Abstract
Objective: To investigate the diagnostic significance of immune cells and biochemical markers in the cerebrospinal fluid and blood of patients with brucella meningitis. Methods: A retrospective study was conducted to analyze the clinical data from 30 patients with Brucella meningitis (Group A), 30 patients with Brucella infection without neurological impairment (Group B), and 30 cases of non-brucella infection (Group C) that were collected from the People’s Hospital of Xinjiang Uygur Autonomous Region between January 2020 and December 2022. The levels of immune cells and biochemical markers in the cerebrospinal fluid and blood were compared between the three groups. Spearman correlation coefficient, logistic regression analysis, and receiver operating characteristic (ROC) curve analysis were used to assess the association between these factors and Brucella meningitis and to determine their diagnostic value. Results: A negative correlation was found between Brucella meningitis and CD3+, CD4+, CD4+/CD8+ T lymphocytes, glucose (C-Glu), and chloride ions (C-Cl) in the cerebrospinal fluid. Conversely, a positive correlation was observed between Brucella meningitis and blood CD4+, CD4+/CD8+ cells, cerebrospinal fluid protein (C-Pro), and lactate dehydrogenase (C-LDH). High levels of C-Glu and C-Cl were identified as protective factors, while elevated C-LDH was considered as a risk factor for Brucella meningitis. The area under the curve (AUC) for C-Glu, C-Cl, C-LDH, and their combination in predicting Brucella meningitis were 0.828, 0.860, 0.869, and 0.971, respectively. Conclusion: The levels of CD3+, CD4+, CD4+/CD8+ cells in the cerebrospinal fluid, as well as the levels of CD4+ and CD4+/CD8+ cells in the blood, are correlated with the occurrence of Brucella meningitis. C-Glu, C-Cl, C-LDH and their combination demonstrate significant potential in aiding the auxiliary diagnosis of Brucellosis meningitis.
Keywords: Human brucellosis, Brucella meningitis, cerebrospinal fluid, blood, immune cells, biochemical levels
Introduction
Brucellosis, a zoonotic disease caused by Brucella bacteria, accounts for over 500,000 new cases worldwide annually [1]. The disease can affect all body systems, with complex and diverse clinical manifestations that vary in severity [2]. When Brucella invades the nervous system, it causes an inflammatory condition known as neurobrucellosis. Although the incidence of neurobrucellosis is only about 4%, it is a significant contributor to mortality among those affected [3]. Meningitis is the most common type of neurobrucellosis, with headache being the typical neurological symptom, with or without meningeal stimulation [4]. In most cases, patients with brucellosis meningitis do not exhibit obvious early clinical symptoms, and the majority of patients are only diagnosed after the occurrence of significant neurological damage.
A positive culture for Brucella in the blood or cerebrospinal fluid is the “gold standard”; however, Brucella is a slow-growing bacterium, typically requiring 5-7 days or longer for cultivation. This process demands highly specialized laboratory training, and improper handling can pose a risk of infection to laboratory personnel [5]. Meanwhile, the cerebrospinal fluid in Brucellosis meningitis lacks specific distinguishing features; it usually shows a mild-to-moderate increase in white blood cell count, predominantly lymphocytes, with protein levels that may be elevated or normal, while glucose and chloride levels may be reduced or normal. Similarly, peripheral blood tests may reveal an increased white blood cell and lymphocyte count [6].
Immune cells play a critical role in the body’s defense system, directly influencing the survival and elimination of Brucella in the central nervous system. The quantity, classification, and functional status of immune cells, along with alterations in biochemical levels, reflect the metabolic and internal environmental imbalances induced by the disease. Integrating these immune cell and biochemical markers may facilitate early diagnosis of Brucella meningitis [7]. Therefore, this study investigated the diagnostic significance of immune cells and biochemical markers in cerebrospinal fluid and blood in patients with Brucella meningitis.
Subjects and methods
Study design and patient inclusion
This retrospective study was conducted at the Xinjiang Uygur Autonomous Region People’s Hospital, involving cases from January 2020 to December 2022. Thirty patients diagnosed with Brucella meningitis (Group A) were selected as the study subjects. Additionally, 30 patients with brucellosis but without neurological impairment (Group B) and 30 non-brucellosis patients without any infectious or central nervous system diseases (Group C) served as the control group.
Inclusion criteria for patients: (1) Clinically diagnosed with brucellosis (Group B) and brucellosis meningitis (Group A); (2) No history of infectious diseases, no central nervous system disorders, no brucellosis infection, and no history of brucellosis exposure (Group C); (3) Age >18 years; (4) Complete medical records available. Exclusion criteria for patients: (1) Presence of other forms of meningitis or neurological disorders; (2) Concomitant hepatic or renal insufficiency, or malignant tumor; (3) Pregnant or lactating women. This study was approved by the Ethics Committee of Xinjiang Uygur Autonomous Region People’s Hospital.
The diagnostic criteria for brucellosis are based on the “Guidelines for Diagnosis and Treatment of Brucellosis (for trial use)” issued by China in 2012. (1) Epidemiological history: a recent history of close contact with livestock or residing in an endemic area; (2) Suspicious clinical symptoms: fever, fatigue, profuse sweating, muscle and joint pain, often accompanied by enlargement of the liver, spleen, lymph nodes, and testes; (3) Laboratory confirmation: presence of one or more positive results in the tube agglutination test, complement fixation test, or brucellosis anti-human immunoglobulin test, or isolation of Brucella.
There is currently no standardized diagnostic criteria for brucellosis meningitis. Based on clinical experience and previous research, the diagnosis was made following the confirmation of brucellosis and the presence of (1) signs and symptoms of suspected meningitis: such as fever, headache, vomiting, seizures, lethargy, disturbance of consciousness, and positive signs of meningeal irritation; (2) Laboratory findings: isolation of Brucella species from cerebrospinal fluid and/or positive anti-brucella antibodies in cerebrospinal fluid; (3) Cerebrospinal fluid analysis: typical meningitis changes, such as increased white blood cell count, elevated protein concentration, and decreased glucose level in cerebrospinal fluid; (4) Imaging findings: abnormalities on head and spinal cord MRI, such as meningeal thickening and hydrocephalus, support a diagnosis of Brucella meningitis [8-10].
Data collection
General data: age, gender, body mass index (BMI), course of disease was collected for three groups of patients. Laboratory data: lumbar puncture was performed within 24 hours after admission, and 5 mL of cerebrospinal fluid was collected. Following centrifugation at 200×g for 10 minutes at 4°C, the supernatant was collected for further analysis. On the morning of the second day after admission, 5 mL of venous blood was collected under fasting condition. The blood was centrifuged at 3000 rpm for 10 minutes, and the supernatant was collected. The levels of CD3+, CD4+, and CD8+ T lymphocytes were detected using Flow cytometry in both cerebrospinal fluid and serum. The biochemical results, including cerebrospinal fluid-protein (C-Pro), cerebrospinal fluid-glucose (C-Glu), cerebrospinal fluid-chloride ion (C-CI), cerebrospinal fluid-lactate dehydrogenase (C-LDH) were detected using an automatic biochemical analyzer.
Outcome measures
(1) The differences in the collected data among the three groups were compared; (2) The correlation between the statistically significant indicators identified in the univariate analysis and Brucella meningitis was analyzed; (3) Independent risk factors for Brucella meningitis were identified from the factors with significant correlation to Brucella meningitis; (4) The diagnostic efficacy of the influencing factors in Brucella meningitis was assessed.
Statistical methods
SPSS 26.0 was used for statistical analysis. Quantitative data following a normal distribution were described as mean ± standard deviation (x̅±s), and analyzed using the independent sample t-test or one-way analysis of variance (ANOVA) with post-hoc LSD-t test or Tamhane’s T2 test. Spearman’s correlation coefficient was used for correlation analysis, and Logistic regression analysis was used to identify influencing factors for Brucella meningitis. The receiver operating characteristic curve (ROC) was used to evaluate the diagnostic performance of influencing factors, with the area under the curve (AUC), sensitivity, specificity and cut-off value being calculated. The AUCs from different ROC curves were compared using the DeLong test. A P value less than 0.05 was considered with statistical difference.
Results
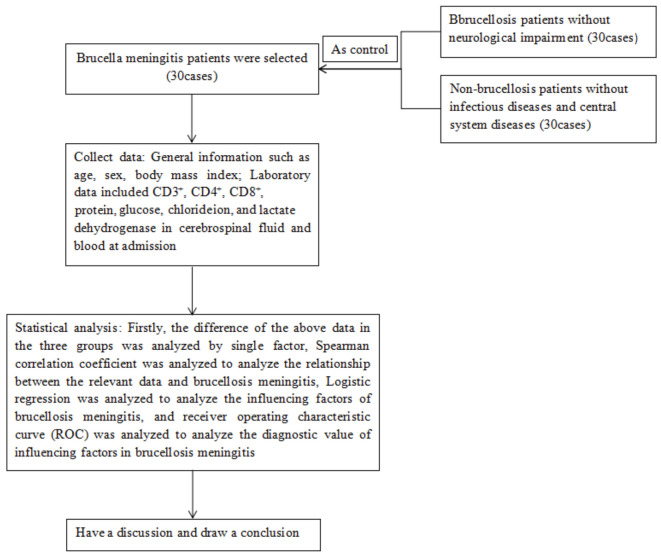
The flow chart of this study is shown in Figure 1.
Figure 1.
Research flow chart.
Comparison of general information among the three groups
There was no significant difference in age, gender, body mass index (BMI), and disease duration among the three groups (P>0.05), as shown in Table 1.
Table 1.
Comparison of general data
| Information | Group A (n=30) | Group B (n=30) | Group C (n=30) | F/χ2/t | P |
|---|---|---|---|---|---|
| Age (years, x̅±s) | 39.10±5.74 | 40.63±3.73 | 37.97±5.51 | 2.088 | 0.130 |
| Gender [n (%)] | 2.551 | 0.279 | |||
| Male | 20 (66.67) | 18 (60.00) | 14 (46.67) | ||
| Female | 10 (33.33) | 12 (40.00) | 16 (53.33) | ||
| Body mass index (kg/m2, x̅±s) | 21.93±2.48 | 22.05±2.08 | 21.62±2.88 | 0.229 | 0.769 |
| Duration (months, x̅±s) | 6.29±2.73 | 5.33±0.99 | - | 1.816 | 0.075 |
Comparison of immune cells
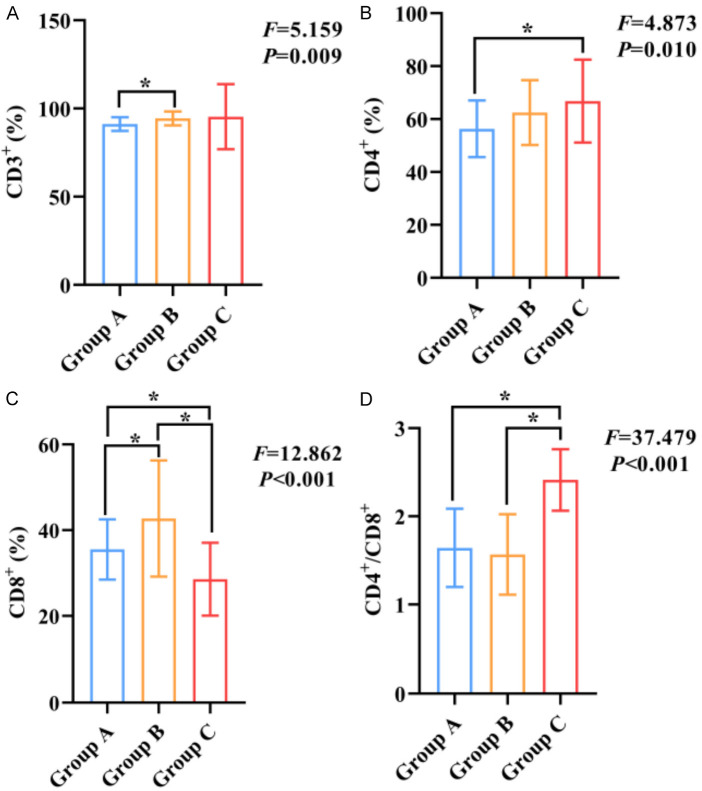
Significant differences were observed in the levels of CD3+, CD4+, CD8+ T lymphocytes and CD4+/CD8+ ratio in the cerebrospinal fluid among the three groups (all P<0.05). The results showed that the levels of CD3+, CD4+, CD8+ and CD4+/CD8+ were lower in group A compared to Group B or group C (all P<0.05), as shown in Figure 2.
Figure 2.
Comparison of cerebrospinal fluid immune cell contents among three groups. A: Comparison of CD3+ levels among three groups; B: Comparison of CD4+ levels among three groups; C: Comparison of CD8+ levels among three groups; D: Comparison of CD4+/CD8+ among three groups. Notes: CD3+: CD3-positive T-lymphocytes; CD4+: CD4-positive T-lymphocytes; CD8+: CD8-positive T-lymphocytes; *: P<0.05.
Significant differences were observed in the levels of CD3+, CD4+, CD8+ T lymphocytes and CD4+/CD8+ ratio in blood samples among all three groups (all P<0.05). Specifically, CD3+ in group B was higher than the group A and C; CD4+ in group A was higher than the group C; CD8+ in group B was higher than the group C; CD4+/CD8+ in group A and C were higher than the group B (all P<0.05), as shown in Figure 3.
Figure 3.
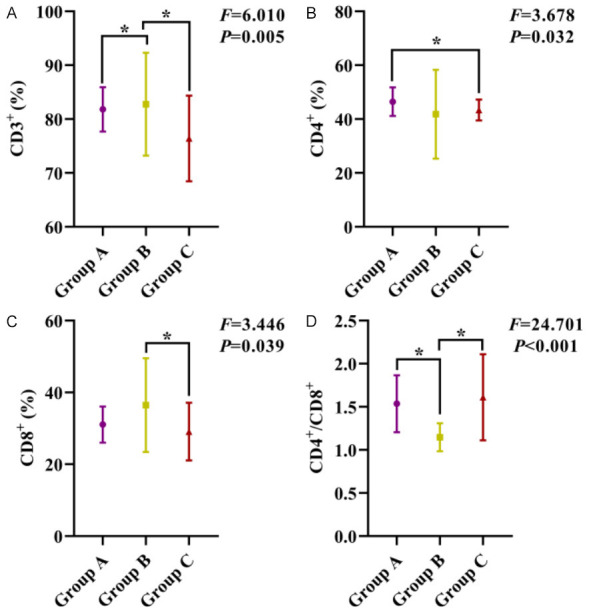
Comparison of immune cell content in the blood among the three groups. A: Comparison of CD3+ levels among the three groups; B: Comparison of CD4+ levels among the three groups; C: Comparison of CD8+ levels among the three groups; D: Comparison of CD4+/CD8+ among the three groups. Notes: CD3+: CD3-positive T-lymphocytes; CD4+: CD4-positive T-lymphocytes; CD8+: CD8-positive T-lymphocytes; *: P<0.05.
Comparison of biochemical levels
The comparison of C-Pro, C-Glu, C-CI, C-LDH levels among the three groups revealed statistically significant differences (all P<0.05). The levels of C-Pro and C-LDH in group A were higher than Group B or Group C, while the levels of C-Glu and C-CI were lower than in Group B and C (all P<0.05), as shown in Figure 4.
Figure 4.
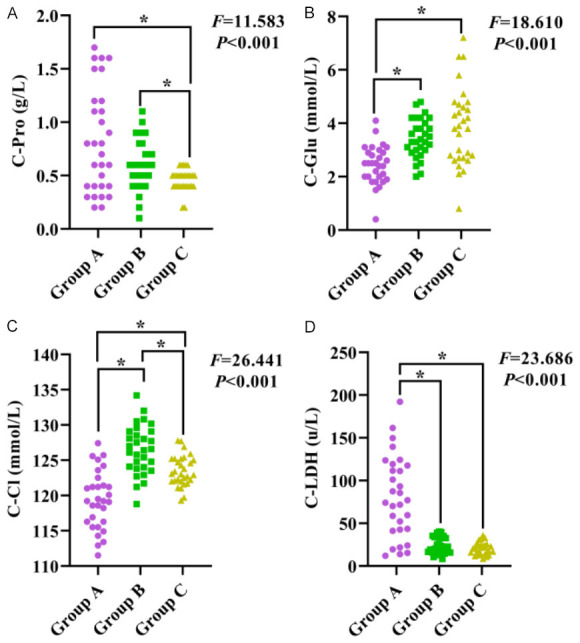
Comparison of biochemical levels in the cerebrospinal fluid among the three groups. A: Comparison of C-Pro levels among the three groups; B: Comparison of C-Glu levels among the three groups; C: Comparison of C-CI level among the three groups; D: Comparison of C-LDH levels among the three groups. Notes: C-Pro: cerebrospinal fluid-protein; C-Glu: cerebrospinal fluid-glucose; C-CI: cerebrospinal fluid-chloride ion; C-LDH: cerebrospinal fluid-lactate dehydrogenase; *: P<0.05.
Correlation analysis
Cerebrospinal fluid-CD3+, cerebrospinal fluid-CD4+, cerebrospinal fluid-CD4+/CD8+, C-Glu, C-CI were negatively correlated with the occurrence of brucellosis meningitis, and blood-CD4, blood-CD4+/CD8+, C-Pro, C-LDH were positively correlated with the occurrence of brucellosis meningitis (P<0.05), as shown in Table 2.
Table 2.
Correlation analysis of the occurrence of brucellosis meningitis
| Indicators | Brucellosis meningitis | |
|---|---|---|
|
| ||
| r | P | |
| Cerebrospinal fluid-CD3+ | 0.233 | 0.027 |
| Cerebrospinal fluid-CD4+ | 0.310 | 0.003 |
| Cerebrospinal fluid-CD8+ | 0.043 | 0.690 |
| Cerebrospinal fluid-CD4+/CD8+ | 0.283 | 0.007 |
| Blood-CD3+ | 0.149 | 0.162 |
| Blood-CD4+ | 0.218 | 0.039 |
| Blood-CD8+ | 0.059 | 0.581 |
| Blood-CD4+/CD8 | 0.320 | 0.002 |
| C-Pro | 0.237 | 0.025 |
| C-Glu | 0.536 | <0.001 |
| C-CI | 0.588 | <0.001 |
| C-LDH | 0.602 | <0.001 |
Notes: CD3+: CD3-positive T-lymphocytes; CD4+: CD4-positive T-lymphocytes; CD8+: CD8-positive T-lymphocytes; C-Pro: cerebrospinal fluid-protein; C-Glu: cerebrospinal fluid-glucose; C-CI: cerebrospinal fluid-chloride ion; C-LDH: cerebrospinal fluid-lactate dehydrogenase.
Logistic regression analysis
Brucella meningitis was used as the dependent variable (0= no, 1= yes), the significant factors (r>0.5/-0.5) in the correlation analysis were used as independent variables. The logistic regression analysis showed that high levels of C-Glu and C-CI were protective factors for the occurrence of Brucella meningitis, while a high C-LDH levels was risk factor for the occurrence of brucella meningitis (P<0.05), as shown in Table 3.
Table 3.
Logistic regression analysis of risk factors for brucellosis meningitis
| Independent variables | Beta. | SE | Waldχ | P | OR (95% CI) |
|---|---|---|---|---|---|
| C-Glu | 1.571 | 0.588 | 7.152 | 0.007 | 0.208 (0.066-0.657) |
| C-CI | 0.296 | 0.137 | 4.701 | 0.030 | 0.744 (0.569-0.972) |
| C-LDH | 0.085 | 0.032 | 4.811 | 0.007 | 1.089 (1.023-1.158) |
Notes: C-Glu: cerebrospinal fluid-glucose; C-CI: cerebrospinal fluid-chloride ion; C-LDH: cerebrospinal fluid-lactate dehydrogenase.
ROC analysis
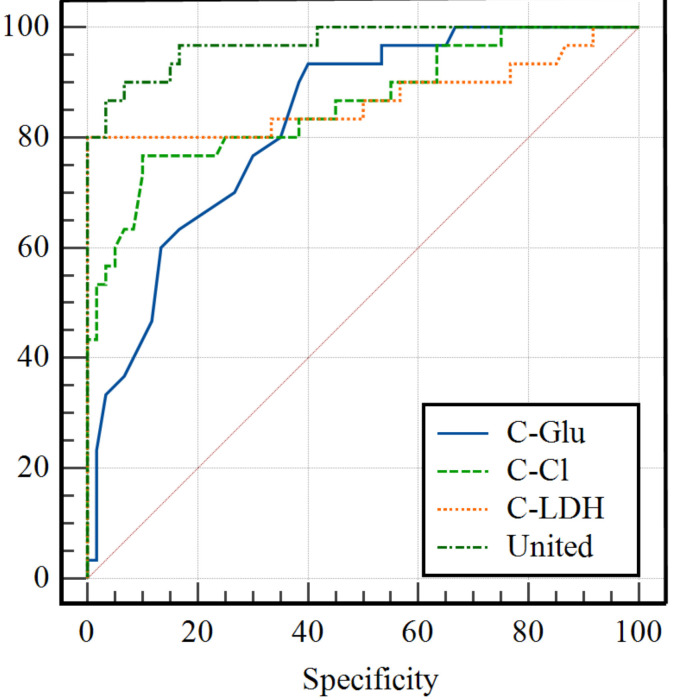
The AUCs of C-Glu, C-CI, and C-LDH for the diagnosis of brucellosis meningitis were 0.828, 0.860, and 0.869, respectively. Their corresponding sensitivities were 93.33%, 76.67%, and 80.00%, and specificities were 60.00%, 90.00%, and 100.00%. The optimal cut-off values were determined to be 3.2 mmol/L for C-Glu, 121.4 mmol/L for C-Cl, and 40.3 u/L for C-LDH. Furthermore, their combination resulted in an AUC value of 0.971, with a sensitivity of 90% and a specificity of 93%, in predicting brucellosis meningitis, which outperformed the AUCs of C-Glu, C-CI, and C-LDH alone (Z=3.376/2.810/2.406, P=0.007/0.005/0.016), see Figure 5 and Table 4.
Figure 5.
ROC analysis of C-Glu, C-CI, and C-LDH for diagnosing Brucella meningitis. Notes: C-Glu: cerebrospinal fluid-glucose; C-CI: cerebrospinal fluid-chloride ion; C-LDH: cerebrospinal fluid-lactate dehydrogenase.
Table 4.
The performance of C-Glu, C-CI, and C-LDH in diagnosing Brucellosis meningitis
| Indicators | AUC | 95% CI | Sensitivity (%) | Specificity (%) | Cut-off value | P |
|---|---|---|---|---|---|---|
| C-Glu | 0.828 | 0.734-0.899 | 93.33 | 60 | 3.2 | <0.001 |
| C-CI | 0.86 | 0.771-0.924 | 76.67 | 90 | 121.4 | <0.001 |
| C-LDH | 0.869 | 0.781-0.931 | 80 | 100 | 40.3 | <0.001 |
| United | 0.971 | 0.912-0.995 | 90 | 93.3 | - | <0.001 |
Notes: C-Glu: cerebrospinal fluid-glucose; C-CI: cerebrospinal fluid-chloride ion; C-LDH: cerebrospinal fluid-lactate dehydrogenase.
Discussion
Brucellosis is the most common zoonotic disease worldwide, mainly distributed in the Mediterranean, the Middle East, Africa, South America, and Asia [11]. In China, the disease is primarily concentrated in areas with developed farming and animal husbandry, including Shanxi, Heilongjiang, Xinjiang Uygur autonomous region, Inner Mongolia, Hebei, Liaoning and Jilin provinces [12]. Humans are infected through direct contact or ingestion of secretions, body fluids, carcasses, or contaminated meat and milk from infected animals. Infection can also occur through inhalation of bacteria-laden dust or bacteria entering the eye conjunctiva. Common clinical symptoms of brucellosis include chills and fever, fatigue, headache, muscle pain, and joint pain [13]. Due to the diversity and nonspecific nature of these symptoms, particularly in non-pastoral areas, the incidence of brucellosis is high, leading to frequent missed or delayed diagnoses. While most of them can be cured by regular treatment, delayed diagnosis and treatment can result in chronic infections, resulting in complex conditions and poor treatment effects [14].
The pathogenesis of neurobrucellosis is very complex, and its specific mechanism remain unclear. It is generally believed that Brucella enters the brain parenchyma through the blood-brain barrier and infects the central nervous system through the blood-borne dissemination [15]. The most important pathological process involves Brucella evading the immune system after infecting cells, leading to a chronic disease course. Macrophages are the main cells that Brucella invades to survive and replicate. The proliferation and replication of Brucella can impair the phagocytic function of macrophages, rendering them unable to kill pathogens or present antigens effectively. This evasion of the host’s immune defense allows Brucella to establish a persistent infection. Additionally, Brucella can inhibit macrophage apoptosis and the secretion of TNF-α, facilitating long-term survival within host cells and contributing to chronic persistent infection. Brucella evades immune clearance by escaping the actions of typical immune components such as neutrophils and NADPH oxidase, which may be the key to its ability to cause infection during the incubation period [16]. Beyond evasion and phagocytosis by innate immune cells, Brucella can inhibit MHC class I and MHC class II antigen-presenting cells (APCs), reducing the ability of T cells to recognize infections [17,18]. Moreover, Brucella affects the maturation of dendritic cells, reducing IL-12 secretion, which halts T lymphocyte activation and weakens the adaptive immune response, thereby establishing a chronic infection. A meta-analysis by Zheng et al. showed that the proportion of CD4+ T cells in the peripheral blood of patients with brucellosis decreased significantly, while the proportion of CD8+ T cells increased, leading to a marked reduction in the CD4+/CD8+ ratio [19]. However, in this study, we found that cerebrospinal fluid levels of CD3+, CD4+ and CD4+/CD8+ were negatively correlated with the occurrence of brucellosis meningitis, indicating that T lymphocyte immune dysfunction in patients with brucellosis meningitis may be primarily reflected at the level of cerebrospinal fluid, rather than in peripheral blood. Therefore, it is crucial to strengthen the detection of these indicators in cerebrospinal fluid of patients with brucellosis. Early identification of T lymphocyte immune dysfunction in the cerebrospinal fluid can aid in the preliminary diagnosis of brucellosis meningitis.
At the biochemical level, this study showed that high levels of C-Glu and C-CI were protective factors for Brucella meningitis, while high C-LDH level was a risk factor. The elevated C-Glu levels in the early stages of infection may be due to the increased energy demands of Brucella as it grows within the cerebrospinal fluid, or due to the heightened activity of immune cells combating the infection. However, as the disease progresses, C-Glu level gradually reduces, eventually falling below normal levels [20]. The inflammatory response triggered by brucellosis increases meningeal permeability, allowing water and electrolytes (including CI) to enter the meningeal space through the blood-cerebrospinal fluid barrier, thereby changing the composition of cerebrospinal fluid and reducing the content of CI [21]. The growth of Brucella and the resulting inflammatory edema in brain tissue, along with increased intracranial pressure, lead to reduced cerebral blood flow. This hypoxia-ischemia condition reinforces local glycolysis, enhancing lactic acid production and catalyzing en-zyme activity, thereby increasing C-LDH levels. Additionally, as granulocyte proliferate, lactic acid, which typically has limited ability to cross the blood-brain barrier, becomes more concentrated within the cerebrospinal fluid, largely independent of blood lactate levels [22].
ROC analysis showed that the AUCs of C-Glu, C-Cl and C-LDH in diagnosing Brucella meningitis were all above 0.8. However, their sensitivity and specificity were relatively low. Notably, when these three indicators were combined, the AUC value increased to an impressive 0.971, with both sensitivity and specificity exceeding 90%. This can be attributed to the fact that C-Glu levels are susceptible to fluctuations in blood sugar levels, potentially masking the decrease in glucose caused by bacterial reproduction in infectious cerebrospinal fluid, leading to falsely normal or elevated C-Glu values [23]. Furthermore, when the blood-brain barrier is compromised, systemic lactate levels can impact cerebrospinal fluid C-LDH levels, potentially resulting in a false positive outcome [24]. Previous studies have indicated that individual cerebrospinal fluid markers exhibit suboptimal sensitivity and specificity for diagnosing bacterial meningitis [25]. Therefore, it is advisable to regularly monitor the levels of C-Glu, C-Cl, and C-LDH in brucellosis patients and perform a combined assessment of the three indicators.
Several limitations exist in this study. Firstly, the cohort was drawn from a single center, potentially introducing selection bias. Secondly, the retrospective nature limited the inclusion parameters to those available in existing medical records. Furthermore, the small sample size restricted the number of significant single factors included in logistic regression analysis, potentially leading to unavoidable confounding bias due to correlations among parameters. Therefore, future research can focus on high-quality studies that explore the roles of C-Glu, C-Cl, and C-LDH in the pathogenesis of Brucella meningitis, providing a more robust scientific basis for effective prevention and treatment strategies.
In summary, the cerebrospinal fluid levels of CD3+, CD4+, CD4+/CD8+, and blood levels of CD4+ and CD4+/CD8+ are all correlated with the occurrence of brucellosis meningitis. C-Glu, C-Cl, C-LDH and their combination demonstrate significant potential for aiding in the auxiliary diagnosis of brucellosis meningitis. These findings offer valuable insights for medical professionals to identify high-risk groups for Brucella meningitis and can therefore be given careful consideration.
Acknowledgements
This study was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (Project Number: 2020D01C108).
Disclosure of conflict of interest
None.
References
- 1.Zhu Y, Shi L, Zeng Y, Piao D, Xie Y, Du J, Gao M, Gao W, Tian J, Yue J, Li M, Guo X, Yao Y, Kang Y. Key immunity characteristics of diverse stages of brucellosis in rural population from Inner Mongolia, China. Infect Dis Poverty. 2022;11:63. doi: 10.1186/s40249-022-00989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu ZG, Song ZY, Wang WX, Xi WN, Jin D, Ai MX, Wu YC, Lan Y, Song SF, Zhang GC, Yao XB, Gao Z, Liu CY, Sun K, Yu DS, Xie BG, Sun SL. Human brucellosis and fever of unknown origin. BMC Infect Dis. 2022;22:868. doi: 10.1186/s12879-022-07872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares CN, da Silva MTT, Lima MA. Neurobrucellosis. Curr Opin Infect Dis. 2023;36:192–197. doi: 10.1097/QCO.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 4.Soares CN, Angelim AIM, Brandão CO, Santos RQ, Mehta R, Silva MTTD. Neurobrucellosis: the great mimicker. Rev Soc Bras Med Trop. 2022;55:e05672021. doi: 10.1590/0037-8682-0567-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouferraa Y, Bou Zerdan M, Hamouche R, Azar E, Afif C, Jabbour R. Neurobrucellosis: brief review. Neurologist. 2021;26:248–252. doi: 10.1097/NRL.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 6.Maji S, Manjunath N, Bahubali VH, Shome R, Palaniappan M, Lahiri S, Ravikumar R, Parthasarathi S, Chandrashekar N. Neurobrucellosis: a neglected entity? An update from tertiary care neurocentre of South East Asia. J Neurol Sci. 2020;411:116683. doi: 10.1016/j.jns.2020.116683. [DOI] [PubMed] [Google Scholar]
- 7.Marques IB, Marto N, Raimundo A, Gil-Gouveia R. Myelitis and polyradiculoneuropathy with severe pain: unusual neurological manifestations as presenting symptoms of brucellosis. Neurologist. 2018;23:131–134. doi: 10.1097/NRL.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 8.Li W, He Y, Li Y, Li X, Bian T, Liu T, Liu X, Jiang W. Metagenomic next-generation sequencing for the diagnosis of neurobrucellosis. Future Microbiol. 2024;19:509–518. doi: 10.2217/fmb-2023-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haripriya S, Malhotra S, Gadepalli R, Tak V, Kumar B. Unveiling neurobrucellosis: a case report emphasizing early diagnosis for better outcomes. Access Microbiol. 2024;6:000705.v3. doi: 10.1099/acmi.0.000705.v3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guven T, Ugurlu K, Ergonul O, Celikbas AK, Gok SE, Comoglu S, Baykam N, Dokuzoguz B. Neurobrucellosis: clinical and diagnostic features. Clin Infect Dis. 2013;56:1407–12. doi: 10.1093/cid/cit072. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi KA, Parvez A, Fahmy NA, Abdel Hady BH, Kumar S, Ganguly A, Atiya A, Elhassan GO, Alfadly SO, Parkkila S, Aspatwar A. Brucellosis: epidemiology, pathogenesis, diagnosis and treatment-a comprehensive review. Ann Med. 2023;55:2295398. doi: 10.1080/07853890.2023.2295398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao Z, Chen Q, Chen Y, Li Y, Mu D, Yang H, Yin W. Epidemiological characteristics of human brucellosis - China, 2016-2019. China CDC Wkly. 2021;3:114–119. doi: 10.46234/ccdcw2021.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Jindan R. Scenario of pathogenesis and socioeconomic burden of human brucellosis in Saudi Arabia. Saudi J Biol Sci. 2021;28:272–279. doi: 10.1016/j.sjbs.2020.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosilkovski M, Siskova D, Spasovska K, Vidinic I, Dimzova M. The influence of illness duration before diagnosis on clinical characteristics and outcome in human brucellosis. Trop Doct. 2019;49:177–181. doi: 10.1177/0049475519846422. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez AM, Delpino MV, Miraglia MC, Giambartolomei GH. Immune mediators of pathology in neurobrucellosis: from blood to central nervous system. Neuroscience. 2019;410:264–273. doi: 10.1016/j.neuroscience.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Jiao H, Zhou Z, Li B, Xiao Y, Li M, Zeng H, Guo X, Gu G. The mechanism of facultative intracellular parasitism of Brucella. Int J Mol Sci. 2021;22:3673. doi: 10.3390/ijms22073673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Acioglu C, Heary RF, Elkabes S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav Immun. 2021;91:740–755. doi: 10.1016/j.bbi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrionuevo P, Giambartolomei GH. Inhibition of antigen presentation by Brucella: many more than many ways. Microbes Infect. 2019;21:136–142. doi: 10.1016/j.micinf.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Zheng R, Xie S, Niyazi S, Lu X, Sun L, Zhou Y, Zhang Y, Wang K. Meta-analysis of the changes of peripheral blood T cell subsets in patients with Brucellosis. J Immunol Res. 2018;2018:8439813. doi: 10.1155/2018/8439813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuang W, He T, Tuerheng J, He G, Wang BL, Yang YH, Zhang L, Dong XZ, Xi SY. Neurobrucellosis: laboratory features, clinical characteristics, antibiotic treatment, and clinical outcomes of 21 patients. BMC Infect Dis. 2024;24:485. doi: 10.1186/s12879-024-09308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Gao J, Liu J, Qi J, Zhang Q. Clinical efficacy of dexamethasone in the treatment of patients with tuberculous meningitis: a meta-analysis. Contrast Media Mol Imaging. 2022;2022:2180374. doi: 10.1155/2022/2180374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh L, Javali M, Mehta A, Pradeep R, Srinivasa R, Acharya PT. Study of cerebrospinal fluid levels of lactate, lactate dehydrogenase and adenosine deaminase in the diagnosis and outcome of acute meningitis. Neurol Res. 2022;44:463–467. doi: 10.1080/01616412.2021.2004366. [DOI] [PubMed] [Google Scholar]
- 23.Zheng G, Ji X, Yu X, Liu M, Huang J, Zhang L, Guo D, Zhang G. Development and verification of a discriminate algorithm for diagnosing post-neurosurgical bacterial meningitis-A multicenter observational study. J Clin Lab Anal. 2020;34:e23069. doi: 10.1002/jcla.23069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozano A, Franchi F, Seastres RJ, Oddo M, Lheureux O, Badenes R, Scolletta S, Vincent JL, Creteur J, Taccone FS. Glucose and lactate concentrations in cerebrospinal fluid after traumatic brain injury. J Neurosurg Anesthesiol. 2020;32:162–169. doi: 10.1097/ANA.0000000000000582. [DOI] [PubMed] [Google Scholar]
- 25.Montes K, Jenkinson H, Habib OB, Esquenazi Y, Hasbun R. Corrected white blood cell count, cell index, and validation of a clinical model for the diagnosis of health care-associated ventriculitis and meningitis in adults with intracranial hemorrhage. Clin Neurol Neurosurg. 2019;178:36–41. doi: 10.1016/j.clineuro.2019.01.012. [DOI] [PubMed] [Google Scholar]