Abstract
Objective: To investigate the effects of Jiannao pills on mice with chronic restraint stress-induced anxiety and its mechanisms. Methods: Anxiety-like behaviors were induced in mice by exposing them to chronic restraint stress (8 h/day for 21 days). Subsequently, Jiannao pills were given to these mice for the treatment of the induced anxiety. Following treatment, the intestinal microflora of the mice were analyzed using the 16S rRNA sequencing method. Results: Under positive electrospray ionization mode, a total of 68 chemical compositions were found in Jiannao pills, and under negative electrospray ionization mode, the number was 18. With these chemical compositions in effect, it was observed that Jiannao pills alleviated chronic restraint induced anxiety-like behaviors in mice by extending their dwelling time, standing time, and grooming time in the central area, as well as the percentage of entries and time spent in the open arms. This effect was similar to that of alprazolam. In addition, Jiannao pills significantly improved neural functions in mice with chronic restraint-induced anxiety, diminished the levels of 5-hydroxytryptamine and glutamate, and increased the levels of γ-aminobutyric acid. Furthermore, Jiannao pills decreased the expressions of the corticotropin-releasing factor and cholecystokinin protein, while elevating neuropeptide Y protein levels. The results of 16S rRNA sequencing analysis revealed both Jiannao pills and alprazolam altered the composition of intestinal microflora in mice, with Jiannao pills exhibiting a more pronounced effect. Specifically, there was a significant increase in the abundance of S24-7 in mice following treatment. Besides, significant differences were observed in a total of 632 operational taxonomic units in mice after Jiannao pill treatment. The functions of the intestinal microflora of mice were primarily associated with their betalain biosynthesis and classification levels. Conclusion: Jiannao pills effectively ameliorated chronic restraint anxiety-like behaviors in mice and enhanced their neural functions potentially through the regulation of their intestinal microflora.
Keywords: Chronic restraint-induced anxiety, Jiannao pills, intestinal microflora
Introduction
Anxiety is one of the most common mental health disorders worldwide, characterized by strong unsettling feelings and fear [1]. Clinically, anxiety manifests as various symptoms, including palpitation, sweating, limb tremor, a sense of impending death, and dyspnea [2,3]. The increasing incidence of anxiety disorders has become a global issue, seriously affecting human health and attracting widespread concern [4]. Hence, the development of effective and safe health strategies for preventing anxiety has become an urgent issue to be addressed.
The pathogenesis of anxiety is closely related to changes in neurotransmitters, neuroendocrine dysfunction, and immune dysfunction, particularly the change in neurotransmitter levels, which is an essential neurobiochemical mechanism that explains the occurrence of anxiety. These neurotransmitters encompass 5-hydroxytryptamine (5-HT), γ-aminobutyric acid (GABA), neuropeptide Y (NPY), glutamate (Glu), cholecystokinin (CCK), and corticotropin-releasing factor (CRF) [5-8]. The intestinal microflora is a complex ecosystem that takes part in various physiological and metabolic activities crucial to human health. If the intestinal microflora becomes imbalanced, psychological and mental disorders may occur [9]. It was found that unhealthy intestinal microflora can induce anxiety and emotional behaviors in animal hosts [10]. Hence, it is speculated that regulation and restoration of the intestinal microflora is conducive to the treatment of anxiety. The regulation of intestinal microflora for the management of anxiety is primarily done through the gut-brain axis, an information exchange system between the gut and brain encompassing immune, vagal, and endocrine pathways. The regulations mediated by the gut-brain axis function bidirectionally, allowing intestinal microflora to exert influence on brain activities to alleviate symptoms of anxiety, while shifts in brain activities can result in disruption and changes in the composition of intestinal microflora [11].
Although there are many chemical anti-anxiety drugs, these medications often come with adverse side effects [12,13]. In contrast, Traditional Chinese Medicine (TCM) offers alternative approaches to managing anxiety with fewer side effects in comparison to the western medicine, showing promising efficacy in the treatment of anxiety disorders. Several studies have demonstrated positive feedback from TCM for anxiety treatment [14-17]. Jiannao pills, as a TCM therapy, comprises 19 TCM herbs, including Angelica sinensis, Geranium, Cistanche deserticola, Chinese yam, Schisandra chinensis, Gastrodia elata, and Salvia miltiorrhiza etc., allowing Jiannao pills to play a critical role in the treatment of various mental disorders, anxiety being one of them [18]. Furthermore, TCM as an important part in life science has garnered increasing interest from around the globe, particularly due to its emphasis on targeting intestinal microflora for treating various illnesses [19]. However, studies on Jiannao pills, a TCM drug, for the treatment of anxiety via regulating intestinal microflora remains limited.
Therefore, this study focused on Jiannao pills and found that the application of the drug diminished chronic restraint stress induced anxiety-like behaviors in mice through regulating intestinal microflora to exert impacts on their nervous systems, suggesting that Jiannao pills are an effective therapeutic strategy for mitigating chronic restraint stress-induced anxiety, providing a preclinical basis for using the drug in the clinical management of anxiety.
Materials and methods
Experimental animals and groups
The six to eight-week-old, male, specific pathogen-free (SPF) grade C57 mice, weighing between 18 and 22 g, were purchased from Suzhou SPF Biotechnology Co., Ltd. (Suzhou, China; production license number: SCXK [Su] 2022-0006; license number: SYXK [Chuan] 2018-100). Prior to the experiment, the mice were housed individually at room temperature ranging from 18°C to 22°C and humidity between 50% and 70%, with a controlled 10-hour light and 14-hour dark cycle (light period: 8:00-18:00; dark period: 18:00-8:00 until the following day). These mice had free access to water, and were fed with a standard and sterilized diet for one week to adapt to the new surroundings. All animal procedures were approved by the Animal Ethics Committee of our hospital.
A total of 12 mice were randomized into the Jiannao pill treatment group (G), the positive control group (P), the anxiety model group (A) and the control group (C), with 3 mice in each group. Mice in the Jiannao pill treatment group were exposed to restraint stress in order to induce anxiety-like behaviors, and were given Jiannao pills (Shanghai Pharma, Shanghai, China; GYZZ Z37020791; Batch number: 1721012) afterwards by gavage. For this purpose, one Jiannao pill was dissolved in 0.5% sodium carboxymethyl cellulose solution to get a final volume of 16 mL, which was then administered at a dose of with 0.2 mL per day, starting one week before the end of the modelling period. Mice in the positive control group experienced restraint stress to build an anxiety model, which were then subsequently treated with alprazolam (dtpharm, China, Hunan; GYZZ H43020578) by gavage. To fulfill this purpose, one tablet of alprazolam was dissolved in 2 mL water, which was given at a dose of 0.2 mL daily, starting one week before the end of the modeling period. Mice in the anxiety model group underwent the same treatment but received 0.2 mL normal saline per day instead. Mice in the control group were free of restraint stress experience, and solely received 0.2 mL normal saline. This experiment was reviewed and approved by the Experimental Animal Ethical Committee of the Chengdu University of Traditional Chinese Medicine (approval number: R20221130-1).
Restraint stress-induced anxiety modeling
Prior to the modeling process, the spontaneous activities of the mice were assessed. After adaptive feeding, the mice were confined in a closed room measuring 3.4 m × 2.3 m × 5 m, which was enclosed by a transparent glass panel measuring approximately 80 × 82 cm, for a duration of 30 min. Before the test, the mice were acclimated in a box (50 cm × 40 cm × 50 cm) for the observation of their independent behaviors, during which their time of being upright, the number of getting upright, and the time spent in the central area within the box were measured for 5 min. Mice with normal spontaneous activities were selected for restraint stress testing. Each morning, the mice were placed head-first into a centrifuge tube fixed horizontally on the table, with the restraint time set to 8 h daily for 21 consecutive days. After 21 days, mice in the model and control groups were employed for behavioral experiments.
Behavioral evaluation of mice
Open field test (OFT)
The OFT serves as a comprehensive assessment tool, evaluating the congruence between anxiety-related behaviors and spontaneous activities in mice [20]. The experimental mice were allowed to move freely in a box measuring 40 cm × 40 cm × 40 cm. During this period, the dwelling time in the central area, standing time, and grooming time of the mice were recorded. The OFT box was cleaned with 75% ethanol after each mouse completed the test.
Elevated plus-maze (EPM) test
The EPM experimental device consisted of two open and two enclosed arms. Before the EPM test, the mice were placed in a dark and quiet behavioral room for 30 min. They were then put into an elevated cross maze with their noses facing an open arm. Their dwelling time in the open arm and the time they spent to enter the open arm were recorded over a 10-min test period. A lower frequency of entering the open arm and a shorter duration of dwelling time in the open arm indicate a higher degree of anxiety [21].
Light dark box (LDB) test
The test mice were placed in an LDB device measuring 40 cm × 30 cm × 35 cm, which contains two equal-sized chambers, one bright and one dark. The mice were allowed to move freely between the light and dark chambers for 10 min [22].
Hole board test (HBT)
The test mice were placed in a HBT apparatus, which measured 40 cm × 40 cm × 35 cm, with 16 circular holes, each with a diameter of 3 cm and a thickness of 1 cm, strategically positioned on the bottom board. The time of the mice spent on burrowing behaviors was recorded over a 10-min period.
Intracardial perfusion
After 4 weeks, the mice were weighed and anesthetized by intraperitoneal injection of 500 mg/kg pentobarbital sodium (Sigma-Aldrich, St. Louis, Missouri, USA) using a 1 mL syringe. Each mouse was positioned on a foam autopsy plate with their limbs secured and the ventral side facing upward. Before dissection, the intended skin area was disinfected by gentle spraying with 75% ethanol. The disinfected area was incised using surgical scissors to expose the thoracic cavity. After which, a scalp needle connected to a perfusion pump was inserted into the cavity, with the chestnut valve adjusted to aspirate 0.9% saline. After the effluent was clarified and the liver turned white, the perfusion pump valve was switched to 4% paraformaldehyde (PFA) solution. The administration of 5 mL of 4% PFA was accompanied by observable limb stiffness and tail reflexes, confirming the successful completion of perfusion completion. The brain tissue was swiftly collected and immersed in 4% PFA at 4°C overnight. Subsequently, dehydration was achieved using 15% (w/v) and 30% (w/v) sucrose solutions until sinking. Coronal sections, each 30 μm in thickness, were obtained by cutting the collected tissue with a cryostat frozen microtome (Thermo Fisher Scientific, NX50, USA).
Hematoxylin and eosin (HE) staining
The mice were euthanized using intraperitoneal injection of 500 mg/kg pentobarbital sodium followed by intracardiac perfusion. Histopathologic changes in hippocampal tissue samples were assessed with HE reagents (C0105S; Beyotime, Shanghai, China) in accordance with the manufacturer’s instructions. From each mouse, one hippocampal tissue sample was obtained, and from this sample, two sections were randomly selected and prepared for further analysis. The sections were stained with hematoxylin for 3-5 min, followed by differentiation in hydrochloric acid alcohol for 1-3 sec. After being rinsed with tap water for 15 min, the sections were stained with eosin for 40 s-1 min. After being successively dehydrated in 80%, 95%, and 100% alcohol, followed by xylene for 2 min, the samples were sealed with neutral gum. The HE results were observed under a light microscope, and photographs were captured for documentation.
Nissl staining
Nessler staining was performed using a Nessler staining solution (C0117, Beyotime) following the manufacturer’s instructions. Nissl-positive cells were observed under a microscope (Olympus, Tokyo, Japan).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining
The hippocampal tissue sections were washed with phosphate-buffered saline (PBS), treated with 0.3% H2O2 for 15 min, and washed again with PBS. Subsequently, the sections were incubated according to the instructions of the TUNEL kit (Roche Corporation, USA). Freshly prepared diaminobenzidine substrate was applied to the hippocampal tissue, whose color development was monitored under a microscope. Finally, images were captured for observation.
Enzyme linked immunosorbent assay (ELISA)
According to the manufacturer’s instructions, the expression levels of 5-TH, GABA, and Glu were determined using the ELISA kits (Esebio, Shanghai, China).
Western blotting
Western blot analysis was conducted as follows: Proteins separated by electrophoresis were transferred to a polyvinylidene fluoride membrane. After incubation with primary antibodies (1:1000; Abcam, Cambridge, UK; CRF [ab184238], NPY [ab133757], CCK [ab225874], and β-Actin [ab7817]), the membranes were incubated with the secondary antibodies (ab6728) at room temperature for 1 h. Protein bands were visualized using electrochemiluminescence reagents (Thermo Fisher Scientific, Inc.), and ImageJ software (National Institutes of Health) was used for analysis.
16s rRNA gene sequencing
The fecal samples of mice in each group were collected, and microbial genomic DNA was extracted using the E.Z.N.A ® Soil DNA Kit (Omega Biotek Norcross, GA, USA). The V3 and V4 regions of the 16S rRNA gene were amplified using an ABI GeneAmp ® 9700 PCR thermal cycler (ABI, CA, USA). A TruSeq Nano DNA LT Library Prep Kit (Illumina) was used to prepare the sequencing library, and double-ended sequencing was performed using a MiSeq sequencer. Overlapping reads were merged using FLASH, and sequence analysis was conducted with the UPARSE software package. Chimeraschimeras were checked and removed during clustering, resulting in high-quality sequences clustered into operational taxonomic units (OTUs) at 97% similarity. The alpha diversity of the gut microbiota was assessed using seven parameters: Chao 1, Simpson, Shannon, Pielou’s evenness, observed species, Faith’s phylogenetic diversity (PD), and Good’s coverage. Principal coordinate analysis (PCoA) and non-metric multidimensional scaling were used to evaluate differences in the complexity of sample species. The relative abundance of bacteria in each sample was classified into distinct taxonomic levels, including phylum, class, order, family, genus, based on comprehensive annotation of the taxa. Linear discriminant analysis coupled with effect size measurements was employed to screen and identify different groups of microbial community markers. Kyoto Encyclopedia of Genes and Genomes (KEGG) was performed for enrichment analysis. Bar plots and PCoA plots were generated in R software (version 4.0.3).
Ultra-performance liquid chromatography-Q exactive-mass spectrometry (UHPLC-QE-MS) analysis
Metabolite extraction
A 100 mg sample was mixed with 500 μL of an extraction solution (methanol: water = 1:1 ratio) containing 10 μg/mL of an internal standard. Following vortex for 30 s, the mixture was homogenized at 45 Hz for 4 min and subsequently sonicated in an ice-water bath for 1 h. The samples were then incubated at -40°C for 1 h, and subsequently centrifuged at 12000 rpm for 15 min under the temperature of 4°C (Heraeus Fresco17; Thermo Fisher Scientific). The clarified supernatants were then filtered through a 0.22 μm microporous membrane and stored at -80°C for further analysis.
Method conditions
Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis was performed using the Vanquish UHPLC 1290 system (Thermo Fisher Scientific), which was outfitted with a Waters UPLC BEH C18 column featuring a 2.1 mm × 100 mm internal diameter and particle size 1.7 μm (Waters, USA). The injection volume for each sample was set at 5 μL. The mobile phase consisted of: (A) water with 0.1% formic acid, and (B) acetonitrile with 0.1% formic acid. Detailed conditions are listed in Supplementary Table 1. The primary and secondary MS/MS data were acquired using a Q Exactive Focus mass spectrometer controlled by Xcalibur software (Thermo Fisher Scientific). The specifications were set as follows: sheath gas flow rate: 30 Arb; auxiliary gas flow rate: 10 Arb; capillary temperature: 350°C; MS resolution: 70000; MS/MS resolution: 17500; collision energy: 15/30/45 NCE; spray voltage: 5.5 kV (positive) or -4.0 kV (negative).
Raw MS data were processed using MS-DIAL for peak alignment, retention time correction and peak area extraction. Ion peaks with missing values exceeding 50% in a group were excluded from the extracted data.
Statistical analysis
All data were analyzed using GraphPad Prism 8 software (San Diego, CA, USA), with each experiment repeated three times. The results were expressed as mean ± standard deviation (SD). Student’s t-test was used for comparison between the two groups. Comparisons among multiple groups were performed using a one-way analysis of variance, followed by Tukey’s multiple comparison test. P < 0.05 was considered statistically significant.
Results
Analysis of chemical composition of Jiannao pills
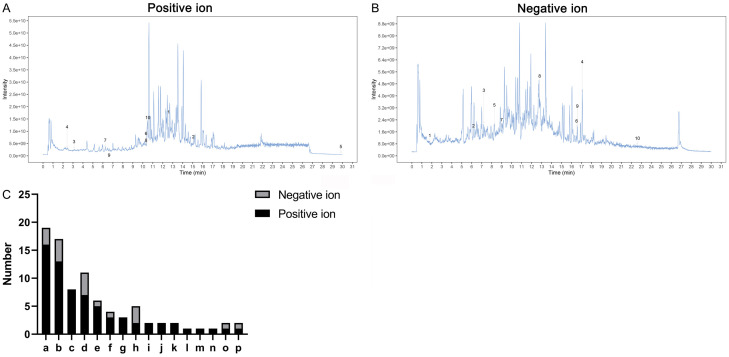
The chemical composition of Jiannao pills was determined through UHPLC-QE-MS assay. A total of 68 chemical components were identified in the positive electrospray ionization modes (Supplementary Table 2), including various compounds such as 16 terpenoids, 13 alkaloids, 8 phenylpropanoids, 7 flavonoids (Figure 1A). In the negative electrospray ionization modes, 18 chemical components were detected (Supplementary Table 3), including 3 terpenoids, 4 alkaloids, 7 flavonoids, 3 phenols (Figure 1B). The active compounds of Jiannao pills were compared between the two ionization modes (Figure 1C). The results suggested that Jiannao Pills might possess multiple pharmacological activities.
Figure 1.
Analysis of the chemical composition of Jiannao pills. A. The total ion chromatography of Jiannao Pills under the positive electrospray ionization mode. B. The total ion chromatography of Jiannao Pills under the negative electrospray ionization mode. C. Comparison between the active compounds in Jiannao Pills under the two ionization modes (a: terpenoids, b: alkaloids, c: phenylpropanoids, d: flavonoids, e: organic acid and its derivatives, f: aromatic compounds, g: others, h: phenols, i: carbohydrate, j: indole and its derivatives, k: lipids, l: centchromans, m: furans, n: quinones, o: carbonyl compounds, p: alcohols).
Jiannao pills improved anxiety-like behaviors in mice
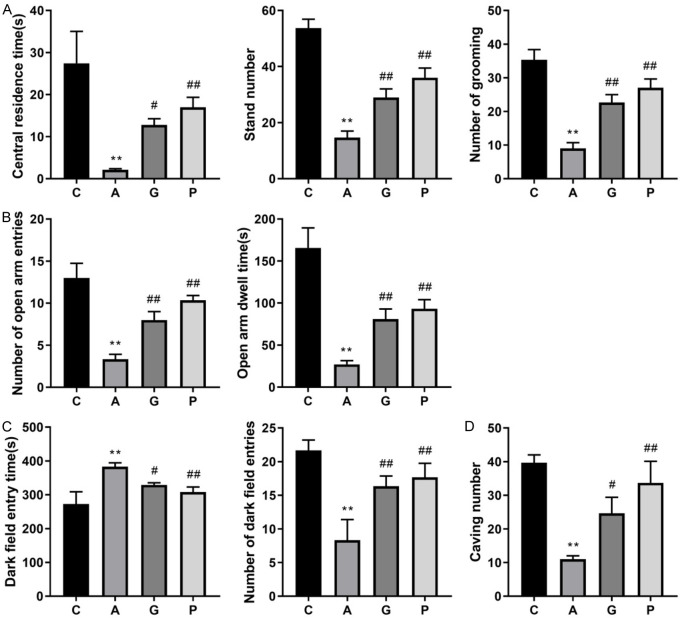
To explore the effect of Jiannao pills on anxiety-like behaviors in mice, anxiety mouse models were constructed. Mice in group A exhibited distinct anxiety-like behaviors in comparison to those in group C, suggesting the success of modeling. The dwelling time in the central area, standing time and grooming time of mice in groups G and P significantly increased when compared to mice in group A (Figure 2A, P < 0.05 or P < 0.01). Additionally, the number of mice entering the open arm and their lingering time in the open arm in groups P and G were significantly elevated in comparison to mice in group A (Figure 2B, P < 0.01). Similarly, mice in groups P and G showed a marked reduction in their lingering time in dark chambers, while their time spent entering the light chambers and exploring the holes significantly increased when compared to mice in group A (Figure 2C, 2D, P < 0.05 or P < 0.01). As a result, the anxiety-like behaviors in groups P and G were markedly mitigated when compared to mice in group A, indicating that Jiannao pills and apuleram could ameliorate anxiety symptoms in mice.
Figure 2.
Jiannao pills improved anxiety-like behaviors in mice. A. The results of dwelling time in the central area, standing time and grooming time of mice by the open field test. B. The number of mice entering the open arm and the lingering time of mice in the open arm by the elevated plus-maze test. C. The time of mice staying in the cassette and the times of entering the cassette by the cassette test. D. The burrowing times of mice by burrowing experiment. C, Control group; A, Anxiety model group; G, Jiannao pills treatment group; P, Positive control group. These data are presented as the mean ± standard (SD). **P < 0.01 when compared to groups C, as measured by Student’s t-test; #P < 0.05, ##P < 0.01 when compared to group A, as detected by one-way analysis of variance, followed by Tukey’s multiple comparison test.
Jiannao pills improved the nervous system in mice with restraint stress induced anxiety
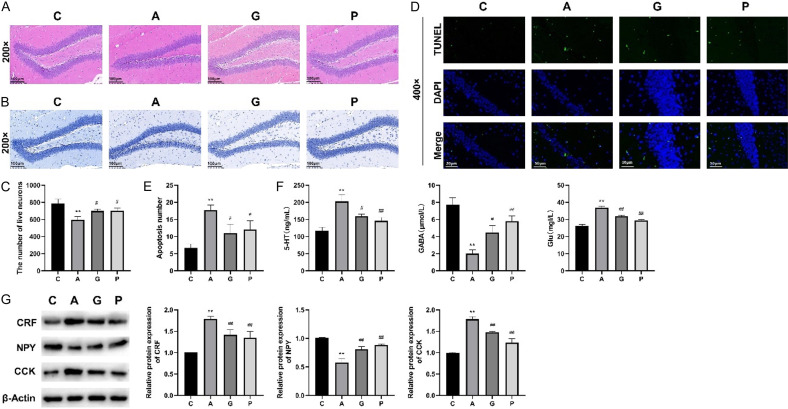
The effects of Jiannao pills on the nervous systems in mice with restraint stress-induced anxiety were investigated in the study. The number of inflammatory cells in mice in group A was elevated and the cell spacing in their hippocampus was reduced compared to mice in group C. However, these changes were significantly reversed with the applications of Jiannao pills and alprazolam (Figure 3A). Additionally, the cellular organization in the dentate gyrus of the hippocampus in group A was markedly disrupted compared to group C, showing numerous Nissl bodies that were pyknotic, hyperchromatic, and irregularly arranged. In contrast, the cells in the dentate gyrus of the hippocampus in groups G and P were relatively regular, with fewer Nissl pyknosis, lighter staining, and a more organized arrangement (Figure 3B, 3C, P < 0.05 or P < 0.01). Furthermore, the cell apoptosis rate of mice was higher in group A than that in group C; however, Jiannao pills and alprazolam significantly reduced cell apoptosis (Figure 3D, 3E, P < 0.05 or P < 0.01). The levels of 5-HT and Glu of mice in group A significantly increased while GABA expression markedly decreased compared to those in group C. However, Jiannao pills and alprazolam significantly decreased the levels of 5-HT and Glu and notably increased the GABA expressions of mice in groups G and P (Figure 3F, P < 0.05 or P < 0.01). Moreover, the levels of CRF and CCK of mice in group A were significantly higher while NPY was notably lower compared to those of mice in group C. With the employment of Jiannao pills and alprazolam, the CRF and CCK levels distinctively reduced and NPY levels increased compared to those of mice in group A (Figure 3G, P < 0.01). These results indicated that Jiannao pills effectively improved the nervous system of mice with restraint stress induced anxiety.
Figure 3.
Jiannao pills improved the nervous system of mice with restraint stress-induced anxiety. A. The structure and cells in the hippocampus of mice by hematoxylin and eosin (HE) staining. Scale: 100 µm; Magnification: 200×. B, C. Cells in dentate gyrus in the hippocampus of mice by Nissl staining method. Scale: 100 µm; Magnification: 200×. D, E. The cell apoptosis rate of mice by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Scale: 50 µm; Magnification: 400×. F. The levels of 5-hydroxytryptamine (5-HT), γ-aminobutyric acid (GABA), and glutamate (Glu)by enzyme linked immunosorbent assay (ELISA). G. The levels of corticotropin-releasing factor (CRF), cholecystokinin (CCK), and neuropeptide Y (NPY) by western blot. Marker: NCM, biotech, P9001; Exposure time: 30 sec. All western blot images were cropped, and full-length blots/gels with exposure times of 30 s and 10 s are presented in Supplementary Figure 2E. C, Control group; A, Anxiety model group; G, Jiannao pills treatment group; P, Positive control group. These data are presented as the mean ± standard (SD). **P < 0.01 when compared to group C, as measured by Student’s t-test; #P < 0.05, ##P < 0.01 when compared to group A, as detected by one-way analysis of variance, followed by Tukey’s multiple comparison test.
Analysis of intestinal microflora species of mice in each group
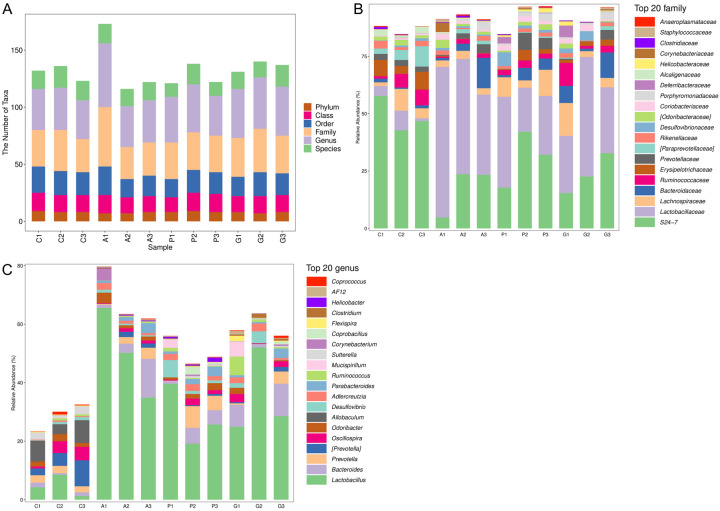
The 16S rRNA sequences from 12 fecal samples collected from mice in each group were analyzed. A total of 707,867 high-quality sequences with an average length of 380.61 base pairs were obtained. The results showed no significant differences in the six taxonomic levels of phylum, class, order, family, genus, and species among the samples (Figure 4A). At the family classification level, the relative abundance of S24-7 was notably lower in group A compared to group C. Conversely, groups G and P demonstrated an increase in the relative abundance of S24-7 when compared to group A. Additionally, the relative abundance of Lactobacillaceae was significantly elevated in group A when compared to group C. Notably, Jiannao pills and alprazolam resulted in a decrease in Lactobacillaceae abundance compared to group A (Figure 4B). Similarly, at the genus level, the relative abundance of Lactobacillus was significantly higher in group A than that in group C. Jiannao pills and alprazolam reduced the relative abundance of Lactobacillus compared to group A (Figure 4C). These results indicated that the abundance of intestinal microflora altered in mice with restraint stress induced anxiety, and that these alterations could be regulated by Jiannao pills and alprazolam.
Figure 4.
Analysis of intestinal microflora species of mice in each group. A. The number of microbial taxons. B. The compositions of species at family level. C. The compositions of species at genus level by 16S rRNA sequencing. C, Control group; A, Anxiety model group; G, Jiannao pills treatment group; P, Positive control group. n = 3.
Analysis of the diversity of intestinal microflora species of mice in each group
The alpha diversity of intestinal microflora species of mice in each group was analyzed. The results showed that based on the Simpson and Shannon indices, the microbial community diversity significantly decreased in group A compared to group C (P < 0.05). Although the microbial community diversity in groups G and P increased compared to group A, the difference was not significant. In terms of the remaining indices (Chao1, Pielou’s evenness, observed species, Faith’s PD, and Good’s coverage), no significant differences were observed among the four groups (Supplementary Figure 1A). The species accumulation curve exhibited a plateauing trend with the inclusion of additional samples, indicating that the sample size was sufficient to reflect all species in the community (Supplementary Figure 1B). The rank-abundance curve results showed that the curve of mice in group A was narrower, with a reduced range along the horizontal axis and a steeper slope, in comparison to that of group C. The rank-abundance curve of mice in groups G and P displayed no discernible changes when compared to those of group A (Supplementary Figure 1C). To determine the differences in the composition of fecal microorganisms among the samples, we conducted a beta diversity analysis as well. The results showed that the plane distribution areas of the samples in group C were distinct from those in groups A, G, and P, featuring relatively clear and well-separated boundaries. The distribution range of the samples from groups G and P surpassed that of group A, yet notably, the separation between groups G and A was substantially wider than the one between groups P and A (Supplementary Figure 1D, 1E). These findings indicated that the composition of the intestinal microflora of mice in groups A, G, and P was different from that of mice in group C. Jiannao pills and alprazolam influenced the intestinal microflora composition to a certain degree, with Jiannao pills having a stronger effect.
Analysis of differences in intestinal microflora species of mice between groups
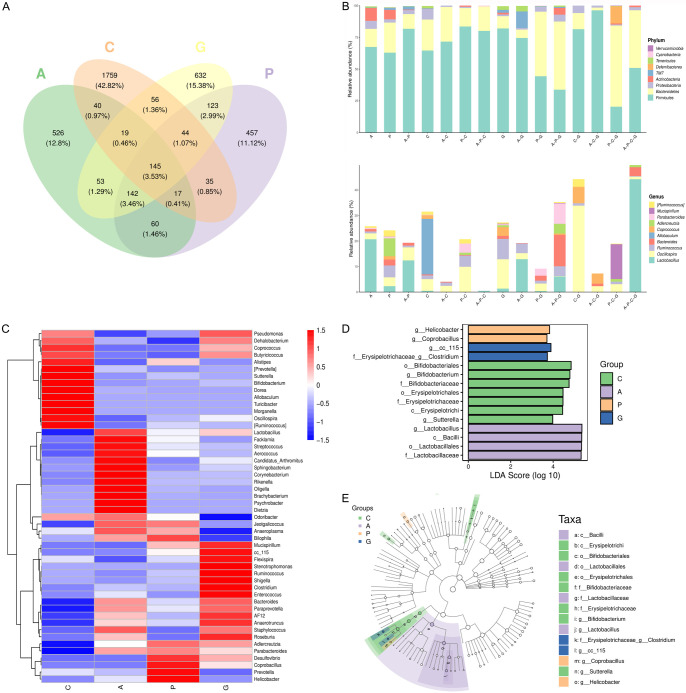
The results of the Venn diagram showed that 145 out of the 5354 OTUs were shared among all groups, with 1759, 526, 632, and 457 OTUs unique to groups C, A, G, and P, respectively (Figure 5A). At the phylum level, the relative abundance of Firmicutes and TM7 in groups A-P was elevated while the levels of Bacteroides and Actinobacteria were significantly reduced when compared to groups A and P. The relative abundance of Firmicutes was found to be increased in groups A-C, P-C, and A-P-C when compared to groups C. Conversely, in comparison with group G, a significant decrease in the relative abundance of Firmicutes was observed in groups A-G, P-G, and A-P-G, with the most pronounced decrease noted in the A-P-G group. In addition, the relative abundance of Firmicutes in group A-C-G were elevated compared to C-G, whereas it decreased significantly in groups P-C-G and A-P-C-G. This trend was reversed for Bacteroides, which showed a decrease in group A-C-G and an increase in groups P-C-G and A-P-C-G compared to groups C-G. At the genus level, the differences between the Lactobacillus and Oscillospira groups were more evident (Figure 5B). Furthermore, the heatmap illustrating the intestinal microflora in mice at the genus level highlighted significant differences among the four groups (Figure 5C). The abundance of Lactobacillus, Bacilli, Lactobacillales, and Lactobacillaceae was significantly elevated in group A. In group G, the abundances of CC-115 and Clostridium were significantly elevated, and in group P the abundances of Helicobacter and Coprobacillus were elevated (Figure 5D, 5E). These results indicated significant differences in the intestinal microbial species of mice among the four groups.
Figure 5.
Analysis of differences in intestinal microflora species of mice in each group. A. Venn diagram of operational taxonomic units (OTUs) for each group; B. Venn Chart OTU abundance histogram at the Phylum and genus levels in different regions; C. The double cluster heatmap of compositions at the genus level; D. The histogram of effect values of marker species by linear discriminant analysis (LDA); E. The chart of classification credit branch. C, Control group; A, Anxiety model group; G, Jiannao pills treatment group; P, Positive control group; The last letter represents the group under comparison.
Analysis of intestinal microflora function in mice of each group
We analyzed the abundance of secondary functional pathways using the KEGG database and identified intergroup differences in KEGG metabolic pathways using the metanomeSeq method. Functional abundance analysis revealed that microbial functions were predominantly associated with metabolism, with metabolic processes showing significantly higher abundance compared to other pathways. Key biological pathways identified in the intestine included carbohydrate metabolism, metabolism of cofactors and vitamins, amino acid metabolism, and metabolism of terpenoids and polyketides (Supplementary Figure 2A). Furthermore, the differences in the KEGG metabolic pathways between groups C and A indicated significant variations in penicillin and cephalosporin biosynthase, Parkinson’s disease, atrazine degradation, hypertrophic cardiomyopathy, and Staphylococcus aureus infection (Supplementary Figure 2B, P < 0.05, P < 0.01 or P < 0.001). Contrasts between groups A and G showed a significant difference in betalain biosynthesis (Supplementary Figure 2C, P < 0.01). These findings underscored the impacts of Jiannao pills on metabolic pathways within the gut microbiota.
Discussion
This study investigated the chemical composition of Jiannao pills and explored their effects and underlying mechanisms in alleviating anxiety. Previous studies have indicated the potential benefits of TCM herbs in treating generalized anxiety disorders [23]. Common mechanisms observed in many plant-based remedies, such as Gastrodia elata, wild jujube, and ginseng, involve the modulation of the GABA system. This modulation can occur through direct receptor binding, ion channels, or cell membrane interactions, as well as through inhibition of GABA transaminase or glutamate decarboxylase [24]. Alprazolam, a well-known anxiolystic, served as a positive control in our study due to its established efficacy in anxiety treatment. Our findings demonstrated that both Jiannao pills and alprazolam effectively improved anxiety-like behaviors in mice. These effects were associated with modulation of the GABA pathway and alterations in intestinal microflora. Specifically, our results suggested that Jiannao pills and alprazolam exerted similar anxiolytic effects, potentially through their influence on intestinal microflora.
Intestinal microflora play crucial roles in various physiological activities, and disruptions in their composition have been linked to psychological and mental disorders [25]. Imbalances in intestinal microflora, characterized by reduced levels of anti-inflammatory bacteria producing butyrate and increased levels of pro-inflammatory bacteria, have been associated with anxiety-related disorders [26]. Additionally, our experimental findings align with previous studies showing anxiety correlates with decreased richness and diversity of intestinal microflora [27]. Therefore, the regulation of intestinal microflora is an important target for anxiety treatment.
Research has underscored significant role of probiotics in alleviating anxiety by regulating intestinal microbiota. For instance, probiotics offer promising therapeutic strategies to counteract the effects of microbiota imbalances in anxiety and eating disorders [28]. Consumption of probiotics has been shown to enhance the diversity of neurotransmitter synthesis pathways and increase levels of microbial neuroactive metabolites, potentially mitigating stress and anxiety in humans [29]. In animal studies, treatment with Bifidobacterium infantis reduced depressive behaviors in rats and restored norepinephrine levels in the brain stem [30]. Clinical research has also supported these findings, demonstrating that treatment with Bifidobacterium strain A-1 improved anxiety and depression scores in patients with schizophrenia [31]. Our study observed that treatment with Jiannao pills and alprazolam significantly increased the relative abundance of S24-7 in anxious mice. S24-7, a dominant family within the Bacteroides group responsible for fermenting carbohydrates in the mouse gut, has been implicated in previous research. Studies have shown that sesquiterpenes attenuates depressive-like behaviors induced by chronic stress by reshaping the gut microbiome and increasing the abundance of S24-7 [32]. Similarly, treatment with Zanthoxylum bungeanum restored gut microbiota dysbiosis induced by chronic unpredictable stress, accompanied by enhanced levels of Bacteroidales_S24-7_group [33]. Based on these findings, we hypothesized that S24-7 played a crucial role in regulating intestinal microflora in anxiety.
Previous studies have highlighted the efficacy of TCM in regulating intestinal microflora and managing anxiety [34,35]. For instance, Fructus gardeniae was reported to have alleviated anxiety and suppressed neuroinflammation in sleep-deprived rats by modulating hippocampal metabolites and intestinal microflora composition [36]. Similarly, Xiaoyao San mitigated anxiety and depression induced by a high-fat diet through modulation of gut microbiota in mice [37]. These findings align with our research, where we observed that treatment with Jiannao pills reversed the trend of S24-7 abundance seen in mice with anxiety, and also increased microbial community diversity. Furthermore, our study identified significant reductions in the abundance of CC-115 and Clostridium in mice treated with Jiannao pills. Additionally, the shuman intestinal bacteria can biotransform active metabolites or alter metabolism following TCM treatment, thereby enhancing their activities and promoting intestinal absorption [38]. In our study, biosynthesis, Parkinson’s disease, and atrazine degradation were significantly altered by penicillin and cephalosporin biosynthesis, and the betalain biosynthase pathway was notably affected following Jiannao pills treatment. Collectively, these studies have underscored the therapeutic potential of TCM in anxiety management through modulation of intestinal microflora. Jiannao pills are a promising therapeutic approach for anxiety disorders as well.
There are some limitations to this study. First, the experimental results were based on the chronic restraint stress-induced anxiety-like mice model, and therefore, require further clinical validation. Second, the molecular mechanism by which Jiannao Pills improve anxiety by regulating intestinal microflora remains unclear and needs further exploration. Additionally, our future research will focus on identifying the specific chemical composition of Jiannao Pills that alleviate anxiety.
Conclusion
Jiannao pills have demonstrated significant efficacy in improving anxiety-like behaviors in mice. This effect appears to be mediated through the regulation of neurotransmitters and the modulation of intestinal microflora. These findings highlight the therapeutic potential of Jiannao pills and suggest that they could represent a novel treatment option for anxiety disorders.
Acknowledgements
This study was supported by the Sichuan Provincial Administration of Traditional Chinese Medicine + Basic research project of traditional Chinese medicine (No. 2021MS449) and the Department of Science and Technology of Sichuan Province + Application basic project (No. 2021JDKY0013).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Leichsenring F, Heim N, Steinert C. A review of anxiety disorders. JAMA. 2023;329:1315–1316. doi: 10.1001/jama.2023.2428. [DOI] [PubMed] [Google Scholar]
- 2.Vu V, Conant-Norville D. Anxiety: recognition and treatment options. Psychiatr Clin North Am. 2021;44:373–380. doi: 10.1016/j.psc.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 3.van Balkom AJLM, Beeres MPJ, Scholten WD. Anxiety. Ned Tijdschr Geneeskd. 2024;168:D7921. [PubMed] [Google Scholar]
- 4.Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, Il Shin J, Kirkbride JB, Jones P, Kim JH, Kim JY, Carvalho AF, Seeman MV, Correll CU, Fusar-Poli P. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2022;27:281–295. doi: 10.1038/s41380-021-01161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rana T, Behl T, Sehgal A, Singh S, Sharma N, Abdeen A, Ibrahim SF, Mani V, Iqbal MS, Bhatia S, Abdel Daim MM, Bungau S. Exploring the role of neuropeptides in depression and anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2022;114:110478. doi: 10.1016/j.pnpbp.2021.110478. [DOI] [PubMed] [Google Scholar]
- 6.Chellappa SL, Aeschbach D. Sleep and anxiety: from mechanisms to interventions. Sleep Med Rev. 2022;61:101583. doi: 10.1016/j.smrv.2021.101583. [DOI] [PubMed] [Google Scholar]
- 7.Pourhamzeh M, Moravej FG, Arabi M, Shahriari E, Mehrabi S, Ward R, Ahadi R, Joghataei MT. The roles of serotonin in neuropsychiatric disorders. Cell Mol Neurobiol. 2022;42:1671–1692. doi: 10.1007/s10571-021-01064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SK, McCarthy DM, Vied C, Stanwood GD, Schatschneider C, Bhide PG. Transgenerational transmission of aspartame-induced anxiety and changes in glutamate-GABA signaling and gene expression in the amygdala. Proc Natl Acad Sci U S A. 2022;119:e2213120119. doi: 10.1073/pnas.2213120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. 2021;78:1343–1354. doi: 10.1001/jamapsychiatry.2021.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Needham BD, Funabashi M, Adame MD, Wang Z, Boktor JC, Haney J, Wu WL, Rabut C, Ladinsky MS, Hwang SJ, Guo Y, Zhu Q, Griffiths JA, Knight R, Bjorkman PJ, Shapiro MG, Geschwind DH, Holschneider DP, Fischbach MA, Mazmanian SK. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature. 2022;602:647–653. doi: 10.1038/s41586-022-04396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Capiau A, Huys L, van Poelgeest E, van der Velde N, Petrovic M, Somers A EuGMS Task, Finish Group on FRIDs. Therapeutic dilemmas with benzodiazepines and Z-drugs: insomnia and anxiety disorders versus increased fall risk: a clinical review. Eur Geriatr Med. 2023;14:697–708. doi: 10.1007/s41999-022-00731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceban F, Rosenblat JD, Kratiuk K, Lee Y, Rodrigues NB, Gill H, Subramaniapillai M, Nasri F, Lui LMW, Lipsitz O, Kumar A, Lee JG, Chau EH, Cao B, Lin K, Ho RC, Mansur RB, Swainson J, McIntyre RS. Prevention and management of common adverse effects of ketamine and esketamine in patients with mood disorders. CNS Drugs. 2021;35:925–934. doi: 10.1007/s40263-021-00846-5. [DOI] [PubMed] [Google Scholar]
- 14.Khan A, Akram M, Thiruvengadam M, Daniyal M, Zakki SA, Munir N, Zainab R, Heydari M, Mosavat SH, Rebezov M, Shariati MA. Anti-anxiety properties of selected medicinal plants. Curr Pharm Biotechnol. 2022;23:1041–1060. doi: 10.2174/1389201022666210122125131. [DOI] [PubMed] [Google Scholar]
- 15.Yang S, Xu Y, Peng W, Han D, Feng F, Wang Z, Gu C, Zhou X, He H. Chinese herbal medicine for symptoms of depression and anxiety in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement Ther Clin Pract. 2021;45:101470. doi: 10.1016/j.ctcp.2021.101470. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Yan Y, Wu Y, Yang H, Zhu P, Yan F, Zhao R, Tian P, Wang T, Fan Q, Su Z. Medicinal herbs for the treatment of anxiety: a systematic review and network meta-analysis. Pharmacol Res. 2022;179:106204. doi: 10.1016/j.phrs.2022.106204. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Teng J, Wang W, Yang N, Tian H, Zhang W, Peng X, Zhang J. Clinical efficacy and safety of traditional Chinese medicine Xiao Yao San in insomnia combined with anxiety. Medicine (Baltimore) 2021;100:e27608. doi: 10.1097/MD.0000000000027608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao CB, Liu J, Shi JL, Wang SN, Ding YS, He S, Lin MX, Luo J, Jiang YN, Bian LH, Yao ZW, Li QY, Wang XM, Guo JY. Anxiolytic effect of alcohol-water extracted Suanzaoren-Wuweizi herb-pair by regulating ECS-BDNF-ERK signaling pathway expression in acute restraint stress male rats. Evid Based Complement Alternat Med. 2020;2020:2078932. doi: 10.1155/2020/2078932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Wu D, Niu J, Sun Y, Wang Q, Yang B, Kuang H. Intestinal flora: a pivotal role in investigation of traditional Chinese medicine. Am J Chin Med. 2021;49:237–268. doi: 10.1142/S0192415X21500130. [DOI] [PubMed] [Google Scholar]
- 20.Gould TD, Dao D, Kovacsics C. Mood and anxiety related phenotypes in mice: characterization using behavioral tests. Springer; 2009. [Google Scholar]
- 21.Fan KQ, Li YY, Wang HL, Mao XT, Guo JX, Wang F, Huang LJ, Li YN, Ma XY, Gao ZJ, Chen W, Qian DD, Xue WJ, Cao Q, Zhang L, Shen L, Zhang L, Tong C, Zhong JY, Lu W, Lu L, Ren KM, Zhong G, Wang Y, Tang M, Feng XH, Chai RJ, Jin J. Stress-induced metabolic disorder in peripheral CD4+ T cells leads to anxiety-like behavior. Cell. 2019;179:864–879. e819. doi: 10.1016/j.cell.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Costall B, Domeney AM, Gerrard PA, Kelly ME, Naylor RJ. Zacopride: anxiolytic profile in rodent and primate models of anxiety. J Pharm Pharmacol. 1988;40:302–305. doi: 10.1111/j.2042-7158.1988.tb05254.x. [DOI] [PubMed] [Google Scholar]
- 23.Kwon CY, Choi EJ, Suh HW, Chung SY, Kim JW. Oriental herbal medicine for generalized anxiety disorder: a systematic review of randomized controlled trials. Eur J Integr Med. 2018;20:36–62. [Google Scholar]
- 24.Sarris J, McIntyre E, Camfield DA. Plant-based medicines for anxiety disorders, part 1: a review of preclinical studies. CNS Drugs. 2013;27:207–219. doi: 10.1007/s40263-013-0044-3. [DOI] [PubMed] [Google Scholar]
- 25.Socala K, Doboszewska U, Szopa A, Serefko A, Wlodarczyk M, Zielinska A, Poleszak E, Fichna J, Wlaz P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. 2021;172:105840. doi: 10.1016/j.phrs.2021.105840. [DOI] [PubMed] [Google Scholar]
- 26.Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. 2021;78:1343–1354. doi: 10.1001/jamapsychiatry.2021.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25:713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 28.Navarro-Tapia E, Almeida-Toledano L, Sebastiani G, Serra-Delgado M, García-Algar Ó, Andreu-Fernández V. Effects of microbiota imbalance in anxiety and eating disorders: probiotics as novel therapeutic approaches. Int J Mol Sci. 2021;22:2351. doi: 10.3390/ijms22052351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma T, Jin H, Kwok LY, Sun Z, Liong MT, Zhang H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol Stress. 2021;14:100294. doi: 10.1016/j.ynstr.2021.100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan T. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Okubo R, Koga M, Katsumata N, Odamaki T, Matsuyama S, Oka M, Narita H, Hashimoto N, Kusumi I, Xiao J, Matsuoka YJ. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: a proof-of-concept study. J Affect Disord. 2019;245:377–385. doi: 10.1016/j.jad.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Jia M, Zhao Y, Hui Y, Pan J, Yu H, Yan S, Dai X, Liu X, Liu Z. Supplementation of sesamin alleviates stress-induced behavioral and psychological disorders via reshaping the gut microbiota structure. J Agric Food Chem. 2019;67:12441–12451. doi: 10.1021/acs.jafc.9b03652. [DOI] [PubMed] [Google Scholar]
- 33.Wei D, Zhao Y, Zhang M, Zhu L, Wang L, Yuan X, Wu C. The volatile oil of Zanthoxylum bungeanum pericarp improved the hypothalamic-pituitary-adrenal axis and gut microbiota to attenuate chronic unpredictable stress-induced anxiety behavior in rats. Drug Des Devel Ther. 2021;15:769–786. doi: 10.2147/DDDT.S281575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Che Q, Luo T, Shi J, He Y, Xu DL. Mechanisms by which traditional Chinese medicines influence the intestinal flora and intestinal barrier. Front Cell Infect Microbiol. 2022;12:863779. doi: 10.3389/fcimb.2022.863779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang HY, Tian JX, Lian FM, Li M, Liu WK, Zhen Z, Liao JQ, Tong XL. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed Pharmacother. 2021;133:110857. doi: 10.1016/j.biopha.2020.110857. [DOI] [PubMed] [Google Scholar]
- 36.Liu D, Wang Q, Li Y, Yuan Z, Liu Z, Guo J, Li X, Zhang W, Tao Y, Mei J. Fructus gardeniae ameliorates anxiety-like behaviors induced by sleep deprivation via regulating hippocampal metabolomics and gut microbiota. Front Cell Infect Microbiol. 2023;13:1167312. doi: 10.3389/fcimb.2023.1167312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Zhong Z, Wang B, Wang Y. Xiaoyao San ameliorates high-fat diet-induced anxiety and depression via regulating gut microbiota in mice. Biomed Pharmacother. 2022;156:113902. doi: 10.1016/j.biopha.2022.113902. [DOI] [PubMed] [Google Scholar]
- 38.Zu X, Nagle DG, Zhou Y, Zhang W. Application of intestinal flora in the study of TCM formulae. In: Systems Biology and its Application in TCM Formulas Research. Elsevier; 2018. pp. 97–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.