Abstract
Objective: To evaluate the prognostic value of serum alpha-fetoprotein (AFP-L3) and Des-γ-carboxy prothrombin (DCP) in advanced primary liver cancer (PLC) undergoing combined treatment with Sorafenib and transarterial chemoembolization (TACE). Methods: This retrospective analysis included 82 patients with advanced PLC treated at the Second Affiliated Hospital, Guangzhou Medical University from January 2018 to January 2020. The patients were divided into an observation group (41 cases) and a control group (41 cases) based on their treatment method. The control group received TACE, while the observation group received a combination of Sorafenib and TACE. Both groups were evaluated after 12 weeks of treatment. Serum AFP-L3 and DCP levels were measured using a chemiluminescence immunoassay with magnetic particles. Short-term efficacy was compared between the two groups after 12 weeks of treatment. Additionally, Karnofsky Performance Status (KPS) scores, serum AFP-L3 and DCP levels before and after 12 weeks of treatment, and the survival rate after 2 years of follow-up were recorded. Serum AFP-L3 and DCP levels were compared between surviving and deceased patients. Results: The objective response rate in the observation group (68.29%) was higher than in the control group (46.34%) (P<0.05). KPS scores in both groups were significantly higher 12 weeks post-treatment compared to pre-treatment (P<0.05); the observation group had higher post-treatment KPS scores than the control group (P<0.05). Serum AFP-L3 and DCP levels were reduced in both groups after 12 weeks of treatment compared to pre-treatment levels (P<0.05). However, post-treatment serum AFP-L3 and DCP levels were lower in the observation group compared to the control group (both P<0.05). After 2 years of follow-up, the survival rate was higher in the observation group compared to the control group (P<0.05). AFP-L3 and DCP levels were higher in deceased patients compared to surviving patients after 2 years of follow-up (both P<0.05). Conclusion: Combination therapy with Sorafenib and TACE is effective for patients with advanced PLC, reducing AFP-L3 and DCP levels and improving patient survival rates. Additionally, higher levels of serum AFP-L3 and DCP are associated with poor prognosis.
Keywords: Alpha-fetoprotein heterosomes, Des-γ-carboxy prothrombin, Sorafenib, percutaneous hepatic arterial chemoembolization, advanced primary liver cancer
Introduction
Primary liver cancer (PLC) has an increasing incidence and high mortality, significantly impacting patients’ quality of life and posing serious threats to their health and survival [1,2]. Due to the insidious nature of PLC symptoms, many patients are diagnosed at advanced stages, missing the optimal window for radical treatment [3]. Comprehensive treatment based on transarterial chemoembolization (TACE) has become a crucial approach for advanced PLC [4,5]. Although TACE can achieve therapeutic effects, repeated procedures and chemotherapy drug infusions can cause severe liver damage. Additionally, TACE is associated with high recurrence and metastasis rates [6,7]. Therefore, identifying an effective combined therapy with TACE for advanced PLC is essential. Sorafenib, an oral multi-kinase and multi-target inhibitor, has been shown to prolong significantly the survival in advanced PLC [8]. Recent studies suggest that alpha-fetoprotein isoforms (AFP-L3) and Des-γ-carboxy prothrombin (DCP) are closely related to the occurrence and progression of PLC. However, there are no relevant research reports on their prognostic value in clinical practice for PLC patients [9]. This study aims to investigate the prognostic value of combining Sorafenib with TACE in advanced PLC patients, using serum AFP-L3 and DCP levels as indicators, to provide a reference for clinical treatment and prognosis.
Materials and methods
General information
This retrospective analysis included 82 patients with advanced PLC treated at the Second Affiliated Hospital, Guangzhou Medical University from January 2018 to January 2020. The patients were divided into an observation group (41 cases) and a control group (41 cases) based on different treatment schemes. The study was approved by the Ethics Committee of the Second Affiliated Hospital, Guangzhou Medical University.
Inclusion criteria
① The disease met the diagnostic criteria for the diagnosis and treatment of primary liver tumor (2017) [11]. ② The disease was confirmed by cytology or pathology and was in the advanced stage. ③ Complete follow-up data were available.
Exclusion criteria
① Patients with other malignant tumors. ② Patients with serious abnormalities of the cardiopulmonary system, renal function, or hematopoietic system. ③ Patients with coagulation dysfunction. ④ Patients with contraindications to TACE or with allergic constitution. ⑤ Patients with severe mental illness. ⑥ Female patients who were pregnant or lactating.
Therapies
The control group was treated with TACE. Under local anesthesia, routine disinfection, and draping, a 5-FRH catheter was inserted into the proper hepatic artery, celiac artery, common hepatic artery, and superior mesenteric artery using the Seldinger method for routine angiography. An SP catheter was used to puncture the blood-supplying artery of the primary lesion according to the lesion’s condition, blood supply, and adjacency. The catheter was fixed with a super-smooth guide wire, and a mixture of epirubicin (Ebewe Pharma Ges.m.b.H.Nfg.KG, HJ20181179) 50 mg/m2, hydroxycamptothecin (Sichuan Kelun Pharmaceutical Co., Ltd., H20051252) 30 mg/m2, oxaliplatin (Qilu Pharmaceutical (Hainan) Co., Ltd., H20203218) 60 mg/m2, and iodized oil was slowly injected into the main artery supplying the lesion for embolization, once every 4 weeks for 3 consecutive times. The observation group was additionally given sorafenib 400 mg orally, twice a day. Both groups were evaluated 12 weeks after treatment.
Evaluation criteria of curative effect
The short-term efficacy in patients, including complete remission (CR), partial remission, stability, and progression, was evaluated according to the mRECIST standard. The objective remission rate was the summation of CR and partial remission rates.
Outcome measures
The changes in KPS scores before treatment and after 12 weeks of treatment in the two groups were observed. The score range was 0-100, with higher scores indicating better quality of life [10].
The changes in serum AFP-L3 and DCP levels before and after 12 weeks of treatment were observed. A 3 ml sample of fasting venous blood was drawn before treatment and 12 weeks post-treatment. Serum was collected after centrifugation for 10 minutes. The levels of serum AFP-L3 and DCP were determined using a magnetic particle chemiluminescence assay, with test kits purchased from Zhengzhou Autobio Lvke Bioengineering Co., Ltd.
The prognosis in both groups was observed. All patients were followed up until April 2022 to record their survival rates.
The changes in serum AFP-L3 and DCP levels in different prognostic conditions were compared.
Statistical methods
SPSS 26 was used for data processing. Enumerated data were expressed as cases (%), and the χ2 test was used for analysis. Measured data conforming to a normal distribution were expressed as mean ± standard deviation (x̅±sd), and the t-test was used for analysis. A P-value of <0.05 was considered significant. The sample size calculation formula was: n = z2σ2/d2, where Z represents the confidence interval, n is the sample size, d is the sampling error range, and σ is the standard deviation, generally taken as 0.5.
Results
Comparison of general data
There were no significant differences in general data between the two groups (all P>0.05) (Table 1).
Table 1.
Comparison of clinical data
| Clinical data | Observation group (n = 41) | Control group (n = 41) | t/χ2 | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 29 | 30 | 0.060 | 0.806 |
| Female | 12 | 11 | ||
| Age (years, x̅±s) | 56.74±8.81 | 55.82±9.29 | 0.460 | 0.647 |
| Weight (kg, x̅±s) | 62.31±9.96 | 61.56±12.14 | 0.306 | 0.761 |
| Tumor diameter (cm, x̅±s) | 6.54±1.73 | 6.23±1.89 | 0.775 | 0.441 |
| Child-Pugh grading | ||||
| A | 30 | 29 | 0.060 | 0.806 |
| B | 11 | 12 | ||
| Child-Pugh score (points, x̅±s) | 4.97±1.52 | 5.03±1.49 | 0.181 | 0.857 |
| Tumor thrombus distribution | ||||
| Portal vein tumor thrombus | 34 | 35 | 0.091 | 0.762 |
| Hepatic vein tumor thrombus | 7 | 6 |
Comparison of efficacy
The objective response rate in the observation group (68.29%) was higher than that of the control group (46.34%) (P<0.05) (Table 2).
Table 2.
Comparison of efficacy between the two groups
| Group | Number of cases | Complete Remission | Partial Remission | Stable | Progressed | Objective Response Rate (%) |
|---|---|---|---|---|---|---|
| Observation group | 41 | 3 | 25 | 10 | 3 | 68.29 |
| Control group | 41 | 1 | 18 | 15 | 7 | 46.34 |
| χ2 | - | - | - | - | - | 4.038 |
| P | - | - | - | - | - | 0.045 |
Comparison of KPS scores
The KPS scores in both groups increased significantly after 12 weeks of treatment compared to pre-treatment (P<0.05). The post-treatment KPS score in the observation group was higher than that in the control group (P<0.05) (Table 3).
Table 3.
Comparison of KPS scores between the two groups (x̅±sd, points)
| Group | Number of Cases | Prior Treatment | After 12 weeks of treatment |
|---|---|---|---|
| Observation group | 41 | 74.32±3.27 | 89.84±6.51* |
| Control group | 41 | 73.48±2.94 | 81.57±5.28* |
| t | - | 1.223 | 6.318 |
| P | - | 0.225 | <0.001 |
Note: Compared to before treatment;
P<0.05.
KPS: Karnofsky Performance Status.
Comparison of serum AFP-L3 and DCP levels
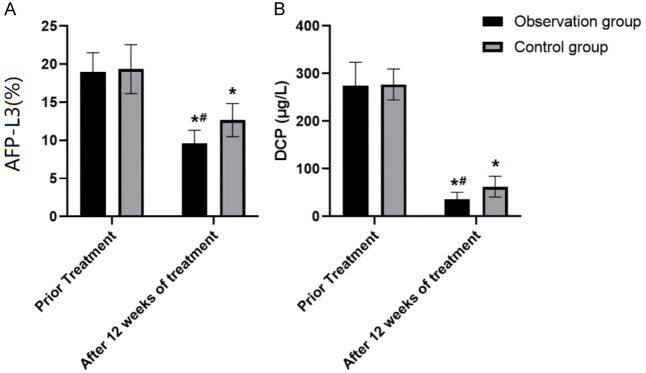
AFP-L3 and DCP levels in both groups after 12 weeks of treatment were lower than those from pre-treatment (both P<0.05). The observation group had lower post-treatment AFP-L3 and DCP levels than the control group (both P<0.05) (Table 4 and Figure 1).
Table 4.
Comparison of serum AFP-L3 and DCP levels between the two groups (x̅±sd)
| Group | Number of Cases | AFP-L3 (%) | DCP (μg/L) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Prior Treatment | After 12 weeks of treatment | Prior Treatment | After 12 weeks of treatment | ||
| Observation group | 41 | 18.94±2.54 | 9.59±1.73* | 274.38±49.21 | 36.23±14.26* |
| Control group | 41 | 19.35±3.21 | 12.64±2.18* | 276.94±32.56 | 62.31±21.82* |
| t | - | 0.641 | 7.017 | 0.278 | 6.407 |
| P | - | 0.0523 | <0.001 | 0.782 | <0.001 |
Note: Compared to before treatment;
P<0.05.
AFP-L3: alpha-fetoprotein; DCP: Des-γ-carboxy prothrombin.
Figure 1.
Comparison of serum AFP-L3 and DCP levels between the two groups. Note: Compared to before treatment, *P<0.05. Compared with Control group, #P<0.05. A: The changes in serum AFP-L3 levels before and after treatment. B: The changes in serum DCP levels before and after treatment. AFP-L3: alpha-fetoprotein; DCP: Des-γ-carboxy prothrombin.
Comparison of prognosis
After 2 years of follow-up, 62 patients survived and 19 died. Among them, 35 patients survived in the observation group and 27 survived in the control group. The survival rate in the observation group was higher than that of the control group (P<0.05) (Table 5).
Table 5.
Comparison of prognosis between the two groups
| Group | Number of Cases | Survival rate (%) |
|---|---|---|
| Observation group | 41 | 35 (85.37) |
| Control group | 41 | 27 (65.85) |
| χ2 | - | 4.232 |
| P | - | 0.040 |
Comparison of serum AFP-L3 and DCP levels in different prognostic conditions
Deceased patients had higher AFP-L3 and DCP levels than surviving patients after 2 years of follow-up (both P<0.05) (Table 6).
Table 6.
Comparison of serum AFP-L3 and DCP levels in different prognostic conditions (x̅±s)
| Prognosis | Number of Cases | AFP-L3 (%) | DCP (μg/L) |
|---|---|---|---|
| Surviving patients | 62 | 11.85±3.25 | 96.42±14.25 |
| Deceased patients | 19 | 25.98±5.51 | 308.29±47.51 |
| t | - | 13.879 | 31.190 |
| P | - | <0.001 | <0.001 |
Note: AFP-L3: alpha-fetoprotein; DCP: Des-γ-carboxy prothrombin.
Discussion
The early clinical manifestations of PLC are insidious, often leading to a diagnosis at an advanced stage, resulting in a low rate of early diagnosis and treatment. Currently, PLC is the third most common malignant tumor in China [12,13]. The specific pathogenesis of PLC has not been completely elucidated, but it is generally considered to be related to chronic liver disease caused by hepatitis viruses (hepatitis B, hepatitis C). Other factors, such as aflatoxin-contaminated food, nonalcoholic fatty liver disease, type 2 diabetes, obesity, and alcoholic liver disease, are also closely associated with the disease [14-16]. Therefore, prompt and effective diagnosis and therapeutic regimens are particularly important to improve the prognosis of the disease.
Sorafenib is an oral molecular targeting drug that can directly inhibit the growth of tumor cells by inhibiting the Raf/MEK/ERK signaling pathway. It also blocks tumor angiogenesis by inhibiting platelet-derived growth factor receptor and vascular endothelial growth factor receptor. TACE is a common and effective palliative treatment for inoperable PLC patients and is widely applied in clinical practice [17,18]. However, the effect of TACE, especially the long-term effect, is not very satisfactory. Additionally, TACE treatment can lead to hypoxia in tumor cells and surrounding tissues, increasing the expression of VEGF by upregulating hypoxia-inducible factor, thus stimulating the growth of hepatocellular carcinoma cells and promoting or causing the progression and metastasis of remaining tumors [19-21]. Sorafenib, on the other hand, can inhibit tumor angiogenesis by acting on VEGFR. Therefore, the combination of TACE and Sorafenib may improve the therapeutic effect and has become a focus of clinical research.
According to this study, the observation group had a higher objective response rate than the control group, indicating that the combination treatment can enhance therapeutic efficacy. The KPS score of the observation group 12 weeks post-treatment was higher than that of the control group, suggesting that the combination treatment improves the quality of life in patients with advanced PLC. Additionally, the survival rate of the observation group was higher than that of the control group, indicating that combined therapy in patients with advanced PLC may improve survival rate.
Alpha-fetoprotein (AFP) is a traditional serum tumor marker, with its expression increasing upon diagnosis of liver disease and significantly elevated in about 80% of liver cancer patients. However, the diagnostic sensitivity of AFP is low [18]. AFP-L3 has emerged as a liver tumor marker in recent years [22,23]. It contains α1-6 fucose residues, binds to the reducing end of N-acetylglucosamine, and is closely associated with the lectin of lentils. AFP-L3 is produced exclusively by liver cancer cells. Bian et al. [24] revealed that AFP-L3 levels in the PLC group were higher than in the chronic hepatitis B or the control groups. Our study demonstrated that after 12 weeks of treatment, the AFP-L3 level in the observation group was lower than in the control group, suggesting that Sorafenib combined with TACE significantly reduces AFP-L3 levels in patients with advanced PLC. Furthermore, AFP-L3 levels were higher in patients who died after 2 years of follow-up compared to those who survived, indicating that a poorer prognosis is associated with higher serum AFP-L3 levels.
Liver cancer can cause γ-glutamate carboxylase to improperly translate the N-terminal amino acid of the prothrombin precursor, preventing it from combining with calcium ions and forming a substance without coagulation function, known as DCP. The formation of CP in the presence of liver cancer due to vitamin K deficiency is not fully understood. The destruction of vitamin K absorption capacity in liver cancer stem cells leads to increased production of DCP [25-27]. Studies have reported that changes induced in primary cell phenotype can reduce DCP produced by cancerous cells, and the surrounding normal tissue can promote DCP formation [28]. Mamdouh et al. [29] demonstrated that DCP levels in liver cancer patients were significantly higher than in those with hepatitis, cirrhosis, and healthy subjects. Logistic regression analysis showed that DCP is closely related to the occurrence of PLC. In this study, the observation group had lower DCP levels than the control group after 12 weeks of treatment, similar to other research findings [30], indicating that the combined therapy can significantly reduce DCP levels in patients with advanced primary liver cancer. The higher levels of serum AFP-L3 in patients who died after 2 years of follow-up, compared to those who survived, further suggest that a worse prognosis is associated with higher serum AFP-L3 levels.
However, due to the relatively small sample size included in this study, the research results may have limitations. It is suggested that the subsequent studies further expand the sample size to obtain more reliable clinical research data.
In conclusion, the combination therapy of Sorafenib and TACE has a beneficial effect on patients with advanced primary liver cancer, reducing AFP-L3 and DCP levels and improving survival rate. Additionally, patients with poorer prognoses have higher levels of serum AFP-L3 and DCP.
Disclosure of conflict of interest
None.
References
- 1.Wada Y, Morine Y, Imura S, Ikemoto T, Saito Y, Takasu C, Yamada S, Shimada M. HIF-1α expression in liver metastasis but not primary colorectal cancer is associated with prognosis of patients with colorectal liver metastasis. World J Surg Oncol. 2020;18:241. doi: 10.1186/s12957-020-02012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. 2021;21:541–557. doi: 10.1038/s41568-021-00383-9. [DOI] [PubMed] [Google Scholar]
- 3.Shao L, Wang X, Yu Y, Xie J. Comparative analysis of the efficacy and accuracy of magnetic resonance imaging (MRI) and contrast-enhanced CT for residual and new lesions after transcatheter arterial chemoembolization (TACE) in patients with primary liver cancer. Transl Cancer Res. 2021;10:3739–3747. doi: 10.21037/tcr-21-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiani A, Narayanan S, Pena L, Friedman M. The role of diagnosis and treatment of underlying liver disease for the prognosis of primary liver cancer. Cancer Control. 2017;24:1073274817729240. doi: 10.1177/1073274817729240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SH, Lee SS, Park SH, Kim KM, Yu E, Park Y, Shin YM, Lee MG. LI-RADS classification and prognosis of primary liver cancers at gadoxetic acid-enhanced MRI. Radiology. 2019;290:388–397. doi: 10.1148/radiol.2018181290. [DOI] [PubMed] [Google Scholar]
- 6.Ye X, Wang L, Xing Y, Song C. Frequency, prognosis and treatment modalities of newly diagnosed small bowel cancer with liver metastases. BMC Gastroenterol. 2020;20:342. doi: 10.1186/s12876-020-01487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, Li HL, Xiang YB. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. 2020;124:330–340. doi: 10.1017/S0007114520001208. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Cui H, Sun M, Liu Q, Liu X, Li G, Wei Y, Fu Q, Liu S, Cao L. Fasting blood glucose, cholesterol, and risk of primary liver cancer: the Kailuan study. Cancer Res Treat. 2021;53:1113–1122. doi: 10.4143/crt.2020.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang HY, Chen J, Xia CC, Cao LK, Duan T, Song B. Noninvasive imaging of hepatocellular carcinoma: from diagnosis to prognosis. World J Gastroenterol. 2018;24:2348–2362. doi: 10.3748/wjg.v24.i22.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo C, Zou X, Hong Z, Sun J, Xiao W, Sun K, Li X, Shen Y, Liang T, Bai X. Preoperative transarterial chemoembolization for barcelona clinic liver cancer stage A/B hepatocellular carcinoma beyond the milan criteria: a propensity score matching analysis. HPB (Oxford) 2021;23:1427–1438. doi: 10.1016/j.hpb.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Medical administration of the health and Family Planning Commission of the People’s Republic of China. Standardization of diagnosis and treatment for hepatocellular carcinoma (2017 edition) Chinese Journal of Digestive Surgery. 2017;16:635–647. [Google Scholar]
- 12.Kim HS, Yi NJ, Kim JM, Joh JW, Lee KW, Suh KS. Clinical impact of the treatment modality on small, solitary, recurrent intrahepatic hepatocellular carcinomas after primary liver resection. Ann Surg Treat Res. 2021;101:85–92. doi: 10.4174/astr.2021.101.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cucchetti A, Russolillo N, Johnson P, Tarchi P, Ferrero A, Cucchi M, Serenari M, Ravaioli M, de Manzini N, Cescon M, Ercolani G. Impact of primary cancer features on behaviour of colorectal liver metastases and survival after hepatectomy. BJS Open. 2018;3:186–194. doi: 10.1002/bjs5.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thistle JE, Petrick JL, Yang B, Bradley MC, Graubard BI, McGlynn KA. Domperidone use and risk of primary liver cancer in the clinical practice research datalink. Cancer Epidemiol. 2018;55:170–175. doi: 10.1016/j.canep.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mejia JC, Pasko J. Primary liver cancers: intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Surg Clin North Am. 2020;100:535–549. doi: 10.1016/j.suc.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Zhao JP, Wang JJ, Chai SS, Zhang YX, Zhang ZG, Xiang S, Chen XP, Zhang WG. The impact of bile leakage on long-term prognosis in primary liver cancers after hepatectomy: a propensity-score-matched study. Asian J Surg. 2020;43:603–612. doi: 10.1016/j.asjsur.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Taylor AC, Maddirela D, White SB. Role of radioembolization for biliary tract and primary liver cancer. Surg Oncol Clin N Am. 2019;28:731–743. doi: 10.1016/j.soc.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Satriano L, Lewinska M, Rodrigues PM, Banales JM, Andersen JB. Metabolic rearrangements in primary liver cancers: cause and consequences. Nat Rev Gastroenterol Hepatol. 2019;16:748–766. doi: 10.1038/s41575-019-0217-8. [DOI] [PubMed] [Google Scholar]
- 19.Lu B, Zhu L, Wang X, Zhong L, Cheng Y, Fan J, Yu L. Effects of radiofrequency ablation combined with transarterial chemoembolization and antiviral therapy on the prognosis and quality of life in primary chronic HBV-related liver cancer. J BUON. 2019;24:1979–1984. [PubMed] [Google Scholar]
- 20.Sun M, Shang P, Bai J, Li S, Li M. High-intensity focused ultrasound ablation combined with transcatheter arterial chemoembolization improves long-term efficacy and prognosis of primary liver cancer. J Clin Lab Anal. 2021;35:e23633. doi: 10.1002/jcla.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao J, Chen Q, Deng Y, Zhao J, Bi X, Li Z, Huang Z, Zhang Y, Zhou J, Zhao H, Cai J. Nomograms predicting primary lymph node metastases and prognosis for synchronous colorectal liver metastasis with simultaneous resection of colorectal cancer and liver metastases. Ann Palliat Med. 2021;10:4220–4231. doi: 10.21037/apm-20-2303. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Li M, Wang J, Cao Y. The effect of nano-albumin paclitaxel on the early postoperative recurrence of primary liver cancer. J Nanosci Nanotechnol. 2020;20:7283–7288. doi: 10.1166/jnn.2020.18710. [DOI] [PubMed] [Google Scholar]
- 23.Zhou D, Wan J, Luo J. Construction and validation of prognostic markers of liver cancer based on autophagy genes. Anticancer Agents Med Chem. 2021;21:1921–1930. doi: 10.2174/1871520621666210329100052. [DOI] [PubMed] [Google Scholar]
- 24.Bian X, He X, Yang L, Wu W, Li L. Prognosis of hepatocellular carcinoma among cancer survivors with other types of primary tumors. Dig Dis Sci. 2020;65:2140–2147. doi: 10.1007/s10620-019-05917-8. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Wu Z, Chang J, Jiang W, Wang Y, Wang H, Li J, Li C, Li X. An updated incidence trends of soft-tissue sarcoma and cancer-specific survival of patients with primary soft-tissue sarcoma of liver: a population-based study. Expert Rev Gastroenterol Hepatol. 2021;15:689–698. doi: 10.1080/17474124.2021.1842193. [DOI] [PubMed] [Google Scholar]
- 26.Liang Y, Wu X, Lu C, Xiao F. Impact of marital status on the prognosis of liver cancer patients without surgery and the critical window. Ann Palliat Med. 2021;10:2990–2999. doi: 10.21037/apm-20-1885. [DOI] [PubMed] [Google Scholar]
- 27.Buisman FE, Galjart B, Buettner S, Groot Koerkamp B, Grünhagen DJ, Verhoef C. Primary tumor location and the prognosis of patients after local treatment of colorectal liver metastases: a systematic review and meta-analysis. HPB (Oxford) 2020;22:351–357. doi: 10.1016/j.hpb.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Chan KS, Tay WX, Cheo FY, Shelat VG. Preoperative transarterial chemoembolization (TACE) + liver resection versus upfront liver resection for large hepatocellular carcinoma (5 cm): a systematic review and meta-analysis. Acta Chir Belg. 2023;123:601–617. doi: 10.1080/00015458.2023.2256539. [DOI] [PubMed] [Google Scholar]
- 29.Mamdouh S, Soliman A, Khorshed F, Saber M. Glypican-3, vascular endothelial growth factor and Golgi protein-73 for differentiation between liver cirrhosis and hepatocellular carcinoma. Asian Pac J Cancer Prev. 2023;24:497–507. doi: 10.31557/APJCP.2023.24.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang MY, Jiang HJ, Jiang H, Zhang RJ, Wang ZC. Micro-positron emission tomography imaging of angiogenesis based on (18)F-RGD for assessing liver metastasis of colorectal cancer. Hepatobiliary Pancreat Dis Int. 2021;20:345–351. doi: 10.1016/j.hbpd.2021.03.001. [DOI] [PubMed] [Google Scholar]