Abstract
Objective: To evaluate the potential of sodium-glucose cotransporter 2 (SGLT2) inhibitors in preventing the progression of diabetic kidney disease and to provide guidance for clinical practice to improve renal health management strategies for diabetic patients. Methods: A retrospective analysis was conducted on 178 patients with diabetic kidney disease admitted to Baoji High Tech Hospital from March 2023 to March 2024. Of these, 88 patients who received early treatment with the SGLT2 inhibitor dapagliflozin were included in the early SGLT2-i group, while 90 patients receiving later treatment with SGLT2 inhibitor dapagliflozin were included in the late SGLT2-i group. Clinical data, overall effectiveness, adverse reactions, blood glucose, renal function, lipid levels, and inflammatory markers were compared between the two groups. Results: Prior to treatment, there were no differences in blood glucose indicators between the two groups (all P > 0.05). Following treatment, both groups showed reductions in 2-hour postprandial blood glucose (2hPG), fasting plasma glucose (FPG), and glycosylated hemoglobin (HbA1c), with the early SGLT2-i group demonstrating significantly lower values compared to the late SGLT2-i group (all P < 0.05). Similarly, there were no differences in renal function indicators between the two groups before treatment (all P > 0.05). However, following treatment, the early SGLT2-i group showed more noticeable improvements compared to the late SGLT2-i group (P < 0.05). Inflammatory markers and lipid levels followed similar patterns. The overall effectiveness of the early SGLT2-i group was higher than that of the late SGLT2-i group (92.05% vs. 78.89%, P < 0.05), while the incidence of adverse reactions did not differ statistically between the two groups (6.82% vs. 10.00%, P > 0.05). Conclusion: Early use of SGLT2 inhibitors in diabetic kidney disease patients effectively controls blood glucose and lipid levels, improves renal function, reduces inflammatory responses, and exhibits a low incidence of adverse reactions. This demonstrates high safety and an important role in delaying disease progression. Therefore, it is worth considering clinical promotion and use for this patient population.
Keywords: Diabetic kidney disease, renal function, glycated hemoglobin, sodium-glucose cotransporter 2 inhibitors, blood glucose
Introduction
Diabetes is a chronic systemic metabolic disease, usually caused by decreased insulin secretion or reduced sensitivity to insulin [1]. Diabetic kidney disease, a common complication of type 2 diabetes, is characterized by an insidious onset and slow progression. Approximately 40-50% of patients may progress to end-stage renal disease, significantly impacting their quality of life [2]. The disease can be divided into five stages, with stages I-III referred to as early-stage diabetic kidney disease. Timely and effective treatment during these stages can delay or even reverse kidney damage, making the treatment of patients in these stages a current research focus. Furthermore, the renal damage in diabetic kidney disease is characterized by chronic progressive injury with no significant early clinical symptoms, underscoring the importance of early treatment.
In recent years, the number of medications for treating diabetic kidney disease has increased, leading to evolving treatment regimens. Throughout the treatment process, it is essential to avoid impacting patients’ physical and mental health while ensuring treatment efficacy and medication safety. In addition to conventional health education, dietary and exercise guidance, blood pressure regulation, lipid control, and glucose management, clinicians have historically chosen renin-angiotensin-aldosterone system (RAAS) blockers for treatment. These agents modulate renal blood flow rhythms, ameliorate the pathological state of glomerular hyperperfusion, and exert a renal-protective effect by inhibiting mesangial cell proliferation, reducing interstitial fibrosis, decreasing basement membrane generation, and slowing kidney function deterioration [3]. RAAS blockade has been clinically confirmed as the standard treatment for diabetic kidney disease. For example, Ahmed OM et al. [4] have demonstrated that RAAS blockade effectively reduces the progression of kidney disease, delaying the onset and mortality rates of cardiovascular and renal complications in diabetic patients. Irbesartan, an RAAS blocker, plays a crucial role in the treatment of diabetic kidney disease. Papakitsou I et al. found that irbesartan positively impacts albuminuria and significantly reduces the progression of diabetic kidney disease [5]. However, RAAS blockade only improves glomerular hyperfiltration in patients with early-stage lesions and can cause severe side effects such as dry cough, hyperkalemia, and acute kidney injury. Therefore, there is a clinical imperative to identify drugs with better indications and tolerability for treating diabetic kidney disease.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors represent a newly discovered class of drugs with the dual potential to lower blood glucose levels and protect renal function in patients. These inhibitors primarily exert renal protection through the tubuloglomerular feedback mechanism. The SGLT2-mediated reabsorption of glucose and sodium demonstrates a synergistic effect, resulting in reduced sodium concentration in the macula densa and promoting glomerular hyperfiltration [6]. In this context, SGLT2 inhibitors achieve renal protection by inhibiting sodium-glucose cotransporters. Based on this theoretical foundation, SGLT2 inhibitors are considered suitable for the early treatment of diabetic kidney disease. Their early use is crucial in slowing the progression of diabetic kidney disease, reducing the incidence of end-stage renal disease, alleviating patient suffering, and ensuring a favorable prognosis. For instance, Kao et al. have suggested that SGLT2 inhibitors not only lower blood glucose levels but also reduce weight, blood pressure, and the risk of diabetic kidney disease [7]. Additionally, Fatima et al. have highlighted the renal protective effects of SGLT2 inhibitors, which are particularly important for type 2 diabetes patients at risk of diabetic kidney disease and chronic kidney disease [8].
In recent years, the effectiveness of SGLT2 inhibitors in managing diabetic kidney disease has been well-established. However, the optimal timing for the initiation of SGLT2 inhibitor therapy in diabetic kidney disease patients is still an area requiring further investigation. This study aims to bridge this gap in knowledge by focusing on the early use of SGLT2 inhibitors in diabetic kidney disease and its impact on disease progression and patient outcomes. By evaluating the potential benefits of early SGLT2 inhibitor intervention, this study aims to provide valuable insights into the timing of SGLT2 inhibitor administration in the management of diabetic kidney disease, thereby contributing to the optimization of clinical practice and treatment strategies for this patient population.
Materials and methods
General information
A total of 178 patients with diabetic kidney disease admitted to Baoji High Tech Hospital from March 2023 to March 2024 were retrospectively included in this study. Among them, 88 patients who received early treatment with the SGLT2 inhibitor dapagliflozin were assigned to the early SGLT2-i group, while 90 patients who received later treatment with the SGLT2 inhibitor dapagliflozin were assigned to the late SGLT2-i group. The treatment regimen was continued for 7 years in both groups. This study followed the relevant statements of the Helsinki Declaration and was approved by the Ethics Committee of Baoji High Tech Hospital. The ethics committee agreed to waive informed consent.
Inclusion criteria: (1) meeting the clinical diagnostic criteria for diabetic kidney disease [9]; (2) patients with type 2 diabetes mellitus; (3) complete clinical data and good compliance; (4) no recent use of other kidney-protective drugs; (5) no use of steroids or immunosuppressive drugs within 8 weeks prior to admission; (6) history of diabetes > 5 years; (7) no history of hypertension; (8) no occurrence of severe diseases such as myocardial infarction or cerebrovascular accidents in the 6 months prior to admission.
Exclusion criteria: (1) history of drug abuse or drug allergies; (2) presence of severe hypoglycemia or acute metabolic disorders in diabetes; (3) pregnancy, planning for pregnancy, or lactation; (4) secondary renal diseases caused by primary hypertension, coronary heart disease, or heart failure; (5) concomitant severe systemic diseases and poor tolerance; (6) incomplete data that could affect the validity of the study.
Methods
Patients in the two groups received routine health education (including an introduction to the pathogenesis, clinical manifestations, hazards, treatment methods, and precautions of the disease), dietary and exercise guidance (advised to follow a low-salt diet, consume high-quality protein, ensure adequate daily calorie intake, avoid overeating; suitable exercise methods based on the patient’s physical condition, avoiding excessive fatigue, gradually increasing exercise duration and intensity), and symptomatic treatment for blood pressure, lipid regulation, and blood sugar control (self-blood glucose monitoring guidance, active control of blood pressure and blood lipid levels within the normal range). Metformin (GuoYaoZhunZi H20023370, manufactured by Shanghai Sine Pharmaceutical Laboratories Co., Ltd.) was administered orally for glycemic control at a dose of 0.5 g twice daily.
The commonly used SGLT2 inhibitors include canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and tofogliflozin. In clinical practice, canagliflozin, empagliflozin, and dapagliflozin are frequently utilized for treatment. This study opted to use dapagliflozin as the SGLT2 inhibitor for both patient groups.
Early SGLT2-i group: In addition to the aforementioned interventions, the early SGLT2-i group received dapagliflozin (AstraZeneca Pharmaceuticals LP, National Medicine Permit HJ20170117, 5 mg) at an initial dose of 5 mg once daily, with the option to increase the dose to 10 mg once daily, based on changes in the patient’s condition.
Late SGLT2-i group: Patients initially received standard metformin (GuoYaoZhunZi H20023370, manufactured by Shanghai Sine Pharmaceutical Laboratories Co., Ltd.) treatment. If after one year they still failed to achieve personalized glycemic control targets, dapagliflozin (AstraZeneca Pharmaceuticals LP, National Medicine Permit HJ20170117, 5 mg) was then administered at the same dosage as in the early treatment group. Both groups continued the medication for 7 years.
Quality control
We ensured that the selected patient population was representative and matched the purpose and hypothesis of the study. We then developed a detailed data collection plan to ensure the completeness, accuracy, and reliability of collected data, including patients’ medical records, medication records, laboratory test data, and adverse reactions. Next, we established a rigorous data collection and recording system to ensure that all relevant data are accurately and completely recorded. This included patients’ clinical indicators, laboratory test results, and other pertinent data. In retrospective cohort studies, confounding factors may interfere with the results so it is necessary to identify and control potential confounding factors, such as patients’ age, gender, duration of disease, and disease staging, to ensure result accuracy. Appropriate statistical methods for data analysis ensure result accuracy and reliability.
Observational indicators
Blood Glucose, Lipids, and Inflammatory Indicators: To compare the blood glucose, blood lipids, and inflammatory indicators of the two groups before and after 1 year of medication, 10 ml of fasting venous blood was drawn from the patients on the day of examination and centrifuged (3000 rpm) for 10 minutes to separate the serum, which was then stored in the refrigerator for later testing. Fasting plasma glucose (FPG) and 2-hour postprandial blood glucose (2hPG) were determined using the glucose oxidase method, and glycosylated hemoglobin (HbA1c) was measured using the Bio-Rad VARIANT II Hemoglobin Testing System with high-performance liquid chromatography. FPG and 2hPG are common methods for blood glucose testing, used to understand fasting and postprandial blood glucose levels. HbA1c is an indicator of the average blood glucose level over the past 2-3 months, assessed by measuring the percentage of glycated hemoglobin in the blood [10]. The Beckman Coulter AU5800 automatic biochemical analyzer was used to measure triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C). These markers reflect the patient’s blood lipid status. The Bio-RAD Model 550 microplate reader was used to measure interleukin-6 (IL-6) (ab178013, Abcam, UK) and monocyte chemoattractant protein-1 (MCP-1) (ab179886, Abcam, UK) through enzyme-linked immunosorbent assay. IL-6 and MCP-1 are markers of the patient’s systemic inflammatory response [11,12].
Renal Function Indicators: To compare the renal function indicators of the two groups before and after 1 year of medication, 24-hour urine collection was conducted to quantitatively determine urine protein using the biuret method and calculate urinary microalbumin (UmALB) and urinary albumin excretion rate (UAER). Before treatment and at 1, 3, 5, and 7 years after treatment, creatinine (Cr) was measured using an automatic biochemical analyzer, and the urine albumin-to-creatinine ratio (UACR) was calculated. Quantitative determination of 24-hour urine protein was performed using the sulfosalicylic acid method. The simplified Modification of Diet in Renal Disease (MDRD) formula [186 × (Cr) - 1.154 × (age) - 0.203 × (0.742 if female)] was used to estimate the glomerular filtration rate (eGFR), where male gender equals 1 and female gender equals 0.742. UAER refers to the excretion rate of albumin in a 24-hour urine sample; 24-hour urine protein quantification refers to the determination of protein in a urine sample collected over 24 hours; UmALB refers to urinary microalbuminuria. eGFR is the estimated glomerular filtration rate, an important indicator for evaluating renal function; UACR is the ratio of albumin to creatinine in urine, a widely used indicator of renal function. Scr is serum creatinine. These markers reflect the patients’ renal function status [13].
Overall Efficacy: After 7 years of medication, the overall efficacy of each group was evaluated as follows: markedly effective - clinical symptoms substantially disappeared, and blood glucose, blood lipids, and renal function indicators returned to normal ranges with a reduction of over 50% in 24-hour urine protein quantitative excretion; effective - evident improvement in clinical symptoms compared to before treatment, tendency toward normal blood lipids and renal function indicators with slight abnormalities, and a reduction of less than 50% but more than 20% in 24-hour urine protein quantitative excretion compared to before treatment; ineffective - no improvement in clinical symptoms or even worsening of the condition, with no change or an increase in 24-hour urine protein quantitative excretion. The formula for calculating the overall efficacy rate is: (markedly effective + effective)/total number of cases × 100.00% [14].
Adverse Reactions: Adverse reactions experienced by patients in each group during the medication period, including hypoglycemia, hyperkalemia, and urinary tract infections, were recorded for statistical analysis.
Statistical analysis
In this study, SPSS 25.0 statistical software was used for data analysis. Descriptive data were presented as counts and percentages [n (%)], and the χ2 test was employed for comparisons. For continuous data, the Shapiro-Wilk test was used to assess the normality of the distribution. If the data were normally distributed, they were presented as mean ± standard deviation (mean ± SD), and the independent sample t-test was used for comparisons between the two groups, while the paired sample t-test was used for within-group comparisons. A significance level of P < 0.05 was considered statistically significant.
Results
Comparison of clinical data
The two groups were compared in terms of clinical data (gender, age, duration of disease, body mass index, and disease staging), and no statistically significant differences were found (all P > 0.05) (Table 1).
Table 1.
Comparison of clinical data
| Clinical Data | Gender | Age (years) | Duration of Disease (years) | Body Mass Index (kg/m2) | Disease Stage | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Male | Female | I | II | III | ||||
| Early SGLT2-i group (n = 88) | 38 | 50 | 66.48±3.19 | 6.89±0.87 | 23.20±1.12 | 33 | 31 | 24 |
| Late SGLT2-i group (n = 90) | 41 | 49 | 65.55±3.26 | 7.12±0.84 | 22.87±1.28 | 36 | 27 | 27 |
| χ2/Z | 0.102 | 1.94 | 1.757 | 1.823 | 0.560 | |||
| P | 0.750 | 0.054 | 0.081 | 0.070 | 0.756 | |||
SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
Comparison of blood glucose indicators
As shown in Table 2, the various blood glucose indicators of both groups before treatment were comparable (all P > 0.05). Following treatment, the 2hPG, FPG, and HbA1c levels in both groups decreased, particularly in patients with diabetic nephropathy treated with early SGLT2 inhibitors, as indicated by the lower 2hPG (7.24±2.21 mmol vs. 8.36±2.65 mmol, P = 0.003), lower FPG (5.58±1.76 mmol vs. 6.24±2.01 mmol, P = 0.021), and lower HbA1c (5.12±1.64% vs. 6.47±2.05%, P < 0.001) compared to before treatment.
Table 2.
Comparison of blood glucose indicators (x̅±sd)
| Group | 2hPG (mmol/L) | FPG (mmol/L) | HbA1c (%) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | |
| Early SGLT2-i group (n = 88) | 13.52±4.26 | 7.24±2.21* | 8.09±2.56 | 5.58±1.76* | 8.34±2.68 | 5.12±1.64* |
| Late SGLT2-i group (n = 90) | 13.81±4.02 | 8.36±2.65* | 8.57±2.75 | 6.24±2.01* | 8.69±2.79 | 6.47±2.05* |
| t | 0.467 | 3.059 | 1.205 | 2.329 | 0.853 | 4.845 |
| P | 0.641 | 0.003 | 0.230 | 0.021 | 0.395 | < 0.001 |
P < 0.05 compared to pre-treatment within the same group;
FPG, fasting plasma glucose; 2hPG, 2-hour postprandial glucose; HbA1c, glycated hemoglobin; SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
Comparison of renal function indicators
As shown in Table 3, the renal function indicators of both groups before treatment were comparable (all P > 0.05). After treatment, both groups showed improvement in UAER, 24-hour urine protein quantification, and UmALB compared to before treatment, particularly in patients with diabetic nephropathy treated with early SGLT2 inhibitors, as indicated by the lower UAER (89.35±9.24 µg/min vs. 97.42±10.03 µg/min, P < 0.001), lower 24-hour urine protein quantification (170.36±17.52 mg/24 h vs. 188.25±20.46 mg/24 h, P < 0.001) and lower UmALB (34.75±10.16 mg/L vs. 48.57±10.29 mg/L, P < 0.001) compared to before treatment.
Table 3.
Comparison of renal function indicators (x̅±sd)
| Group | UAER (µg/min) | 24 h Urinary Protein (mg/24 h) | UmALB (mg/L) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | |
| Early SGLT2-i group (n = 88) | 155.36±16.38 | 89.35±9.24* | 263.57±28.41 | 170.36±17.52* | 66.32±11.42 | 34.75±10.16* |
| Late SGLT2-i group (n = 90) | 155.81±16.20 | 97.42±10.03* | 265.14±28.03 | 188.25±20.46* | 66.58±11.72 | 48.57±10.29* |
| t | 0.184 | 5.580 | 0.371 | 6.260 | 0.147 | 9.023 |
| P | 0.854 | < 0.001 | 0.711 | < 0.001 | 0.883 | < 0.001 |
P < 0.05 compared to pre-treatment within the same group;
UAER, urinary albumin excretion rate; UmALB, uric micro-albuminiuria; SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
Comparison of long-term renal function indicators
As shown in Table 4, the renal function indicators of both groups before treatment were comparable. After treatment, both groups showed improvement in UACR, eGFR, and Scr compared to before treatment, particularly in patients with diabetic nephropathy treated with early SGLT2 inhibitors (all P < 0.001).
Table 4.
Comparison of long-term renal function indicators (x̅±sd)
| Parameter | Early SGLT2-i group (n = 88) | Late SGLT2-i group (n = 90) | t | P | |
|---|---|---|---|---|---|
| UACR (mg/g) | Before treatment | 161.25±12.36 | 160.78±12.81 | 0.252 | 0.801 |
| After 1 year treatment | 85.39±7.62* | 91.74±8.93* | 5.108 | < 0.001 | |
| After 3 years treatment | 72.46±7.52* | 82.41±8.12* | 8.480 | < 0.001 | |
| After 5 years treatment | 54.29±6.58* | 71.53±7.51* | 16.305 | < 0.001 | |
| After 7 years treatment | 41.87±7.23* | 60.25±7.16* | 17.047 | < 0.001 | |
| eGFR [ml/(min·1.73 m2)] | Before treatment | 73.25±8.38 | 73.69±8.02 | 0.359 | 0.720 |
| After 1 year treatment | 85.36±10.25* | 79.11±9.45* | 4.230 | < 0.001 | |
| After 3 years treatment | 96.14±10.36* | 88.52±9.47* | 5.120 | < 0.001 | |
| After 5 years treatment | 105.42±10.56* | 94.59±9.13* | 7.309 | < 0.001 | |
| After 7 years treatment | 113.38±10.51* | 101.19±9.43* | 8.139 | < 0.001 | |
| Scr (μmol/L) | Before treatment | 121.46±10.37 | 121.59±11.65 | 0.078 | 0.938 |
| After 1 year treatment | 109.12±10.14 | 118.75±10.19 | 6.323 | < 0.001 | |
| After 3 years treatment | 98.52±10.58 | 109.73±10.42 | 7.115 | < 0.001 | |
| After 5 years treatment | 84.53±9.54 | 97.16±9.13 | 9.017 | < 0.001 | |
| After 7 years treatment | 75.49±8.79 | 89.43±8.42 | 10.809 | < 0.001 | |
P < 0.05 compared to pre-treatment within the same group;
UACR, urine albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; Scr, serum creatinine; SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
Comparison of inflammatory markers
As shown in Table 5, the inflammatory markers of both groups before treatment were comparable (all P > 0.05). After treatment, both groups showed a decrease in IL-6 and MCP-1 compared to before treatment, particularly in patients with diabetic nephropathy treated with early SGLT2 inhibitors, as indicated by the lower IL-6 (11.07±2.59 pg/ml vs. 12.04±2.60 pg/ml, P < 0.001) and lower MCP-1 (51.28±6.49 pg/ml vs. 63.79±7.33 pg/ml, P < 0.001) levels.
Table 5.
Comparison of inflammatory markers (x̅±sd, pg/ml)
| Group | IL-6 | MCP-1 | ||
|---|---|---|---|---|
|
|
|
|||
| Before Treatment | After Treatment | Before Treatment | After Treatment | |
| Early SGLT2-i group (n = 88) | 19.68±3.25 | 11.07±2.59* | 142.36±15.75 | 51.28±6.49* |
| Late SGLT2-i group (n = 90) | 20.45±3.71 | 12.04±2.60* | 142.95±16.02 | 63.79±7.33* |
| t | 1.472 | 2.493 | 0.248 | 12.046 |
| P | 0.143 | 0.014 | 0.805 | < 0.001 |
P < 0.05 compared to pre-treatment within the same group;
IL-6, Interleukin-6; MCP-1, Monocyte Chemoattractant Protein-1; SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
Comparison of blood lipid levels
As depicted in Table 6, the blood lipid levels of both groups before treatment were comparable (all P > 0.05). Following treatment, both groups demonstrated improvement in TG, TC, LDL-C, and HDL-C compared to before treatment, particularly in patients with diabetic nephropathy treated with early SGLT2 inhibitors, as indicated by the lower TG (1.15±0.28 mmol/L vs. 1.43±0.37 mmol/L, P < 0.001), lower TC (3.27±1.06 mmol/L vs. 3.81±1.25 mmol/L, P = 0.002), lower LDL-C (1.63±0.45 mmol/L vs. 1.84±0.51 mmol/L, P = 0.004), and higher HDL-C (1.30±0.41 mmol/L vs. 1.14±0.37 mmol/L, P = 0.007).
Table 6.
Comparison of blood lipid levels (x̅±sd, mmol/L)
| Group | TG | TC | LDL-C | HDL-C | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | Before Treatment | After Treatment | |
| Early SGLT2-i group (n = 88) | 1.70±0.46 | 1.15±0.28* | 4.68±1.45 | 3.27±1.06* | 2.58±0.74 | 1.63±0.45* | 0.82±0.26 | 1.30±0.41* |
| Late SGLT2-i group (n = 90) | 1.68±0.44 | 1.43±0.37* | 4.42±1.37 | 3.81±1.25* | 2.66±0.80 | 1.84±0.51* | 0.79±0.23 | 1.14±0.37* |
| t | 0.296 | 5.684 | 1.230 | 3.105 | 0.692 | 2.911 | 0.816 | 2.735 |
| P | 0.767 | < 0.001 | 0.220 | 0.002 | 0.490 | 0.004 | 0.416 | 0.007 |
P < 0.05 compared to pre-treatment within the same group;
TG, Triglycerides; TC, Total Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol; HDL-C, High-Density Lipoprotein Cholesterol; SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
Comparison of overall effective rate
The overall effective rate was used to evaluate the effectiveness of drug therapy. The early SGLT2-i group demonstrated a higher overall effective rate of 92.05% (81 patients) compared to the late SGLT2-i group, which had an overall effective rate of 78.89% (71 patients) (χ2 = 6.335, P = 0.042). Specifically, within the early SGLT2-i group, 39 (44.32%) experienced a marked effect, 42 (47.73%) were deemed effective, and only 7 (7.95%) were categorized as ineffective. Conversely, in the late SGLT2-i group, 31 (34.44%) experienced a marked effect, 40 (44.44%) were effective, and 19 (21.11%) were deemed ineffective (Table 7).
Table 7.
Comparison of effective rates [n (%)]
| Group | Marked Effect (n, %) | Effective (n, %) | Ineffective (n, %) | Overall effective rate |
|---|---|---|---|---|
| Early SGLT2-i group (n = 88) | 39 (44.32) | 42 (47.73) | 7 (7.95) | 81 (92.05) |
| Late SGLT2-i group (n = 90) | 31 (34.44) | 40 (44.44) | 19 (21.11) | 71 (78.89) |
| χ2 | 6.335 | |||
| P | 0.042 |
SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
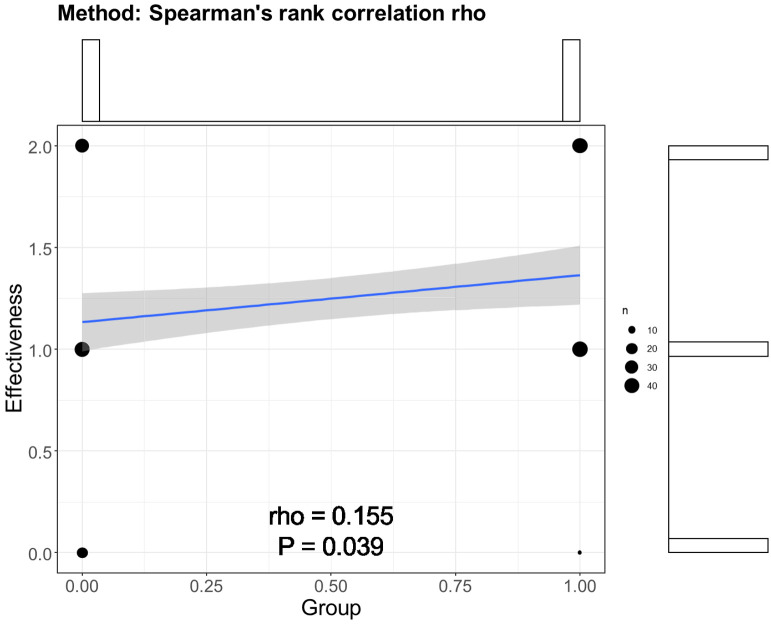
To determine the relation between early SGLT2-i intervention and overall effectiveness, Spearman correlation analysis was performed. The rho was 0.155 with a p-value of 0.039 (Figure 1). This result emphasized the potential clinical significance of early SGLT2-i intervention.
Figure 1.
Spearman correlation analysis between early SGLT2-i intervention and overall effectiveness. SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
Adverse reactions comparison
The incidence of adverse reactions is used to assess the safety of drug therapy. As indicated in Table 8, there was no statistically significant difference in the incidence of adverse reactions between the early SGLT2-i group and the late SGLT2-i group (6.82% vs. 10.00%, χ2 = 0.584, P = 0.445).
Table 8.
Comparison of adverse reactions [n (%)]
| Group | Hypoglycemia (n, %) | Hyperkalemia (n, %) | Urinary Tract Infection (n, %) | Incidence Rate |
|---|---|---|---|---|
| Early SGLT2-i group (n = 88) | 1 (1.14) | 1 (1.14) | 4 (4.55) | 6 (6.82) |
| Late SGLT2-i group (n = 90) | 2 (2.22) | 2 (2.22) | 5 (5.56) | 9 (10.00) |
| χ2 | 0 | 0 | 0 | 0.584 |
| P | 1 | 1 | 1 | 0.445 |
SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
Discussion
Diabetic nephropathy is a long-term complication of diabetes mellitus caused by hemodynamic changes and endocrine metabolic disorders in the kidneys due to chronic hyperglycemia. This condition leads to increased glomerular pressure, hyperfiltration, and hyperperfusion, resulting in thickening of the basement membrane, increased mesangial matrix, and progressive renal tissue changes that can lead to renal failure. Essentially, it represents a dynamic process of renal fibrosis, which includes interstitial fibrosis and glomerulosclerosis, marking irreversible damage to the kidneys [15]. As one of the main microvascular complications of diabetes, the incidence of diabetic nephropathy is increasing annually, and once it progresses to end-stage renal disease, its treatment becomes more challenging compared to other kidney diseases [16]. Therefore, it is evident that the early selection of appropriate treatment modalities plays a crucial role in reducing the progression of diabetic nephropathy.
Due to the frequent presence of renal impairment in diabetic nephropathy patients, it can directly impact the kidney’s clearance capacity for blood glucose and HbA1c. As renal function declines, the retention time of blood glucose and HbA1c in the body increases, leading to elevated levels. In an experimental study involving diabetic animal models, it was found that SGLT2 inhibitors reduced 2hPG, FPG, and HbA1c levels and improved glucose intolerance [17]. This conclusion is similar to the results of this study. In this study, after medication, both groups showed a decrease in 2hPG, FPG, and HbA1c levels compared to before medication, with the early SGLT2-i group exhibiting lower levels than the late SGLT2-i group, confirming the positive impact of SGLT2 inhibitors on reducing blood glucose levels.
Dapagliflozin belongs to the class of SGLT2 inhibitors, with its primary function being the inhibition of SGLT2 in the kidneys. SGLT2 is expressed in the proximal renal tubules and is primarily responsible for the reabsorption of glucose by the renal tubules [18]. When dapagliflozin inhibits the function of SGLT2, the reabsorption of glucose by the kidneys is reduced, leading to increased urinary glucose excretion, thus achieving a reduction in 2hPG and FPG. Additionally, the HbA1c level reflects the average blood glucose level over a period of time. By lowering blood glucose, dapagliflozin indirectly reduces the binding of glucose to hemoglobin, thereby lowering the HbA1c level [19].
The levels of UAER, 24-hour urinary protein quantification, UmALB, eGFR, UACR, and Scr are important indicators for evaluating renal function, and their abnormal levels are mainly associated with kidney damage caused by diabetes [20]. Prolonged hyperglycemia damages the glomerular basement membrane and mesangial cells, leading to microalbuminuria and subsequently raising UAER, UmALB, UACR, and Scr levels. Moreover, 24-hour urinary protein quantification reflects the patient’s total daily protein excretion. Typically, patients with diabetic nephropathy excrete large amounts of protein in the urine due to impaired glomerular filtration, resulting in abnormally elevated 24-hour urinary protein quantification levels. A decrease in eGFR levels indicates a decline in renal filtration function. Due to the associated renal microvascular disease and glomerulosclerosis in diabetic nephropathy patients, a reduction in glomerular filtration rate is likely, further contributing to the decline in eGFR levels [21].
In this study, treatment with dapagliflozin resulted in improvements in UAER, 24-hour urinary protein quantification, UmALB, eGFR, UACR, and Scr in both groups after medication, with the early SGLT2-i group showing more significant improvements compared to the late SGLT2-i group, suggesting a beneficial effect of dapagliflozin on renal function. Similar to the findings of Zhankui et al. [22], in a retrospective analysis of 176 cases of diabetic nephropathy patients, the observation group treated with dapagliflozin exhibited lower levels of UAER, UACR, and 24-hour urinary protein quantification than the control group. Dapagliflozin increases the transport of sodium ions to the macula densa, promotes afferent arteriolar vasoconstriction, reduces renal blood flow, improves the glomerular hyperfiltration state, restores tubuloglomerular feedback, lowers glomerular pressure, and consequently reduces proteinuria. Additionally, the drug can ameliorate renal hypoxia, inhibit oxidative stress reactions, and thus reduce inflammation-induced renal fibrosis. Furthermore, dapagliflozin can inhibit sodium-hydrogen exchange proteins in the kidneys, reduce endothelial damage, and glomerulosclerosis, ultimately improving renal function indicators.
Research has shown [23] that SGLT2 inhibitors have an anti-inflammatory effect, with inflammatory reactions playing a crucial role in the occurrence and progression of diabetic nephropathy. Through in-depth clinical analysis, it has been discovered that inhibiting inflammatory reactions has a renal protective effect on diabetic nephropathy patients. Ruscica M et al. [24] conducted a study on type 2 diabetes mice, revealing that SGLT2 inhibitors could reduce the levels of IL-6 and MCP-1. Additionally, Tinti et al. [25] demonstrated the mechanism of action of SGLT2 inhibitors in reducing diabetic renal inflammation, particularly dapagliflozin’s ability to lower the levels of inflammatory markers and weaken histological evidence of nephropathy, preventing the enhanced expression of inflammatory factors in an animal model. This study also found that after medication, both IL-6 and MCP-1 levels decreased in both groups, with the early SGLT2-i group showing a greater reduction compared to the late SGLT2-i group, further confirming the role of dapagliflozin in reducing inflammatory responses. IL-6 and MCP-1, as inflammatory factors, play a crucial role in the pathogenesis of diabetic nephropathy, participating in renal inflammatory response, fibrotic processes, and cell damage at multiple levels. This underscores the significant importance of reducing the levels of these inflammatory factors in improving the prognosis of diabetic nephropathy patients [26]. IL-6 is a cytokine belonging to the interleukin family, involved in regulating immune and inflammatory responses; MCP-1 is a monocyte chemotactic protein that plays a crucial role in inflammation and immune responses. Dapagliflozin exhibits a direct anti-inflammatory effect, reducing the infiltration of inflammatory cells in renal tissue, decreasing the release of inflammatory mediators, thereby lowering the levels of IL-6 and MCP-1, and alleviating renal inflammation.
Firstly, diabetes itself exerts a negative impact on lipid metabolism. Secondly, as a chronic complication of diabetes, diabetic nephropathy also affects lipid metabolism, and as the condition progresses, the decline in glomerular filtration rate and impaired tubular reabsorption function lead to reduced clearance of lipids in the body, ultimately triggering abnormal blood lipids [27,28]. In this study, treatment with SGLT2 inhibitors showed that after medication, both groups exhibited improvements in TG, TC, LDL-C, and HDL-C levels, with more significant improvements observed in the early SGLT2-i group, a conclusion similar to that of Shaheer et al. [29]. TG is an indicator in lipid tests, mainly responsible for providing energy for cellular metabolism; TC is an important item in blood lipids, representing the total cholesterol content of all lipoproteins in the serum; LDL-C is a type of lipoprotein in the blood, responsible for transporting cholesterol and other lipids to various parts of the body; HDL-C is a lipid component in human blood, with the function of transporting phospholipids and cholesterol. These four markers reflect the patient’s blood lipid status. Due to the ability of SGLT2 inhibitors to alleviate internal inflammatory and oxidative stress responses, both of which are closely associated with abnormal blood lipids, and as SGLT2 inhibitors can also reduce body weight and lower blood pressure, they have a positive impact on improving blood lipid levels. Additionally, weight loss can reduce the accumulation of adipose tissue, lowering lipid synthesis and release, while the reduction in blood pressure can decrease damage to the blood vessel wall, lowering the risk of atherosclerosis. Therefore, SGLT2 inhibitors (dapagliflozin) can improve the levels of TG, TC, LDL-C, and HDL-C in diabetic nephropathy patients through various mechanisms, including reducing inflammatory and oxidative stress responses, weight loss, and lowering blood pressure [30]. Bessho R et al. [31] stated that the earlier the use of SGLT2 inhibitors begins and the longer the treatment duration, the greater the therapeutic effect on diabetic nephropathy. Furthermore, Baviera M et al. [32] found that SGLT2 inhibitors can reduce the risk of major adverse cardiovascular events, all-cause mortality, and worsening of kidney disease. This study, based on conventional treatment, adopted SGLT2 inhibitors (dapagliflozin), with the results indicating that the total effective rate in the early SGLT2-i group was higher than that in the late SGLT2-i group, and the incidence of adverse reactions in both groups showed no statistically significant difference, indicating that the drug not only demonstrated significant efficacy but also had a high level of safety and a low incidence of adverse reactions.
This study addresses a critical knowledge gap regarding the timing of SGLT2 inhibitor use in diabetic kidney disease and adds to the existing body of evidence supporting the effectiveness of SGLT2 inhibitors in renal health management. By demonstrating the favorable effects of early SGLT2 inhibitor intervention on blood glucose control, renal function improvement, reduction in inflammatory responses, and minimal adverse effects, this study provides valuable insights into the optimal timing of SGLT2 inhibitor administration. The findings underscore the importance of considering early SGLT2 inhibitor use as a viable strategy for delaying the progression of diabetic kidney disease and improving patient outcomes. Further research and clinical initiatives focusing on the temporal aspects of SGLT2 inhibitor therapy in diabetic kidney disease are warranted to build upon the implications of this study and inform evidence-based treatment guidelines.
This study has certain limitations. Although the research spanned seven years - a relatively long period for the progression of chronic diseases - it may still not be sufficient to comprehensively assess the long-term impact of SGLT2 inhibitors on diabetic nephropathy progression. The study’s retrospective, observational nature limits its ability to directly establish a causal relationship between SGLT2 inhibitors and diabetic nephropathy progression. Future clinical studies should conduct prospective, randomized controlled trials to better control potential confounding factors and accurately assess treatment effects. Additionally, extending the study period and conducting multicenter, large-sample, long-term follow-up studies would be beneficial for more accurately evaluating the long-term impact of SGLT2 inhibitors on diabetic nephropathy progression.
In conclusion, for patients with diabetic nephropathy, early administration of SGLT2 inhibitors effectively controls blood glucose and lipid levels, improves renal function, reduces inflammatory responses, and exhibits minimal adverse effects, demonstrating high safety. These inhibitors play a crucial role in delaying disease progression and are worthy of clinical promotion and use.
Disclosure of conflict of interest
None.
References
- 1.Lee SH, Yoon KH. A century of progress in diabetes care with insulin: a history of innovations and foundation for the future. Diabetes Metab J. 2021;45:629–640. doi: 10.4093/dmj.2021.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yi X, Yan W, Guo T, Liu N, Wang Z, Shang J, Wei X, Cui X, Sun Y, Ren S, Chen L. Erythropoietin mitigates diabetic nephropathy by restoring PINK1/Parkin-mediated mitophagy. Front Pharmacol. 2022;13:883057. doi: 10.3389/fphar.2022.883057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell DSH. Combine and conquer: with type 2 diabetes polypharmacy is essential not only to achieve glycemic control but also to treat the comorbidities and stabilize or slow the advancement of diabetic nephropathy. J Diabetes Res. 2022;2022:7787732. doi: 10.1155/2022/7787732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed OM, Ali TM, Abdel Gaid MA, Elberry AA. Effects of enalapril and paricalcitol treatment on diabetic nephropathy and renal expressions of TNF-α, p53, caspase-3 and Bcl-2 in STZ-induced diabetic rats. PLoS One. 2019;14:e0214349. doi: 10.1371/journal.pone.0214349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papakitsou I, Vougiouklakis G, Elisaf MS, Filippatos TD. Differential pharmacology and clinical utility of dapagliflozin in type 2 diabetes. Clin Pharmacol. 2019;11:133–143. doi: 10.2147/CPAA.S172353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song P, Huang W, Onishi A, Patel R, Kim YC, van Ginkel C, Fu Y, Freeman B, Koepsell H, Thomson S, Liu R, Vallon V. Knockout of Na+-glucose cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase NOS1 in the macula densa and glomerular hyperfiltration. Am J Physiol Renal Physiol. 2019;317:F207–F217. doi: 10.1152/ajprenal.00120.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao TY, Wu HW, Lee SS, Liang PH, Guh JH, Hsu LC. Characterization of a fluorescent glucose derivative 1-NBDG and its application in the identification of natural SGLT1/2 inhibitors. J Food Drug Anal. 2021;29:521–532. doi: 10.38212/2224-6614.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatima A, Rasool S, Devi S, Talha M, Waqar F, Nasir M, Khan MR, Ibne Ali Jaffari SM, Haider A, Shah SU, Sapna F, Varrassi G, Khatri M, Kumar S, Mohamad T. Exploring the cardiovascular benefits of sodium-glucose cotransporter-2 (SGLT2) inhibitors: expanding horizons beyond diabetes management. Cureus. 2023;15:e46243. doi: 10.7759/cureus.46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liew A, Bavanandan S, Prasad N, Wong MG, Chang JM, Eiam-Ong S, Hao CM, Lim CY, Lim SK, Oh KH, Okada H, Susantitaphong P, Lydia A, Tran HTB, Villanueva R, Yeo SC, Tang SCW. Asian Pacific Society of Nephrology clinical practice guideline on diabetic kidney disease. Nephrology (Carlton) 2020;25(Suppl 2):12–45. doi: 10.1111/nep.13785. [DOI] [PubMed] [Google Scholar]
- 10.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, Metzger BE, Nathan DM, Kirkman MS. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2023;69:808–868. doi: 10.1093/clinchem/hvad080. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Anshita D, Ravichandiran V. MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101:107598. doi: 10.1016/j.intimp.2021.107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang S, Narazaki M, Metwally H, Kishimoto T. Historical overview of the interleukin-6 family cytokine. J Exp Med. 2020;217:e20190347. doi: 10.1084/jem.20190347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang G, Li S, Zhang C, Chen H, Wang N, Feng Y. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management. Acta Pharm Sin B. 2021;11:2749–2767. doi: 10.1016/j.apsb.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simes BC, MacGregor GG. Sodium-glucose cotransporter-2 (SGLT2) inhibitors: a clinician’s guide. Diabetes Metab Syndr Obes. 2019;12:2125–2136. doi: 10.2147/DMSO.S212003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naas S, Schiffer M, Schödel J. Hypoxia and renal fibrosis. Am J Physiol Cell Physiol. 2023;325:C999–C1016. doi: 10.1152/ajpcell.00201.2023. [DOI] [PubMed] [Google Scholar]
- 16.Guo H, Yan Z, Hu Y, Huang X, Pan C. Complement C7 is specifically expressed in mesangial cells and is a potential diagnostic biomarker for diabetic nephropathy and is regulated by miR-494-3p and miR-574-5p. Diabetes Metab Syndr Obes. 2021;14:3077–3088. doi: 10.2147/DMSO.S311725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sano R, Shinozaki Y, Ohta T. Sodium-glucose cotransporters: functional properties and pharmaceutical potential. J Diabetes Investig. 2020;11:770–782. doi: 10.1111/jdi.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan T, Liu S, Dong Y, Fu Y, Tang Y, Zhao W. Effects of dapagliflozin on serum and urinary uric acid levels in patients with type 2 diabetes: a prospective pilot trial. Diabetol Metab Syndr. 2020;12:92. doi: 10.1186/s13098-020-00600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suchy Y, Butner J, Wiebe DJ, Campbell M, Turner SL, Berg CA. Executive cognitive functions and behavioral control differentially predict HbA1c in type 1 diabetes across emerging adulthood. J Int Neuropsychol Soc. 2020;26:353–363. doi: 10.1017/S1355617719001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Pan J, Li B, Tian H, Zhu Y, Liao Z, Kou L, Tang C, Wang M, Ye G, Wang M. Positive correlation between cognitive impairment and renal microangiopathy in patients with type 2 diabetic nephropathy: a multicenter retrospective study. J Int Med Res. 2018;46:5040–5051. doi: 10.1177/0300060518789299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Liu Z. Mechanistic pathogenesis of endothelial dysfunction in diabetic nephropathy and retinopathy. Front Endocrinol (Lausanne) 2022;13:816400. doi: 10.3389/fendo.2022.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin ZJ, Wang GZ. Clinical efficacy of dapagliflozin in the treatment of patients with diabetic nephropathy and its effect on proteinuria level. Diabetes Metab Syndr Obes. 2023;16:2167–2175. doi: 10.2147/DMSO.S421579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Wang Y, Zhu C, Yang Y, Long C, Chen Q. Identification of Ribonuclease 6 as an immunoinflammatory key gene associated with the glomerular injury in diabetic nephropathy. Sci Rep. 2022;12:19709. doi: 10.1038/s41598-022-24289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruscica M, Corsini A, Ferri N, Banach M, Sirtori CR. Clinical approach to the inflammatory etiology of cardiovascular diseases. Pharmacol Res. 2020;159:104916. doi: 10.1016/j.phrs.2020.104916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinti F, Lai S, Noce A, Rotondi S, Marrone G, Mazzaferro S, Di Daniele N, Mitterhofer AP. Chronic kidney disease as a systemic inflammatory syndrome: update on mechanisms involved and potential treatment. Life (Basel) 2021;11:419. doi: 10.3390/life11050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Li L, Zhang Z, Chen P, Shu H, Yang C, Chu Y, Liu J. Ferroptosis: an important player in the inflammatory response in diabetic nephropathy. Front Immunol. 2023;14:1294317. doi: 10.3389/fimmu.2023.1294317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qudus B Aroyehun A, Abdul Razak S, Palaniveloo K, Nagappan T, Suraiza Nabila Rahmah N, Wee Jin G, Chellappan DK, Chellian J, Kunnath AP. Bioprospecting cultivated tropical green algae, Caulerpa racemosa (Forsskal) J. Agardh: a perspective on nutritional properties, antioxidative capacity and anti-diabetic potential. Foods. 2020;9:1313. doi: 10.3390/foods9091313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bankole T, Winn H, Li Y. Dietary impacts on gestational diabetes: connection between gut microbiome and epigenetic mechanisms. Nutrients. 2022;14:5269. doi: 10.3390/nu14245269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaheer A, Kumar A, Menon P, Jallo M, Basha S. Effect of add-on therapy of sodium-glucose cotransporter 2 inhibitors and dipeptidyl peptidase 4 inhibitors on adipokines in type 2 diabetes mellitus. J Clin Med Res. 2021;13:355–362. doi: 10.14740/jocmr4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17:761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 31.Bessho R, Takiyama Y, Takiyama T, Kitsunai H, Takeda Y, Sakagami H, Ota T. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep. 2019;9:14754. doi: 10.1038/s41598-019-51343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baviera M, Foresta A, Colacioppo P, Macaluso G, Roncaglioni MC, Tettamanti M, Fortino I, Genovese S, Caruso I, Giorgino F. Effectiveness and safety of GLP-1 receptor agonists versus SGLT-2 inhibitors in type 2 diabetes: an Italian cohort study. Cardiovasc Diabetol. 2022;21:162. doi: 10.1186/s12933-022-01572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]