Abstract
Objective: To investigate the clinical characteristics of 21 patients with drug-resistant epilepsy (DRE) and evaluate the therapeutic outcome of guided resection of epileptic foci by stereotactic electroencephalography (SEEG). Methods: The clinical data of 21 patients with DRE treated in the Brain Hospital of Guangxi Zhuang Autonomous Region from April 2022 to April 2024 were retrospectively analyzed. All patients underwent multimodal imaging assessment before surgery, and the SEEG electrode implantation scheme was designed based on clinical data. The etiology was determined via SEEG. Intraoperative resection of epileptogenic foci was guided by SEEG, followed by a postoperative follow-up to evaluate the therapeutic effect. Patients’ prognosis was assessed according to the Engle Seizure Control Scale, with Engel grade I indicating a good prognosis and grades II to IV indicating a poor prognosis. Logistic regression analysis was used to further explore the influencing factors of surgical prognosis. Results: A total of 240 SEEG electrodes were implanted in 21 patients, ranging from 8 to 17 per patient, with an average of (11.43±2.77) electrodes. There were a total of 1472 contact points, ranging from 31 to 118 per patient, with an average of (70.10±21.32). The postoperative follow-up time varied from 2 to 15 months. One patient experienced memory decline. Of the 21 patients, 11 (52.38%) had a good prognosis and 10 (47.62%) had a poor prognosis. Multivariate logistic regression analysis showed that long course of disease was an independent risk factor for poor postoperative prognosis. Conclusion: In the treatment of drug-resistant epilepsy, multimodal imaging based SEEG can effectively detect epileptogenic foci, guiding the surgical excision safely and efficiently. This method holds promise for enhancing surgical outcomes in the treatment of DRE.
Keywords: Drug-resistant epilepsy, stereotactic electroencephalogram, epileptotomy
Introduction
Clinical treatment of drug-resistant epilepsy (DRE) predominantly involves surgical resection of the epileptogenic focus, with accurate location of the originating region of epilepsy being the key to successful outcomes [1]. Most patients have their epileptogenic origin accurately identified through non-invasive examination. Commonly utilized preoperative non-invasive evaluation methods include symptomology, long-term video electroencephalogram, head MRI, Positron emission tomography (PET)-CT, and magnetoencephalogram [2,3]. However, when non-invasive examinations fail to locate the epileptogenic region or clarify the relationship between the functional region and epileptogenic region, invasive intracranial electrode examination becomes necessary. Stereotactic electroencephalography (SEEG) is one such method, offering advantages over traditional subdural EEG by enabling the detection of focal cortical disturbances in deep brain structures, thereby aiding in the precise localization of epilepsy origins [4].
With the continuous update and development of multimodal imaging technology, SEEG has achieved good results in preoperative assessment of refractory epilepsy [5]. Despite its potential, research on SEEG’s role in managing DRE remains limited. Based on this, this study explored the clinical characteristics of DRE and evaluated the therapeutic effect of SEEG - guided resection of epileptic foci, thereby contributing valuable evidence for clinical treatment of drug-resistant epilepsy.
Data and methods
Study design and patients
The clinical data of 21 patients with DRE treated in the Brain Hospital of Guangxi Zhuang Autonomous Region from April 2022 to April 2024 were retrospectively analyzed. Inclusion criteria: (1) Poor response to anti-epileptic drugs. (2) Variable number and frequency of seizures, occurring daily or monthly. (3) Inconclusive localization of the epileptogenic zone after a Phase I assessment on the Engle scale of seizure control, necessitating a Phase II SEEG evaluation in all eligible patients. (4) Availability of complete clinical data. Exclusion criteria: (1) Progressive central nervous system diseases such as Alzheimer’s disease and Parkinson’s disease. (2) Nervous system seizures caused by immunity, severity, infection, etc. (3) Cognitive impairment or mental illness that preclude cooperation with the preoperative SEEG assessment. (4) Presence of intracranial space-occupying lesions such as malignant tumors. The study protocol was approved by the Ethics Committee of the Brain Hospital of Guangxi Zhuang Autonomous Region (2024-032).
Methods
Non-invasive assessment
① A 3.0T MRI scanner was used for scanning in coronal, sagittal, and axial planes using T1 weighted imaging (T1WI) and T2 weighted imaging (T2WI). Fluid attenuated inversion recovery (FLAIR) imaging was performed in the axial position parallel to the long axis of the hippocampus and in the oblique coronal plane perpendicular to the right long axis of the hippocampus. The layer thickness was set to 3 mm. ② Multi-modal image fusion: the T1WI enhancement in the axial position was used for front and rear thin-layer scanning with the layer thickness set to 1 mm. AMIRA image processing software was used to subtract the data and extract the blood vessel information. Thin-layer scanning was performed on T2 FLAIR in the coronal position of lipid compression, with a layer thickness of 1 mm. Brainsuite software was used to automatically extract imaging data of cerebral cortex, and the acquired multi-modal images were fused in SINOPLAN software system. Initially, the PET and T1WI images were fused to identify the epileptogenic region. Subsequently, the extracted T2 FLAIR cerebral cortex imaging data was fused with subtracted vascular images to construct a three-dimensional visual model of the cerebral cortex, incorporating vascular structures. ③ Electrodes were positioned according to the international 10-20 system. A video electroencephalogram detection system captured the EEG activity and seizure episodes during at least three regular seizures. The lens was not be blocked during the monitoring. The caregiver removed the patient’s body covering in time to mark the seizure time.
Epileptogenic focus localization and SEEG electrode implantation
After comprehensive analysis of the patient’s seizure symptoms, seizure period and inter-seizure intervals, and a variety of imaging data, including video-electroencephalography (VEEG), head CT, MRI, PET-CT and image fusion results, the initial epilepsy network was described before surgery, and the SEEG electrode implantation scheme was developed. All 21 patients were implanted with electrodes guided by Sino-Plan neurosurgical robot [Huake Precision (Beijing) Medical Technology Co., LTD.]. Under general anesthesia, a robotic arm guided the process which involved drilling the skull, electro-coagulating and puncturing the dura, and installing the guide screw. The electrode insertion depth was calculated, and electrodes were inserted using a probe provided by Beijing Huake Hundsun Medical Technology Co., Ltd. Each electrode cap was then securely fixed. After positioning the electrodes, they were wrapped with an iodophor-soaked cotton sheet for sterility. Intracranial electrodes were monitored by video EEG to determine the origin of epilepsy, and cortical electrical stimulation was employed to record functional maps of brain areas, which were crucial in planning the surgical resection and guiding the surgical procedure.
SEEG-guided radiofrequency thermocoagulation
SEEG monitoring results were used to determine the epileptic origin location and the epileptic network, and the epileptic focus was determined by induction test, and the epileptic focus was performed by heat coagulation, carefully avoiding the mapped critical functional areas. If the initial ablation did not yield satisfactory results, the procedure was repeated until the desired effect was achieved.
Surgical excision
Postoperative SEEG data guided the identification of onset area. The excision value of the irritable area (evidenced by abnormal discharge during intermittent seizures) and the PET low metabolic area was evaluated, and the functional preservation areas were identified according to the results of electrical stimulation. This allowed for the complete resection of degenerated brain tissue surrounding the non-functional area of epileptic focus and areas treated with thermal coagulation. Based on this, the resection plan was designed, and the surgical resection was completed under the guidance of navigation.
Data collection
Clinical data of patients were collected by consulting hospital electronic medical records, including gender, age, course of disease, types of anti-epileptic drugs used before surgery, lesion locations, MRI evaluation results, surgical resection scope, and consistency between VEEG and MRI findings regarding lesion location.
Outcome measures
The primary outcome measure was treatment effectiveness. The efficacy of seizure control was evaluated by comparing the frequency of seizures one month post-surgery to the month prior to surgery. Outcomes were assessed using the Engel criteria: Grade I: Seizures were either markedly improved, occurring only as threats, or were completely absent; Grade II: Seizure frequency reduced by more than 90%; Grade III: Seizure frequency reduced by at least 75%; Grade IV: Seizure frequency reduced by less than 75%. Engel Grade I was indicative of good epilepsy control, whereas Grades II to IV were categorized as poor control. The secondary measures included adverse reactions.
Statistical method
SPSS 23.0 software was used for data analysis. Counting data were expressed as percentage (%) and analyzed using Chi-square test (Fisher exact test was used for samples less than 40). Measurement data conforming to normal distribution were expressed as mean ± standard deviation (x̅±s) and analyzed using independent sample t test. Factors influencing patient’s prognosis were analyzed by binary Logistic regression analysis. P<0.05 was considered statistically significant.
Results
Clinical features
The study cohort consisted of 12 males and 9 females. The average age was (27.10±6.24) years, ranging from 14 to 39 years. The course of disease ranged from 5 to 25 years, with an average of (14.52±4.92) years. Patients had been treated with between one and four antiepileptic drugs, averaging 2.29±0.85 drugs per patient. Lesion localization varied among the patients: 8 cases had lesions in the left temporal and medial structures, 4 in the right temporal and medial structures, 2 in the hippocampus (one left and right), 1 in bilateral temporal lobes, 2 in parieto-occipital cortex (one left and right), 1 in left frontal lobe, 1 at the left temporoparietal junction (roughly in the supramarginal gyrus area), and 2 in the insula (one on each side) (Table 1).
Table 1.
Clinical features [n (%), x̅±s]
| Gender | |
| Female | 9 (42.86) |
| Male | 12 (57.14) |
| Age | 27.10±6.24 |
| Course of disease | 13.98±8.59 |
| Antiepileptic drugs were used preoperatively | 2.29±0.85 |
| Focal location | |
| Left temporal lobe | 8 (38.10) |
| Right temporal lobe | 4 (19.05) |
| Bilateral temporal lobe | 1 (4.76) |
| Hippocampal area | 2 (9.52) |
| Parieto-occipital cortex | 2 (9.52) |
| Left frontal lobe | 1 (4.76) |
| Left temporoparietal junction | 1 (4.76) |
| Left insula | 1 (4.76) |
| Right insula | 1 (4.76) |
SEEG electrode implantation
A total of 240 SEEG electrodes were implanted in 21 patients, and 8 to 17 SEEG electrodes were implanted in each patient, with an average of (11.43±2.77). There were a total of 1472 contact points, and each patient had 31 to 118 contact points, with an average of (70.10±21.32). All electrodes were successfully implanted in their preoperatively planned location, and the procedure was well-tolerated by the patients. There were no complications such as electrode displacement or fracture, intracranial hemorrhage, infection or cerebrospinal fluid leakage. More than 3 regular episodes were detected during monitoring.
Surgical resection
Among the 21 patients, 9 underwent localized resection at the site of the epileptogenic focus, and the remaining 12 underwent extended area resection. The postoperative hospital stay ranged from 9 to 31 days, with an average of (18.53±6.01) days. The postoperative follow-up period varied from 2 to 15 months. Outcomes were favorable in 11 cases (52.38%), categorized as Engel grade I, whereas the other 10 cases (47.62%) exhibited a poor prognosis, including 5 cases (23.81%) of grade II, 2 cases (9.52%) of grade III, and 3 cases (14.29%) of grade IV. After surgery, one patient experienced memory loss, and no cases reported intracranial hemorrhage, infection, hemiplegia or other complications.
Analysis of influencing factors
Fisher’s exact test results showed that the disease course in the poor prognosis group was significantly longer than that in the good prognosis group, as shown in Table 2. Logistic regression analysis showed that long course of disease was an independent risk factor for poorer outcomes in surgeries for drug-refractory epilepsy [OR=2.768 (95% CI: 1.027-7.461), P=044].
Table 2.
Analysis of factors influencing the prognosis of patients with drug-resistant epilepsy [x̅±s, n (%)]
| Factors | Good prognosis (n=11) | Poor prognosis (n=10) | t/χ2 | P |
|---|---|---|---|---|
| Gender | ||||
| Female | 3 (27.27) | 6 (60.00) | - | 0.198 |
| Male | 8 (72.73) | 4 (40.00) | ||
| Age | 27.82±6.52 | 26.30±6.15 | 0.547 | 0.591 |
| Course of disease | 11.18±3.28 | 18.20±3.61 | 4.665 | <0.001 |
| Drugs for epilepsy control | ||||
| ≤2 | 7 (63.64) | 5 (50.00) | - | 0.670 |
| >2 | 4 (36.36) | 5 (50.00) | ||
| Focal location | ||||
| Temporal lobe | 9 (81.82) | 6 (60.00) | - | 0.361 |
| Nontemporal lobe | 2 (18.18) | 4 (40.00) | ||
| MRI | ||||
| Negative | 0 (0.00) | 1 (10.00) | - | 0.476 |
| Positive | 11 (100.00) | 9 (90.00) | ||
| Consistency of VEEG and MRI localization | ||||
| Consistent | 8 (72.73) | 3 (30.00) | - | 0.086 |
| Inconsistent | 3 (27.27) | 7 (70.00) | ||
| Scope of resection | ||||
| Local excision | 3 (27.27) | 6 (60.00) | - | 0.198 |
| Extended excision | 8 (72.73) | 4 (40.00) |
VEEG, video-electroencephalography.
Typical case
A 28-year-old female suffered from seizures for more than 3 years, and worsened 1 day prior to the consultation. Patients exhibited three distinct types of seizure manifestations: ① Lip smacking, each episode lasted about 1-2 minutes; Post-ictal amnesia is noted, where the patient cannot recall the event. ② Automatisms and abnormal behaviors: This included sudden mumbling, abnormal behavior such as walking, repeatedly folding a quilt, jumping, and occasionally groping or grasping with the hands. These episodes vary in duration from more than 10 seconds to 10 minutes, with partial consciousness sometimes retained. ③ Staring episodes: Characterized by a sudden onset where the patient appears to stare blankly, with clenched hands and both lower limbs extended. These episodes last from a few seconds to over 10 seconds. Her medication regimen comprised Sodium Slow-Release Tablets, 0.5 g, twice daily; Oxcarbazepine, 450 mg, twice daily; Clonazepam, 2 mg, once daily. Physical examination revealed no abnormalities in the nervous system.
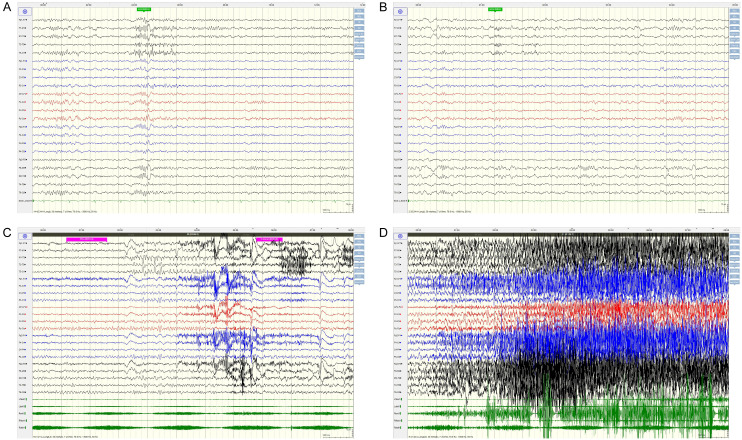
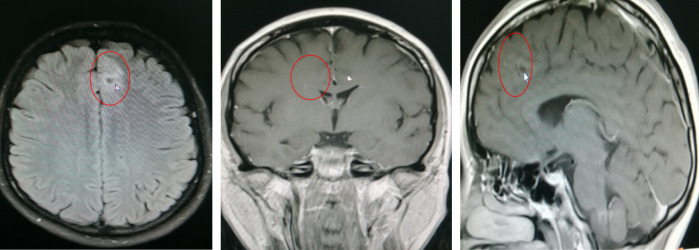
As shown by EEG (Figure 1) findings, during the intermittent phase, epileptiform discharges localized to the left temporal and occipital area; and during the seizure phase, sequence initiating with lip-smacking, followed by head and eye deviation to the right, vocalization from the larynx, right limb tonus, and culminating in a Generalized Tonic-Clonic Seizure (GTCS), the EEG indicated diffuse activity, primarily involving the left frontal and temporal lobes. Head MRI examination revealed abnormal signal focus in the left frontal lobe, raising suspicions of possible oligodendroglioma or astrocytoma (Figure 2). PET examination showed a nodular low-density shadow in the left frontal lobe, with localized hypometabolism in the left cingulate gyrus, suggesting a secondary epileptic focus associated with a space-occupying lesion in the left frontal lobe. Fusion of preoperative PET and MRI is shown in Figure 3.
Figure 1.
Preoperative EEG examination of the typical case. A, B: Preoperative intermittent VEEG figures; C, D: VEEG in the preoperative episode. VEEG, video-electroencephalography.
Figure 2.
Preoperative head MRI of the typical case.
Figure 3.
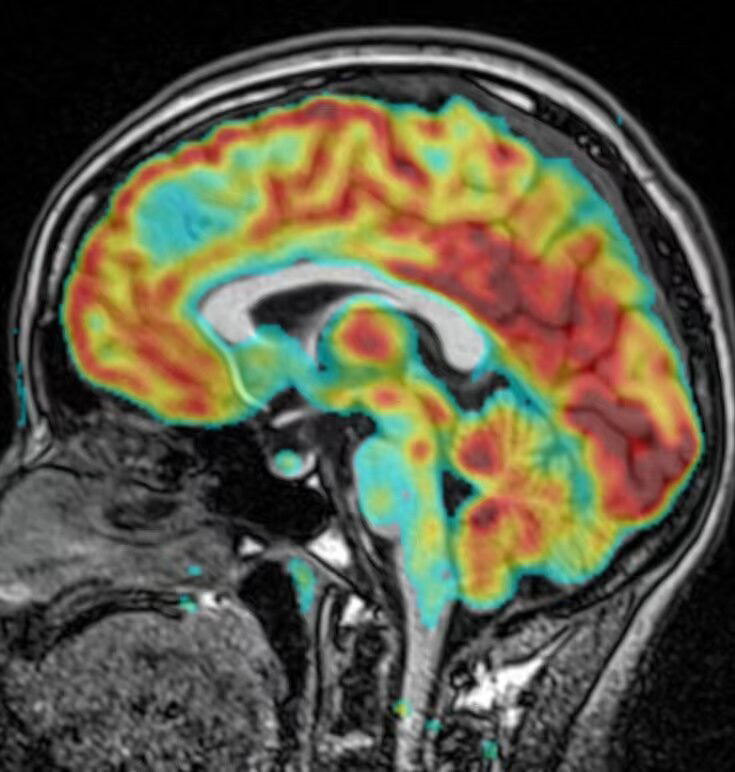
Preoperative fusion of PET and MRI. PET, Positron emission tomography.
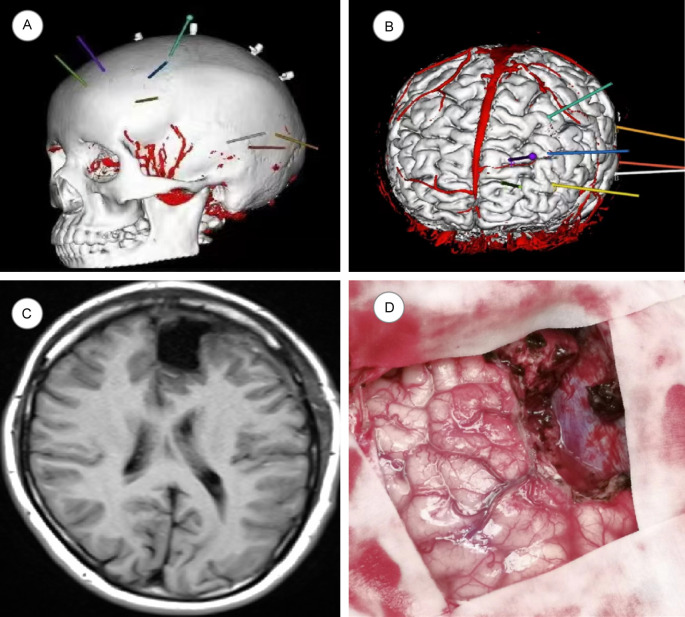
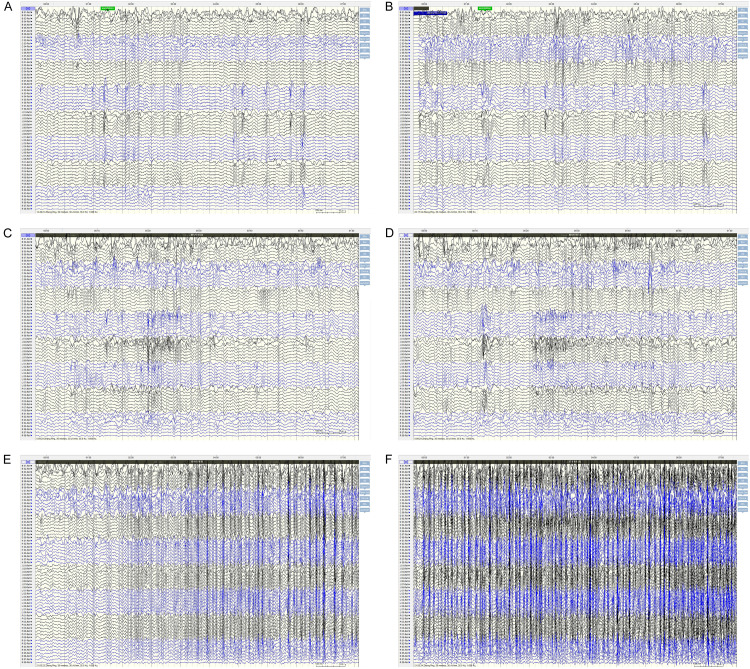
SEEG targets and pathway design: (1) Middle temporal gyrus to hippocampal head; (2) Middle temporal gyrus to hippocampal tail; (3) Posterior middle temporal gyrus to cingulate isthmus; (4) Inferior margin of middle frontal gyrus to anterior inferior margin of the lesion; (5) Lateral frontal pole to anterior cingulate gyrus; (6) Inferior margin of middle frontal gyrus to posterior margin of the lesion; (7) Supra frontal gyrus, running through the lesion to dorsal side of the anterior cingulate gyrus; (8) Posterior portion of middle frontal gyrus to pre-supplementary motor area (PRE-SSMA). Two months after SEEG implantation, the patient was readmitted to the hospital for surgical resection of the epileptogenic focus in the left frontal lobe. During the 15-month follow-up, the patient did not experience any further seizures. The precise location of electrode placement and the extent of resection are shown in Figure 4, and the postoperative EEG after SEEG electrode implantation is shown in Figure 5.
Figure 4.
Electrode placement and resection range in the typical case. A, B: Illustration of electrode implantation in the skull; C: MRI after excision of left frontal lobe epileptic focus under SEEG guidance; D: Surgical removal of left perifrontal brain tissue.
Figure 5.
EEG after SEEG implantation in the typical case. A, B: Intermittent EEG after electrode implantation; C, D: EEG at the beginning of SEEG attack; E, F: Epileptic EEG after electrode implantation.
Discussion
Currently, many non-invasive localization methods are employed to localize epileptogenic focus, including PET, magnetoencephalgraphy, and head MRI. However, studies have shown that about 30% of drug-refractory epilepsy patients cannot have their epileptogenic focus accurately located by non-invasive examination [6,7]. For such patients, clinical use of subdural EEG technology is common. This method involves implanting electrodes onto the cerebral surface beneath the intracranial dural via a craniotomy hole to locate the epileptogenic focus [8]. However, subdural EEG needs to expose a large area of the cerebral cortex, which is relatively invasive and prone to postoperative complications. Moreover, the scope of exploration is relatively limited, lacking the capacity to thoroughly investigate subcortical and the deep cortical regions. In contrast, SEEG technology uses a network to locate epileptogenic foci in the brain, offering the advantage of prolonged monitoring without the need for a craniotomy [9,10]. Despite its potential, there remains a paucity of research on the application of SEEG technology in the contest of drug-refractory epilepsy. This study highlights the significant clinical importance and innovation of SEEG-guided resection of epileptic foci. The application of SEEG technology, combined with multimodal imaging, provides a powerful tool for accurately locating and removing epileptic foci, thus significantly improving surgical success and treatment outcomes. The combination of multimodal imaging with SEEG not only improves the localization accuracy, but also provides more comprehensive brain information for surgery. In addition, the prognostic evaluation model established by Fisher accurate test and logistic regression analysis provided a scientific basis for predicting surgical outcomes and guiding clinical treatment decisions.
The rapid development of multi-modal imaging techniques such as MRI and PET fusion, plays an important role in the localization of epileptogenic foci. The fused images not only allow for the observation of cortical metabolism but also compensate for the lack of detailed anatomical structure displayed by PET/CT, thereby enhancing the detection of epileptogenic foci [11,12]. In this study, all patients underwent both PET examination and MRI examinations before surgery. The fused imaging data further provided a reference for the design of SEEG electrode placement scheme, enabling the identification of surgical targets in drug-refractory epilepsy patients, whose epileptogenic foci could not previously be accurately located.
Current clinical multimodal imaging techniques mostly use T1WI enhancement for anteroposterior thin-layer scanning in the axial plane and T2 fluid attenuated inversion recovery (FLAIR) imaging for thin-layer scanning in the compressed-lipid coronal plane. In this process, the T1WI enhancements are subtracted using the AMIRA software to extract information about the vasculature, while T2 FLAIR imaging data from the cerebral cortex are automatically extracted and fused using Brainsuite software. This approach not only allows for 3D visualization of the cerebral cortex, but also aids in planning the placement of intracranial SEEG electrodes, providing a clear basis for surgical resection [13,14]. In this study, a total of 240 SEEG electrodes were implanted in 21 patients, all of whom underwent epileptogenic focectomy. No patient reported complications such as severe hemorrhage, cerebrospinal fluid leakage, infection, and SEEG electrode displacement or fracture, while only one patient experienced postoperative memory loss. This aligns with findings of Lamberink et al. [15], indicating that SEEG based on multimodal images has good safety profile. In addition, the results of this study showed that patients with a long course of disease had a higher probability of poor postoperative prognosis, which was a risk factor for poor prognosis after resection of epileptic foci, consistent with previous literature reports [16]. Delev et al. [17] showed that the duration of the disease was negatively correlated with the patient’s surgical prognosis; the prolongation of the disease duration not only necessitates increased postoperative use of antiepileptic drugs but may also exacerbate the expansion of abnormal tissue areas and seizure signaling pathways, increasing the likelihood of secondary epilepsy-associated damage in patients with a longer course of disease. Therefore, for patients with drug-refractory epilepsy, surgical treatment should be actively taken if conditions permit.
Compared with traditional subdural EEG, SEEG has the following advantages [18,19]: (1) SEEG facilitates the detection of deep structures that are otherwise challenging to reach, such as the medial side of the frontal lobe, the medial sulci cortex, the insula, and the medial temporal lobe. (2) Compared with subdural EEG, SEEG does not require craniotomy. This minimally invasive nature stems from precise preoperative planning, sophisticated electrode fabrication technology, and delicate operation. (3) SEEG is particularly beneficial for patients requiring extensive, bilateral electrode implantation or patients with severe adhesion who may need secondary surgeries for SEEG electrode placement. The absence of a craniotomy with SEEG saves significant time, allowing for more thorough analysis of SEEG results. This method also permits initial therapeutic interventions such as thermocoagulation in the epilepsy-induced area before electrode removal or additional surgical interventions if the initial resection is not effective [20,21]. In addition, postoperative outcomes in this study have shown excellent seizure control, with a significant improvement in seizure frequency compared to pre-surgery conditions. The proportion of Engel grade I patients was 52.38%, aligning with findings from previous studies like Yao et al. [22], which underscores utility of SEEG guided by multimodal imaging in the clinical management of drug-resistant epilepsy.
In summary, SEEG based on multimodal images can effectively detect epileptogenic foci with high safety profile. It holds substantial promise in the application for the surgical treatment of drug-resistant epilepsy. Nonetheless, the relatively small sample size of this study suggests a need for further research with a larger cohort to validate these findings comprehensively.
Disclosure of conflict of interest
None.
References
- 1.Bullinger KL, Alwaki A, Gross RE. Surgical treatment of drug-resistant generalized epilepsy. Curr Neurol Neurosci Rep. 2022;22:459–465. doi: 10.1007/s11910-022-01210-w. [DOI] [PubMed] [Google Scholar]
- 2.Tóth M, Barsi P, Tóth Z, Borbély K, Lückl J, Emri M, Repa I, Janszky J, Dóczi T, Horváth Z, Halász P, Juhos V, Gyimesi C, Bóné B, Kuperczkó D, Horváth R, Nagy F, Kelemen A, Jordán Z, Újvári Á, Hagiwara K, Isnard J, Pál E, Fekésházy A, Fabó D, Vajda Z. The role of hybrid FDG-PET/MRI on decision-making in presurgical evaluation of drug-resistant epilepsy. BMC Neurol. 2021;21:363. doi: 10.1186/s12883-021-02352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernasconi A, Bernasconi N. The role of MRI in the treatment of drug-resistant focal epilepsy. Eur Neurol. 2022;85:333–341. doi: 10.1159/000525262. [DOI] [PubMed] [Google Scholar]
- 4.Doss DJ, Johnson GW, Englot DJ. Imaging and stereotactic electroencephalography functional networks to guide epilepsy surgery. Neurosurg Clin N Am. 2024;35:61–72. doi: 10.1016/j.nec.2023.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gholipour T, Koubeissi MZ, Shields DC. Stereotactic electroencephalography. Clin Neurol Neurosurg. 2020;189:105640. doi: 10.1016/j.clineuro.2019.105640. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Fresnedo A, Domingo RA, McGeary RC, Sirven JI, Feyissa AM, Tatum W, Ritaccio AL, Middlebrooks EH, Grewal SS. Encephalocele-associated drug-resistant epilepsy of adult onset: diagnosis, management, and outcomes. World Neurosurg. 2021;151:91–101. doi: 10.1016/j.wneu.2021.04.121. [DOI] [PubMed] [Google Scholar]
- 7.Missey F, Rusina E, Acerbo E, Botzanowski B, Trébuchon A, Bartolomei F, Jirsa V, Carron R, Williamson A. Orientation of temporal interference for non-invasive deep brain stimulation in epilepsy. Front Neurosci. 2021;15:633988. doi: 10.3389/fnins.2021.633988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frauscher B, Mansilla D, Abdallah C, Astner-Rohracher A, Beniczky S, Brazdil M, Gnatkovsky V, Jacobs J, Kalamangalam G, Perucca P, Ryvlin P, Schuele S, Tao J, Wang Y, Zijlmans M, McGonigal A. Learn how to interpret and use intracranial EEG findings. Epileptic Disord. 2024;26:1–59. doi: 10.1002/epd2.20190. [DOI] [PubMed] [Google Scholar]
- 9.Mallela AN, Abou-Al-Shaar H, Nayar GM, Luy DD, Barot N, González-Martínez JA. Stereotactic electroencephalography implantation through nonautologous cranioplasty: proof of concept. Oper Neurosurg (Hagerstown) 2021;21:258–264. doi: 10.1093/ons/opab260. [DOI] [PubMed] [Google Scholar]
- 10.Kandregula S, Matias CM, Malla BR, Sperling MR, Wu C, Sharan AD. Accuracy of electrode insertion using frame-based with robot guidance technique in stereotactic electroencephalography: supine versus lateral position. World Neurosurg. 2021;154:e325–e332. doi: 10.1016/j.wneu.2021.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Pérez Hinestroza J, Mazo C, Trujillo M, Herrera A. MRI and CT fusion in stereotactic electroencephalography (SEEG) Diagnostics (Basel) 2023;13:3420. doi: 10.3390/diagnostics13223420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Ellenrieder N, Dubeau F, Dudley RWR, Dufresne D, Gotman J, Baillet S, Moreau JT, Bernasconi N, Bernasconi A, Osterman B, Simard-Tremblay E, Myers KA. “Generalized-to-focal” epilepsy: stereotactic EEG and high-frequency oscillation patterns. Epileptic Disord. 2022;24:1087–1094. doi: 10.1684/epd.2022.1489. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira LP, Pérez-Enríquez C, Barguilla A, Langohr K, Conesa G, Infante N, Principe A, Rocamora R. Stereo-electroencephalography-guided radiofrequency thermocoagulation in patients with MRI-negative focal epilepsy. J Neurosurg. 2022;138:837–846. doi: 10.3171/2022.6.JNS22733. [DOI] [PubMed] [Google Scholar]
- 14.Du C, Jin W, Wang L, Yan J, Li G, Wu Y, Zhao G, Cui D, Yin S. Stereoelectroencephalography-guided radiofrequency thermocoagulation of the epileptogenic zone: a potential treatment and prognostic indicator for subsequent excision surgery. Acta Neurochir (Wien) 2024;166:210. doi: 10.1007/s00701-024-06106-x. [DOI] [PubMed] [Google Scholar]
- 15.Lamberink HJ, Otte WM, Blümcke I, Braun KPJ European Epilepsy Brain Bank writing group; study group; European Reference Network EpiCARE. Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. 2020;19:748–757. doi: 10.1016/S1474-4422(20)30220-9. [DOI] [PubMed] [Google Scholar]
- 16.Barba C, Cossu M, Guerrini R, Di Gennaro G, Villani F, De Palma L, Grisotto L, Consales A, Battaglia D, Zamponi N, d’Orio P, Revay M, Rizzi M, Casciato S, Esposito V, Quarato PP, Di Giacomo R, Didato G, Pastori C, Pavia GC, Pellacani S, Matta G, Pacetti M, Tamburrini G, Cesaroni E, Colicchio G, Vatti G, Asioli S, Caulo M TLE Study Group. Marras CE, Tassi L. Temporal lobe epilepsy surgery in children and adults: a multicenter study. Epilepsia. 2021;62:128–142. doi: 10.1111/epi.16772. [DOI] [PubMed] [Google Scholar]
- 17.Delev D, Oehl B, Steinhoff BJ, Nakagawa J, Scheiwe C, Schulze-Bonhage A, Zentner J. Surgical treatment of extratemporal epilepsy: results and prognostic factors. Neurosurgery. 2019;84:242–252. doi: 10.1093/neuros/nyy099. [DOI] [PubMed] [Google Scholar]
- 18.Narasimhan S, Kundassery KB, Gupta K, Johnson GW, Wills KE, Goodale SE, Haas K, Rolston JD, Naftel RP, Morgan VL, Dawant BM, González HFJ, Englot DJ. Seizure-onset regions demonstrate high inward directed connectivity during resting-state: an SEEG study in focal epilepsy. Epilepsia. 2020;61:2534–2544. doi: 10.1111/epi.16686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagata K, Kunii N, Shimada S, Saito N. Utilizing excitatory and inhibitory activity derived from interictal intracranial electroencephalography as potential biomarkers for epileptogenicity. Neurol Med Chir (Tokyo) 2024;64:65–70. doi: 10.2176/jns-nmc.2023-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Wang J, Zhao Q, Wang T, Wang P. MRI-negative medial temporal lobe epilepsy can benefit from stereotactic radiofrequency thermocoagulation applied to the amygdalohippocampal complex. Curr Med Imaging. 2022;18:712–718. doi: 10.2174/1573405617666211005144936. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Jin W, Zhang Y, Wang S, Li Q, Qin J, Li Z, Cheng Y, Feng K, Yin S. Stereoelectroencephalography-guided radiofrequency thermocoagulation in drug-resistant focal epilepsy. Ann Transl Med. 2022;10:192. doi: 10.21037/atm-21-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Y, Hu W, Zhang C, Wang X, Zheng Z, Sang L, Shao X, Zhang K. A comparison between robot-guided and stereotactic frame-based stereoelectroencephalography (SEEG) electrode implantation for drug-resistant epilepsy. J Robot Surg. 2023;17:1013–1020. doi: 10.1007/s11701-022-01504-8. [DOI] [PubMed] [Google Scholar]