Abstract
This study used meta-analysis to examine the role of baseline absolute lymphocyte count (ALC) in the prognosis of advanced breast cancer (ABC) or metastatic breast cancer (MBC). A comprehensive search encompassing PubMed, The Cochrane Library, Embase, and Web of Science databases was undertaken to identify and screen literature based on predefined inclusion and exclusion criteria. Progression-free survival (PFS), time to treatment failure (TTF), post-progression survival (PPS), and overall survival (OS) were selected as outcome measures. A meta-analysis of 14 studies, involving 2,540 patients and employing Review Manager 5.3 and Stata 14.0, was conducted. Notably, 12 of these studies originated from Japan. The findings indicated that patients with ABC or MBC exhibiting high ALC had significantly improved PFS, TTF, PPS (hazard ratio [HR] = 0.53, 95% confidence interval [CI]: 0.45-0.62, P < 0.00001; HR = 0.57, 95% CI: 0.51-0.64, P < 0.00001), and OS (HR = 0.44, 95% CI: 0.33-0.58, P < 0.00001; HR = 0.68, 95% CI: 0.60-0.77, P < 0.00001) juxtaposed with low ALC individuals. These findings were corroborated by both univariate and multivariate analyses. Furthermore, subgroup analysis based on breast cancer subtype unveiled that high ALC was associated with prolonged PFS (HR = 0.35, 95% CI: 0.21-0.56, P < 0.0001), TTF, and PPS (HR = 0.45, 95% CI: 0.29-0.71, P = 0.0006) in both human epidermal growth factor receptor 2 (HER-2)-positive and -negative ABC or MBC patients. Additionally, high ALC correlated with prolonged OS in all BC subtypes (HR = 0.73, 95% CI: 0.61-0.88, P = 0.0008) and HER-2-negative ABC or MBC patients (HR = 0.65, 95% CI: 0.55-0.78, P < 0.00001). Subgroup analysis was conducted on chemotherapy regimens, with and without eribulin. Despite variations in chemotherapy regimens, patients with ABC or MBC and high ALC exhibited longer PFS and PPS (HR = 0.45, 95% CI: 0.30-0.67, P < 0.0001), PFS and TTF (HR = 0.39, 95% CI: 0.20-0.78, P = 0.008), and OS (HR = 0.71, 95% CI: 0.62-0.82, P < 0.00001; HR = 0.5, 95% CI: 0.35-0.70, P < 0.0001). The results of this meta-analysis suggest that baseline ALC, as an immune marker, can serve as an effective prognostic indicator for ABC or MBC.
Keywords: Advanced breast cancer, metastatic breast cancer, absolute lymphocyte count, eribulin, meta-analysis
Introduction
According to recent global cancer burden data, breast cancer (BC) has now surpassed lung cancer as the most common malignancy worldwide [1,2]. Therefore, the development of early diagnostic techniques and effective treatments for BC is critical. Approximately 5%-10% of patients receive a diagnosis of advanced BC (ABC) or metastatic BC (MBC) at initial presentation [3], and 20%-30% of BC cases eventually develop metastasis or recurrence [4]. ABC, or MBC, is characterized by the proliferation of tumor cells from the original breast tissue to secondary sites such as the skin, lymph nodes, and vital organs, including the liver, lungs, or bones. Patients diagnosed with ABC or MBC generally face a poor prognosis and present challenges in terms of treatment options. Although recent advances in treatment have improved prognosis, the survival rates for these patients remain low, with 5-year and 10-year survival rates at 27% and 13%, respectively [5]. Several factors, including the BC subtype, presence of distant metastases, and treatment strategy, influence the prognosis of patients with ABC or MBC [6]. Treatment strategies and prognoses differ among patients due to heterogeneity in clinical characteristics and BC subtype. Despite a range of available treatment options, selecting the most appropriate strategy can be challenging, as patients with ABC or MBC might exhibit dissimilar responses to identical treatments. Thus, there is an urgent need to identify effective and easily accessible predictive markers to assess the prognosis of patients with ABC or MBC.
The immune system plays a crucial role in controlling malignant tumors, affecting both tumor progression and treatment response. An activated immune system may target and induce tumor cells death or, through inflammatory pathways, assist in creating a tumor microenvironment that supports tumor cell growth [7,8]. Tumeh et al. demonstrated that the immune system significantly influences the prognosis of malignant tumors [9]. Pre-treatment systemic immune markers, such as absolute lymphocyte count (ALC), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR), have been identified as reliable predictors of tumor prognosis. ALC, in particular, is an absolute count that may provide a more accurate reflection of disease status and prognosis compared to NLR and PLR [10-12]. Jimbo et al. reported an association between decreased ALC and the progression of MBC [12]. Lymphocytes, including T cells, B cells, and natural killer cells, are crucial immune cells involved in both humoral and cellular anti-tumor immune responses [13-15]. ALC reflects the proliferation capacity of lymphocytes and plays an essential role in regulating immunity, targeted killing of tumor cells, and inhibiting tumor cell proliferation [8,16].
Additionally, lymphocytes can migrate to tumor sites through humoral factors [17]. Both ALC and tumor-infiltrating lymphocytes (TILs) promote the antitumor immune response. Moreover, ALC influences the formation of TILs, thereby indirectly reflecting the immune microenvironment of tumor tissue [18]. Savas et al. reported that patients with BC exhibiting high levels of TILs had better prognosis [19]. Therefore, ALC may serve as a valuable immune marker for evaluating the prognosis of BC.
Recently, ALC has been recognized as an important immune marker for evaluating the prognosis of malignant tumors [20]. However, the clinical significance of baseline ALC in ABC or MBC has not been thoroughly investigated. This study aimed to categorize the immune status of ABC or MBC based on baseline ALC levels and determine the predictive value of these levels for survival outcomes through meta-analysis.
Materials and methods
Methods
This study adhered to the reporting specifications for meta-analysis of observational studies and followed the implementation steps outlined in the Cochrane guidelines [21,22].
Search strategy
A comprehensive search for relevant articles from four databases was carried out, namely PubMed (https://pubmed.ncbi.nlm.nih.gov/), The Cochrane Library (https://www.cochranelibrary.com/), Embase (www.embase.com/), and Web of Science (https://www.webofknowledge.com/), spanning from the inception of the databases to June 2023. The search strategy employed in PubMed was based on the following search string: ((Breast Neoplasms[Mesh]) OR (((((Breast Tumors[Title/Abstract]) OR (Mammary Cancers[Title/Abstract])) OR (Breast Carcinomas[Title/Abstract])) OR (Breast Malignant Neoplasms[Title/Abstract])) OR (Breast cancer[Title/Abstract]))) AND (Absolute lymphocyte count[Title/Abstract]). The retrieval approach combined subject words and free words. Simultaneously, a meticulous manual retrieval approach was employed to meticulously trace and analyze the references included in the selected studies. This meta-analysis was registered in INPLASY (INPLASY202470038).
Inclusion and exclusion criteria
The inclusion criteria for this study were as follows: (1) publication in the English language; (2) cohort studies; (3) focus on the correlation between baseline ALC and ABC or MBC; (4) provision of outcome indicators such as hazard ratio (HR) and 95% confidence interval (CI).
Exclusion criteria were as follows: (1) reviews, case reports, animal studies, conference abstracts, and other studies that did not meet the specified requirements; (2) studies where data extraction was not possible; (3) studies containing repeated data; (4) studies in which no specific cut-off point for baseline ALC threshold is mentioned.
Data extraction and quality of the included studies
Independent screening of titles and abstracts was carried out by two researchers in accordance with the aforementioned inclusion and exclusion criteria. Following initial screening, the full texts were re-evaluated, and results were cross-checked. Discrepancies were discussed and resolved collaboratively to decide on inclusion. If an article lacked complete information, the authors of the original studies were contacted to obtain missing data. The reasons for inclusion and exclusion at each step were documented throughout the screening process. Data collected included various variables such as the first author’s name, year of publication, country of publication, total patients sample size, BC subtype, chemotherapy regimen, ALC threshold, survival data, etc. In cases where individual articles did not report clear HR data, data extraction from survival curves was performed using Engauge Digitizers 4.1 software. Subsequently, HR and 95% CI values were calculated using Tierney tables [23].
Quality evaluation of the included studies
The quality of the included studies was assessed utilizing the Newcastle-Ottawa scale (NOS) [24]. The evaluation considered three aspects: selection of the research population, comparability between groups, and outcome measurement. The scale was divided into three levels: low risk, unclear, and high risk. A higher score reflects superior study quality. Scores ranging from 0 to 3 indicate low quality, scores ranging from 4 to 6 indicate moderate quality, and scores ranging from 7 to 9 indicate high quality.
Statistical methods
Data analysis was conducted employing the inverse variance method utilizing both Review Manager (version 5.3; Cochrane) and Stata (version 14.0; StataCorp LP). The prognostic correlation between baseline ALC and patients with ABC or MBC was determined by pooling HR and 95% CI values from the respective studies. The significance level for the outcome index was set at α = 0.05. Heterogeneity between studies was assessed utilizing Cochran’s Q test and the I2 test [25]. P > 0.10 or I2 < 50% indicated low heterogeneity among the studies, and the fixed-effect model was utilized for data analysis. In addition, P < 0.10 or I2 > 50% suggested significant heterogeneity among the studies, prompting the utilization of the random-effects model. Forest plots were employed to present the findings of the meta-analysis, and the stability of these findings was assessed via sensitivity analysis. Subgroup analysis was conducted to investigate factors affecting heterogeneity and to evaluate the impact of grouping factors on the results. Publication bias was assessed visually using funnel plots and formally evaluated through the Egger and Begg tests [26,27]. P ≤ 0.05 indicated publication bias.
Results
Screening process for the studies
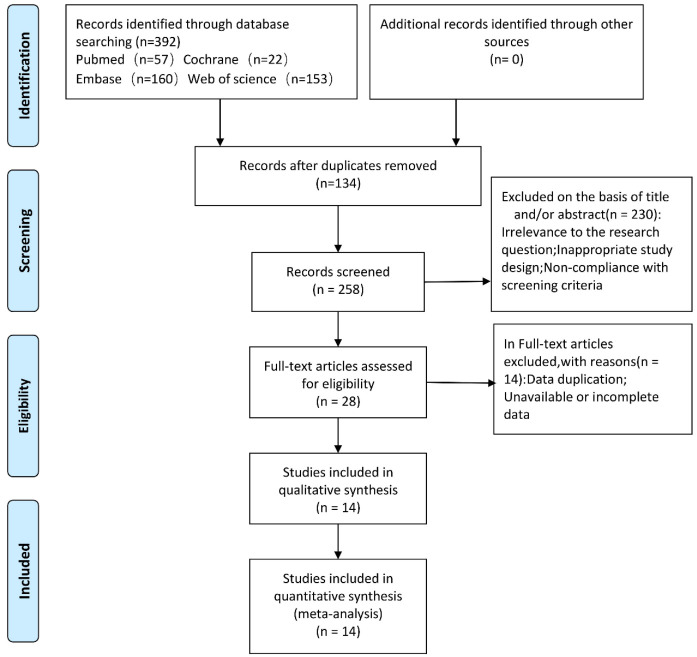
Following the retrieval strategy outlined in Methods section, 392 articles were initially identified. After removing literature that lacked data or failed to meet the data analysis requirements, 14 studies involving a total of 2,540 patients were included in the final analysis (Figure 1).
Figure 1.
PRISMA flow diagram of study selection.
Basic information about the studies
All studies were designed as retrospective cohort studies, with 12 conducted in Japan. The baseline ALC thresholds applied in the studies were 1500/µL, 1258/µL, and 1000/µL. Baseline ALC is determined using laboratory data obtained before the initiation of treatment, ensuring that patients have not yet been affected by myelotoxicity from treatments, particularly chemotherapy. Therefore, baseline ALC may provide a more accurate representation of the immune status of the patient (Table 1).
Table 1.
Characteristics of the included studies
| First author | Country | Sample size | Type of tumor | Threshold | Diagnosis | Treatment | Outcome | NOS |
|---|---|---|---|---|---|---|---|---|
| Araki_1 2018 [10] | Japan | 51 | HER-2+ | 1000/µL | ABC | ERI + PT/Nab-PTX + PT | PFS | 7 |
| Araki_2 2018 [10] | Japan | 51 | HER-2+ | 1500/µL | ABC | ERI + PT/Nab-PTX + PT | PFS | 7 |
| Che 2019 [48] | China | 68 | HER-2+ | 1000/µL | MBC | T + Chemotherapy | PFS and OS | 8 |
| EMILE 2021 [49] | France | 114 | HER-2- | 1500/µL | MBC | CDK4/6i + ET | PFS and OS | 7 |
| Goto 2022 [33] | Japan | 97 | tBC | 1500/µL | MBC | ERI | OS | 8 |
| Koyama 2021 [28] | Japan | 120 | HER-2- | 1258/µL | MBC | ERI | PFS and OS | 7 |
| Miyagawa 2020 [50] | Japan | 179 | tBC | 1500/µL | MBC | BP | PFS and OS | 7 |
| Miyoshi 2020 [47] | Japan | 500 | tBC | 1500/µL | MBC | ERI/TPC | OS | 7 |
| Morisaki 2021 [51] | Japan | 88 | tBC | 1500/µL | MBC | ERI | OS | 7 |
| Nakamoto_1 2021 [34] | Japan | 114 | HER-2- | 1500/µL | ABC | BP | TTF and OS | 7 |
| Nakamoto_2 2021 [52] | Japan | 94 | HER-2- | 1500/µL | ABC | ERI | PPS and OS | 7 |
| Sata 2020 [35] | Japan | 243 | tBC | 1500/µL | MBC | ERI | PFS and OS | 7 |
| Sawa 2022 [36] | Japan | 74 | tBC | 1500/µL | MBC | Anthracyclines/Taxanes or 5-FU/HER2-targeted therapy/Others | OS | 6 |
| Takahashi 2021 [53] | Japan | 565 | HER-2- | 1500/µL | ABC and MBC | ERI | OS | 6 |
| Ueno 2020 [54] | Japan | 125 | tBC | 1500/µL | MBC | ERI | PFS and OS | 6 |
| Watanabe_1 2020 [44] | Japan | 108 | HER-2- | 1500/µL | ABC | ERI | OS | 8 |
| Watanabe_2 2020 [44] | Japan | 108 | HER-2- | 1000/µL | ABC | ERI | OS | 8 |
HER-2, human epidermal growth factor receptor 2; tBC, total breast cancer; ABC, advanced breast cancer; MBC, metastatic breast cancer; ERI, eribulin; PT, pertuzumab and trastuzumab; Nab-PTX, nab-paclitaxel; CDK4/6i, cyclin-dependent-kinase 4-6 inhibitors; ET, endocrine therapy; TPC, treatment of physician’s choice; BP, bevacizumab and paclitaxel; PFS, progression-free survival; OS, overall survival; TTF, time to treatment failure; PPS, post-progression survival. tBC was divided into two subtypes: (1) (HER-2)-positive BC and (2) (HER-2)-negative BC.
Results of the meta-analyses
Following data extraction from the literature, four outcome indicators, namely, baseline ALC level and progression-free survival (PFS), time to treatment failure (TTF), post-progression survival (PPS), and overall survival (OS), were selected for analysis. Univariate and multivariate analyses were conducted to examine the relationships among these indicators.
Univariate analysis of PFS, TTF, and PPS
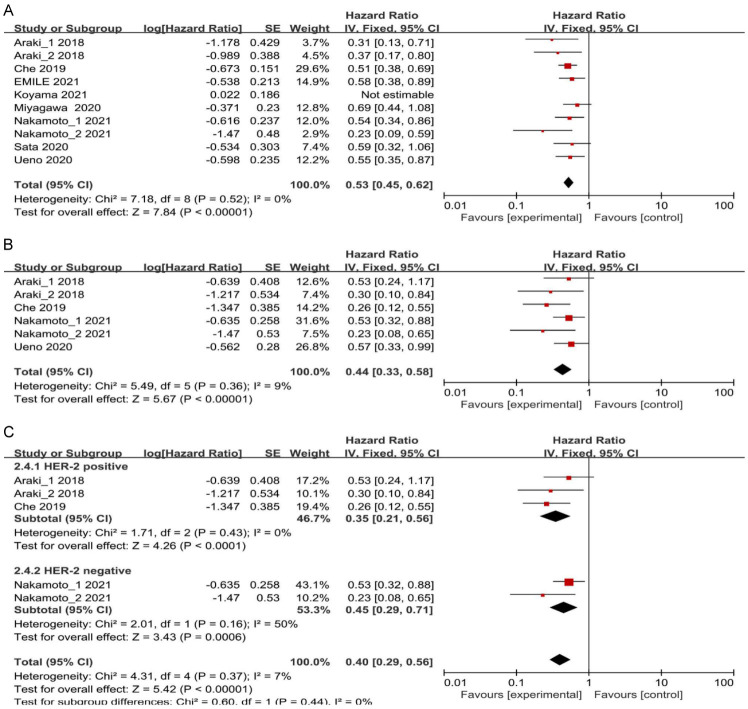
In total, nine studies reported the univariate analysis results of PFS, TTF, and PPS. These data were pooled and analyzed without considering confounding factors. Strong heterogeneity among the studies was revealed (I2 = 50%; P = 0.04); therefore, a random-effects model was utilized to combine the results. Alternatively, the exploration of the underlying causes of heterogeneity could be further explored. Sensitivity analysis was conducted on the results of the selected random-effects model, using a method of sequentially excluding studies to determine their impact on heterogeneity. It was found that the study by Koyama 2021 [28] had a significant influence on the heterogeneity. After excluding this study, heterogeneity was significantly reduced (I2 = 0%; P = 0.52). A fixed-effects model was then used to combine effect sizes, demonstrating that patients with high ALC had longer PFS, TTF, and PPS compared to those with low ALC (HR = 0.53, 95% CI: 0.45-0.62, P < 0.00001) (Figure 2A).
Figure 2.
A. Forest plots for the fixed-effect meta-analysis of the association between high ALC and PFS, TTF, and PPS by univariate analysis. B. Forest plots for the fixed-effect meta-analysis of the association between high ALC and PFS, TTF, and PPS by multivariate analysis. C. Forest plots for the fixed-effect meta-analysis of the association between high ALC and PFS, TTF, and PPS by BC subtype. HR, hazard ratio; CI, confidence interval; ALC, absolute lymphocyte count; PFS, progression-free survival; TTF, time to treatment failure; PPS, post-progression survival; BC, breast cancer.
Multivariate and subgroup analyses of PFS, TTF, and PPS
A total of five studies were conducted with multivariate analysis on PFS, TTF, and PPS results, taken into consideration various confounding factors. A heterogeneity test revealed no significant heterogeneity between the studies (I2 = 9%; P = 0.36). Therefore, a fixed-effect model was utilized to combine all the effect sizes. These findings indicated that patients with ABC or MBC with high ALC had longer PFS, TTF, and PPS (HR = 0.44, 95% CI: 0.33-0.58, P < 0.00001) (Figure 2B). Sensitivity analysis was carried out by individually eliminating studies. The results showed that the combined effect size remained stable even when studies were arbitrarily eliminated, indicating result robustness.
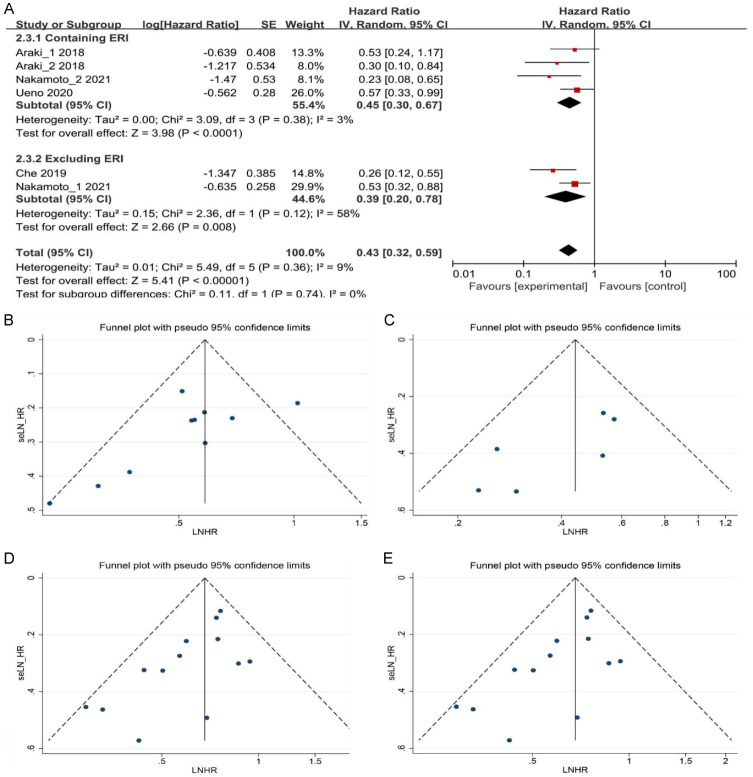
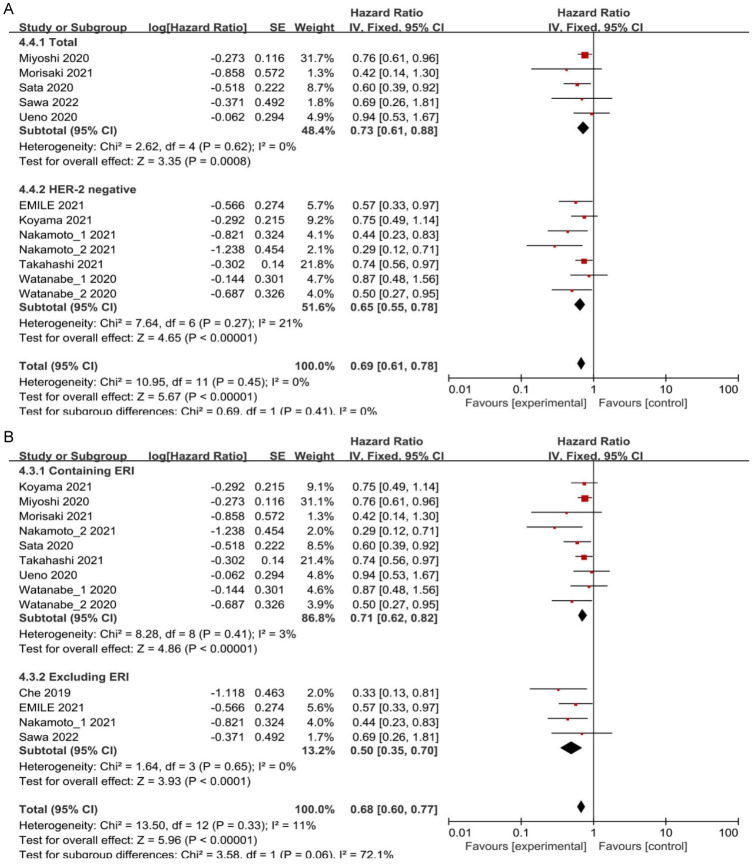
Further subgroup analysis was performed based on the BC subtype. BC classification was based on the expression levels of human epidermal growth factor receptor 2 (HER-2), distinguishing between HER-2-positive and HER-2-negative subtypes. In patients with HER-2-positive BC, results from the three studies demonstrated that patients with ABC or MBC with high ALC experienced prolonged PFS (HR = 0.35, 95% CI: 0.21-0.56, P < 0.0001) compared to those with low ALC (Figure 2C). In patients with HER-2-negative BC, the two studies demonstrated that individuals with high ALC exhibited longer TTF and PPS (HR = 0.45, 95% CI: 0.29-0.71, P = 0.0006) compared to those with low ALC (Figure 2C). Subgroup analysis was also conducted based on chemotherapy regimens. In patients treated with eribulin (ERI), it is clear from the three studies that patients with ABC or MBC who had high ALC experienced longer PFS and PPS (HR = 0.45, 95% CI: 0.30-0.67, P < 0.0001) as opposed to those with low ALC (Figure 3A). Among patients not treated with ERI in the two studies, individuals with high ALC exhibited longer PFS and TTF (HR = 0.39, 95% CI: 0.20-0.78, P = 0.008) compared to those with low ALC (Figure 3A).
Figure 3.
A. Forest plots for the fixed-effect meta-analysis of the association between high ALC and PFS, TTF, and PPS by different chemotherapy regimens. B and C. Funnel plot for publication bias in the association between high ALC and PFS, TTF, and PPS by univariate and multivariate analyses. D and E. Funnel plot for publication bias in the association between high ALC and OS by univariate and multivariate analyses. Each study is represented by one circle. A vertical line represents the pooled effect estimate. HR, hazard ratio; CI, confidence interval; ALC, absolute lymphocyte count; PFS, progression-free survival; TTF, time to treatment failure; PPS, post-progression survival; OS, overall survival; LNHR, Logarithm of the Negative Hazard Ratio; seLN_HR, Standard Error of the Logarithm of the Negative Hazard Ratio.
Univariate analysis of OS
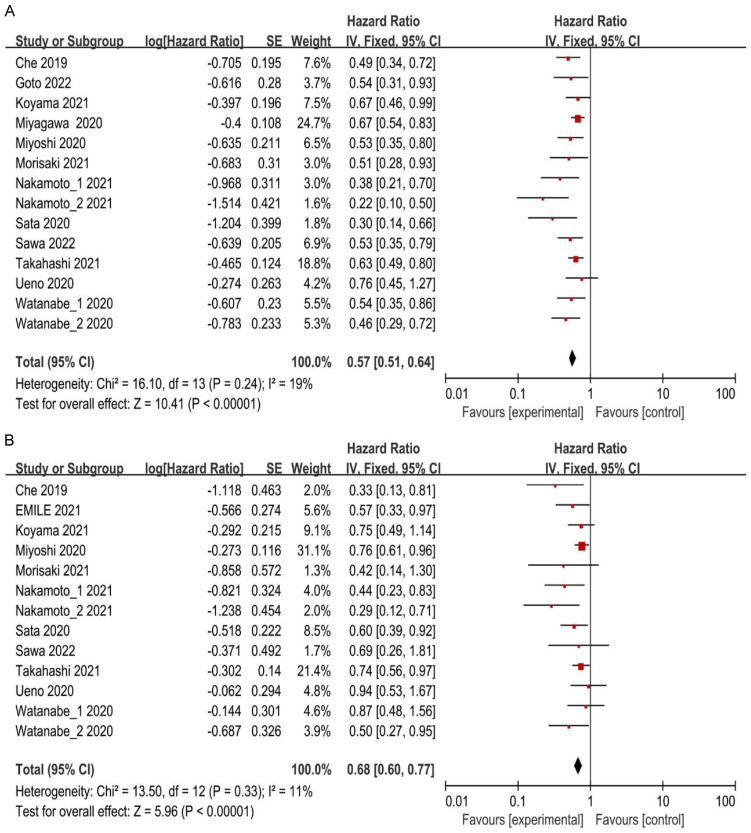
Thirteen studies conducted a univariate analysis of OS and performed a pooled analysis without considering confounding factors. No considerable heterogeneity was found among the studies (I2 = 19%; P = 0.24), allowing for the use of a fixed-effects model to combine the effect sizes. The findings indicated that patients with ABC or MBC with high ALC exhibited prolonged OS (HR = 0.57, 95% CI: 0.51-0.64, P < 0.00001) relative to those with low ALC (Figure 4A). Sensitivity analysis was conducted by systematically eliminating studies; the combined effect size remained unchanged, demonstrating result robustness.
Figure 4.
A. Forest plots for the fixed-effect meta-analysis of the association between high ALC and OS by univariate analysis. B. Forest plots for the fixed-effect meta-analysis of the association between high ALC and OS by multivariate analysis. HR, hazard ratio; CI, confidence interval; ALC, absolute lymphocyte count; OS, overall survival.
Multivariate and subgroup analyses of OS
A total of 12 studies conducted a multivariate analysis of OS, adjusting for various confounding factors prior to data pooling. The heterogeneity test (I2 = 11%; P = 0.33) revealed no considerable heterogeneity among the studies. Therefore, the fixed-effect model was utilized to combine the effect size. The analysis revealed that patients with high ALC had longer OS (HR = 0.68, 95% CI: 0.60-0.77, P < 0.00001) (Figure 4B). Sensitivity analysis was carried out by systematic exclusion of studies. The pooled effect size consistently remained stable throughout study elimination, indicating the robustness of the analysis. Five studies demonstrated longer OS for individuals with ABC or MBC with high ALC by subgroup analysis considering all BC subtypes (HR = 0.73, 95% CI: 0.61-0.88, P = 0.0008) (Figure 5A). For patients with HER-2-negative BC, six studies showed longer OS for individuals with ABC or MBC with high ALC (HR = 0.65, 95% CI: 0.55-0.78, P < 0.00001) (Figure 5A). Subgroup analysis was conducted based on chemotherapy regimens. Findings from the eight studies indicated that individuals with ABC or MBC who exhibited high ALC and were treated with ERI had experienced prolonged OS (HR = 0.71, 95% CI: 0.62-0.82, P < 0.00001) (Figure 5B). Similarly, in the four studies, individuals who did not receive ERI, exhibited correlation of high ALC to prolonged OS (HR = 0.5, 95% CI: 0.35-0.70, P < 0.0001) (Figure 5B).
Figure 5.
A. Forest plots from the fixed-effect meta-analysis of the association between high ALC and OS in different subtypes of BC. B. Forest plots from the fixed-effect meta-analysis of the association between high ALC and OS by different chemotherapy regimens. HR, hazard ratio; CI, confidence interval, ALC, absolute lymphocyte count; OS, overall survival; BC, breast cancer.
Quality of the studies included for analysis
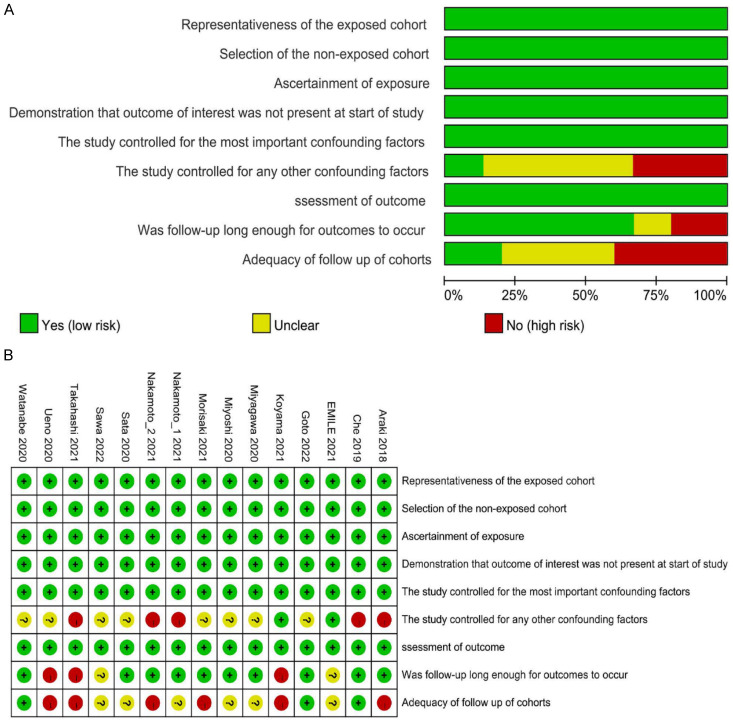
The NOS scores chart indicated that the overall quality of the studies was satisfactory (Figure 6A and 6B). Scores for the included research ranged from 6 to 8. The scores of the included studies are illustrated in Table 1.
Figure 6.
A. Risk of bias of the included studies. B. Risk of bias summary.
Publication bias
In the case of PFS, TTF, and PPS, the funnel plots exhibited visual symmetry in both univariate and multivariate analyses (Figure 3B and 3C). Additionally, the P-values obtained from Begg’s and Egger’s tests were 0.032 and 0.452, and 0.091 and 0.069, respectively. These results collectively indicate the absence of significant publication bias. However, for OS, funnel plot symmetry was not satisfactory in both univariate and multivariate analyses (Figure 3D and 3E). P-values from Begg’s and Egger’s tests were 0.012 and 0.024, and 0.002 and 0.018, respectively, suggesting publication bias.
Discussion
Declining immune function is closely linked to tumor immune escape, leading to recurrence and metastasis of malignant tumors [29]. Assessing immune status through ALC constitutes an important and convenient method. Low ALC is associated with an overall suppression of the immune system in cancer patients and often linked to poor prognosis [30]. Reduction in ALC has been identified as an adverse prognostic factor in various cancer types [30-32]. ALC is related to the prognosis of ABC or MBC. Some studies have already demonstrated the potential of ALC as an immune marker for patients with ABC or MBC [33-36]. However, the specific mechanisms by which ALC in patients with ABC or MBC influences tumor cell growth, angiogenesis, and distant metastasis through the secretion of cytokines and chemokines remain under investigation [37]. As the studies analyzed here are individual clinical studies, we conducted a systematic review and meta-analysis using rigorous inclusion and exclusion criteria to compile relevant literature and analyze data related to HR to assess the prognostic impact of baseline ALC in patients with ABC or MBC. The included studies, all published since 2018, highlight recent interests in utilizing baseline ALC as a potential prognostic marker for patients with ABC or MBC. Due to limitations in the number and content of the studies, the short-term outcome indicators selected included PFS, TTF, and PPS. Patients with ABC or MBC with high ALC had longer univariate PFS, TTF, and PPS than those with low ALC (HR = 0.53, 95% CI: 0.45-0.62). Consistent results were obtained from multivariate analysis after confounding factors were excluded (HR = 0.44, 95% CI: 0.33-0.58). Moreover, OS was considered a long-term outcome indicator. Risks of death in patients with ABC or MBC with high ALC appeared lower. The results of both univariate and multivariate analyses were consistent (HR = 0.57, 95% CI: 0.51-0.64; HR = 0.68, 95% CI: 0.60-0.77), and meta-analysis found strong associations between baseline ALC and the prognosis of individuals with ABC or MBC. Despite such findings, it is important to note that not all of the results reached statistical significance.
Subgroup analysis was conducted to investigate the effect of BC subtypes and chemotherapy regimens on the prognosis of patients with ABC or MBC. HER-2, a member of the epidermal growth factor receptor family, plays a crucial role in cell differentiation, proliferation, and survival. Approximately 20%-30% of BC cases exhibit HER-2 overexpression, which is associated with aggressive tumors and poor prognosis [38,39]. Subgroup analysis revealed that high ALC significantly correlated with improved survival in patients with ABC or MBC, regardless of tumor subtype (P < 0.05). ALC serves as an indirect indicator of the tumor immune microenvironment and has potential predictive value for drug sensitivity. Chemotherapy remains an important treatment option for patients with ABC or MBC, with the selection of chemotherapy regimen requiring the consideration of multiple factors such as BC subtype, metastasis site, and prior adjuvant therapy [40]. Some chemotherapeutic drugs can induce immunogenic death of tumor cells or activate immune effector mechanisms, leading to tumor cell-specific apoptosis [41,42]. ERI, a tubulin polymerization inhibitor, has been approved for use in patients with ABC or MBC after failure of anthracycline and taxane therapy [43,44]. ERI enhances antitumor immune responses by reducing the expression of programmed death-death-ligand 1 and forkhead box P3, and by increasing the expression of cluster of differentiation 8 [45]. In this meta-analysis, ten articles examined the use of single-drug or combined ERI chemotherapy regimens. In this meta-analysis, ten articles examined the use of single-drug or combined ERI chemotherapy regimens, showing that ERI-containing regimens could significantly extend PFS, PPS, and OS in patients with ABC or MBC who had high ALC (P < 0.05). However, the ALC threshold was not completely consistent between studies. Interestingly, Cortes and Miyoshi et al. found that ERI had a more significant impact on OS than PFS [46,47]. The hypothesis proposed in this study suggests that a high baseline ALC indicates a favorable immune microenvironment. This may increase the immune regulatory potential, strengthen the antitumor immune response triggered by chemotherapeutic drugs, and ultimately contribute to improved patient prognosis in ABC or MBC. Therefore, baseline ALC can potentially serve as an indicator to evaluate drug sensitivity before initiating chemotherapy in patients with ABC or MBC, thereby predicting their therapeutic outcome.
Limitation of this study
The current study has several limitations. First, the search source was relatively limited as all 12 studies had been conducted in Japan. Moreover, the small sample size in these studies could potentially introduce the possibility of selection or bias. Second, only baseline ALC status was assessed in the included studies. Given the dynamic nature of the immune system, further evaluation is needed to fully understand the impact of dynamic changes in ALC on prognosis, especially given the direct effects of chemotherapy on ALC. A detailed analysis of peripheral blood lymphocyte subsets may provide additional predictive values. Furthermore, variations in BC subtypes, sites of distant metastasis, chemotherapy regimens, and follow-up durations among the studies might have impacted patient survival. Consequently, some degree of heterogeneity among the studies could potentially affect the stability of the meta-analysis results.
Conclusion
In summary, the outcomes of this study indicate that baseline ALC can serve as a reliable immune marker that can be monitored without additional invasive procedures. Baseline ALC can reflect the immune status and predict the prognosis of individuals with ABC or MBC. Therefore, baseline ALC monitoring should be an important part of clinical practices when evaluating the prognosis of individuals with ABC or MBC. These studies are retrospective and further larger prospective studies are required to verify the findings of this study.
Disclosure of conflict of interest
None.
References
- 1.Pondé NF, Zardavas D, Piccart M. Progress in adjuvant systemic therapy for breast cancer. Nat Rev Clin Oncol. 2019;16:27–44. doi: 10.1038/s41571-018-0089-9. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br J Radiol. 2022;95:20211033. doi: 10.1259/bjr.20211033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L, Boyle F, Cardoso MJ, Carey LA, Cortés J, El Saghir NS, Elzayat M, Eniu A, Fallowfield L, Francis PA, Gelmon K, Gligorov J, Haidinger R, Harbeck N, Hu X, Kaufman B, Kaur R, Kiely BE, Kim SB, Lin NU, Mertz SA, Neciosup S, Offersen BV, Ohno S, Pagani O, Prat A, Penault-Llorca F, Rugo HS, Sledge GW, Thomssen C, Vorobiof DA, Wiseman T, Xu B, Norton L, Costa A, Winer EP. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuji W, Teramukai S, Ueno M, Toi M, Inamoto T. Prognostic factors for survival after first recurrence in breast cancer: a retrospective analysis of 252 recurrent cases at a single institution. Breast Cancer. 2014;21:86–95. doi: 10.1007/s12282-012-0358-x. [DOI] [PubMed] [Google Scholar]
- 5.Eng LG, Dawood S, Sopik V, Haaland B, Tan PS, Bhoo-Pathy N, Warner E, Iqbal J, Narod SA, Dent R. Ten-year survival in women with primary stage IV breast cancer. Breast Cancer Res Treat. 2016;160:145–152. doi: 10.1007/s10549-016-3974-x. [DOI] [PubMed] [Google Scholar]
- 6.Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F, Birtwisle-Peyrottes I, Balu-Maestro C, Marcy PY, Raoust I, Lallement M, Chamorey E. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19:2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki K, Ito Y, Fukada I, Kobayashi K, Miyagawa Y, Imamura M, Kira A, Takatsuka Y, Egawa C, Suwa H, Ohno S, Miyoshi Y. Predictive impact of absolute lymphocyte counts for progression-free survival in human epidermal growth factor receptor 2-positive advanced breast cancer treated with pertuzumab and trastuzumab plus eribulin or nab-paclitaxel. BMC Cancer. 2018;18:982. doi: 10.1186/s12885-018-4888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong J, Chen X, Gao W, Zhu S, Wu J, Huang O, He J, Zhu L, Chen W, Li Y, Fei X, Lin L, Shen K. A high absolute lymphocyte count predicts a poor prognosis in HER-2- positive breast cancer patients treated with trastuzumab. Cancer Manag Res. 2019;11:3371–3379. doi: 10.2147/CMAR.S187233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimbo H, Horimoto Y, Ishizuka Y, Nogami N, Shikanai A, Saito M, Watanabe J. Absolute lymphocyte count decreases with disease progression and is a potential prognostic marker for metastatic breast cancer. Breast Cancer Res Treat. 2022;196:291–298. doi: 10.1007/s10549-022-06748-4. [DOI] [PubMed] [Google Scholar]
- 13.Andre F, Dieci MV, Dubsky P, Sotiriou C, Curigliano G, Denkert C, Loi S. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013;19:28–33. doi: 10.1158/1078-0432.CCR-11-2701. [DOI] [PubMed] [Google Scholar]
- 14.Ownby HE, Roi LD, Isenberg RR, Brennan MJ. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer. 1983;52:126–130. doi: 10.1002/1097-0142(19830701)52:1<126::aid-cncr2820520123>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20:218–232. doi: 10.1038/s41568-019-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Y, Jin Y, Ding J, Yujie W, Shi Q, Qu X, Zhao S, Li J, Lijuan C. Low absolute CD4(+) T cell counts in peripheral blood predict poor prognosis in patients with newly diagnosed multiple myeloma. Leuk Lymphoma. 2020;61:1869–1876. doi: 10.1080/10428194.2020.1751840. [DOI] [PubMed] [Google Scholar]
- 17.Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74:7168–7174. doi: 10.1158/0008-5472.CAN-14-2458. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 19.Savas P, Salgado R, Denkert C, Sotiriou C, Darcy PK, Smyth MJ, Loi S. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol. 2016;13:228–241. doi: 10.1038/nrclinonc.2015.215. [DOI] [PubMed] [Google Scholar]
- 20.Postow MA, Chasalow SD, Kuk D, Panageas KS, Cheng ML, Yuan J, Wolchok JD. Absolute lymphocyte count as a prognostic biomarker for overall survival in patients with advanced melanoma treated with ipilimumab. Melanoma Res. 2020;30:71–75. doi: 10.1097/CMR.0000000000000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells G. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. Symposium on Systematic Reviews: Beyond the Basics. 2014:2014. [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyama Y, Kawai S, Uenaka N, Okazaki M, Asaoka M, Teraoka S, Ueda AI, Miyahara K, Kawate T, Kaise H, Yamada K, Ishikawa T. Absolute lymphocyte count, platelet-to-lymphocyte ratio, and overall survival in eribulin-treated HER2-negative metastatic breast cancer patients. Cancer Diagn Progn. 2021;1:435–441. doi: 10.21873/cdp.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, Chouaib S. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11:824–846. doi: 10.1002/1878-0261.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I, Guastalla JP, Bachelot T, Perol D, Chabaud S, Hogendoorn PC, Cassier P, Dufresne A, Blay JY European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cézé N, Thibault G, Goujon G, Viguier J, Watier H, Dorval E, Lecomte T. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol. 2011;68:1305–1313. doi: 10.1007/s00280-011-1610-3. [DOI] [PubMed] [Google Scholar]
- 32.Feng JF, Liu JS, Huang Y. Lymphopenia predicts poor prognosis in patients with esophageal squamous cell carcinoma. Medicine (Baltimore) 2014;93:e257. doi: 10.1097/MD.0000000000000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goto W, Kashiwagi S, Takada K, Asano Y, Morisaki T, Shibutani M, Tanaka H, Hirakawa K, Ohira M. Utility of follow-up with absolute lymphocyte count in patients undergoing eribulin treatment for early detection of progressive advanced or metastatic breast cancer. Anticancer Res. 2022;42:939–946. doi: 10.21873/anticanres.15553. [DOI] [PubMed] [Google Scholar]
- 34.Nakamoto S, Ikeda M, Kubo S, Yamamoto M, Yamashita T, Notsu A. Systemic immunity markers associated with lymphocytes predict the survival benefit from paclitaxel plus bevacizumab in HER2 negative advanced breast cancer. Sci Rep. 2021;11:6328. doi: 10.1038/s41598-021-85948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sata A, Fukui R, Miyagawa Y, Bun A, Ozawa H, Fujimoto Y, Higuchi T, Imamura M, Miyoshi Y. C-reactive protein and absolute lymphocyte count can predict overall survival of patients treated with eribulin. Anticancer Res. 2020;40:4147–4156. doi: 10.21873/anticanres.14414. [DOI] [PubMed] [Google Scholar]
- 36.Sawa A, Bando H, Kamohara R, Takeuchi N, Terasaki A, Okazaki M, Iguchi-Manaka A, Hara H. Absolute lymphocyte count as an independent prognostic factor in metastatic breast cancer: a retrospective study. Oncology. 2022;100:591–601. doi: 10.1159/000526963. [DOI] [PubMed] [Google Scholar]
- 37.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 38.Luque-Cabal M, García-Teijido P, Fernández-Pérez Y, Sánchez-Lorenzo L, Palacio-Vázquez I. Mechanisms behind the resistance to trastuzumab in HER2-amplified breast cancer and strategies to overcome it. Clin Med Insights Oncol. 2016;10(Suppl 1):21–30. doi: 10.4137/CMO.S34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pondé N, Brandão M, El-Hachem G, Werbrouck E, Piccart M. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev. 2018;67:10–20. doi: 10.1016/j.ctrv.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Isakoff SJ, Krishnamurthy J, Lyons J, Marcom PK, Matro J, Mayer IA, Moran MS, Mortimer J, O’Regan RM, Patel SA, Pierce LJ, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Young JS, Burns JL, Kumar R. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 41.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 42.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 43.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, Biganzoli L, Boers-Doets CB, Cardoso MJ, Carey LA, Cortés J, Curigliano G, Diéras V, El Saghir NS, Eniu A, Fallowfield L, Francis PA, Gelmon K, Johnston SRD, Kaufman B, Koppikar S, Krop IE, Mayer M, Nakigudde G, Offersen BV, Ohno S, Pagani O, Paluch-Shimon S, Penault-Llorca F, Prat A, Rugo HS, Sledge GW, Spence D, Thomssen C, Vorobiof DA, Xu B, Norton L, Winer EP. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe J, Saito M, Horimoto Y, Nakamoto S. A maintained absolute lymphocyte count predicts the overall survival benefit from eribulin therapy, including eribulin re-administration, in HER2-negative advanced breast cancer patients: a single-institutional experience. Breast Cancer Res Treat. 2020;181:211–220. doi: 10.1007/s10549-020-05626-1. [DOI] [PubMed] [Google Scholar]
- 45.Goto W, Kashiwagi S, Asano Y, Takada K, Morisaki T, Fujita H, Takashima T, Ohsawa M, Hirakawa K, Ohira M. Eribulin promotes antitumor immune responses in patients with locally advanced or metastatic breast cancer. Anticancer Res. 2018;38:2929–2938. doi: 10.21873/anticanres.12541. [DOI] [PubMed] [Google Scholar]
- 46.Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389) investigators. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 47.Miyoshi Y, Yoshimura Y, Saito K, Muramoto K, Sugawara M, Alexis K, Nomoto K, Nakamura S, Saeki T, Watanabe J, Perez-Garcia JM, Cortes J. High absolute lymphocyte counts are associated with longer overall survival in patients with metastatic breast cancer treated with eribulin-but not with treatment of physician’s choice-in the EMBRACE study. Breast Cancer. 2020;27:706–715. doi: 10.1007/s12282-020-01067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Che YQ, Zhang Y, Wang D, Liu HY, Shen D, Luo Y. Baseline lymphopenia: a predictor of poor outcomes in HER2 positive metastatic breast cancer treated with trastuzumab. Drug Des Devel Ther. 2019;13:3727–3734. doi: 10.2147/DDDT.S212610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emile G, Penager S, Levy C, Johnson A, Allouache D, Lequesne J, Hrab I, Segura C, Morel A, Gunzer K, Faveyrial A, Cherifi F, Da Silva A. Baseline lymphopenia as prognostic factor in patients with metastatic breast cancer treated with palbociclib. Oncol Lett. 2022;23:25. doi: 10.3892/ol.2021.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyagawa Y, Yanai A, Yanagawa T, Inatome J, Egawa C, Nishimukai A, Takamoto K, Morimoto T, Kikawa Y, Suwa H, Taji T, Yamaguchi A, Okada Y, Sata A, Fukui R, Bun A, Ozawa H, Higuchi T, Fujimoto Y, Imamura M, Miyoshi Y. Baseline neutrophil-to-lymphocyte ratio and c-reactive protein predict efficacy of treatment with bevacizumab plus paclitaxel for locally advanced or metastatic breast cancer. Oncotarget. 2020;11:86–98. doi: 10.18632/oncotarget.27423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morisaki T, Kashiwagi S, Asano Y, Goto W, Takada K, Ishihara S, Shibutani M, Tanaka H, Hirakawa K, Ohira M. Prediction of survival after eribulin chemotherapy for breast cancer by absolute lymphocyte counts and progression types. World J Surg Oncol. 2021;19:324. doi: 10.1186/s12957-021-02441-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamoto S, Ikeda M, Kubo S, Yamamoto M, Yamashita T. Dynamic changes in absolute lymphocyte counts during eribulin therapy are associated with survival benefit. Anticancer Res. 2021;41:3109–3119. doi: 10.21873/anticanres.15095. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi M, Inoue K, Mukai H, Yamanaka T, Egawa C, Miyoshi Y, Sakata Y, Muramoto K, Ikezawa H, Matsuoka T, Tsurutani J. Indices of peripheral leukocytes predict longer overall survival in breast cancer patients on eribulin in Japan. Breast Cancer. 2021;28:945–955. doi: 10.1007/s12282-021-01232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ueno A, Maeda R, Kin T, Ito M, Kawasaki K, Ohtani S. Utility of the absolute lymphocyte count and neutrophil/lymphocyte ratio for predicting survival in patients with metastatic breast cancer on eribulin: a real-world observational study. Chemotherapy. 2019;64:259–269. doi: 10.1159/000507043. [DOI] [PubMed] [Google Scholar]