Abstract
Objective: To investigate SUI (stress urinary incontinence) and POP (pelvic organ prolapse) in women after childbirth by transperineal ultrasonography. Methods: In this retrospective study, 107 six-week postpartum primiparous mothers and 42 healthy nulliparous women were selected during the period from January 2021 to March 2023, in Pudong New Area People’s Hospital. Among the postpartum mothers, 54 delivered vaginally and 53 underwent cesarean section. Various parameters such as bladder detrusor muscle thickness, urethrovesical angle, bladder neck mobility, puborectalis muscle hiatus area, and puborectalis muscle hiatus circumference were collected and analyzed. Results: During pregnancy and childbirth, several parameters underwent significant increases, including bladder detrusor muscle thickness, urethrovesical angle, bladder neck mobility, puborectalis muscle hiatus area, and puborectalis muscle hiatus circumference. Furthermore, vaginal delivery led to a notably more pronounced elevation in these indicators compared to other delivery methods (all P<0.05). Our findings revealed that the risk of pelvic organ prolapse (POP) escalated with an increasing number of pregnancies (P<0.05). Moreover, obese pregnant women, defined as having a body mass index (BMI) of 25 kg/m2 or higher, exhibited a heightened risk of developing POP. Conclusions: Perineal ultrasound provides reliable imaging evidence, treatment theory basis, and evaluation value for women with pelvic floor dysfunction after childbirth.
Keywords: Transperineal pelvic floor ultrasound, postpartum pelvic organ injury, prolapse
Introduction
Pelvic floor dysfunction (PFD) refers to a range of conditions caused by various factors that alter the position and function of pelvic floor organs [1]. It primarily results in damage to female pelvic floor tissues and weakened supporting tissues, and it is generally classified into anterior (e.g., stress urinary incontinence (SUI)), middle (e.g., pelvic organ prolapse (POP)), and posterior pelvic floor dysfunctions (e.g., outlet obstructive constipation (OOC)) [2-5]. Numerous studies have shown that age, pregnancy, childbirth, obesity, genetics, pelvic surgery, and chronic cough are all risk factors for PFD [7], with pregnancy and childbirth being independent risk factors for PFD [8], leading to substantial disruptions in the physical and mental health and daily lives of female patients at different stages. Common clinical examination methods for diagnosing PFD include POP-Q classification, cotton swab test and urodynamic test. However, accurately diagnosing and evaluating the efficacy of treatments remains challenging. Diagnosis and evaluation mainly rely on patients’ clinical symptoms, physical examination, and other physiological indices. These approaches are often subjective and limited by the interpretation of results. While magnetic resonance imaging (MRI) can provide more accurate and reliable results, its high cost limits its applicability for post-natal examinations [9].
Transperineal pelvic floor ultrasound is a non-invasive imaging technique increasingly used to assess pelvic floor dysfunction, particularly in the context of postpartum pelvic floor injuries. This imaging modality allows for the visualization of the pelvic floor muscles, urethra, bladder, and rectum, providing valuable information on the structure and function of the pelvic floor [10]. In the context of postpartum pelvic floor injuries, transperineal pelvic floor ultrasound is instrumental in diagnosing and evaluating conditions such as POP, urinary incontinence, and anal sphincter injuries. It can reveal the extent of muscle damage, the presence of tears or defects in the pelvic floor muscles, and the position of pelvic organs [11,12]. Zhuo et al. found that transperineal pelvic floor ultrasound can detect SUI early in middle-aged and elderly individuals facilitating lifestyle treatment [13]. Isabelle M.A declared that transperineal pelvic floor ultrasound could help diagnose posterior pelvic floor disorders in women with obstructed defecation syndrome [14]. Transperineal pelvic floor ultrasound holds broad application prospects in pelvic floor morphology examination. Its powerful data post-processing capacity allow for clear visualization of pelvic floor anatomy, making it applicable to pelvic floor dysfunction diagnosis. The spatial resolution of this ultrasound technique is comparable to that of magnetic resonance imaging (MRI), and its real-time imaging feature speeds up the examination process, enhancing its desirability as a diagnostic tool. However, there is limited research on transperineal pelvic floor ultrasound in the diagnosis of pelvic organ injury and prolapse in postpartum women, mainly due to the potentially inadequate resolution of the ultrasound images and its reliance on the skill and experience of the operator.
Therefore, in this study, we investigated SUI and POP in postpartum women by transperineal ultrasonography, with the objectives of: 1) to evaluate the therapeutic effect of pelvic floor rehabilitation on postpartum SUI; 2) to explore the efficacy of three-dimensional pelvic floor ultrasound in detecting POP-related parameters in women with different pregnancies/BMI; 3) to visually evaluate the injury and prolapse of posterior pelvic floor organs in patients with OOC; 4) to provide reliable imaging basis and theoretical basis for treatment of postpartum SUI, POP and OOC.
Methods
Patient enrollment
This retrospective study was conducted between January 2021 and March 2023 at Pudong New Area People’s Hospital. Study included 107 six-week postpartum primiparous mothers and 42 healthy nulliparous women. Among the postpartum participants, 54 delivered vaginally and 53 underwent cesarean section. The research project was approved by the Medical Ethics Committee of Pudong New Area People’s Hospital.
Inclusive criteria: 1) diagnosed as PFD by a pelvic gynecologist based on the patient’s clinical performance, physical examination and specialized tests (e.g., pad test, acupressure test) [15]; 2) age>18 years old; 3) 6 weeks after delivery; 4) no history of pelvic organ prolapse surgery, no current or past symptoms of severe pelvic pain, or known pelvic floor anomalies that could confound the assessment of POP.
Exclusive criteria: 1) previous pelvic surgery, urinary tract infections, kidney disease, and organic pelvic lesions; 2) persistent vaginal bleeding or postpartum lochia; 3) urinary incontinence caused by nervous system; 4) inability to complete effective pelvic floor muscle contraction or Valsalva maneuver.
Transperineal ultrasonography
The ultrasonic images were collected using a GE Voluson E8Color Doppler ultrasound machine (Siemens, Acuson S2000) equipped with RIC 5-9-D and RAB 6-D probes. The working frequency was 5 to 10 MHz. Prior to image collection, patients were instructed to empty their bladder and rectum, ensuring that the amount of residual urine in the bladder was less than 50 mL. The supine bladder lithotomy position was chosen, with the hips flexed and gently abducted. During the examination, the perineum of the subject was exposed. The vaginal probe was evenly coated with coupling agent and covered with a condom. The probe was then slowly inserted into the perineum until it was closely attached to the lower edge of the pubic symphysis, maintaining a distance of less than 10 mm between the probe surface and the lower edge of the pubic symphysis. Ultrasound images of the pelvic floor at rest and during Valsalva maneuver were collected for all subjects.
Pelvic rehabilitation training
All parturients were instructed to empty their bladder and rectum before treatment. Using the PHENIX pelvic floor therapy device, an electromyography probe (muscle therapy head) was gently inserted into the parturient’s vagina. Additionally, three electrode pads were placed on both sides of the parturient’s anterior superior iliac spine and lower abdomen. Rehabilitation training was conducted using a combination of electrical stimulation and biofeedback (30 minutes per session, twice a week, 16 sessions per course). Furthermore, all parturients underwent pelvic floor muscle training. They were guided to lie flat with legs slightly apart and perform pelvic floor muscle contraction exercises (8-12 times per session, for a period of 6 months).
Observation indicators
The primary indicators utilized in this study were pelvic floor ultrasound parameters, specifically including bladder detrusor muscle thickness, urethrovesical angle, bladder neck mobility, puborectalis hiatus area, and puborectalis hiatus circumference. Additionally, POP-related indicators such as the bladder urethral posterior angle at rest and the urethral inclination angle during the Valsalva maneuver were also considered.
The secondary indicators encompassed demographic information and clinical characteristics, namely educational level, age, and occupation, among others.
Statistical analysis
SPSS 22.0 was used for data analysis. The estimation of the number of patients in the three groups was performed using the G*Power tool [16]. All result data were presented as mean ± SD. For comparison between two groups, a t-test was used, while for comparison among three or more groups, a one-way ANOVA was used. P<0.05 was considered statistically significant.
Results
Basic characteristics
In this study, there were no significant differences in age, education level, and occupation among the three groups (P>0.05) (Table 1).
Table 1.
Basic characteristics of research subjects
| Group | Control group (n=42) | Vaginal delivery group (n=54) | Caesarean section group (n=53) | F/χ2 | p |
|---|---|---|---|---|---|
| Age | 28.83±6.27 | 30.12±7.94 | 33.14±9.23 | 0.73 | P>0.05 |
| Education level | 1.13 | P>0.05 | |||
| Junior high school | 8 (19.05%) | 10 (18.52%) | 12 (22.64%) | ||
| High school | 16 (38.09%) | 21 (38.89%) | 19 (35.85%) | ||
| College or above | 18 (42.86%) | 23 (42.59%) | 22 (41.51%) | ||
| Occupation | 0.91 | P>0.05 | |||
| Physical type | 6 (14.29%) | 11 (20.37%) | 14 (26.42%) | ||
| Brain type | 13 (30.95%) | 16 (29.63%) | 13 (24.53%) | ||
| Comprehensive type | 23 (54.76%) | 27 (50.00%) | 26 (49.06%) |
Pelvic floor ultrasound parameters
Compared with the control group, the bladder detrusor muscle thickness (5.27±0.89 mm/4.06±0.67 mm vs. 2.32±0.52 mm), urethrovesical angle ((172.19±8.16)°/(142.72±7.02)° vs. (107.58±6.88)°), bladder neck mobility ((34.27±6.04) mm/(24.18±5.31) mm vs. (14.8±2.92) mm), puborectalis hiatus area ((32.16±6.12) cm2/(26.25±5.03) cm2 vs. (16.09±4.21) cm2), and puborectalis hiatus circumference ((21.19±2.16) cm/(17.24±1.66) cm vs. (13.52±2.62) cm) in the vaginal delivery group/cesarean section group were all significantly increased (F=262.35/517.43/462.54/271.16/243.14, all P<0.01; Table 2). At the same time, the results also showed that all indicators in the vaginal delivery group were higher than those in the cesarean section group, suggesting that during pregnancy and childbirth, all indicators may increase, and vaginal delivery may lead to a more severe increase in all indicators.
Table 2.
Correlation between pelvic floor ultrasound indicators of the study subjects
| Group | Control group (n=42) | Vaginal delivery group (n=54) | Caesarean section group (n=53) | F | P |
|---|---|---|---|---|---|
| Bladder detrusor muscle thickness | 2.32±0.52 | 5.27±0.89 | 4.06±0.67 | 262.35 | P<0.01 |
| Urethrovesical angle | 107.58±6.88 | 172.19±8.16 | 142.72±7.02 | 517.43 | P<0.01 |
| Bladder neck mobility | 14.8±2.92 | 34.27±6.04 | 24.18±5.31 | 462.54 | P<0.01 |
| Anal sphincter hiatus area | 16.09±4.21 | 32.16±6.12 | 26.25±5.03 | 271.16 | P<0.01 |
| Anal sphincter hiatus circumference | 13.52±2.62 | 21.19±2.16 | 17.24±1.66 | 243.14 | P<0.01 |
Pelvic floor rehabilitation efficacy
In the vaginal delivery group (41 cases), 26 cases were completely cured, 11 cases showed significant improvement, and 4 cases had almost no effect, with a total effective rate of 90.24%. Meanwhile, in the cesarean section group (29 cases), 20 cases were completely cured, 7 cases showed significant improvement, and 3 cases had almost no effect, with a total effective rate of 93.1% (Table 3). These results suggest that pelvic floor rehabilitation treatment was effective in both groups. Although the total effective rate in the cesarean section group was slightly higher than that in the vaginal delivery group, the difference was not statistically significant (χ2=0.134, P=0.66).
Table 3.
Study on the efficacy of pelvic floor rehabilitation in SUI patients
| Group | Vaginal delivery group | Caesarean section group | χ2 | P |
|---|---|---|---|---|
| Cases | 41 | 29 | 0.134 | 0.66 |
| Cure | 26 | 20 | ||
| Effective | 11 | 7 | ||
| Invalid | 4 | 2 |
Pelvic floor ultrasound-related indicators before and after pelvic floor rehabilitation
Pelvic floor rehabilitation therapy significantly and effectively improved the thickness of the detrusor muscle, urethrovesical angle, bladder neck mobility, anal sphincter hiatus area, and anal sphincter hiatus circumference after cesarean section and vaginal delivery (all P<0.05) (Table 4). Transperineal pelvic floor ultrasound can be effectively used to evaluate the treatment outcomes of SUI in patients after cesarean section and vaginal delivery.
Table 4.
Comparison of relevant indicators before and after treatment in SUI patients between the vaginal delivery group and the cesarean section group
| Group | Treatment situation | Vaginal delivery group (n=54) | Caesarean section group (n=53) |
|---|---|---|---|
| Bladder detrusor muscle thickness | Before treatment | 5.27±0.89 | 4.06±0.67 |
| After treatment | 2.46±0.61 | 3.24±0.49 | |
| t | 9.32 | 8.16 | |
| p | P<0.01 | P<0.01 | |
| Urethrovesical angle | Before treatment | 172.19±8.16 | 142.72±7.02 |
| After treatment | 127.95±9.83 | 129.26±6.91 | |
| t | 13.81 | 9.52 | |
| p | P<0.01 | P<0.01 | |
| Bladder neck mobility | Before treatment | 34.27±6.04 | 24.18±5.31 |
| After treatment | 24.26±6.19 | 19.38±6.12 | |
| t | 9.14 | 7.43 | |
| p | P<0.01 | P<0.01 | |
| Anal sphincter hiatus area | Before treatment | 32.16±6.12 | 26.25±5.03 |
| After treatment | 23.81±4.06 | 18.16±4.57 | |
| t | 8.84 | 8.42 | |
| p | P<0.01 | P<0.01 | |
| Anal sphincter hiatus area | Before treatment | 21.19±2.16 | 17.24±1.66 |
| After treatment | 17.86±1.78 | 16.73±1.64 | |
| t | 7.93 | 8.93 | |
| p | P<0.01 | P<0.01 |
The impact of multiparity on POP-related indicators
The incidence of POP differed significantly among patients with different parities (χ2=32.531, P<0.05). Ultrasound detection revealed that the urethrovesical angle at rest in POP patients with different parities (1, 2, and 3 or more) showed no significant difference (t=4.102, P>0.05). However, there were significant differences in the urethral inclination angle and urethrovesical angle during Valsalva maneuver, showing an increasing trend with increasing parity (F=2.743, F=3.253, all P<0.05) (Table 5). However, in patients who had experienced 3 or more pregnancies, there was an observed increase in the incidence of bladder prolapse, uterine prolapse, lower rectocele position, and an expanded pelvic floor hiatus area (Figure 1). These results suggest that the more parities a woman has, the greater the risk of developing POP.
Table 5.
The impact of multiparity on POP-related indicators
| Parity | Cases | Patient index | Bladder urethral posterior angle in resting state | Urethral inclination angle under Valsalva action | Posterior angle of the bladder and urethra under Valsalva maneuver | |
|---|---|---|---|---|---|---|
|
| ||||||
| POP positive | POP negative | |||||
| 1 time | 21 | 17 | 4 | 105.72±14.91 | 54.18±21.27 | 152.33±26.10 |
| 2 times | 14 | 9 | 5 | 103.67±15.83 | 55.91±22.41 | 157.48±21.07 |
| ≥3 times | 3 | 3 | 0 | 104.07±20.11 | 56.11±30.11 | 199.15±40.94 |
| χ2/F | - | 2.531 | 4.102 | 2.743 | 3.253 | |
| P | - | P>0.05 | P>0.05 | P<0.05 | P<0.05 | |
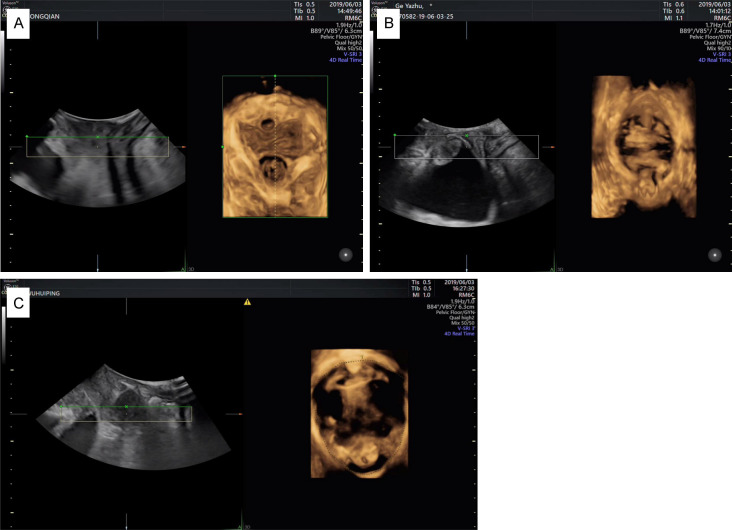
Figure 1.
The impact of multiparity on POP-related indicators. A: Mild pelvic organ prolapse in a woman with one parity. During the Valsalva maneuver, the bladder neck, cervix, and rectovaginal pouch positions were assessed. The bladder neck mobility was 20 mm, the cervix was 15 mm above the reference line, and the rectovaginal pouch protrusion height was 10 mm; B: Moderate pelvic organ prolapse in a woman with two parities, with bladder neck, cervix, and rectocele protruding during the Valsalva maneuver. The bladder neck displacement was 30 mm, cervix was 2 mm below the reference line, and rectocele protrusion height was 15 mm; C: Severe pelvic organ prolapse in a woman with three parities, with descent of the bladder neck, cervix, and rectocele during the Valsalva maneuver. The bladder neck descent was 38 mm, cervix descended 21 mm below the reference line, and rectocele protrusion height was 32 mm.
The impact of BMI on POP-related indicators in postpartum women
The incidence of POP differed significantly in patients with different BMI (χ2=3.774, P<0.05). BMI exhibited significant influence on the urethral inclination angle during Valsalva maneuver, as well as the urethrovesical angle during Valsalva maneuver (t=2.574, t=2.863, all P<0.05). However, there was no impact on the urethrovesical angle at rest (t=1.04, P>0.05). Specifically, the urethral inclination angle and urethrovesical angle during Valsalva maneuver of women with BMI≥25 kg/m2 (-40.99±20.22°, 138.15±13.54°) were significantly greater than those with BMI<25 kg/m2 (-50.76±19.75°, 112.48±15.87°) (P<0.05) (Table 6). Further examination found that BMI had an impact on the bladder neck, cervix, and rectocele position during Valsalva maneuver, and compared to women with BMI<25 kg/m2, women with BMI≥25 kg/m2 have increased bladder neck protrusion, more severe cervix descent, lower rectocele position, and larger pelvic floor hiatus area (Figure 3), suggesting that obese women may have an increased risk of pelvic organ prolapse.
Table 6.
Ultrasound evaluation of the impact of different BMI on pelvic organ prolapse (POP) related indicators in postpartum women
| Parity | Cases | Patient index | Bladder urethral posterior angle in resting state | Urethral inclination angle under Valsalva action | Posterior angle of the bladder and urethra under Valsalva maneuver | |
|---|---|---|---|---|---|---|
|
| ||||||
| POP positive | POP negative | |||||
| <25 kg/m2 | 22 | 10 | 12 | 112.48±15.87 | 50.76±19.75 | 161.77±21.53 |
| ≥25 kg/m2 | 16 | 6 | 16 | 138.15±13.54 | 40.99±20.22 | 149.12±23.59 |
| Χ2/t | - | 1.774 | 1.94 | 3.574 | 2.863 | |
| P | - | 0.02 | 0.01 | 0.008 | P<0.05 | |
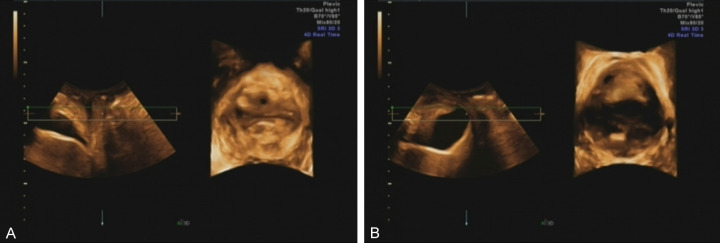
Figure 3.
Ultrasound evaluation of the impact of different BMI on pelvic organ prolapse (POP) related indicators in postpartum women. A: In postpartum women with BMI<25 kg/m2, the positions of the bladder neck, cervix, rectocele, and pelvic floor hiatus during the Valsalva maneuver were evaluated. The bladder neck mobility was 13 mm, the cervix was 20 mm above the reference line, there was no rectocele, and the pelvic floor hiatus area was 17 cm2; B: In postpartum women with BMI≥25 kg/m2, the positions of the bladder neck, cervix, and rectocele during the Valsalva maneuver, as well as the area of the pelvic floor hiatus, were measured. The bladder neck displacement was 36 mm, the cervix descended 8 mm below the reference line, the rectocele protruded 21 mm, and the area of the pelvic floor hiatus was 32 cm2.
Ultrasound evaluation of parameters related to posterior pelvic floor dysfunction
Transperineal ultrasound diagnosed 21 cases of posterior pelvic floor dysfunction, OOC, in postpartum patients. The results showed rectal prolapse in 16 cases, with 9 cases also accompanied by bladder prolapse (Figure 2A). Compared to the control group (Figure 2B), it was found that in patients with pelvic floor dysfunction, during the Valsalva maneuver, the vaginal posterior wall of the rectum prolapsed, with a continuous interruption of the anal canal rectal internal sphincter, forming a 90° angle between the anal canal and the rectal prolapse. The prolapsed part extended vertically below the level of the pubic symphysis, and the height of the prolapsed part exceeded 15 mm (Figure 2C).
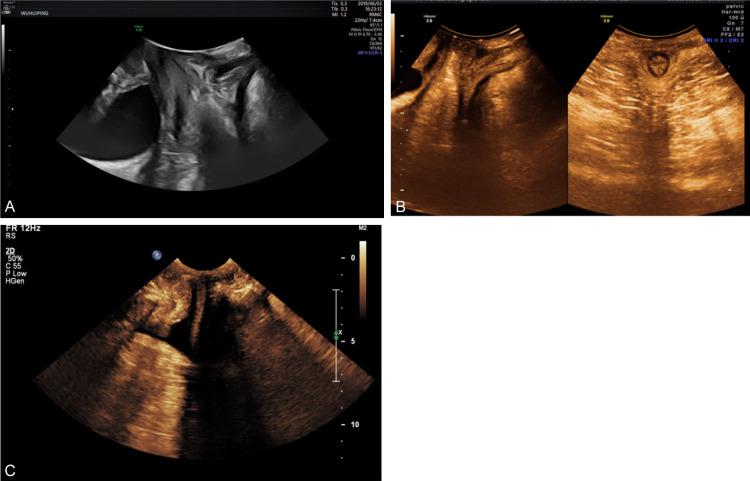
Figure 2.
Ultrasound evaluation of parameters related to pelvic floor dysfunction after OOC. A: Postpartum women with pelvic floor dysfunction may present with rectal prolapse accompanied by bladder prolapse. The lowest point of the bladder is located 5 mm below the reference line, with a rectal prolapse height of 18 mm; B: Pelvic floor anal canal and rectal ampulla of normal control group; C: Patients with functional pelvic floor disorders after OOC have intestinal herniation in the pelvic floor anal canal and rectal ampulla, with obvious discontinuity of the anal rectal muscle. The depth of the herniation is about 25 mm, and the length is about 43 mm.
Discussion
In this study, we utilized transperineal ultrasound to detect pelvic floor changes in postpartum women who had undergone either vaginal delivery or cesarean section. Compared to normal healthy nulliparous women, the bladder detrusor muscle thickness, urethrovesical angle, bladder neck mobility, puborectalis hiatus area, and puborectalis hiatus circumference were significantly increased in both postpartum groups. These increases are likely attributable to the effects of pregnancy and childbirth, with vaginal delivery leading to more pronounced changes in these indicators. Subsequently, we selected 70 cases of stress urinary incontinence (SUI) patients who had vaginal delivery or cesarean section for pelvic floor rehabilitation and ultrasound detection. The results showed that both groups achieved significant therapeutic effects after pelvic floor rehabilitation. Transperineal pelvic floor ultrasound assessment of SUI-related indicators before and after treatment revealed that the bladder detrusor muscle thickness, urethrovesical angle, bladder neck mobility, puborectalis hiatus area, and puborectalis hiatus circumference were significantly improved after pelvic floor rehabilitation, suggesting that transperineal pelvic floor ultrasound is an effective tool for evaluating various SUI indicators and providing a theoretical basis for rehabilitation treatment.
Transperineal pelvic floor ultrasound provides an intuitive view of the anatomical structure of the pelvic floor, allowing real-time dynamic observation of the pelvic floor in three states: rest, maximum Valsalva maneuver, and contraction. In this study, we examined the impact of parity on pelvic floor function. We found that number of parities had no significant impact on the urethral inclination angle and urethrovesical angle at rest; however, the impact was significant during the Valsalva maneuver, with an increasing trend as parity increased. For women with less than three parities, the impact of parity on the bladder neck, cervix, and rectocele position was minimal during the Valsalva maneuver; conversely, women with three or more parities exhibited bladder prolapse, uterine prolapse, lower rectocele position, and increased pelvic floor hiatus area, indicating that the risk of pelvic organ prolapse with increased parity. During childbirth, the pelvic floor muscles and connective tissues are stretched and weakened, leading to a loss of support for the pelvic organs, resulting in prolapse of the bladder, uterus, or rectum [17,18]. Furthermore, the nerves controlling the pelvic floor muscles can be damaged during childbirth, affecting their ability to provide proper support to the pelvic organs [19]. Additionally, hormonal changes during pregnancy and childbirth may affect the strength and elasticity of the pelvic floor muscles and connective tissues, increasing the risk of prolapse [20]. Transperineal pelvic floor ultrasound plays a crucial role in assessing pelvic organ prolapse in women with a history of multiple childbirths. This imaging technique allows for a detailed visualization of the pelvic floor structures, including the position and support of the pelvic organs. By providing a real-time assessment of the pelvic floor during rest and maximum Valsalva maneuver (bearing down), transperineal pelvic floor ultrasound can help in diagnosing and monitoring pelvic organ prolapse, guiding treatment decisions, and evaluating the effectiveness of interventions such as pelvic floor exercises or surgical repair [21].
Transvaginal pelvic floor ultrasound can observe the specific location of organ descent in patients with pelvic organ prolapse, providing crucial guidance for clinical treatment. Posterior pelvic floor dysfunction mainly involves rectocele and enterocele, where the rectum protrudes into the vagina posterior wall [22-24]. Currently, the focus of pelvic floor ultrasound has shifted to the posterior pelvic floor. In this study, through transvaginal ultrasound diagnosis, we identified 21 cases with obstetric anal sphincter injury-related posterior pelvic floor dysfunction. Among these, 16 cases had rectocele, and 9 of them were also accompanied by cystocele. During the Valsalva maneuver, the rectum protruded into the vagina posterior wall at the rectocele position, with continuous interruption of the rectal internal sphincter, forming a 90° angle between the anal canal and the rectal protrusion, with the protrusion extending vertically below the level of the pubic symphysis and a height exceeding 15 mm. This suggests that transvaginal two-dimensional ultrasound can effectively observe and analyze the ultrasound manifestations of rectocele.
Transvaginal pelvic floor ultrasound is a valuable tool in diagnosing pelvic organ injuries in postpartum women. This imaging technique allows for detailed visualization of the pelvic floor muscles, ligaments, and organs, providing valuable information on the extent and location of any damage [25-27]. One of the key advantages of transvaginal pelvic floor ultrasound is its non-invasive nature, making it a safe and comfortable option for women who have recently given birth. It also offers real-time imaging, allowing for immediate assessment and diagnosis [28]. Additionally, transvaginal pelvic floor ultrasound can help guide treatment decisions by providing information on the optimal approach for repair or rehabilitation. This can lead to more targeted and effective interventions, ultimately improving outcomes for postpartum women with pelvic organ injuries [29].
This study had a few limitations. The data were obtained from a single center, and the study population was small. Future studies should expand on these findings by examining transperineal pelvic floor ultrasound in postpartum pelvic organ injury and prolapse in a larger, more diverse population.
In summary, we have shown that transperineal pelvic floor ultrasound parameters can aid in the diagnosis of pelvic organ injury in postpartum women. This imaging modality offers a reliable, non-invasive method for evaluating and managing pelvic floor dysfunction, ultimately contributing to better treatment outcomes.
Acknowledgements
This work was supported by the Youth project of Shanghai Pudong New Area Municipal Health Burean (PW2022B-15).
Disclosure of conflict of interest
None.
References
- 1.Sam E, Cinislioglu AE, Yilmazel FK, Demirdogen SO, Yilmaz AH, Karabulut I. Is biofeedback-assisted pelvic floor muscle training superior to pelvic floor muscle training alone in the treatment of dysfunctional voiding in women? A prospective randomized study. Int Braz J Urol. 2022;48:501–511. doi: 10.1590/S1677-5538.IBJU.2021.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bondurri A, Maffioli A, Danelli P. Pelvic floor dysfunction in inflammatory bowel disease. Minerva Gastroenterol Dietol. 2015;61:249–59. [PubMed] [Google Scholar]
- 3.Preda A, Moreira S. Stress urinary incontinence and female sexual dysfunction: the role of pelvic floor rehabilitation. Acta Med Port. 2019;32:721–726. doi: 10.20344/amp.12012. [DOI] [PubMed] [Google Scholar]
- 4.Sims L, Hay-Smith J, Dean S. Pelvic floor exercises and female stress urinary incontinence. Br J Gen Pract. 2022;72:185–187. doi: 10.3399/bjgp22X719033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagen S, Stark D, Glazener C, Dickson S, Barry S, Elders A, Frawley H, Galea MP, Logan J, McDonald A, McPherson G, Moore KH, Norrie J, Walker A, Wilson D POPPY Trial Collaborators. Individualised pelvic floor muscle training in women with pelvic organ prolapse (POPPY): a multicentre randomised controlled trial. Lancet. 2014;383:796–806. doi: 10.1016/S0140-6736(13)61977-7. [DOI] [PubMed] [Google Scholar]
- 6.Chilaka C, Toozs-Hobson P, Chilaka V. Pelvic floor dysfunction and obesity. Best Pract Res Clin Obstet Gynaecol. 2023;90:102389. doi: 10.1016/j.bpobgyn.2023.102389. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Wang X, Ma T, Lu Y, Yan Z, Wang J, Hao Q. A visualization analysis of hotspots and global trends on pelvic floor dysfunction in cervical cancer. J Cancer Res Clin Oncol. 2024;150:54. doi: 10.1007/s00432-023-05531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng C, Guo B, Li R, Wu W, Mi C, Li X. Correlation between postpartum pelvic floor dysfunction and vaginal microecological imbalance in late pregnancy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47:1608–1614. doi: 10.11817/j.issn.1672-7347.2022.220100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng S, Jiang Q, Zhu W, Wang M, Zhang Y. Transperineal pelvic floor ultrasound for assessing posterior pelvic injury and prolapse in postpartum women. Am J Transl Res. 2023;15:6170–6179. [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, He H, Yu B, Jin H, Zhao Y, Zhou X, Huang H. Application of transperineal pelvic floor ultrasound in changes of pelvic floor structure and function between pregnant and non-pregnant women. Int J Womens Health. 2022;14:1149–1159. doi: 10.2147/IJWH.S361755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nygaard IE, Clark E, Clark L, Egger MJ, Hitchcock R, Hsu Y, Norton P, Sanchez-Birkhead A, Shaw J, Sheng X, Varner M. Physical and cultural determinants of postpartum pelvic floor support and symptoms following vaginal delivery: a protocol for a mixed-methods prospective cohort study. BMJ Open. 2017;7:e014252. doi: 10.1136/bmjopen-2016-014252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vellucci F, Regini C, Barbanti C, Luisi S. Pelvic floor evaluation with transperineal ultrasound: a new approach. Minerva Ginecol. 2018;70:58–68. doi: 10.23736/S0026-4784.17.04121-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhuo Z, Ye Z, Zhang J, Yu H. Correlation between three-dimensional transperineal ultrasound and pelvic floor electromyography in women with stress urinary incontinence. Ginekol Pol. 2023;94:25–32. doi: 10.5603/GP.a2022.0117. [DOI] [PubMed] [Google Scholar]
- 14.van Gruting IMA, Kluivers K, Sultan AH, De Bin R, Stankiewicz A, Blake H, Thakar R. Does 4D transperineal ultrasound have additional value over 2D transperineal ultrasound for diagnosing posterior pelvic floor disorders in women with obstructed defecation syndrome? Ultrasound Obstet Gynecol. 2018;52:784–791. doi: 10.1002/uog.19105. [DOI] [PubMed] [Google Scholar]
- 15.Chi N, Lozo S, Rathnayake RAC, Botros-Brey S, Ma Y, Damaser M, Wang RR. Distinctive structure, composition and biomechanics of collagen fibrils in vaginal wall connective tissues associated with pelvic organ prolapse. Acta Biomater. 2022;152:335–344. doi: 10.1016/j.actbio.2022.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Rong Q, Liu Y, Wang J, Xie B, Ren S. Relationship between high intra-abdominal pressure and compliance of the pelvic floor support system in women without pelvic organ prolapse: a finite element analysis. Front Med (Lausanne) 2022;9:820016. doi: 10.3389/fmed.2022.820016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muldoon J. Uterine prolapse: impact of the condition and practical advice. Br J Nurs. 2022;31:S8–S14. doi: 10.12968/bjon.2022.31.18.S8. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Zhao Z, Wen J, Ling W, Miao Y, Wu J. Cellular senescence: a pathogenic mechanism of pelvic organ prolapse (Review) Mol Med Rep. 2020;22:2155–2162. doi: 10.3892/mmr.2020.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagen S, Glazener C, McClurg D, Macarthur C, Elders A, Herbison P, Wilson D, Toozs-Hobson P, Hemming C, Hay-Smith J, Collins M, Dickson S, Logan J. Pelvic floor muscle training for secondary prevention of pelvic organ prolapse (PREVPROL): a multicentre randomised controlled trial. Lancet. 2017;389:393–402. doi: 10.1016/S0140-6736(16)32109-2. [DOI] [PubMed] [Google Scholar]
- 20.DeLancey JOL, Masteling M, Pipitone F, LaCross J, Mastrovito S, Ashton-Miller JA. Pelvic floor injury during vaginal birth is life-altering and preventable: what can we do about it? Am J Obstet Gynecol. 2024;230:279–294. e2. doi: 10.1016/j.ajog.2023.11.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng S, Jiang Q, Zhu W, Wang M, Zhang Y. Transperineal pelvic floor ultrasound for assessing posterior pelvic injury and prolapse in postpartum women. Am J Transl Res. 2023;15:6170–6179. [PMC free article] [PubMed] [Google Scholar]
- 22.Peinado-Molina RA, Hernández-Martínez A, Martínez-Vázquez S, Rodríguez-Almagro J, Martínez-Galiano JM. Pelvic floor dysfunction: prevalence and associated factors. BMC Public Health. 2023;23:2005. doi: 10.1186/s12889-023-16901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quaghebeur J, Petros P, Wyndaele JJ, De Wachter S. Pelvic-floor function, dysfunction, and treatment. Eur J Obstet Gynecol Reprod Biol. 2021;265:143–149. doi: 10.1016/j.ejogrb.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Riaz H, Nadeem H, Rathore FA. Recent advances in the pelvic floor assessment and rehabilitation of Women with Pelvic Floor Dysfunction. J Pak Med Assoc. 2022;72:1456–1459. doi: 10.47391/JPMA.22-83. [DOI] [PubMed] [Google Scholar]
- 25.Dietz HP. Diagnosis of maternal birth trauma by pelvic floor ultrasound. Eur J Obstet Gynecol Reprod Biol. 2023;285:86–96. doi: 10.1016/j.ejogrb.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Vellucci F, Regini C, Barbanti C, Luisi S. Pelvic floor evaluation with transperineal ultrasound: a new approach. Minerva Ginecol. 2018;70:58–68. doi: 10.23736/S0026-4784.17.04121-1. [DOI] [PubMed] [Google Scholar]
- 27.Sun S, Li H, Liu M, Shang Q, Tan Q, Yin W. An evaluation of the effects of gestational weight gain on the early postpartum pelvic floor using transperineal ultrasound. J Ultrasound Med. 2023;42:2331–2338. doi: 10.1002/jum.16257. [DOI] [PubMed] [Google Scholar]
- 28.Xu Z, He H, Yu B, Jin H, Zhao Y, Zhou X, Huang H. Application of transperineal pelvic floor ultrasound in changes of pelvic floor structure and function between pregnant and non-pregnant women. Int J Womens Health. 2022;14:1149–1159. doi: 10.2147/IJWH.S361755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao L, Li F, Wang D, Sheng S. Evaluation of acupuncture treatments of postpartum female pelvic floor dysfunction by four-dimensional transperineal pelvic floor ultrasound. Medicine (Baltimore) 2021;100:e27236. doi: 10.1097/MD.0000000000027236. [DOI] [PMC free article] [PubMed] [Google Scholar]