches in combination with moderate post-operative rehabilitations. Additionally, the prediction model, built by the joint employment of the risk factors, can also be applied to assess the risk factors of other diseases in relation to wound healing. Overall, the results in this research have provided important references for the prediction and assessment of keloid scar recurrence both in clinical settings and for future research.
Limitations
This study employed logistic regression analysis to examine the influencing factors of keloid scar recurrence in patients after surgery. However, it still has certain limitations. The data obtained in this study might be incomplete or subject to bias due to the retrospective nature. Additionally, the sample size is relatively small. Therefore, the results should be further validated through large-scale, multicenter, randomized controlled trials.
Summary
The presence of infection, a family history of keloid scars, a relatively large scar size, the absence of radiation therapy, and the absence of local hormone treatment were identified as independent risk factors for postoperative recurrence of keloid scars. Patients with these risk factors should receive special attention and meticulous wound care to minimize the risk of recurrence. Moreover, for patients who have undergone surgery for keloid scars and do not have contraindications, considering the use of hormone therapy or radiation therapy may be beneficial in reducing the recurrence rate.
Disclosure of conflict of interest
None.
References
- 1.Hawash AA, Ingrasci G, Nouri K, Yosipovitch G. Pruritus in keloid scars: mechanisms and treatments. Acta Derm Venereol. 2021;101:adv00582. doi: 10.2340/00015555-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekstein SF, Wyles SP, Moran SL, Meves A. Keloids: a review of therapeutic management. Int J Dermatol. 2021;60:661–671. doi: 10.1111/ijd.15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naik PP. Novel targets and therapies for keloid. Clin Exp Dermatol. 2022;47:507–515. doi: 10.1111/ced.14920. [DOI] [PubMed] [Google Scholar]
- 4.Ojeh N, Bharatha A, Gaur U, Forde AL. Keloids: current and emerging therapies. Scars Burn Heal. 2020;6:2059513120940499. doi: 10.1177/2059513120940499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeschke MG, Wood FM, Middelkoop E, Bayat A, Teot L, Ogawa R, Gauglitz GG. Scars. Nat Rev Dis Primers. 2023;9:64. doi: 10.1038/s41572-023-00474-x. [DOI] [PubMed] [Google Scholar]
- 6.Walsh LA, Wu E, Pontes D, Kwan KR, Poondru S, Miller CH, Kundu RV. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi: 10.1186/s13643-023-02192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Z, Kong W, Wang H, Xiao Y, Shi Y, Gan L, Sun Y, Tang H, Xia Z. Clinical status of hospitalized keloid cases from 2013 to 2018. Burns. 2022;48:1874–1884. doi: 10.1016/j.burns.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Nangole FW, Ouyang K, Anzala O, Ogeng’o J, Agak GW, Zuriel D. Does keloid histology influence recurrence? Am J Dermatopathol. 2021;43:642–646. doi: 10.1097/DAD.0000000000001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai CH, Ogawa R. Keloid research: current status and future directions. Scars Burn Heal. 2019;5:2059513119868659. doi: 10.1177/2059513119868659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao H, Zheng L, Hsu P, He J, Wu R, Xu S, Zeng R, Zhou Y, Ma H, Liu H, Tang Q. IL-13RA2 downregulation in fibroblasts promotes keloid fibrosis via JAK/STAT6 activation. JCI Insight. 2023;8:e157091. doi: 10.1172/jci.insight.157091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, Ding J, Jin J, Song N, Liu Y. Continuous tension reduction to prevent keloid recurrence after surgical excision: preliminary experience in Asian patients. Dermatol Ther. 2020;33:e13553. doi: 10.1111/dth.13553. [DOI] [PubMed] [Google Scholar]
- 12.Huang C, Liu L, You Z, Du Y, Ogawa R. Managing keloid scars: from radiation therapy to actual and potential drug deliveries. Int Wound J. 2019;16:852–859. doi: 10.1111/iwj.13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang NH, Chang JH, Lee NK, Yang KS. Effect of the biologically effective dose of electron beam radiation therapy on recurrence rate after keloid excision: a meta-analysis. Radiother Oncol. 2022;173:146–153. doi: 10.1016/j.radonc.2022.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Madni TD, Lu K, Nakonezny PA, Imran JB, Cunningham HB, Clark AT, Taveras L, Hoopman JE, Wolf SE, Kenkel JM, Phelan HA. Treating hypertrophic burn scar with 2940-nm Er:YAG laser fractional ablation improves scar characteristics as measured by noninvasive technology. J Burn Care Res. 2019;40:416–421. doi: 10.1093/jbcr/irz056. [DOI] [PubMed] [Google Scholar]
- 15.Liang Z, Zhang M, Hao Y, Shan M, Liu H, Xia Y, Chen Q, Chang G, Wang Y. Risk factors associated with keloid infections: a five-year retrospective study. Int Wound J. 2023;20:2215–2223. doi: 10.1111/iwj.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Direder M, Weiss T, Copic D, Vorstandlechner V, Laggner M, Pfisterer K, Mildner CS, Klas K, Bormann D, Haslik W, Radtke C, Farlik M, Shaw L, Golabi B, Tschachler E, Hoetzenecker K, Ankersmit HJ, Mildner M. Schwann cells contribute to keloid formation. Matrix Biol. 2022;108:55–76. doi: 10.1016/j.matbio.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Shan M, Liu H, Hao Y, Meng T, Feng C, Song K, Wang Y. IL-4 and CCR7 play an important role in the development of keloids in patients with a family history. Am J Transl Res. 2022;14:3381–3394. [PMC free article] [PubMed] [Google Scholar]
- 18.Maemoto H, Ishigami K, Iraha S, Arashiro K, Kusada T, Ganaha F, Murayama S. Analyses of size and computed tomography densitometry parameters for prediction of keloid recurrence after postoperative electron beam radiation therapy. Skin Res Technol. 2020;26:125–131. doi: 10.1111/srt.12775. [DOI] [PubMed] [Google Scholar]
- 19.Maemoto H, Iraha S, Arashiro K, Ishigami K, Ganaha F, Murayama S. Risk factors of recurrence after postoperative electron beam radiation therapy for keloid: comparison of long-term local control rate. Rep Pract Oncol Radiother. 2020;25:606–611. doi: 10.1016/j.rpor.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C. Risk factors for recurrence after keloid surgery with electron radiotherapy. Medicine (Baltimore) 2023;102:e35683. doi: 10.1097/MD.0000000000035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deliaert AEK, Mermans JF, Schop SJ, Dormaar TS, Heerdt EM, Xanthoulea SA, Maessen JG, van den Kerckhove E, van der Hulst R. The effect of smoking on sternal scar healing: a prospective cohort study. Wounds. 2019;31:200–204. [PubMed] [Google Scholar]
- 22.Petrou IG, Jugun K, Rüegg EM, Zilli T, Modarressi A, Pittet-Cuénod B. Keloid treatment: what about adjuvant radiotherapy? Clin Cosmet Investig Dermatol. 2019;12:295–301. doi: 10.2147/CCID.S202884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leszczynski R, da Silva CA, Pinto A, Kuczynski U, da Silva EM. Laser therapy for treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2022;9:CD011642. doi: 10.1002/14651858.CD011642.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZC, Zhao WY, Cao Y, Liu YQ, Sun Q, Shi P, Cai JQ, Shen XZ, Tan WQ. The roles of inflammation in keloid and hypertrophic scars. Front Immunol. 2020;11:603187. doi: 10.3389/fimmu.2020.603187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng K, Xiao H, Liu X, Ogawa R, Xu X, Liu Y. Strontium-90 brachytherapy following intralesional triamcinolone and 5-fluorouracil injections for keloid treatment: a randomized controlled trial. PLoS One. 2021;16:e0248799. doi: 10.1371/journal.pone.0248799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijers S, Meijers R, van der Veen E, van den Aardweg M, Bruijnzeel H. A systematic literature review to compare clinical outcomes of different surgical techniques for second branchial cyst removal. Ann Otol Rhinol Laryngol. 2022;131:435–444. doi: 10.1177/00034894211024049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma QY, Yang YT, Chen ZA, Xie CH, Wang WB, Lin X, Xia LL, Zhao Q, Gao Z, Wu XL. Laser combined with radiotherapy for keloid treatment: a novel and efficient comprehensive therapy with a lower recurrence rate. Plast Reconstr Surg. 2023;152:1022e–1029e. doi: 10.1097/PRS.0000000000010376. [DOI] [PubMed] [Google Scholar]