Abstract
Objectives: There is some debate about the link between vitamin C and depressive risk. This study utilized data from the National Health and Nutrition Examination Survey (NHANES) and Mendelian randomization (MR) methodology to investigate the association between the two. Methods: We obtained serum vitamin C levels through laboratory data and determined the intake of vitamin C through a 24-hour dietary recall method on the first day from NHANES 2017 to 2018. Assessment of depressive risk was employed by the 9-item Patient Health Questionnaire (PHQ-9). The association between serum vitamin C levels and depressive risk was examined using logistic regression. Furthermore, the research utilized a two-sample MR methodology to investigate the potential causal connection between vitamin C and depressive risk. Results: Three thousand four hundred and thirty-four participants aged 20+ with serum vitamin C levels and depressive risk data was included. Among the participants, 18.7% had low serum vitamin C levels and 25.2% had self-reported depressive risk. Serum vitamin C levels were associated with depressive risk [OR 1.64, (95% CI: 1.36-1.97), P < 0.01], which remained significant [OR 1.32, (95% CI: 1.08-1.61), P < 0.01] after adjustments. Distinct genetic SNPs were identified for serum vitamin C levels and depressive risk, allowing detailed analysis. No additional influences were observed between genetic variations. IVW and MR Egger analysis showed a non-causal association between vitamin C levels and depressive risk (All P > 0.05). Conclusions: Our findings of the nationally representative survey revealed a strong correlation between serum levels, intake as a supplement or medication of vitamin C and depressive risk, however, without a unidirectional causal association.
Keywords: Vitamin C, depressive risk, mental health, National Health and Nutrition Examination Survey, Mendelian randomization study
Introduction
Depression is a common mental disorder characterised by feelings of sadness or a lack of interest or pleasure in activities over a long period of time, with around 280 million people suffering from depression worldwide; additionally, over 700,000 persons die from suicide each year. Unfortunately, depression not only gives rise to compromised physical well-being and reduced quality of life but also results in higher rates of illness and a substantial economic burden [1]. Moreover, socioeconomic circumstances, lifestyle habits, physical activity levels, sleep quality etc., significantly contribute to maintaining optimal mental well-being [2]. Meanwhile, numerous studies also have established a connection between nutrition and the likelihood of experiencing depression [3]. Diet and the accompanying eating habits assume a safeguarding function against psychiatric disorders. In contrast, personal views on diet and nutritional well-being are closely linked to the emergence, severity, and persistence of depression [4,5]. Numerous studies have shown the beneficial impact of healthy eating patterns on reducing depression risk, particularly by increasing fruit and vegetable consumption [6]. Notably, the pivotal role of vitamins, in particular, has been shown to significantly impact ameliorating depressive symptoms [7].
Vitamin C, a water-soluble vitamin not produced by the body, must be obtained from fruits and vegetables. It acts as a powerful antioxidant, aids neurotransmission, supports neuron structure, and plays a role in neuronal differentiation, maturation, and survival [8]. Juliet M. Pullar’s study found a noteworthy correlation between vitamin C levels and current mood in young adult men, suggesting that maintaining optimal vitamin C levels may enhance mood in this group. Moreover, many older patients have inadequate vitamin C levels, and those with biochemical depletion exhibit significantly higher depressive symptoms than those with higher concentrations [9,10]. However, previous studies were limited by small sample sizes, and they neither investigated the cross-sectional correlation with serum vitamin C levels and depressive risk via logistic regression analysis nor explored the causality between serum vitamin C levels and depressive risk which might provide a theoretical basis for natural drug treatment of depression.
The National Health and Nutrition Examination Survey (NHANES) is a US survey providing representative data on the population. It includes questionnaire surveys, physical examinations, and laboratory tests with strict quality controls. The database is publicly available, providing extensive and high-quality multidimensional data for free. Mendelian randomization (MR) serves as a technique to delve into the evidence supporting causality [11]. It capitalizes on genetic variants as instrumental variables (IVs) that bear significant correlation with risk factors, thereby furnishing a dependable estimate of causality [12]. MR has been employed to scrutinize the causal relationship between serum vitamin C levels and various health outcomes, including nonalcoholic fatty liver disease [13], digestive system cancers [14], hyperglycemia and metabolic syndrome [15], and atrial fibrillation [16]. However, the exact causal link between serum vitamin C levels and depression remains elusive.
So, in the present study, our objective was to probe the association and causality between serum vitamin C levels and depressive risk using data from NHANES taking advantage of both observation and MR analysis from the IEU open gwas project. With over 3,000 participants included, this survey may represent the largest serologic study conducted on the relationship between vitamin C and depressive risk.
Material and methods
Subjects
Our research utilizes publicly accessible data obtained from NHANES 2017-2018 including the contents of both serum vitamin C levels and depressive risk, with all relevant information sourced from the official website (https://www.cdc.gov/nchs/nhanes/index.htm). Participants who were less than 20 years old (n = 3685), and pregnant women (n = 55), with missing depressive risk scores (n = 735) were excluded. Participants without relevant covariates (age, sex, marital status, education level, BMI, PIR, smoking and drinking status) were not included. Ultimately, 3,434 eligible individuals were included in this study (Figure 1). Data on the participants’ demographics, examination findings, medical history, dietary habits, as well as laboratory results were collected, and the NHANES study have received ethical approval from the Ethics Review Board of the National Centre for Health Statistics in the United States.
Figure 1.
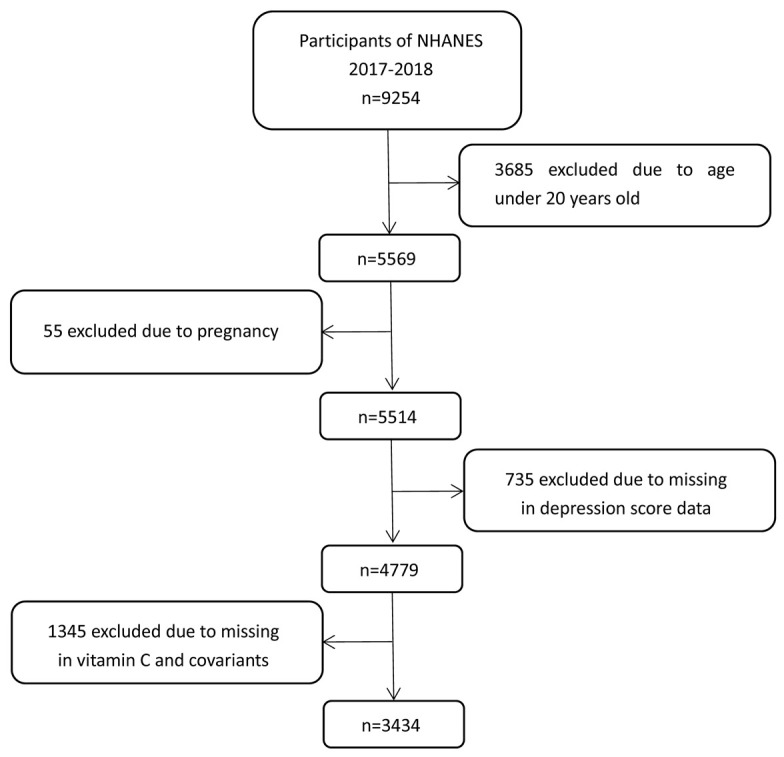
Flow chart of study participants from NHANES 2017-2018.
Measures
Assessment of depressive risk
The PHQ-9 questionnaire is a tool used to measure the severity of depressive risk. It has nine questions, each with four answer options. The scoring ranges from 0-27, with higher scores indicating more severe symptoms. Scores between 5-9 are considered mild, 10-14 suggest moderate, 15-19 indicate moderate to severe, and 20 and above indicate severe symptoms. In this study, depressive risk is defined as a PHQ-9 score of five or more.
Assessment of vitamin C
Serum vitamin C levels are determined using laboratory data. To quantify vitamin C in the serum, an isocratic ultrahigh performance liquid chromatography (UPLC) technique is employed, coupled with electrochemical detection at 450 mV (with a detection range of 200 mA). To stabilize and acidify the serum, one part of serum is mixed with four parts of 6% metaphosphoric acid (MPA). The sample is then frozen at -70°C until it is ready for analysis. Serum levels < 23 µmol/L were defined as vitamin C deficiency [17]. During the 2017-2018 survey period, information about the intake of vitamin C through both food and supplements was collected using a 24-hour diet recall method. All participants were required to undergo two 24-hour dietary recall interviews. The first interview was conducted face-to-face at the MEC, while the second interview was conducted by telephone within 3-10 days. To obtain more accurate data, we chose to rely on the first dietary recall interview. According to the Dietary Guidelines for Americans 2020-2025 [18], we define vitamin C intake deficiency in men who consume less than 75 grams and in women less than 90 grams of vitamin C per day.
Assessment of covariates
Possible confounders of vitamin C and depressive risk have been investigated as follows: gender (male, female), age (participants over the age of 20 are divided into three categories: 20-39, 40-59 and 60 or greater), race/ethnicity (including five categories, Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black and other race), education (high school or below, greater than high school), smoking and drinking status (Yes and no), diabetes, hypertension, marital status, BMI and family income to poverty ratio (the higher the PIR, the better, PIR stands for the ratio of household income to the poverty line) from NHANES. Marital status was categorized into two levels. In particular, divorced, widowed and living with a partner are also classified as married. BMI is classified into four groups based on the cutoff points of 18.5, 25, and 30, representing underweight, normal weight, overweight, and obesity. Similarly, PIR (0-5) is divided into four groups based on the cutoff points of 1, 2, and 3, representing below poverty, low-middle income, upper-middle income, and high income. Blood pressure is measured three times at a mobile examination centre with a 60-second interval between each reading. Using the Omron HEM-907XL device, the second reading is selected for analysis. Hypertension is defined as having a systolic blood pressure of 140 mmHg or higher, and/or a diastolic blood pressure of 90 mmHg or higher [19]. NHANES lab data provides diabetes information. Hemoglobin A1c reflects average blood sugar levels for the past 2-3 months. It is determined by the formation of a glycation product due to high blood glucose levels. Glycoprotein HbA1c (%) > 6.5 is the diabetes standard for this study [20].
Statistical analysis
Descriptive analysis investigated associations between serum vitamin C levels, confounders and depressive risk. Continuous variables that followed a normal distribution were presented as means and standard deviations (SDs), while categorical variables were summarized using frequencies (n) and percentages (%). Differences in socioeconomic factors, lifestyle and health status among participants who were depressed and those with depressive risk were compared using χ2 tests. Correlations between the different variables of the study were established using the Spearman’s correlation test. To examine the association between vitamin C and depressive risk in patients, taking into account confounding factors, we constructed the five distinct models using binary logistic regression models, the effect sizes were recorded with a 95% confidence interval. Additional subgroup analysis was performed to investigate the ORs and 95% CIs for Covariates differences in the impact on depressive risk in the condition of vitamin C deficiency using the Chi-square test. All data processing and analyses were performed using SPSS 26.0, and a two-sided P < 0.05 was considered meaningful.
MR analysis
Our study utilized a two-sample MR analysis of the potential causal connection between serum levels and intake as a supplement or medication of vitamin C and depression which was considered exposure to depression as an outcome. MR is based on three main assumptions [21]: the instrumental variables should be correlated with the exposure; the instrumental variables should not be associated with confounders; and the instrumental variables should influence the outcome only through the exposure (no horizontal pleiotropy). In order to select instrumental variables that satisfy the three assumptions of the MR analysis above, we performed the following steps: To minimize confounding factors, we selected both the exposed and outcome populations from the IEU Open GWAS project and used single nucleotide polymorphisms (SNPs) from genome-wide association studies as instrumental variables; we chose independent SNPs associated with serum levels and intake as a supplement or medication of vitamin C and depressive risk at a significant level and excluded weak instrumental variables. Genetic variants (i.e., SNPs) related to serum levels and intake as a supplement or medication of vitamin C and depressive risk at P < 5×10-8 were identified from a genome-wide association meta-analysis; however, with less ten SNPs, P < 5×10-6 at least was selected [22]. The linkage disequilibrium across these SNPs were calculated using the 10000 genomes linkage disequilibrium European panel as the reference population in moderate linkage disequilibrium (r2 > 0.001) [23].
We used MR analysis with five different methods: inverse variance weighted (IVW), MR-Egger, Weighted median, Weighted mode and Simple mode. Above all, the IVW method assumes no pleiotropy and that all instrumental variables are valid, the MR-Egger approach can provide a valid estimate even when instrumental variables are invalid, and these two methods are the most important to determine the causal association. We conducted sensitivity analyses to evaluate the resilience of the MR findings. In the IVW analysis, we employed fixed-effect or random-effect models depending on the heterogeneity observed. To ascertain the absence of horizontal pleiotropy, we scrutinized the MR-Egger intercept and conducted the IVW global test. Additionally, we performed a leave-one-out analysis to assess the influence of individual SNPs on the causal relationship. All statistical analyses were conducted in R, using the “TwoSampleMR” packages. A significance level of P < 0.05 was considered statistically significant.
Results
Characteristics of the participants
Three thousand four hundred thirty-four participants in total (male:female 1720:1714) were included in the study, of whom 867 (25.25%) had depressive risk if their PHQ-9 score was ≥ 5. A flowchart showing how to select a sample is shown in Figure 1. Six hundred and forty-two (18.7%) individuals were serum vitamin C deficient, and 1,886 people (54.92%) did not meet the vitamin C intake recommended by the Dietary Guidelines for US residents. No significant associations with depressive risk were found for marital status and hypertension. However, other socio-demographic and health variables, including sex, age, race, education, PIR, alcohol/smoking, BMI and diabetes were obviously associated with depressive risk (all P < 0.05) (Table 1). Meanwhile, serum vitamin C levels were 51.80 ± 27.60 in no depressive risk group and 46.16 ± 30.02 in depressive risk group, difference has statistical significance (P < 0.001) (Supplementary Figure 1).
Table 1.
Baseline characteristics of depressive risk group versus no depressive risk group (n = 3434)
| Variable | No depressive risk (n = 2567) | Depressive risk (n = 867) | P value |
|---|---|---|---|
| Sex | P < 0.001 | ||
| Male | 1355 (52.8) | 365 (42.1) | |
| Female | 1212 (47.2) | 502 (57.9) | |
| Age | P = 0.010 | ||
| 20-39 | 770 (30.0) | 235 (27.1) | |
| 40-59 | 802 (31.2) | 319 (36.8) | |
| 60- | 995 (38.8) | 313 (36.1) | |
| Race/ethnicity | P = 0.003 | ||
| Mexican American | 295 (11.5) | 99 (11.4) | |
| Other Hispanic | 209 (8.1) | 78 (9.0) | |
| Non-Hispanic White | 981 (38.2) | 385 (44.4) | |
| Non-Hispanic Black | 589 (22.9) | 179 (20.6) | |
| Other race including multi-racial | 493 (19.2) | 126 (14.5) | |
| Education | P < 0.001 | ||
| ≤ High school | 979 (38.1) | 419 (48.3) | |
| 1588 (61.9) | 448 (51.7) | ||
| PIR | P < 0.001 | ||
| < 1 | 374 (14.6) | 224 (25.8) | |
| 1-2 | 675 (26.3) | 281 (32.4) | |
| 2-3 | 412 (16.0) | 137 (15.8) | |
| > 3 | 1106 (43.1) | 225 (26.0) | |
| Marital status | P = 0.057 | ||
| Married | 2145 (83.6) | 700 (80.7) | |
| Never married | 422 (16.4) | 167 (19.3) | |
| Drinking status | P = 0.001 | ||
| Yes | 2327 (90.7) | 817 (94.2) | |
| No | 240 (9.3) | 50 (5.8) | |
| Smoking status | P < 0.001 | ||
| Yes | 400 (15.6) | 241 (27.8) | |
| No | 640 (30.0) | 224 (25.8) | |
| Unspecified | 1527 (59.5) | 402 (46.4) | |
| BMI | P < 0.001 | ||
| < 18.5 | 33 (1.3) | 20 (2.3) | |
| 18.5-24.9 | 755 (29.4) | 194 (22.4) | |
| 25-29.9 | 882 (34.4) | 253 (29.2) | |
| ≥ 30 | 898 (35.0) | 400 (46.1) | |
| Diabetes | P = 0.001 | ||
| Yes | 342 (13.3) | 154 (17.8) | |
| No | 2225 (86.7) | 713 (82.2) | |
| Hypertension | P = 0.629 | ||
| Yes | 604 (23.5) | 211 (24.3) | |
| No | 1963 (76.5) | 656 (75.7) | |
| Vitamin C intake (mg/d) | P = 0.019 | ||
| < 75 mg/d women | 1380 (53.8) | 506 (58.4) | |
| < 90 mg/d men | |||
| ≥ 75 mg/d women | 1187 (46.2) | 361 (41.6) | |
| ≥ 90 mg/d men | |||
| Serum vitamin C (μmol/L) | P < 0.001 | ||
| < 23 μmol/L | 428 (17.7) | 214 (24.7) | |
| ≥ 23 μmol/L | 2139 (83.3) | 653 (75.3) |
Correlation of various covariates
We conducted a correlation analysis to determine the relationship between vitamin C and depressive risk. However, there are many other factors that can influence depressive risk, such as socioeconomic and health-related factors. Hence, we also analyzed the relationship between covariates and depressive risk, as well as the relationship between various variables. The results of these analyses are presented in Table 2. The correlation coefficient used to measure the strength of the relationship between variables ranges from -1 to +1. A value closer to +1 indicates a stronger positive correlation, while a value closer to -1 indicates a stronger negative correlation. We found that the correlation among the variables was statistically significant (P < 0.05). It’s obvious in the table, not only serum vitamin C but also vitamin C intake is negative related with depressive risk, as well as education. Furthermore, PIR had the strongest correlation.
Table 2.
Correlation plot of various covariates
| Gender | Age | Race | Education | PIR | Marital status | Alcohol | Smoking | BMI | Diabetes | Hypertension | Vitamin C intake | Serum Vitamin C | Depressive risk | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | r | 1 | -0.032 | -0.011 | 0.062** | -0.029 | -0.009 | 0.105** | 0.184** | -0.008 | -0.034* | -0.023 | -0.003 | 0.176** | 0.093** |
| p | 0.063 | 0.503 | 0 | 0.089 | 0.588 | 0 | 0 | 0.651 | 0.046 | 0.178 | 0.881 | 0 | 0 | ||
| Age | r | 1 | -0.036* | -0.078** | 0.051** | -0.365** | 0.022 | -0.050** | 0.032 | 0.242** | 0.257** | 0.135** | 0.071** | -0.001 | |
| p | 0.033 | 0 | 0.003 | 0 | 0.199 | 0.003 | 0.059 | 0 | 0 | 0 | 0 | 0.966 | |||
| Race | r | 1 | 0.205** | 0.114** | 0.080** | 0.109** | 0.027 | -0.121** | 0.012 | 0.027 | -0.011 | -0.013 | -0.052** | ||
| p | 0 | 0 | 0 | 0 | 0.109 | 0 | 0.481 | 0.109 | 0.523 | 0.430 | 0.002 | ||||
| Education | r | 1 | 0.359** | 0.050** | -0.036* | 0.143** | -0.016 | -0.056** | -0.056** | 0.122** | 0.118** | -0.091** | |||
| p | 0 | 0.004 | 0.035 | 0 | 0.352 | 0.001 | 0.001 | 0 | 0 | 0 | |||||
| PIR | r | 1 | -0.089** | -0.018 | 0.189** | -0.024 | -0.019 | -0.049** | 0.146** | 0.134** | -0.174** | ||||
| p | 0 | 0.294 | 0 | 0.164 | 0.268 | 0.004 | 0 | 0 | 0 | ||||||
| Marital status | r | 1 | -0.002 | 0.031 | -0.042* | -0.079** | -0.063** | -0.096** | -0.021 | 0.033 | |||||
| p | 0.904 | 0.065 | 0.013 | 0 | 0 | 0 | 0.213 | 0.057 | |||||||
| Alcohol | r | 1 | 0.200** | -0.066** | 0.039* | 0.037* | 0.032 | 0.063** | -0.056** | ||||||
| p | 0 | 0 | 0.022 | 0.029 | 0.059 | 0 | 0.001 | ||||||||
| Smoking | r | 1 | -0.005 | 0.003 | -0.031 | 0.123** | 0.222** | -0.135** | |||||||
| p | 0.765 | 0.859 | 0.067 | 0 | 0 | 0 | |||||||||
| BMI | r | 1 | 0.162** | 0.049** | -0.067** | -0.177** | 0.090** | ||||||||
| p | 0 | 0.004 | 0 | 0 | 0 | ||||||||||
| Diabetes | r | 1 | 0.080** | 0.020 | -0.069** | 0.055** | |||||||||
| p | 0 | 0.234 | 0 | 0.001 | |||||||||||
| Hypertension | r | 1 | -0.002 | -0.049** | 0.008 | ||||||||||
| p | 0.930 | 0.004 | 0.629 | ||||||||||||
| Vitamin C intake | r | 1 | 0.494** | -0.066** | |||||||||||
| p | 0 | 0 | |||||||||||||
| Serum Vitamin C | r | 1 | -0.100** | ||||||||||||
| p | 0 |
p value 0.01 (two-tailed), the correlation was significant;
p value 0.05 (two-tailed), the correlation was significant;
r, correlation coefficient; p, significance; p is significant at the 0.05 level; Negative values indicate the opposite correlation.
Association between vitamin C and depressive risk
The logistic regression analysis of depressive risk before and after adjustment for covariates is presented in Table 3. Four multivariable models were constructed to adjust for potential covariates, besides the unadjusted model: a rude model (no covariates were adjusted), the depressive risk is significantly associated with serum vitamin C [OR 1.64 (95% CI: 1.36-1.97)]. Model 1 (only sociodemographic variables, including sex, race and age) [OR 1.74 (95% CI: 1.44-2.10)]; Model 2 socioeconomic status variables, including education level, marital status and the ratio of family income to poverty (PIR), were adjusted) [OR 1.55 (95% CI: 1.28-1.88)]; Model 3 (covariates in Model 2 and health-related variables, including drinking status, ever cigarette smoking and BMI, were included) [OR 1.32 (95% CI: 1.08-1.62)]; Model 4 (covariates in Model 3 and Past medical history, including diabetes and hypertension), [OR 1.32 (95% CI: 1.08-1.61)]. To sum up, after adjusting for these all covariates, the association remained significant (P < 0.01). Surprisingly, comparable findings were observed when examining the impact of both dietary intake and dietary supplements of vitamin C on depressive risk (Crude model: [OR 1.21 (95% CI: 1.03-1.41)]; Model 1: [OR 1.24 (95% CI: 1.06-1.45)]) (Supplementary Table 1).
Table 3.
Association between serum vitamin C levels and depressive risk
| Crude OR | Mode l | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|
| Total | 1.64 (1.36-1.97) | 1.74 (1.44-2.10) | 1.55 (1.28-1.88) | 1.32 (1.08-1.62) | 1.32 (1.08-1.61) |
Figures are expressed as odds ratio (95% confidence interval). Model 1: Adjusted for gender, race and age. Model 2: Further adjusted for socioeconomic factors including education level, marital status and ratio of family income to poverty (PIR). Model 3: Further adjusted for drinking status, ever cigarette smoking and BMI. Model 4: Further adjusted for diabetes and hypertension.
Subgroup analysis
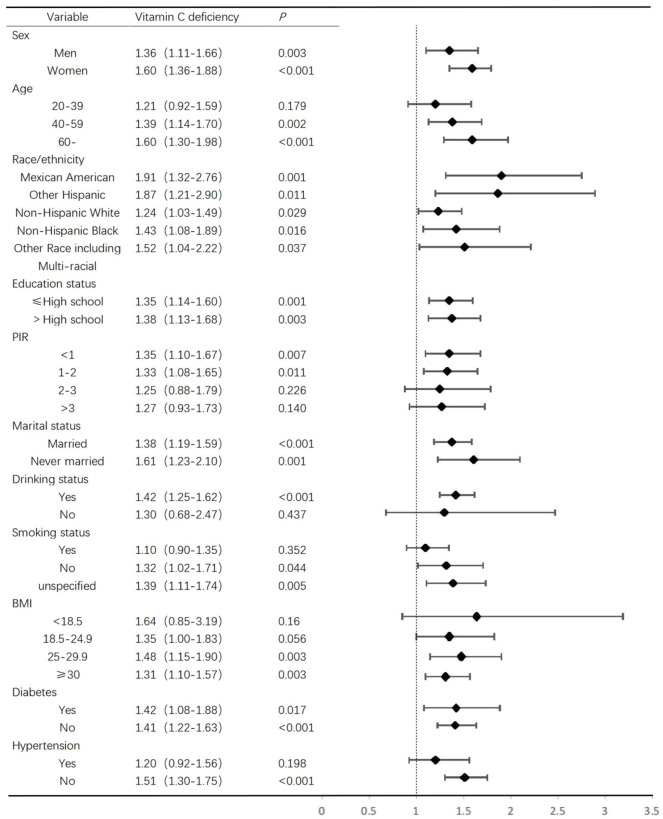
The relationship between serum vitamin C levels and depressive risk was influenced by many other characteristics of the participants (n = 3,434), to further confirm the relationship between them, the subgroup analyzed by sex, age, race/ethnicity, educational status, marital status, PIR, BMI, drinking and smoking status, hypertension and diabetes. Among participants with vitamin C deficiency, the association was significant in both men and women, whereas there was no significant association in the 20-39 [OR 1.21 (95% CI: 0.92-1.59)]. In the 40-59 [OR 1.39 (95% Cl: 1.14-1.70)] and the 60+ [OR 1.60 (95% CI: 1.30-1.98)] age group, depressive risk was correlated with vitamin C. Compared to people without depressive risk, the depressive participants were more likely to have lower PIR [OR 1.35 (95% CI: 1.10-1.67)]. Higher alcohol and extremely high BMI are more likely to be associated with depressed people under the condition of vitamin C deficiency (Figure 2).
Figure 2.
Stratified analyses of the association of depressive risk and vitamin C deficiency.
Unidirectional causal association between vitamin C and depressive risk
This research utilized a two-sample MR methodology to investigate the potential unidirectional causal connection between vitamin C and depressive risk (Table 4; Supplementary Table 2). Distinct genetic SNPs were discovered for various forms of serum vitamin C levels (GWAS ID: ukb-b-19390, from UK Biobank) and depression (GWAS ID: finn-b-F5_DEPRESSIO, from FinnGen Biobank), enabling a more comprehensive analysis. The scholars observed heterogeneity and horizontal pleiotropy (All P > 0.05), implying that the genetic variations exerted no additional influences through alternative pathways. Moreover, the IVW and MR Egger analysis indicated a non-unidirectional causal association between serum vitamin C levels and depression (IVW, [OR 1.21 (95% Cl: 0.93-1.57)], P = 0.154); MR Egger, [OR: 1.41 (95% Cl: 0.82-2.43)], P = 0.247). Still, further examinations specifically concentrated on the unidirectional association between intake of vitamin C as supplement (GWAS ID: ukb-b-15175, from UK Biobank) or medication (GWAS ID: ukb-b-488, from UK Biobank) and depression, also consistently revealed non-causal association between them (Supplementary Table 3). Furthermore, the leave-one-out analysis demonstrated that the overall outcomes were not influenced by any individual genetic variation in these all examinations (Supplementary Figure 2).
Table 4.
Causal effects of serum vitamin C levels on depressive risk estimated by MR
| Exposure/Outcome | nSNP | IVW | MR Egger | Weighted median | Weighted mode | Simple mode | Heterogeneity P | Pleiotropy P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||||
| OR (95% Cl) | P | OR (95% Cl) | P | OR (95% Cl) | P | OR (95% Cl) | P | OR (95% Cl) | P | IVW | MR Egger | |||
| Serum vitamin C levels/Depression | 11 | 1.21 (0.93-1.57) | 0.154 | 1.41 (0.82-2.43) | 0.247 | 1.37 (1.01-1.86) | 0.044 | 1.59 (1.09-2.31) | 0.035 | 1.61 (1.00-2.57) | 0.077 | 0.102 | 0.084 | 0.539 |
Abbreviations: IVW, inverse variance weighted; MR, Mendelian randomization; SNP, Single nucleotide polymorphisms.
Discussion
This is the first study to investigate the relationship between serum vitamin C and depressive risk in Americans using the NHANES database. Our study found a potent association between serum vitamin C levels and depressive risk, and lower serum vitamin C levels and self-reported depressive risk remained statistically significant after adjusting for covariates. Several studies show that low vitamin C concentrations may cause depression, leading to a 65.1% incidence of conditions including depression. Consuming vitamin C from food, supplements, and vegetables is negatively correlated with the risk of depression in the general population [24-26]. A meta-analysis of observational studies concluded that patients with depression had lower dietary intake of vitamin C compared to the control group [27]. Moreover, patients who received fluoxetine combined with vitamin C for 6 months showed a significantly greater reduction in depression compared to those in the fluoxetine plus placebo group [28]. In addition, in mouse models, vitamin C and escitalopram were able to correct elevated levels of FKBPL in stress-induced depression, the overexpression of which has been linked to emotional disorders like anxiety and depression [29]. This article supports the same perspective, finding a negative correlation between serum levels and acceptable daily intake of vitamin C and depressive risk in NHANES, suggesting that low serum vitamin C levels might contribute to the development of depression.
In our current study, we found that with vitamin C deficiency, older participants were more likely to self-report depressive risk. Jia X et al., support our point of view, depressive patients with vitamin deficiency are more likely to be observed in women and overweight older adults [26]. Besides, vitamin C deficiency is more severe in older hospitalized patients and can increase the likelihood of frailty in older patients [30]. In a word, hypovitaminosis C is more common in the elderly [31]. However, a double-blind randomized controlled trial in nursing and residential homes found 29% of residents had depressive symptoms and 67% had low vitamin C levels, but did not find a significant negative correlation between vitamin C levels and low mood [32]. This could be due to differences in sample size; when the sample size is not large enough, some relationships cannot be discovered. Additionally, as many previous studies have reported, female and overweight participants with depressive risk were also found to consume significantly fewer vitamins in our study. Researchers strongly encourage proper vitamin C intake and supplementation among patients with varying BMI levels, which leads to a shorter duration until viral clearance [33]. The relationship between smoking and depressive risk is controversial. Higher smoking frequency and amount are linked to a higher onset of depressive risk. Conversely, quitting smoking reduces the chance of developing depressive risk, with longer cessation durations lowering the risk further [34]. However, due to a significant number of individuals not providing their smoking history in our study, there may be a bias in the results, therefore further research is needed to investigate the relationship between the two. Besides, we did not find that serum levels, and intake as a supplement or medication of vitamin C had a unidirectional causal relationship with depression in the IVW and MR-Egger approach. To the best of our knowledge, this was the first study to evaluate the non-causal association between serum levels, intake as supplement or medication of vitamin C and depression in the IEU open GWAS project.
The pathogenesis of depression is complex, neurotransmitter deficiency may play an important role in the regulation of the stress response and depression-like behaviours [35]. Previous studies have shown that serotonin and noradrenaline reuptake inhibitors (SNRIs) and dopamine stimulants (DSAs) are believed to have broad potential in the management of depression [36,37]. Vitamin C regulates neurotransmitter biosynthesis [11], and it may reduce the risk of depression by regulating the biosynthesis of catecholamines, dopamine, norepinephrine, and epinephrine. Besides, inflammation, oxidative stress, and neurotrophic factor dysfunction are associated with depression symptoms [38]. A study showed seahorse diet reduces depression and anxiety-like behaviour. Effects are attributed to decreased neuroinflammation and oxidation, and restored neurotrophin and neurotransmitter function in the hippocampus [39]. At the same time, a review also demonstrated the therapeutic potential of antioxidant doses in psychopathology related to anxiety and stress in the brain [40]. Vitamin C is an important antioxidant that fights reactive oxygen species and nitrogen species that are naturally produced during cellular metabolism, for proper neurogenesis and differentiation, vitamin C is essential as an enzymatic cofactor, gene expression modulator, and antioxidant [41]. It can be inferred that the possible mechanism by which vitamin C reduces the occurrence of depression may be through its antioxidant properties. To sum up, both our large cross-sectional study and many previous studies have confirmed the link of vitamin C to depressive risk, and this relationship was also retained even after adjusting for many relevant variables. However, the mechanism remains to be further elucidated.
Limitations of this study include that the extensive sample database of the US is not universally representative of other countries. In addition, our research is made from publicly available data over the last ten decades, however, we only include available NHANES 2017-2018 data. In the SNP analysis of the MR study, a less strict P value threshold was used, potentially influencing the results, and the generalizability of our MR results is limited to populations of European ancestry. Due to time and conditions and other constraints, we have not carried out the mechanism by which vitamin C affects depressive risk, which still needs to be further explored. These constraints should be considered when interpreting the findings of our study, and further research is crucial to address these limitations and gain a more comprehensive understanding of the topic. In the end, the lack of randomized control trials (RCT) studies on the relationship between vitamin C and depression is indeed a limitation of this article, and we are still planning to conduct an RCT study in our upcoming research.
Conclusions
Our findings of the nationally representative survey revealed a strong correlation between serum levels, intake as a supplement or medication of vitamin C and depressive risk, however, without a unidirectional causal association. In clinical nursing, nurses may incorporate vitamin C monitoring into assessments, especially for high-risk patients, to help regulate mood-related issues. Collaboration with dietitians and physicians ensures comprehensive care. Additionally, nurses can encourage patients to maintain adequate vitamin C intake, particularly through diet or supplements, which is especially important for vulnerable populations such as the elderly or those with chronic illnesses.
Acknowledgements
We thank the National Health and Nutrition Examination Survey, the IEU Open GWAS project and the developers of the MR-Base platform. This study was supported by Guiding Project of Qinghai Provincial Health Commission (2023-wjzdx-99), and Science Popularization Work Creation Project of Wuxi Municipal Health Commission (P202310).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Somoza-Moncada MM, Turrubiates-Hernández FJ, Muñoz-Valle JF, Gutiérrez-Brito JA, Díaz-Pérez SA, Aguayo-Arelis A, Hernández-Bello J. Vitamin D in depression: a potential bioactive agent to reduce suicide and suicide attempt risk. Nutrients. 2023;15:1765. doi: 10.3390/nu15071765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zielińska M, Łuszczki E, Dereń K. Dietary nutrient deficiencies and risk of depression (Review Article 2018-2023) Nutrients. 2023;15:2433. doi: 10.3390/nu15112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman-Hasbun J, Nosova E, DeBeck K, Dahlby L, Kerr T. Food insufficiency is associated with depression among street-involved youth in a Canadian setting. Public Health Nutr. 2019;22:115–121. doi: 10.1017/S1368980018002574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackburn TP. Depressive disorders: treatment failures and poor prognosis over the last 50 years. Pharmacol Res Perspect. 2019;7:e00472. doi: 10.1002/prp2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quirk SE, Williams LJ, O’Neil A, Pasco JA, Jacka FN, Housden S, Berk M, Brennan SL. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry. 2013;13:175. doi: 10.1186/1471-244X-13-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardinet J, Pouchieu C, Chuy V, Helmer C, Etheve S, Gaudout D, Samieri C, Berr C, Delcourt C, Cougnard-Grégoire A, Féart C. Plasma carotenoids and risk of depressive symptomatology in a population-based cohort of older adults. J Affect Disord. 2023;339:615–623. doi: 10.1016/j.jad.2023.07.076. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Fan Y, Han X, Huang Z, Yu M, Zhang Y, Xu Q, Li X, Wang X, Lu C, Xia Y. Association between serum vitamin levels and depression in U.S. adults 20 years or older based on national health and nutrition examination survey 2005-2006. Int J Environ Res Public Health. 2018;15:1215. doi: 10.3390/ijerph15061215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travica N, Ried K, Sali A, Scholey A, Hudson I, Pipingas A. Vitamin C status and cognitive function: a systematic review. Nutrients. 2017;9:960. doi: 10.3390/nu9090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gariballa S. Poor vitamin C status is associated with increased depression symptoms following acute illness in older people. Int J Vitam Nutr Res. 2014;84:12–17. doi: 10.1024/0300-9831/a000188. [DOI] [PubMed] [Google Scholar]
- 11.Pullar JM, Carr AC, Bozonet SM, Vissers MCM. High vitamin C status is associated with elevated mood in male tertiary students. Antioxidants (Basel) 2018;7:91. doi: 10.3390/antiox7070091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. 2019;10:486–496. doi: 10.1002/jrsm.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H, Guo JL, Yao JJ, Yu JJ, Xia RY, Huang WQ, Tang X, He GM. Serum vitamin C levels and risk of non-alcoholic fatty liver disease: results from a cross-sectional study and Mendelian randomization analysis. Front Nutr. 2023;10:1162031. doi: 10.3389/fnut.2023.1162031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson SC, Mason AM, Vithayathil M, Carter P, Kar S, Zheng JS, Burgess S. Circulating vitamin C and digestive system cancers: Mendelian randomization study. Clin Nutr. 2022;41:2031–2035. doi: 10.1016/j.clnu.2022.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Park S. A causal relationship between vitamin C intake with hyperglycemia and metabolic syndrome risk: a two-sample Mendelian randomization study. Antioxidants (Basel) 2022;11:857. doi: 10.3390/antiox11050857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemilä H, Chalker E. Vitamin C and the risk of atrial fibrillation: Mendelian randomization study may be misleading. Clin Nutr. 2022;41:1446–1447. doi: 10.1016/j.clnu.2022.04.029. [DOI] [PubMed] [Google Scholar]
- 17.Hui S, Lim A, Koh E, Abasszade J, Morgan A, Tan PY, Lemoh C, Robertson M. Prevalence and prognostic significance of vitamin C deficiency in patients with acute upper gastrointestinal bleeding: a prospective cohort study. Aliment Pharmacol Ther. 2023;57:313–322. doi: 10.1111/apt.17359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shams-White MM, Pannucci TE, Lerman JL, Herrick KA, Zimmer M, Meyers Mathieu K, Stoody EE, Reedy J. Healthy eating index-2020: review and update process to reflect the dietary guidelines for Americans, 2020-2025. J Acad Nutr Diet. 2023;123:1280–1288. doi: 10.1016/j.jand.2023.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theodoridis X, Chourdakis M, Chrysoula L, Chroni V, Tirodimos I, Dipla K, Gkaliagkousi E, Triantafyllou A. Adherence to the DASH diet and risk of hypertension: a systematic review and meta-analysis. Nutrients. 2023;15:3261. doi: 10.3390/nu15143261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu B, Lin ZY, Zou RP, Gan YW, Ji JM, Guo JX, Li WG, Guo YJ, Xu HQ, Sun DL, Yi M. Dietary zinc intake affects the association between dietary vitamin a and depression: a cross-sectional study. Front Nutr. 2022;9:913132. doi: 10.3389/fnut.2022.913132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Q, Qian X, Sun F, Liu H, Dou Z, Zhang J. Independent and joint associations of dietary antioxidant intake with risk of post-stroke depression and all-cause mortality. J Affect Disord. 2023;322:84–90. doi: 10.1016/j.jad.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Islam MR, Ali S, Karmoker JR, Kadir MF, Ahmed MU, Nahar Z, Islam SMA, Islam MS, Hasnat A, Islam MS. Evaluation of serum amino acids and non-enzymatic antioxidants in drug-naïve first-episode major depressive disorder. BMC Psychiatry. 2020;20:333. doi: 10.1186/s12888-020-02738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia X, Wang Z, Zhang B, Su C, Du W, Zhang J, Zhang J, Jiang H, Huang F, Ouyang Y, Wang Y, Li L, Wang H. Food sources and potential determinants of dietary vitamin C intake in Chinese adults: a cross-sectional study. Nutrients. 2018;10:320. doi: 10.3390/nu10030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding J, Zhang Y. Associations of dietary vitamin C and E intake with depression. a meta-analysis of observational studies. Front Nutr. 2022;9:857823. doi: 10.3389/fnut.2022.857823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amr M, El-Mogy A, Shams T, Vieira K, Lakhan SE. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: a randomized, double-blind, placebo-controlled pilot study. Nutr J. 2013;12:31. doi: 10.1186/1475-2891-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gammoh O, Ibrahim A, Qnais E, Alqudah A, Altaber S, Aljabali AAA, Tambuwala MM. Vitamins C and D exhibit similar antidepressant effects to escitalopram mediated by NOx and FKBPL in a stress-induced mice model. Nutrients. 2023;15:2692. doi: 10.3390/nu15122692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma Y, Popescu A, Horwood C, Hakendorf P, Thompson C. Prevalence of hypovitaminosis C and its relationship with frailty in older hospitalised patients: a cross-sectional study. Nutrients. 2021;13:2117. doi: 10.3390/nu13062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr AC, Rowe S. Factors affecting vitamin C status and prevalence of deficiency: a global health perspective. Nutrients. 2020;12:1963. doi: 10.3390/nu12071963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gosney MA, Hammond MF, Shenkin A, Allsup S. Effect of micronutrient supplementation on mood in nursing home residents. Gerontology. 2008;54:292–299. doi: 10.1159/000131886. [DOI] [PubMed] [Google Scholar]
- 33.Hafez W, Saleh H, Abdelshakor M, Ahmed S, Osman S, Gador M. Vitamin C as a potential interplaying factor between obesity and COVID-19 outcome. Healthcare (Basel) 2022;11:93. doi: 10.3390/healthcare11010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z, Yue Q, Zhao Z, Wen J, Tang L, Zhong Z, Yang J, Yuan Y, Zhang X. A cross-sectional study of smoking and depression among US adults: NHANES (2005-2018) Front Public Health. 2023;11:1081706. doi: 10.3389/fpubh.2023.1081706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurban N, Qin Y, Zhao HL, Hu X, Chen X, Zhao YY, Peng YS, Wang HB, Cui SY, Zhang YH. Chronic stress-induced elevation of Melanin-concentrating hormone in the locus coeruleus inhibits norepinephrine production and associated with depression-like behaviors in rats. Int J Neuropsychopharmacol. 2024;27:pyad069. doi: 10.1093/ijnp/pyad069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tundo A, Betro’ S, de Filippis R, Marchetti F, Nacca D, Necci R, Iommi M. Pramipexole augmentation for treatment-resistant unipolar and bipolar depression in the real world: a systematic review and meta-analysis. Life (Basel) 2023;13:1043. doi: 10.3390/life13041043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeuring HW, D’Angremont E, Tol JMH, Risselada AJ, Sommer IEC, Oude Voshaar RC. The effectiveness of off-label dopamine stimulating agents in depressive disorder: a systematic review and meta-analysis. Psychiatry Res. 2023;319:115010. doi: 10.1016/j.psychres.2022.115010. [DOI] [PubMed] [Google Scholar]
- 38.Catena-Dell’Osso M, Bellantuono C, Consoli G, Baroni S, Rotella F, Marazziti D. Inflammatory and neurodegenerative pathways in depression: a new avenue for antidepressant development? Curr Med Chem. 2011;18:245–255. doi: 10.2174/092986711794088353. [DOI] [PubMed] [Google Scholar]
- 39.Li K, Yan L, Zhang Y, Yang Z, Zhang C, Li Y, Kalueff AV, Li W, Song C. Seahorse treatment improves depression-like behavior in mice exposed to CUMS through reducing inflammation/oxidants and restoring neurotransmitter and neurotrophin function. J Ethnopharmacol. 2020;250:112487. doi: 10.1016/j.jep.2019.112487. [DOI] [PubMed] [Google Scholar]
- 40.Zalachoras I, Hollis F, Ramos-Fernández E, Trovo L, Sonnay S, Geiser E, Preitner N, Steiner P, Sandi C, Morató L. Therapeutic potential of glutathione-enhancers in stress-related psychopathologies. Neurosci Biobehav Rev. 2020;114:134–155. doi: 10.1016/j.neubiorev.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Salazar K, Jara N, Ramírez E, de Lima I, Smith-Ghigliotto J, Muñoz V, Ferrada L, Nualart F. Role of vitamin C and SVCT2 in neurogenesis. Front Neurosci. 2023;17:1155758. doi: 10.3389/fnins.2023.1155758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.