Abstract
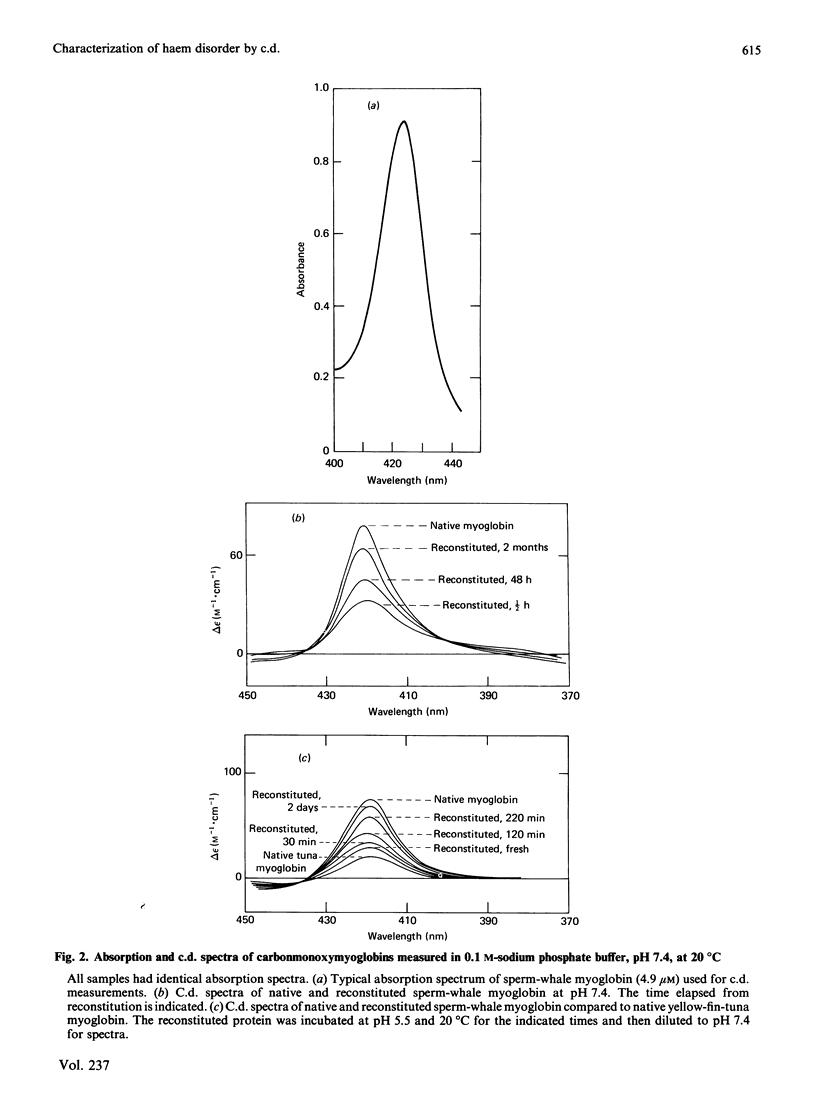
Native and reconstituted myoglobin were prepared and their c.d. spectra recorded in the Soret region. Time-dependent changes in dichroism following reconstitution were observed and related to haem orientational disorder. Comparative c.d. studies, in agreement with n.m.r. studies, reveal that the degree and nature of this disorder are species-dependent.
Full text
PDF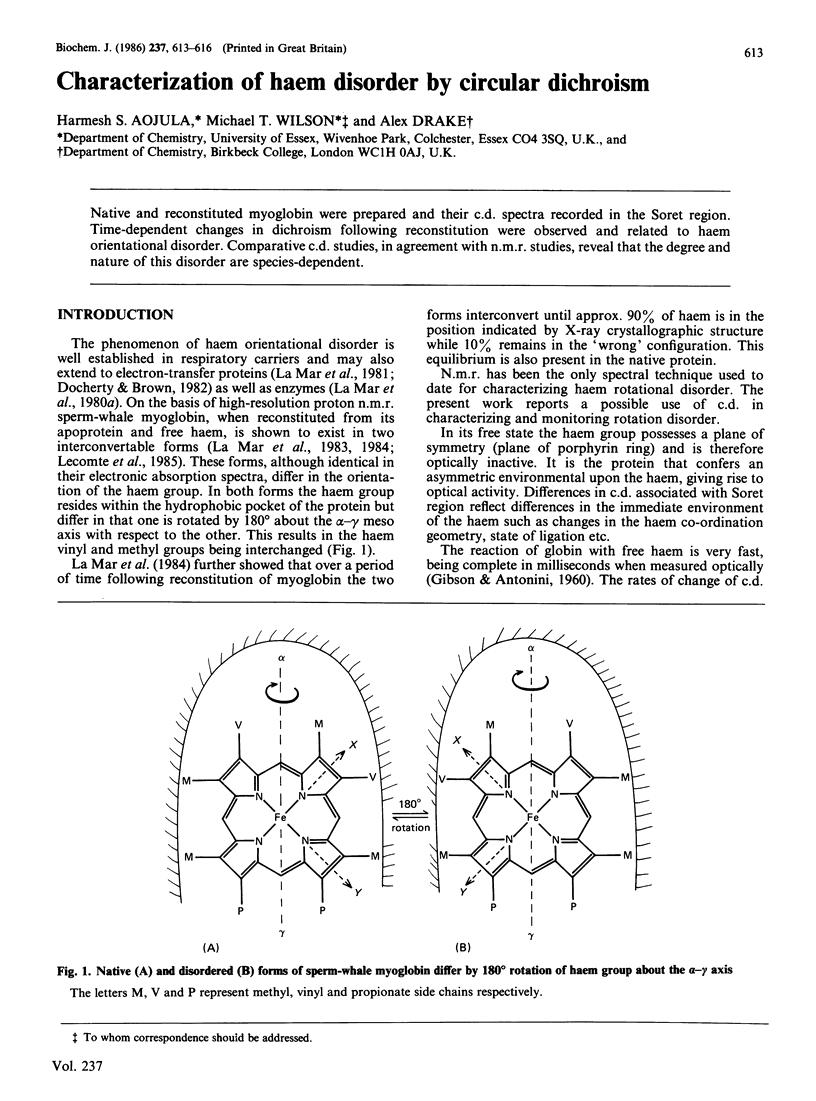


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad M. B., Kincaid J. R. Haem disorder in modified myoglobins. Effect of reconstitution procedures. Biochem J. 1983 Oct 1;215(1):117–122. doi: 10.1042/bj2150117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. C., Brown S. B. Haem disorder in reconstituted human haemoglobin. Biochem J. 1982 Dec 1;207(3):583–587. doi: 10.1042/bj2070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formanek H., Engel J. Optical rotatory dispersion of a respiratory hemeprotein of Chironomus thummi. Biochim Biophys Acta. 1968 Jun 26;160(2):151–158. doi: 10.1016/0005-2795(68)90081-0. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ANTONINI E. Kinetic studies on the reaction between native globin and haem derivatives. Biochem J. 1960 Nov;77:328–341. doi: 10.1042/bj0770328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. C., Woody R. W. The origin of the heme Cotton effects in myoglobin and hemoglobin. J Am Chem Soc. 1971 Jul 14;93(14):3515–3525. doi: 10.1021/ja00743a036. [DOI] [PubMed] [Google Scholar]
- Kawamura-Konishi Y., Suzuki H. Binding reaction of hemin to globin. J Biochem. 1985 Nov;98(5):1181–1190. doi: 10.1093/oxfordjournals.jbchem.a135384. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., Burns P. D., Jackson J. T., Smith K. M., Langry K. C., Strittmatter P. Proton magnetic resonance determination of the relative heme orientations in disordered native and reconstituted ferricytochrome b5. Assignment of heme resonances by deuterium labeling. J Biol Chem. 1981 Jun 25;256(12):6075–6079. [PubMed] [Google Scholar]
- La Mar G. N., Davis N. L., Parish D. W., Smith K. M. Heme orientational disorder in reconstituted and native sperm whale myoglobin. Proton nuclear magnetic resonance characterizations by heme methyl deuterium labeling in the Met-cyano protein. J Mol Biol. 1983 Aug 25;168(4):887–896. doi: 10.1016/s0022-2836(83)80080-1. [DOI] [PubMed] [Google Scholar]
- La Mar G. N., Smith K. M., Gersonde K., Sick H., Overkamp M. Proton nuclear nagnetic resonance characterization of heme disorder in monomeric insect hemoglobins. J Biol Chem. 1980 Jan 10;255(1):66–70. [PubMed] [Google Scholar]
- Lecomte J. T., Johnson R. D., La Mar G. N. Characterization of heme orientational disorder in myoglobin by proton nuclear Overhauser effects. Biochim Biophys Acta. 1985 Jun 10;829(2):268–274. doi: 10.1016/0167-4838(85)90197-9. [DOI] [PubMed] [Google Scholar]
- Levy M. J., La Mar G. N., Jue T., Smith K. M., Pandey R. K., Smith W. S., Livingston D. J., Brown W. D. Proton NMR study of yellowfin tuna myoglobin in whole muscle and solution. Evidence for functional metastable protein forms involving heme orientational disorder. J Biol Chem. 1985 Nov 5;260(25):13694–13698. [PubMed] [Google Scholar]
- Myer Y. P. Circular dichroism spectroscopy of hemoproteins. Methods Enzymol. 1978;54:249–284. doi: 10.1016/s0076-6879(78)54019-6. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Watts D. A., Brown W. D. Sequences of the soluble tryptic peptides from myoglobin of yellowfin tuna (Thunnus albacares). Comp Biochem Physiol B. 1979;62(4):481–487. doi: 10.1016/0305-0491(79)90121-4. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]


