Abstract
Objective: To investigate the pharmacological mechanisms and clinical effects of Traditional Chinese Medicine (TCM) wet compresses on pain, inflammation, and quality of life in patients with soft tissue injuries. Methods: First, the network pharmacology of the active ingredients from TCM wet compresses were identified using the TCMSP (Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform) database (Common monkshood mother root, Two-toothed achyranthes root, Sour orange, Garden balsam stem, Safflower, Ground beetle, Zingiber, Haichow elsholtzia herb). Relevant targets for soft tissue injuries were obtained from GeneCards, revealing 20 key therapeutic targets. Pathway clustering highlighted inflammation-related pathways. Then we conducted clinical investigation by retrospectively analyzing the data of 80 patients with soft tissue injury treated in our hospital from January 2023 to December 2023. The data from healthy subjects who had physical examination in our hospital during the same period served as the control group (n=40). According to the treatment plan, the patients were divided into study group A (n=40, external treatment with Voltaalin ointment), and study group B (n=40, treatment with TCM wet compress). Pain intensity, inflammatory factor levels, and quality of life of the patients between the two groups were compared. Results: Analysis of TCM wet compresses revealed 86 active ingredients and 259 intersecting targets, with key ingredients including sitogluside, beta-sitosterol, and quercetin. Key targets included IL-6, IL-1B, TNF, CXCL8, and IL10. Clinically, both patient groups showed significant reductions in pain and inflammatory markers, with greater improvements in study group B. In addition, quality of life improved notably post-treatment in study group B. Conclusion: TCM wet compress patches are effective in reducing pain, modulating inflammation, and improving the quality of life in patients with soft tissue injury. These findings support TCM wet compresses as a promising alternative treatment.
Keywords: Chinese herbal wet compress patch, soft tissue injury, pain symptoms, inflammatory factors, quality of life, network pharmacology
Introduction
Soft tissue injuries, equivalent to tendon injuries in Chinese medicine, encompass a variety of conditions affecting the skin, subcutaneous tissues, muscles, tendons, joint capsules, synovial sacs, peripheral nerves, blood vessels, intervertebral discs, and other tissues. These injuries can result from violence, chronic strain injury, and invasion by external evils [1,2]. Soft tissue injuries often manifest as localized pain, swelling, and impaired function, significantly impacting patients’ quality of life.
Western medicine approaches typically involve the administration of oral non-steroidal anti-inflammatory analgesics, which may lead to gastrointestinal adverse effects and compromise treatment adherence [3,4]. Conversely, external Chinese medicine therapies offer a viable alternative for managing soft tissue injuries. These modalities encompass a range of interventions, including acupuncture, moxibustion, myofascial manipulation, and the external application of traditional Chinese medicine formulations. These methods provide promising avenues for alleviating symptoms and promoting recovery, while minimizing the risk of adverse reactions associated with conventional treatments.
Aseptic inflammatory reactions can occur locally after soft tissue damage, and the release of relevant inflammatory mediators can further aggravate the inflammatory reaction [5]. Early manifestations of soft tissue typically include local edema, congestion. As the injury progresses, inflammatory adhesions and fibrous tissue proliferation may develop, and ultimately leading to fibrous degeneration [6]. Effectively managing soft tissue injuries involves controlling inflammation and preventing tissue adhesions or contractures [7]. Historical texts like the General Records of Shengji emphasize the importance of addressing meridian and collateral disruptions, blood stagnation, and pain following injuries. Treatment strategies aim to relax tendons and collaterals, improve blood circulation, resolve blood stasis, and alleviate swelling and pain. Wet compress therapy is commonly used during the acute phase of soft tissue injuries, with prescriptions tailored to different stages of injury progression. Early interventions focus on regulating qi, promoting blood circulation, and resolving blood stasis, while mid-stage treatments aim to harmonize bodily functions and alleviate tendon tension. In the late stage of injury, therapies focus on strengthening tendons and bones to facilitate recovery and functional restoration [8].
Previous studies have concluded that wet compresses enable effective transdermal absorption, maintaining a stable local blood drug concentration, which can effectively relieve local pain, swelling and dysfunction along with other symptoms [9]. However, the traditional formula for wet compress paste is complex and cumbersome to prepare. Given the complexity of current technology and the limited research available, this study simplifies the composition of TCM wet compresses. Our primary objective is to investigate the therapeutic mechanism of this composition and assess its clinical efficacy in alleviating pain symptoms, reducing inflammatory factor levels, and enhancing quality of life for patients.
Materials and methods
Ethics statement
This study was approved by the Medical Ethics Committee of the Sixth Medical Center of PLA General Hospital.
Mechanism of action of Chinese medicine wet compress patch
The traditional Chinese medicine wet compress patch (Common monkshood mother root, Two-toothed achyranthes root, Sour orange, Garden balsam stem, Safflower, Ground beetle, Zingiber, Haichow elsholtzia herb) used in this research was searched in the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) and the comprehensive chemical database of Shanghai Institute of Organics. We then employed the GeneCards database to identify targets related to soft-tissue injury and screened effective ingredients for further study. These targets were annotated using the Uniprot database. We analyzed the intersection of targets from the TCM wet compress and soft tissue injury targets, identified 20 key targets using Cytoscape 3.9.1, and plotted the protein-protein interaction (PPI) map. This allowed us to visualize the network map of TCM action and perform cluster analysis to discern key pathways enriched by the TCM wet compress’s action, offering insights into its therapeutic potential for soft tissue injuries.
General information
Eighty patients with soft tissue injuries, who underwent treatment at the Sixth Medical Center, Chinese PLA General Hospital between January 2023 and December 2023, were chosen for retrospective examination. Inclusion criteria: ① fulfilling the diagnostic standards for qi stagnation and blood stasis as outlined in the Guiding Principles for Clinical Research of New Chinese Medicines, as well as meeting the diagnostic criteria for soft tissue contusion in Western medicine; ② aged 18-45 years old; ③ injury occurring within 48 h; ④ complete clinical data. Exclusion criteria: ① accompanied by limb fracture, dislocation, vascular and nerve injury; ② combined with serious cardiovascular, cerebrovascular or other primary diseases or psychiatric diseases; ③ presence of allergies or allergic to the ingredients of the medicinal wine; ④ pregnant or breastfeeding individuals. The clinical data from healthy subjects aged between 18 and 45 who underwent physical examination in our hospital from January 2023 to December 2023 served as the controls (n=40). According to different treatment plans, the patients were divided into study group A (n=40, treated with voltaalin ointment) and study group B (n=40, treated with TCM wet compress).
Methods of treatment
(1) Study group A received conventional treatment, which involved elevating the injured limb appropriately and immobilizing it with splints; applying Futhalin ointment externally for pain relief, and inserting diclofenac suppositories as directed by the physician in case of severe pain; applying ice packs for cold compression, with 20-minute intervals on and 10-minute intervals off, then switching to heat packs after 24 hours, with each application lasting 20 minutes once a day; and administering safflower injection to dilate blood vessels and improve microcirculation. One treatment course lasted for 14 days.
(2) Study group B was treated with TCM wet compress. The injured limb was properly elevated and the brace was fixed. The TCM wet compress was applied using a specific blend of herbs: Common monkshood mother root (15 g), Two-toothed achyranthes root (15 g), Sour orange (15 g), Garden balsam stem (15 g), safflower (10 g), Ground beetle (10 g), Zingiber (10 g) and Haichow elsholtzia herb (10 g). The Chinese medicine composition has excellent analgesic effect and excellent therapeutic effect on soft tissue injury. These ingredients were boiled in 1000 ml of water for 20 minutes, followed by adding 3 towels and simmering for an additional 10 minutes at a relative density of 1.3 (80°C). The infused towels were then removed, placed in a basin, and applied to the affected area once they reached a suitable temperature. Patients were instructed to lie in a comfortable position, exposing the affected area. The first towel was applied, quickly followed by the remaining two towels on top, and then covered with a treatment towel. The wet hot compress was maintained at a tolerable temperature to avoid scalding and was applied for 30 minutes per session, twice daily, with the treatment course spanning 14 days.
Detection methods
Before treatment and after one course of treatment, 5 ml fasting venous blood was collected from participants in both Study group A and Study group B, and centrifuged for 10 min at a centrifugation radius of 10 cm at 3000 r/min. Serum levels of IL-6 (interleukin-6), CRP (C-reactive protein), tumor necrosis factor alpha (TNF-alpha), IL-1β (interleukin-1β), prostaglandin E2 (PEG2), IL-10 (interleukin-10) and IL-4 (interleukin-4) were measured by enzyme-linked immunoassay. Identical blood collection and analysis procedures were employed for the control group, ensuring consistency across all measurements.
Observation indicators
(1) The Visual Analog Scale (VAS) was utilized to assess the pain experienced by patients in both groups. Scores ranged from 0-10 points, with lower scores indicating less severe pain. Score 0: no pain, score 1-3: mild pain, score 4-6: moderate pain, and score 7-10: severe pain.
(2) Collection of inflammation-related factors: Inflammatory factors: IL-6, CRP, TNF-α, IL-1β, PEG2, and anti-inflammatory factors: IL-10, IL-4 were examined in both groups before and after one course of treatment.
(3) Patient quality of life indicators: The Health Status Questionnaire (36-item Short-Form, SF-36), a universal measurement scale developed by the American Medical Outcomes Research Group (AMORG), was used to assess the quality of life in patients before and after one course of treatment. The scale consists of 36 items, including general health status, social functioning, somatic roles, somatic pain, somatic functioning, vitality, mental health, and emotional roles. Each domain’s score contributes to a cumulative total score ranging from 0 to 100, with higher scores indicating better quality of life.
Statistical methods
SPSS 27.0 was employed for data analysis. Data following a normal distribution were expressed using mean and standard deviation (x±s), while non-normally distributed data were represented as medians (lower quartile, upper quartile). For single factor analysis, the t-test was utilized for normally distributed continuous data, whereas the chi-square test was applied for comparisons across categorical data, which were expressed as percentages (%). Statistical significance was set at P < 0.05.
Results
Network pharmacology results
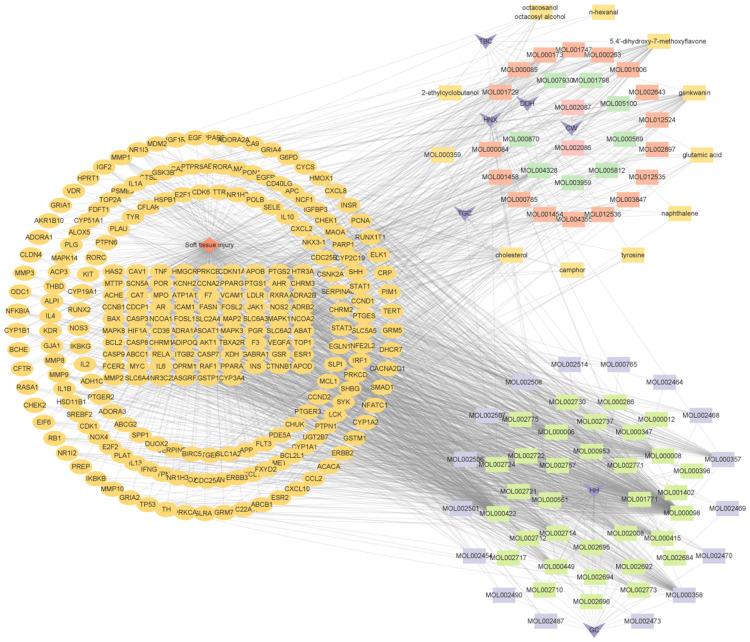
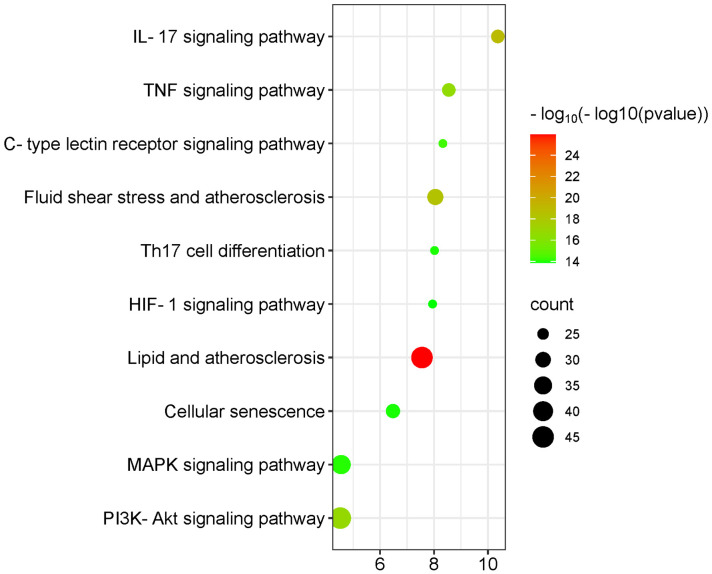
A total of 335 effective targets were collected from TCM wet compresses, and 5,564 relevant targets were collected for soft tissue injury, with 259 intersecting targets, see Figure 1. The network diagram of TCM wet compresses-active ingredients-soft tissue injury was constructed, and the results showed that there were 354 nodes in the network, including 1 disease node, 8 drug nodes, 86 active herb ingredient nodes, and 259 target protein nodes, involving 1,704 interactions. Among the 86 effective TCM ingredients, Sitogluside (MOL000357), beta-sitosterol (MOL000358), baicalein (MOL002714), rutin (MOL000415), kaempferol (MOL000422), Stigmasterol (MOL000449), Astragalin (MOL000561), apigenin (MOL000008), and quercetin (MOL000098) were the common chemical constituents of the herbs, as shown in Figure 2. Sitogluside, beta-sitosterol, rutin, quercetin and kaempferol are the key active ingredients of TCM wet compresses for treating soft tissue injury, as shown in Table 1. The network analysis of the PPI diagrams identified 20 core targets for the treatment of soft tissue injury by TCM wet dressing, with IL-6, IL-1B, TNF, CXCL8, IL10 being crucial for treatment efficacy, see Figure 3. Additionally, 10 signaling pathways significantly impacted by the drug treatment were visualized through target clustering analysis, as shown in Figure 4.
Figure 1.
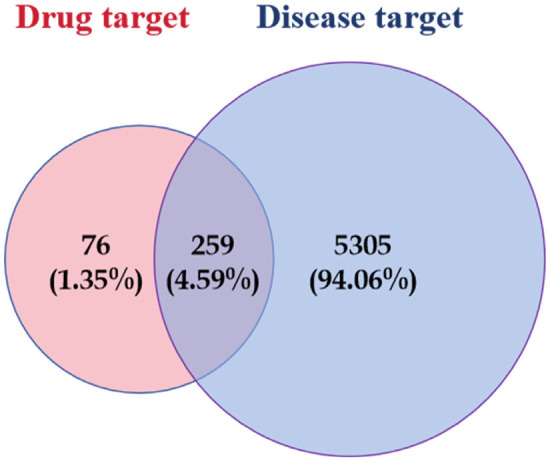
Intersecting targets of traditional Chinese medicine wet compress patches and soft tissue injuries.
Figure 2.
Diagram of the mechanism of soft tissue injury by Chinese medicine wet compress patch. Note: Orange rhombus is the node of soft tissue injury; dark purple quadrilateral is the node of TCM. TBC: Ground beetle, TGC: Garden balsam stem, HNX: Two-toothed achyranthes root, DDH: Sour orange, CW: Common monkshood mother root, HH: Safflower, GJ: Zingiber. The rectangle is the active ingredient of TCM, in which orange is the active ingredient of Garden balsam stem and Ground beetle, orange-red is the active ingredient of Two-toothed achyranthes root, green is the active ingredient of Sour orange, pink is the active ingredient of Common monkshood mother root, cyan green is the active ingredient of Safflower, and lavender is the active ingredient of Zingiber. The orange oval was the soft tissue injury target of wet application of traditional Chinese medicine.
Table 1.
Main active ingredients of traditional Chinese medicine wet compress in the treatment of soft tissue injury
| Active ingredient of Chinese medicine | MOL number | Chinese medicine ingredient | The active ingredient corresponds to the target |
|---|---|---|---|
| Sitogluside | MOL000357 | Zingiber, Safflower, Two-toothed achyranthes root | PGR, PTGS1, CHRM3, KCNH2, CHRM1, SCN5A, PTGS2, HTR3A, RXRA, ADRB2, NCOA2 |
| Beta-sitosterol | MOL000358 | Zingiber, Safflower, Two-toothed achyranthes root | PGR, NCOA2, PTGS1 |
| PTGS2, KCNH2, CHRM3, CHRM1, SCN5A, ADRA1A, CHRM2, ADRB2, SLC6A4, OPRM1, GABRA1, BCL2, BAX, CASP9, CASP3, CASP8 | |||
| PRKCA, PON1, MAP2 | |||
| Kaempferol | MOL000422 | Safflower, Two-toothed achyranthes root, Garden balsam stem | NOS2, PTGS1, AR, PTGS2, NCOA2, PGR, CHRM1, ACHE, SLC6A2, CHRM2, GABRA1, F7, RELA, IKBKB |
| AKT1, BCL2, BAX, TNF, CASP3, MAPK8, XDH, MMP1, STAT1, PPARG, HMOX1, CYP3A4, CYP1A2, CYP1A1, ICAM1, SELE, VCAM1, NR1I2, CYP1B1, HAS2, GSTP1, AHR, SLC2A4, NR1I3, INSR, GSTM1, SLPI | |||
| Quercetin | MOL000098 | Safflower, Two-toothed achyranthes root, Garden balsam stem | PTGS1, AR, PTGS2, NCOA2, KCNH2, SCN5A, ADRB2, MMP3, F7, RXRA, ACHE, GABRA1, RELA, EGFR, AKT1, VEGFA, CCND1, BCL2, BCL2L1, CDKN1A, EIF6, BAX, CASP9, PLAU, MMP2, MMP9, MAPK1, IL10, EGF, RB1, TNF, IL6, CASP3, TP53, ELK1, NFKBIA, POR, ODC1, XDH, CASP8, TOP1, RAF1, PRKCA, MMP1, HIF1A, STAT1, RUNX1T1, ERBB2, PPARG, ACACA, HMOX1, CYP3A4, CYP1A2, CAV1, MYC, F3, GJA1, CYP1A1, ICAM1, IL1B, CCL2, SELE, VCAM1, PTGER3, CXCL8, PRKCB, BIRC5, DUOX2, NOS3, HSPB1, IL2, NR1I2, CYP1B1, CCNB1, PLAT, THBD, SERPINE1, IFNG, IL1A, MPO, TOP2A, NCF1, HAS2, GSTP1, NFE2L2, AHR, SLC2A4, CXCL11, CXCL2, NR1I3, CHEK2, INSR, CLDN4, PPARA, PPARD, CRP, CXCL10, CHUK, SPP1, RUNX2, E2F1, E2F2, ACP3, CTSD, IGFBP3, IGF2, CD40LG, IRF1, ERBB3, PON1, NKX3-1, RASA1, GSTM1 |
| Rutin | MOL000415 | Safflower, Two-toothed achyranthes root, Garden balsam stem | RELA, TNF, IL6, CASP3, POR, CAT, IL1B, CXCL8, PRKCB, HMGCR, HAS2, GSTP1, INS, FCER2, ITGB2, TBXA2R |
Figure 3.
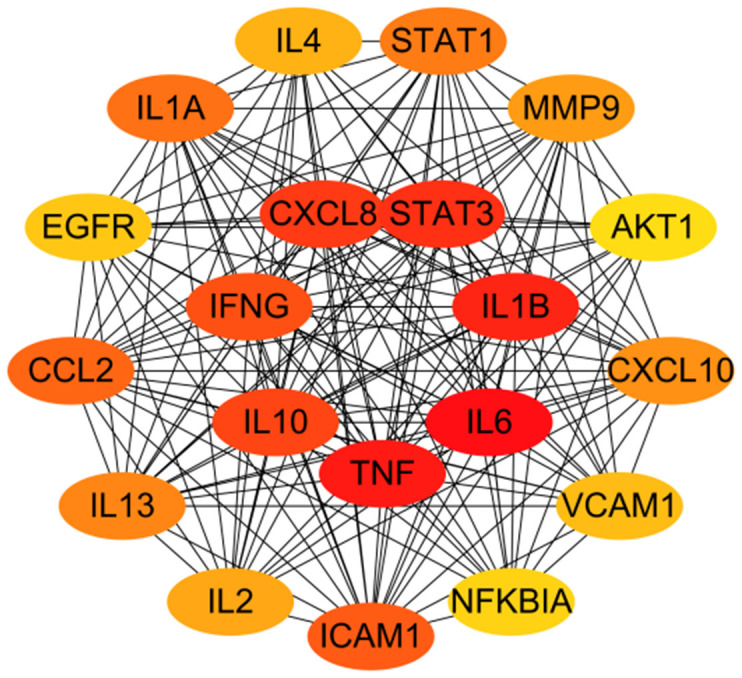
Key targets of Chinese medicine wet compress paste for soft tissue injury treatment. Note: The darker the color of the node, the more critical the target is.
Figure 4.
Disease-enriched signaling pathways in Chinese medicine wet compress paste action.
Comparison of patients’ general information
There were no significant differences in gender, age and BMI and personal income between the patients in the study group and the healthy subjects (all P > 0.05), as shown in Table 2.
Table 2.
General data of the two groups of patients
| General information | Control (n=40) | Study Group A (n=40) | Study Group B (n=40) | Χ2/F | P |
|---|---|---|---|---|---|
| Gender [n (%)] | 0.897 | 0.639 | |||
| Male | 22 (55.00) | 26 (65.00) | 23 (57.50) | ||
| Female | 18 (45.00) | 14 (35.00) | 17 (42.50) | ||
| Age (years, x̅±s) | 26.68±6.28 | 27.13±6.22 | 26.19±6.59 | 0.108 | 0.898 |
| BMI (kg/m2, x̅±s) | 21.98±2.79 | 21.54±2.49 | 21.79±2.25 | 0.348 | 0.707 |
| Personal income [Yuan, n (%)] | 0.699 | 0.705 | |||
| ≤ 3500 | 7 (17.50) | 7 (17.50) | 5 (12.50) | ||
| 3500-8000 | 22 (55.00) | 19 (47.50) | 21 (52.50) | ||
| ≥ 8000 | 11 (27.50) | 14 (35.00) | 14 (35.00) |
Note: BMI: body mass index.
Comparison of pain symptoms
Before treatment, there was no significant difference in VAS score between study group A and study group B (P > 0.05). After one course of treatment, both groups experienced a reduction in VAS scores compared to their initial scores, indicating improvement in pain symptoms. Notably, the VAS scores in Study Group B were significantly lower than that in Study Group A (P < 0.05), suggesting a more pronounced pain relief in the group treated with the TCM wet compress. These findings are summarized in Table 3.
Table 3.
Comparison of VAS scores before and after treatment [score, x̅±s]
| Group | VAS | |||
|---|---|---|---|---|
|
| ||||
| Before treatment | After treatment | t | P | |
| Study Group B (n=40) | 6.30±1.18 | 1.85±1.27 | 24.881 | < 0.001 |
| Study Group A (n=40) | 6.75±1.56 | 3.25±1.30 | 18.812 | < 0.001 |
| t | 415.657 | 4.876 | ||
| P | < 0.001 | < 0.001 | ||
Note: VAS: Pain score.
Inflammatory factor levels
There was no significant difference in inflammatory factors between group A and Group B before treatment (P > 0.05). However, post-treatment comparisons revealed significant differences between the two groups (P < 0.05). After one course of treatment, both groups exhibited reduced levels of inflammatory factors compared to baseline, with Study Group B showing a more pronounced reduction (P < 0.05). These changes are detailed in Table 4.
Table 4.
Comparison of inflammatory factors before and after treatment (x̅±s)
| Group | IL-6 (pg/ml) | CRP (mg/L) | TNF-α (ng/L) | IL-1β (ng/L) | PGE2 (μg/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Study Group B (n=40) | 53.28±6.36 | 32.52±4.78 | 2.31±0.42 | 1.57±0.20 | 25.26±3.77 | 10.34±1.78 | 86.31±17.56 | 50.12±10.22 | 366.54±27.68 | 226.78±19.67 |
| Study Group A (n=40) | 53.42±6.71 | 37.45±5.23 | 2.28±0.37 | 1.98±0.22 | 25.34±3.68 | 17.25±2.13 | 87.26±17.88 | 56.92±12.81 | 364.31±29.60 | 255.74±23.55 |
| Control group (n=40) | 3.59±1.12a,b | / | 1.26±0.18a,b | / | 0.91±0.12a,b | / | 4.31±1.26a,b | / | 43.78±10.57a,b | / |
| T/F | 1139.282 | 4.764 | 120.507 | 8.818 | 859.278 | 15.783 | 426.776 | 2.630 | 2364.750 | 6.032 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.010 | < 0.001 | < 0.001 |
Note: IL-6: Interleukin-6, CRP: C-reactive protein, TNF-α: tumor necrosis factor alpha, IL-1β: Interleukin-1β, PEG2: prostaglandin E2.
P < 0.05, compare with group B;
P < 0.05, compare with group A.
There were no significant differences in anti-inflammatory factors between the two groups (P > 0.05). Post-treatment, significant differences emerged (P < 0.05). The level of the anti-inflammatory factor IL-10 increased in both groups compared to before treatment, with a more significant up-regulation observed in Study Group A (P < 0.05). Conversely, IL-4 levels decreased after treatment, with a notable normalization in Study Group B (P < 0.05). These changes are detailed in Table 5.
Table 5.
Comparison of anti-inflammatory factors before and after treatment (x̅±s)
| Group | IL-10 (ng/L) | IL-4 (ng/L) | ||
|---|---|---|---|---|
|
|
|
|||
| Before treatment | After treatment | Before treatment | After treatment | |
| Study Group B (n=40) | 10.45±2.56 | 14.26±2.33 | 14.73±2.14 | 5.78±1.66 |
| Study Group A (n=40) | 10.34±2.74 | 18.35±2.67 | 14.06±2.21 | 9.98±1.93 |
| Control group (n=40) | 3.26±0.86a,b | 4.97±1.37a,b | ||
| T/F | 136.973 | 7.074 | 333.267 | 10.410 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Note: IL-10: Interleukin-10, IL-4: Interleukin-4.
P < 0.05, compare with group B;
P < 0.05, compare with group A.
Comparison of patients’ quality of life
There was no significant difference in quality-of-life score between group A and Group B before treatment (P > 0.05); however, these scores were significantly lower than that in the control group (P < 0.05). After treatment, the quality of life of patients in the study group was significantly improved (P < 0.05), with significantly higher score in the Group B compared with Group A (P < 0.05), as shown in Table 6.
Table 6.
Comparison of quality-of-life scores between the two groups before and after treatment (score, x̅±s)
| Group | SF-36 | |
|---|---|---|
|
| ||
| Before treatment | After treatment | |
| Study Group A (n=40) | 49.05±6.01 | 67.40±8.16 |
| Study Group B (n=40) | 48.35±5.35 | 76.03±8.75 |
| Control group (n=40) | 81.55±6.56a,b | / |
| t/F | 400.486 | 4.558 |
| P | < 0.001 | < 0.001 |
Note: SF-36: 36-item Short-Form.
P < 0.05, compare with group B;
P < 0.05, compare with group A.
Discussion
Acute soft tissue injury is a prevalent condition in orthopedics and traumatology, characterized by various local symptoms such as pain, swelling, dysfunction, bruises, and ecchymosis. Within the framework of Traditional Chinese Medicine (TCM), soft tissue injuries are primarily conceptualized as manifestations of blood stasis. When such injuries occur, damage to veins and collateral vessels can lead to blood flow obstruction or stagnation. This stagnation often transforms into heat within the affected area. Accordingly, TCM therapeutic strategies focus on enhancing blood circulation, dispersing stagnation, and reducing heat to restore blood balance within the body [10].
As documented in the Yizong Jinjian, symptoms such as swelling and pain following an injury are attributed to blood stasis and accumulation, causing discomfort. The Miscellaneous Diseases Source and Flow Rhinoceros Candle recorded that injuries resulting from falls or blunt trauma harm both the external and internal aspects of the body, impacting qi and blood, the main symptoms being pain and swelling. Qi and blood, which originate from the same source, are both compromised when injuries occur, leading to stagnation and obstruction in the veins and blood vessels, thus impeding the smooth flow of qi. TCM has a long history of effectively treating acute closed soft tissue injuries externally [11]. The primary treatment focus in TCM involves enhancing blood circulation, relieving pain and fatigue, clearing heat, and cooling the blood, strategies that have shown substantial clinical efficacy [12]. Nonetheless, the preparation of traditional wet compresses is time-consuming, and the formulas are complex. Therefore, this study seeks to assess the clinical effectiveness of simplified TCM wet compresses, aiming to offer a practical solution for clinical treatment.
In this comprehensive investigation, we identified key therapeutic targets of TCM wet compresses, including IL-6, IL-1B, TNF, CXCL8, and IL-10. These targets are intricately involved in various inflammation-related signaling pathways, with particular emphasis on the IL-17 signaling pathway, which emerges as a crucial mediator. It is plausible that the anti-inflammatory effects of TCM wet compresses are mediated, at least in part, through the modulation of the IL-17 signaling pathway [13].
Soft tissue injury, characterized primarily by an inflammatory response within the damaged tissue, triggers a cascade of events marked by the enhanced release of vasoactive substances, histamine, and other inflammatory mediators. This cascade leads to the accumulation of acidic byproducts and initiates a series of microcirculatory changes, including increased capillary permeability, decreased vascular tone, and impaired blood flow. These alterations exacerbate local inflammation, contributing to the progression of inflammatory lesions [14].
Our investigation has extensively explored the formulation of a TCM wet compress paste, with Common monkshood mother root playing a central role as the monarch ingredient. Historically recognized for its potent heating properties, Common monkshood mother root was documented in the “Pu Ji Fang” for its efficacy in alleviating various types of pain, including wind-cold and dampness paralysis, joint pain, cardiac and abdominal pain, and anesthesia-related discomfort. These historical records underscore the analgesic potential of Common monkshood mother root.
Recent research has focused on the anti-inflammatory properties of a refined polysaccharide, RG-II, extracted from Huanghua aconite. Studies have demonstrated its ability to inhibit the KMPS-2E inflammatory signaling pathway, effectively suppressing inflammation induced by macrophages and carrageenan foot swelling in a dose-dependent manner. Additionally, the root of Common monkshood has proven to have notable anti-inflammatory effects, making it a promising therapeutic option for managing soft tissue injuries [15].
Traditionally, Common monkshood mother root has been employed to alleviate a spectrum of symptoms, including wind-cold dampness paralysis, joint pain, limb numbness, hemiplegia, wind-induced headaches, abdominal and cardiac cold pain, hernia discomfort, bruising, gangrene, swelling, and poisoning. It is used in conjunction with Two-toothed achyranthes root and Sour orange, tonifying liver and kidney, strengthening bones, and promoting blood circulation to remove blood stasis. Additionally, Garden balsam stem can dispel wind and humidity, relax the muscles and promote blood circulation, disperse blood stasis and relieve pain; safflower can promote blood circulation, reduce swelling and relieve pain; Ground beetle can promote blood circulation, reduce swelling and relieve pain; Zingiber can dispel cold, restore Yang, and promote the free flow through the meridians; an Haichow elsholtzia herb can dispel wind and cold, and act as an analgesic and antispasmodic. Combined, these ingredients synergistically enhance the analgesic effects and promote the smooth flow of energy and blood.
In this study, the decrease in VAS score in Study group B markedly outperformed Study group A, signifying the notable efficacy of the TCM wet compress in alleviating patients’ pain symptoms. Moreover, the level of PGE2 in Study group B exhibited a significant decrease compared to Study group A, underscoring the noteworthy analgesic potential of the TCM formula. PGE2 is an inflammatory mediator known for its role in sensitizing nociceptive receptors during inflammation, enhancing tissue sensitivity to pain-inducing substances like serotonin (5-HT) and histamine (HIS), while reducing the effectiveness of analgesic agents. This often results in prolonged and intensified pain perception on sensory nerve endings [16]. In addition, previous studies on the pressure pain threshold of rats after administration of different doses of eupolyphaga enzyme showed that high dosage of Ground beetle administration can significantly increase the pressure pain threshold of rats, implying the analgesic effect of the enzymatic action of the Ground beetle component [17].
In this study, the levels of IL-6, CRP, TNF-α, IL-1β and PGE2 were significantly decreased after treatment in group B, while the anti-inflammatory factor IL-4 was significantly decreased. The efficacy in regulating inflammatory factor levels was markedly superior to that observed in Group A. According to Western medicine, soft tissue injury primarily arises from external trauma, precipitating acute aseptic inflammation in soft tissues. Its pathological mechanism involves the impact of external forces on the body, leading to local capillary rupture, hemorrhage, and infiltration of intracellular fluid into tissue spaces, resulting in congestion, swelling, and subsequent release of inflammatory mediators such as IL-6. This process leads to the accumulation of inflammatory metabolites, including PGE2 and PGD2, which drive an inflammatory response that stimulates nerve endings, causing pain and restricting movement [18]. IL-β is an important inflammatory transmitter in the inflammatory response, which can gather at the injury site to cause local vasodilatation and increased capillary permeability, causing local edema and tissue damage, pain, swelling, and functional impairment.
Research conducted on lipopolysaccharide-induced acute lung injury in mice revealed that injections of saffron yellow pigment significantly ameliorated lung symptoms. The treatment led to reduced expression of inflammatory factors such as TNF-α, IL-1β, and IL-6, while promoting the expression of IL-10 in mice. Further mechanistic studies suggested that this effect might be linked to the inhibition of p38MAPK phosphorylation [19]. Research indicates that saffron extract can significantly lower whole blood viscosity, extend activated partial thromboplastin time (APTT), and inhibit platelet aggregation in a dose-dependent manner [20]. In addition, destruction, degeneration, and necrosis of local muscle fiber structure are recognized as fundamental pathological bases of soft tissue injury [21].
The TCM wet compress paste exerts a localized stimulating effect primarily through factors that induce localized dilation of blood vessels. This dilation promotes blood circulation and enhances the surrounding tissue’s nutrient supply, facilitating the activation of qi, promoting blood circulation, anti-inflammatory action, and reduction of swelling [22]. Moreover, the external application allows medical agents to penetrate the skin directly, reaching close to the lesion and increasing the local concentration of the drugs. This facilitates blood activation, removal of blood stasis, improvement of qi and blood circulation, clearance of stagnation, cooling of the blood, reduction of swelling, alleviation of pain, and promotion of vascular neovascularization efficacy [23]. Skin absorption plays a crucial role in this process. As the primary peripheral barrier of the human body, the skin functions in excretion and absorption, besides protecting against external pathogens [24]. The drug’s permeability and skin’s absorption capacity collectively allow the drugs to enter the body, where they traverse the meridians to regulate and balance internal organs or directly target local lesions, exerting either systemic or local therapeutic effects. This method bypasses the liver and kidneys’ detoxification and excretion processes, preserving the active herb ingredients and maximizing their efficacy [25]. Additionally, patients show greater acceptance of traditional Chinese medicine, leading to a significant improvement in their quality of life. In this study, post-treatment observations indicated that IL-4 levels in study group B gradually returned to normal. As the damage is repaired and inflammation subsides, the body’s requirement for this anti-inflammatory regulator decreases, leading to a normalization of IL-4 secretion. Conversely, IL-10 levels showed a downward trend after treatment, reflecting the effective management of inflammation and the restoration of immune balance, thereby reducing the demand for this powerful immunosuppressive and anti-inflammatory factor.
This study evaluated for the first time the clinical efficacy of a simplified traditional Chinese medicine (TCM) wet compress paste, designed to address the complexities and time-consuming nature associated with traditional formulations, thereby offering a more convenient treatment approach. Simultaneously, it systematically assessed the comprehensive effects of the TCM wet compress paste in relieving pain, modulating inflammatory factors, and improving patients’ quality of life, highlighting its unique advantages stemming from multiple therapeutic mechanisms. The study’s limitations include a relatively small sample size predominantly focused on specific populations, which may not adequately represent all individuals with soft tissue injuries. Future research should expand sample sizes and diversify samples to enhance the generalizability and reliability of the results. Additionally, this study focused on the short-term efficacy and safety of the TCM wet compress paste, lacking evaluation of its long-term efficacy and potential side effects. Subsequent research should include longer follow-up periods to assess its long-term effects and safety profile.
Conclusion
In summary, the pharmacological mechanism of the traditional Chinese medicine wet compress patch is characterized by its comprehensive approach. Primarily, it enhances circulatory function, mitigates inflammatory exudation, and provides analgesic effects, all of which collectively contribute to its therapeutic efficacy in managing soft tissue injuries. From a clinical perspective, this study highlights the significant impact of Chinese medicine wet compress paste in treating soft tissue injuries. It particularly excels in pain relief, regulation of inflammatory factors, and improving the overall quality of life for patients. These findings validate the effectiveness of this treatment modality in addressing the complex challenges associated with soft tissue injuries.
Disclosure of conflict of interest
None.
References
- 1.Osmani HT, Nicolaou N, Anand S, Gower J Steering Group. Metcalfe A, McDonnell S, Siddiqui F, Carter H, Room J, Kirbyshire H, Hay L, Iqbal S, Gerleman S. Future research priorities for soft-tissue knee injuries. Bone Joint J. 2024;106-B:232–239. doi: 10.1302/0301-620X.106B3.BJJ-2023-0946.R1. [DOI] [PubMed] [Google Scholar]
- 2.Zhu C, He L, He T, Liang Y, Zhang BW, Zhao HY, Guan H, Yang XK, Hu DH, Han JT, Liu JQ. Clinical effects of early rehabilitation treatment after repair surgery of skin and soft tissue defects accompanied by extensor tendon injury on the back of hand. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2024;40:365–372. doi: 10.3760/cma.j.cn501225-20230820-00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsafty O, Berkey CA, Dauskardt RH. Insights and mechanics-driven modeling of human cutaneous impact injuries. J Mech Behav Biomed Mater. 2024;153:106456. doi: 10.1016/j.jmbbm.2024.106456. [DOI] [PubMed] [Google Scholar]
- 4.Stephens A, Searle H, Carlos W, Gomindes A, Pilarski A, Syed F, Smith N, Khatri C. Diagnostic impacts on management of soft tissue injuries associated with tibial plateau fractures: a narrative review. Injury. 2024;55:111546. doi: 10.1016/j.injury.2024.111546. [DOI] [PubMed] [Google Scholar]
- 5.Wang K, Xia Z, Yu R, Zhang W, Wang Z, Zhu M, Li R, Hu Z, Chen Z, Xu K, Mu C. Novel hydrogel adjuvant of Chinese medicine external preparations for accelerated healing of deep soft tissue injuries. ACS Biomater Sci Eng. 2024;10:4425–4436. doi: 10.1021/acsbiomaterials.4c00165. [DOI] [PubMed] [Google Scholar]
- 6.Suero-Pineda A, Oliva-Pascual-Vaca Á, Durán MR, Sánchez-Laulhé PR, García-Frasquet MÁ, Blanquero J. Response to letter to the editor on “effectiveness of a telerehabilitation evidence-based tablet app for rehabilitation in traumatic bone and soft-tissue injuries of the hand, wrist and fingers”. Arch Phys Med Rehabil. 2024;105:1212–1214. doi: 10.1016/j.apmr.2024.02.718. [DOI] [PubMed] [Google Scholar]
- 7.Arosarena OA, Eid IN. Mechanisms of soft tissue injury. Facial Plast Surg. 2021;37:424–431. doi: 10.1055/s-0041-1727247. [DOI] [PubMed] [Google Scholar]
- 8.Singh D, Khan MA, Akhtar K, Rehman S, Parveen S, Amin KMY, Siddique HR. Protective effects of a polyherbal medicine, Majoon Suranjan against bisphenol-A induced genetic, oxidative and tissue damages. Drug Chem Toxicol. 2023;46:1057–1069. doi: 10.1080/01480545.2022.2124519. [DOI] [PubMed] [Google Scholar]
- 9.Bobrov N, Morochovič R, Mandelík J. Scope assessment of soft tissue injury within polytrauma for needs of fortis system injury classification. J Biomed Phys Eng. 2021;11:315–324. doi: 10.31661/jbpe.v0i0.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bittenbinder MA, van Thiel J, Cardoso FC, Casewell NR, Gutiérrez JM, Kool J, Vonk FJ. Tissue damaging toxins in snake venoms: mechanisms of action, pathophysiology and treatment strategies. Commun Biol. 2024;7:358. doi: 10.1038/s42003-024-06019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu WH, Shen Y, Xiao Y, Shi Q, Fan ZX, Feng YQ, Wan HB, Qu B, Zhao J, Zhang WQ, Xu GH, Wu XQ, Tang DZ. Efficacy and safety of Wuhu oral liquid in treating acute soft tissue injuries: a multicenter, randomized, double-blind, double-dummy, parallel-controlled trial. Front Pharmacol. 2024;15:1335182. doi: 10.3389/fphar.2024.1335182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwee E, Borgdorff M, Schepers T, Halm JA, Winters HAH, Weenink RP, Ridderikhof ML, Giannakópoulos GF. Adjunctive hyperbaric oxygen therapy in the management of severe lower limb soft tissue injuries: a systematic review. Eur J Trauma Emerg Surg. 2024;50:1093–1100. doi: 10.1007/s00068-023-02426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann-Langen MV, Eggeling L, Glaab R, von Rehlingen-Prinz F, Kösters C, Herbst E. Hoffa fractures are associated with concomitant soft tissue injures and a high postoperative complication rate. Arch Orthop Trauma Surg. 2024;144:747–754. doi: 10.1007/s00402-023-05133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu YP, Zou YF, Lei FY, Wangensteen H, Inngjerdingen KT. Aconitum carmichaelii Debeaux: a systematic review on traditional use, and the chemical structures and pharmacological properties of polysaccharides and phenolic compounds in the roots. J Ethnopharmacol. 2022;291:115148. doi: 10.1016/j.jep.2022.115148. [DOI] [PubMed] [Google Scholar]
- 15.Dong SH, Peng J, Wu MH, Ma ZG, Xu CL, Cao H, Zhang Y. Herbalogical analysis of aconiti carmichaeli radix. Zhongguo Zhong Yao Za Zhi. 2021;46:3156–3164. doi: 10.19540/j.cnki.cjcmm.20210314.101. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Li R, Hu R, Yao J, Yang Y. PEG2-induced pyroptosis regulates the expression of HMGB1 and promotes hEM15A migration in endometriosis. Int J Mol Sci. 2022;23:11707. doi: 10.3390/ijms231911707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Ren C, Bi W, Batu W. Glycyrrhizin mitigates acute lung injury by inhibiting the NLRP3 inflammasome in vitro and in vivo. J Ethnopharmacol. 2023;303:115948. doi: 10.1016/j.jep.2022.115948. [DOI] [PubMed] [Google Scholar]
- 18.Pan F, Zeng F, Chen Y, Zheng Y, Chen Z, Zhu X, Yin MF, Huang Y, Liu Z. Warm acupuncture reduces pain and inflammation in rats with lumbar disc herniation induced by autologous nucleus pulposus transplantation via regulating p38MAPK/NF-κB pathway. J Acupunct Meridian Stud. 2024;17:28–37. doi: 10.51507/j.jams.2024.17.1.28. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Ke T, Zhang Y, Guo L, Chen F, He W. Danshensu inhibits the IL-1β-induced inflammatory response in chondrocytes and osteoarthritis possibly via suppressing NF-κB signaling pathway. Mol Med. 2021;27:80. doi: 10.1186/s10020-021-00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian D, Zhang Q, He CX, Guo J, Huang XT, Zhao J, Zhang H, Xu C, Peng W. Hai-Honghua medicinal liquor is a reliable remedy for fracture by promotion of osteogenic differentiation via activation of PI3K/Akt pathway. J Ethnopharmacol. 2024;330:118234. doi: 10.1016/j.jep.2024.118234. [DOI] [PubMed] [Google Scholar]
- 21.Xiao B, Li Q, Han N, Zhang CL, Yin J. Soft tissue contusion repairing effects of Hong Yao with different penetration enhancers. J Ethnopharmacol. 2013;148:610–616. doi: 10.1016/j.jep.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Zheng ZX, Wang J, Hou JS, Ma L, Jiang CB. A traditional Chinese medicine therapy warming meridians to nourish blood in treating chronic pain due to soft tissue injury of the neck and shoulder: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2011;9:153–157. doi: 10.3736/jcim20110207. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Li M, Zheng F, Jiang W, Lei K, Li H, Liu D, Zhang B, He M. Pharmacological mechanisms of Shangke Huangshui against skin and soft tissue infection. Evid Based Complement Alternat Med. 2022;2022:9312611. doi: 10.1155/2022/9312611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan ZY, Zhang ZH, Sun RH, Wu YL, Nan JX, Lian LH. A therapeutic strategy of parthenolide in improving imiquimod-induced psoriasis-like skin inflammation targeting IL-36/NETs through skin transdermal therapeutic system. Int Immunopharmacol. 2024;131:111824. doi: 10.1016/j.intimp.2024.111824. [DOI] [PubMed] [Google Scholar]
- 25.Wang WJ, Duan XY, Wang W. Mechanism of Taohong Siwu Decoction in treating soft tissue injury based on UPLC-Q-TOF-MS,network pharmacology and experimental verification. Zhongguo Zhong Yao Za Zhi. 2021;46:3043–3051. doi: 10.19540/j.cnki.cjcmm.20210311.403. [DOI] [PubMed] [Google Scholar]