Abstract
Objective: To investigate the short-term efficacy of combining flexible cystoscopy with flexible ureteroscopy in the treatment of complex renal stones. Methods: The medical records of 145 patients with complex renal stones admitted to Yan’an People’s Hospital from February 2020 to February 2022 were retrospectively analyzed. Among these, 65 patients treated with flexible ureteroscopy alone constituted the control group. The research group consisted of 81 patients receiving both flexible cystoscopy and flexible ureteroscopy. Outcomes compared between the two groups included stone removal rate, operative time, time to ambulation, hospitalization duration, and intraoperative bleeding. Logistic regression analysis was used to assess the risk of stone retention. Results: In the research group, the stone removal rate was 85.19% and the residual stone rate was 14.81%, compared to a stone removal rate of 70.77% and a residual rate of 29.23% in the control group, with a statistically significant difference (P<0.05). The research group had a significantly longer operative time than the control group (P<0.05). However, intraoperative bleeding and hospitalization duration were significantly lower in the research group (P<0.05). There was no statistically significant difference in time to ambulation between the groups (P>0.05). Multivariate logistic regression analysis identified multiple stones (OR=3.581, P=0.013) as an independent risk factor for residual stones, while stone location outside the lower calyx (OR=0.305, P=0.021) and treatment with combined flexible cystoscopy and ureteroscopy (OR=0.398, P=0.160) were independent protective factors against residual stones. The area under the curve for predicting stone retention based on the number of stones, stone location, and treatment modality were 0.647, 0.642, and 0.606, respectively. Conclusion: The combination of flexible cystoscopy and flexible ureteroscopy in treating patients with complex renal stones significantly improves the stone clearance rate and postoperative patient recovery.
Keywords: Flexible cystoscopy, flexible ureteroscopy, complex kidney stones, stone retention
Introduction
Kidney stones are a prevalent condition within the urinary system, accounting for approximately 40% of urological interventions and affecting 8 out of every 1,000 adults annually [1]. Over the past few decades, their prevalence and incidence have consistently increased. A survey conducted in China revealed a prevalence rate of 6.4%, with a gender breakdown of 6.5% for men and 5.1% for women [2].
Complex renal stones, often characterized by their staghorn shape or multiple stones exceeding 2.5 cm in diameter [3], are increasingly common in clinical settings, likely occurring due to shifts in dietary habits [4]. Their intricate distribution, irregular shape, and considerable size make them prone to complications such as urinary tract obstruction and infection, potentially leading to renal failure and kidney damage, posing significant health risks [5]. Due to their size, high stone burden, or abnormal renal anatomy, complex renal stones present a significant challenge for surgical management, making them a recognized technical obstacle in clinical practice [6].
With advancements in endoscopic technology, percutaneous nephrolithotripsy has become a widely used treatment for complex kidney stones. However, this procedure carries risks, including significant intraoperative and postoperative bleeding, which can lead to complications such as nephrectomy and liver, pleural, and intestinal injuries [7].
As medical equipment continues to improve and surgeons gain more experience, minimally invasive procedures such as flexible ureteroscopy, laparoscopic lithotripsy, and combined endoscopic surgeries have gained traction due to their benefits of causing less trauma and enabling quicker recovery [8,9]. The advantages of these minimally invasive surgeries include reduced postoperative pain, shorter hospital stays, and a faster return to normal activities, making them increasingly preferred for treating complex renal stones.
The flexible ureteroscope and flexible cystoscope are valuable tools in urological procedures due to their unique characteristics [10]. The flexible ureteroscope, which can be maneuvered through the urethra, bladder, and ureter, has a bendable lens that allows it to navigate the natural curves of the urinary tract to access different parts of the kidneys, particularly the upper pole and calyces [11]. This makes it especially useful for examining and treating kidney stones in these areas.
On the other hand, the flexible cystoscope is designed to access the bladder through the urethra. Its soft, flexible body and bendable lens enable it to rotate within the urethra and bladder, allowing visualization of various parts of the bladder lining, including areas that are difficult to reach with a rigid scope [12,13].
While these instruments offer distinct advantages in their respective applications, the potential benefits of combining their use in treating complex renal stones or other urological conditions have yet to be fully explored. Therefore, this retrospective study collected data from patients with complex kidney stones treated with two different regimens to observe patient outcomes.
Materials and methods
In this retrospective study, we selected 168 patients from Yan’an People’s Hospital who met the inclusion criteria from February 2020 to February 2022. Considering the exclusion criteria, 23 cases were excluded. Ultimately, data from 145 patients with complex renal stones were included. Based on the surgical approach, 65 patients treated with flexible ureteroscopy alone were designated as the control group, and 81 patients treated with a combination of flexible cystoscopy and flexible ureteroscopy were designated as the research group. The study was conducted with the approval of the Medical Ethics Committee of Yan’an People’s Hospital.
Inclusion criteria
Patients diagnosed with complex kidney stones based on clinical history and imaging findings [14]. Patients with preoperative urinary tract infections treated with anti-infective therapy and well-controlled. Patients treated with cystoscopy combined with ureteroscopy or ureteroscopy alone. Age ≥18 years old.
Exclusion criteria
Patients with a combination of acute and chronic infectious diseases. Patients with a previous surgical history of kidney stones. Patients with severe cardiovascular, cerebrovascular, pulmonary, hepatic, or other vital organ diseases. Patients with severe upper or lower urinary tract infections. Patients with systemic hemorrhagic disorders where coagulation had not been corrected.
All patients underwent ureteroscopy and double J stent placement (Shenzhen Kangyibo Company, China) prior to surgery. After general anesthesia, the control group was placed in the lithotomy position. A rigid ureteroscope (German Wolf, size F8/9.8) was inserted into the bladder to remove the double J stent. The ureteral opening was located, and a zebra guidewire was inserted into the ureter on the affected side. The rigid ureteroscope was advanced into the affected ureter and then removed. A flexible ureteroscope (German Wolf, size F12/14) sheath was then introduced to the renal pelvis. Upon locating the stone, the zebra guidewire was withdrawn, and a holmium laser fiber (Lumenis, USA) with a frequency and power setting of 20 Hz and 1 J was used to break the stone.
In the research group, a flexible cystoscope (OLYMPUS, EVIS EXERA III CLV-190) was additionally used to explore each calyx. Residual stones were located and fragmented until no residual stones were detected in the calyx after repeated exploration. Both the double J stent and nephrostomy tube were left in place, and the operation was concluded.
The following factors were analyzed from the collected patient data: gender, age, stone diameter, duration of disease, BMI, site, number of stones, location of stones, degree of hydronephrosis, blood creatinine levels, stone clearance rate, operation time, intraoperative bleeding, time to ambulation, and length of hospital stay. The study flow is illustrated in Figure 1.
Figure 1.
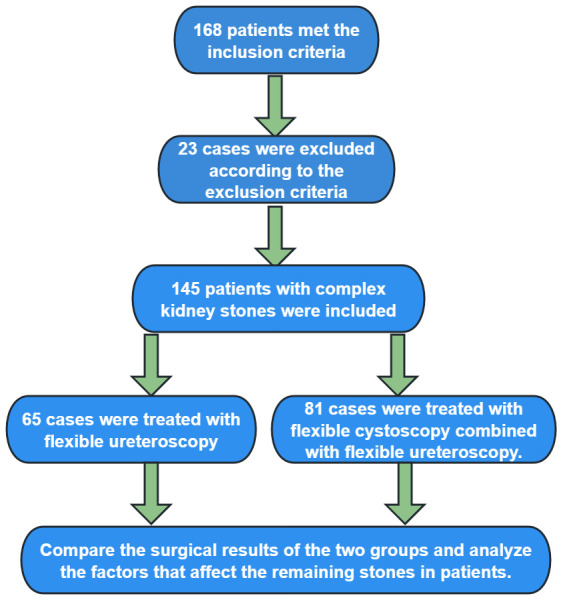
Flowchart.
The one-time stone clearance rate was determined postoperatively. The criteria for one-stage stone clearance were as follows: if the diameter of the residual stone was ≤4 mm and the anatomy of the upper urinary tract was normal on the 2nd and 3rd postoperative days, it was considered completely cleared. Otherwise, residual stones were noted.
Observation indicators
The primary observation indicators were the postoperative stone clearance rate and residual stone rate for both groups. Secondary indicators included surgery-related metrics such as operation time, time to ambulation, length of hospital stay, and blood loss. The risk of residual stones was evaluated using logistic regression analysis, and the Receiver Operating Characteristic (ROC) curve was drawn to assess the predictive value for residual stones.
Statistical methods
Based on previous data and literature [15,16], we estimated the stone-free rate of the control group to be approximately 68% and that of the research group to be about 88%. Using the formula n = (Zα/2 + Zβ)2 × [p1 (1 - p1) + p2 (1 - p2)]/(p1 - p2)2 estimate the sample size, where α=0.05, Zα/2=1.96, β=0.20, Zβ=0.84, p1=0.88, p2=0.68, we calculated that at least 64 subjects were needed per group. Accordingly, 65 individuals were included in the control group and 81 in the research group, based on the hospital’s capacity. All statistical analyses were performed using IBM SPSS software version 20.0 (IBM Corp, Armonk, NY, USA). A p-value of less than 0.05 was considered statistically significant. For normally distributed measurement variables, data were described using the median ± standard deviation, and comparisons between two independent groups were made using the independent samples t-test. For categorical variables, chi-square analysis was employed. Logistic regression analysis was used to assess the risk of stone retention, with odds ratio (OR) values calculated for risk assessment. The ROC curve was utilized to analyze the best cutoff value for measures and the predictive value for the risk of stone retention.
Results
Comparison of baseline information
The baseline data of the two groups were compared, revealing no statistically significant differences in gender, age, stone diameter, disease duration, BMI, site, number of stones, stone location, degree of hydronephrosis, and blood creatinine levels (P>0.05), as shown in Table 1.
Table 1.
Comparison of baseline data
| Control group (n=65) | Research group (n=81) | t/χ2 | P | |
|---|---|---|---|---|
| Gender | ||||
| Male | 35 | 52 | ||
| Female | 30 | 29 | 1.605 | 0.205 |
| Age (years) | 45.14±9.80 | 46.70±10.09 | 0.947 | 0.345 |
| Stone diameter (mm) | 15.42±6.16 | 14.22±5.41 | 1.227 | 0.222 |
| Duration of the disease (months) | 26.45±4.86 | 27.63±4.40 | 1.521 | 0.131 |
| BMI (kg/m2) | 23.24±2.01 | 23.13±2.52 | 0.276 | 0.783 |
| Site | ||||
| Left kidney | 34 | 39 | ||
| Right kidney | 31 | 42 | 0.250 | 0.617 |
| Number of stones | ||||
| Single-shot | 26 | 36 | ||
| Frequent | 39 | 45 | 0.292 | 0.589 |
| Location of stones | ||||
| Renal calyces | 40 | 45 | ||
| Non-renal calyces | 25 | 36 | 0.531 | 0.466 |
| Degree of hydronephrosis | ||||
| No or slight water accumulation | 42 | 44 | ||
| Moderate or severe water accumulation | 23 | 37 | 1.579 | 0.209 |
| Blood creatinine (μmol/L) | 64.90±8.25 | 63.51±8.16 | 1.016 | 0.312 |
BMI: Body mass index.
Comparison of surgical outcomes
Comparing the stone removal and residual rates between the two groups, the research group had a residual rate of 14.81%, significantly lower than the control group’s residual rate of 29.23% (P<0.05), as shown in Table 2.
Table 2.
Results of surgical outcomes
| Stone removal | Clearance | Stones remain | Residual rate | |
|---|---|---|---|---|
| Control group (n=65) | 46 | 70.77% | 19 | 29.23% |
| Research group (n=81) | 69 | 85.19% | 12 | 14.81% |
| χ2 | 4.481 | |||
| P | 0.034 |
Comparison of surgical and postoperative recovery
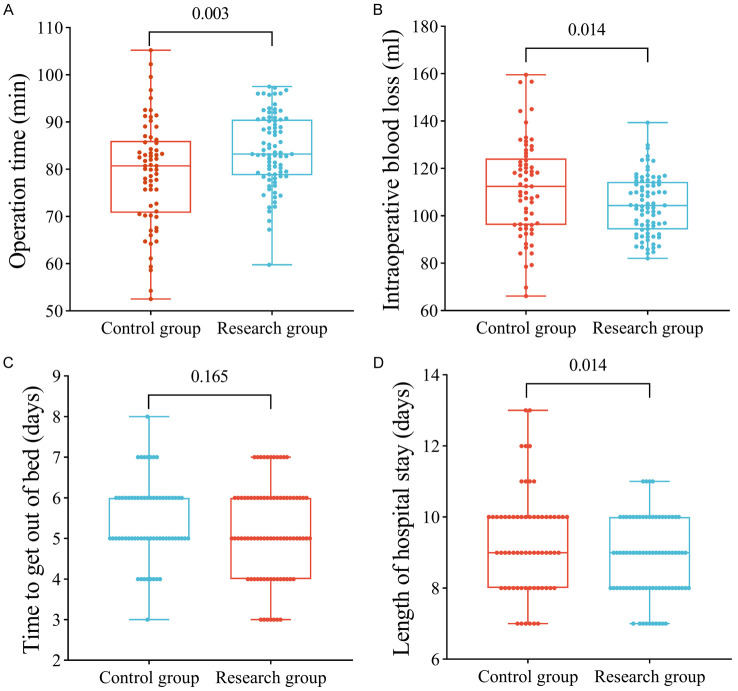
Comparing the operation and postoperative recovery metrics between the two groups, the research group had a significantly longer operation time than the control group (P<0.05). Besides, the research group experienced significantly lower intraoperative bleeding and shorter hospitalization times compared to the control group (P<0.05). There was no statistical difference between the groups regarding the time to ambulation (P>0.05), as illustrated in Figure 2.
Figure 2.
Comparison of surgical and postoperative recovery. A. The operation time was significantly higher in the research group than that in the control group. B. Intraoperative bleeding was significantly lower in the research group than that in the control group. C. The comparison of time to ambulation between the two groups was not statistically significant. D. The length of hospital stay was significantly lower in the research group than that in the control group. ns indicates P>0.05, * indicates P<0.05, and ** indicates P<0.01.
Analysis of optimal cutoff value for the measures
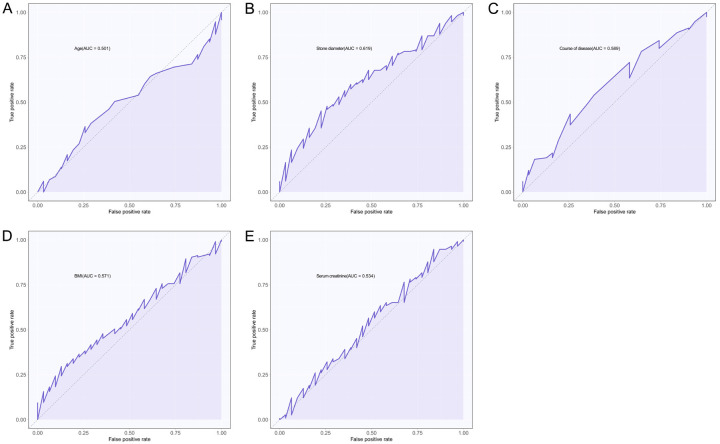
Since logistic regression requires dichotomous information analysis, we used the ROC curve to determine the optimal cutoff values for age, stone diameter, disease duration, BMI, and blood creatinine. These measures were classified as dichotomous information based on the optimal cutoff values. The area under the curve (AUC) was 0.501 for age, 0.619 for stone diameter, 0.589 for disease duration, 0.571 for BMI, and 0.534 for blood creatinine. The optimal cutoff values were 41.5 years, 25.835 mm, 13.5 months, 21.705 kg/m2, and 50.77 µmol/L, respectively, as shown in Figure 3 and Table 3.
Figure 3.
ROC curves of metrological data in predicting stone retention. A. ROC curve for age in predicting stone retention. B. Stone diameter in the ROC curve is used to predict stone retention. C. Disease duration in predicting ROC curves for stone retention. D. ROC curve of BMI in predicting stone retention. E. ROC curve of blood creatinine in predicting stone retention. ROC: Receiver Operating Characteristic, BMI: Body mass index.
Table 3.
ROC curve results
| AUC | 95% CI | Specificity | Sensitivity | Youden index | Cut off | |
|---|---|---|---|---|---|---|
| (A person’s) age | 0.501 | 0.393-0.609 | 74.19% | 36.52% | 10.72% | 41.5 |
| Stone diameter | 0.619 | 0.513-0.724 | 77.42% | 45.22% | 22.64% | 25.835 |
| Course of disease | 0.589 | 0.479-0.699 | 74.19% | 43.48% | 17.67% | 13.5 |
| BMI | 0.571 | 0.464-0.678 | 87.10% | 29.57% | 16.66% | 21.705 |
| Creatinine | 0.534 | 0.415-0.652 | 16.13% | 94.78% | 10.91% | 50.77 |
BMI: Body mass index.
Analysis of single factors affecting stone retention
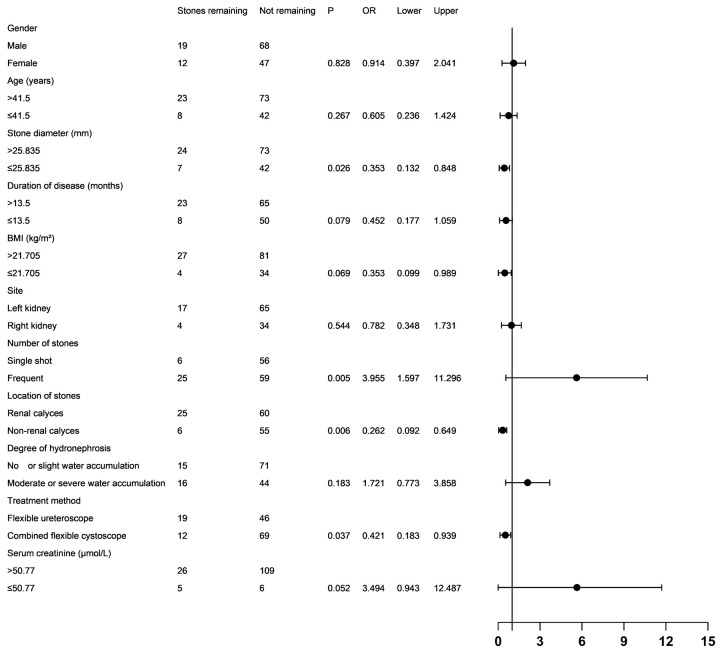
Univariate analysis of 31 patients with residual stones and 115 patients without residual stones showed that stone diameter, number of stones, stone location, and treatment method were significantly associated with residual stones (P<0.05). In contrast, gender, age, disease duration, BMI, stone site, and degree of hydronephrosis were not significantly associated with residual stones (all P>0.05), as shown in Figure 4.
Figure 4.
Forest plot of the results of the single-factor analysis. BMI: Body mass index.
Multifactorial analysis
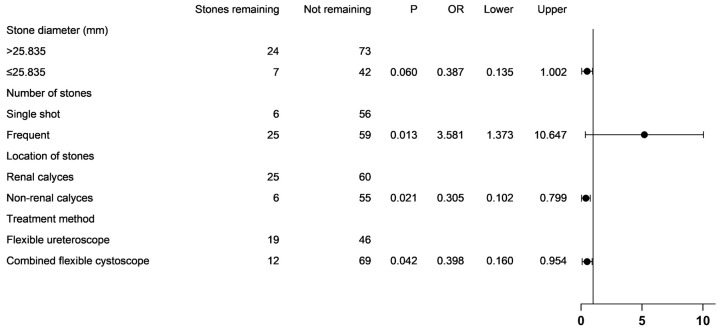
Multifactorial logistic regression analysis revealed that having multiple stones (OR=3.581, P=0.013) was an independent risk factor for residual stones. Conversely, stone location outside the inferior calyces (OR=0.305, P=0.021) and treatment with a combination of cystoscopy and ureteroscopy (OR=0.398, P=0.042) were independent protective factors against residual stones, as shown in Figure 5.
Figure 5.
Forest plot of the results of the multifactor analysis.
Predictive value of independent influences on stone retention
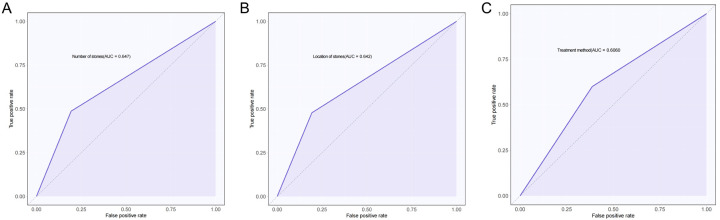
The predictive value of stone number, stone location, and treatment modality for stone retention was evaluated by plotting ROC curves. The AUC for stone number, stone location, and treatment modality were 0.647, 0.642, and 0.606, respectively, indicating modest predictive value, as shown in Figure 6 and Table 4.
Figure 6.
ROC curves for independent influences to predict stone retention. A. Number of stones predicts the ROC curve for stone retention. B. Location of stones predicts ROC curves for stone retention. C. Treatment method predicts the ROC curve for stone retention. ROC: Receiver Operating Characteristic.
Table 4.
ROC curve results
| AUC | 95% CI | Specificity | Sensitivity | Youden index | Cut off | |
|---|---|---|---|---|---|---|
| Number of stones | 0.647 | 0.562-0.731 | 80.65% | 48.70% | 29.34% | 0.5 |
| Location of stones | 0.642 | 0.558-0.727 | 80.65% | 47.83% | 28.47% | 0.5 |
| Treatment method | 0.606 | 0.508-0.705 | 61.29% | 60.00% | 21.29% | 0.5 |
AUC: area under the curve.
Discussion
Recent advances in medical technology have enabled the development of minimally invasive strategies for managing kidney stones, generally yielding favorable outcomes [17,18]. However, complex renal stones still pose significant challenges due to their size, the number of affected calyces, and often associated renal anatomical and functional abnormalities [19]. Key factors in managing these complex kidney stones include enhancing stone removal efficiency, reducing surgical trauma, and facilitating patient recovery [20].
This study compared the surgical outcomes of two groups and found that the stone clearance rate using ureteroscopy alone for complex renal stones was 70.77% [21]. However, when combined with cystoscopy, the clearance rate increased significantly to 85.19%. This improvement in stone clearance may be because complex renal stones are often large and irregularly shaped, making them difficult to remove entirely with ureteroscopy alone [22]. Takazawa et al. [23] suggested that ureteroscopes are more suitable for stones smaller than 20 mm and that staged procedures should be considered for larger stones. Lv et al. [24] found that when the stone’s long diameter is greater than 25 mm, the effectiveness of ureteroscopy diminishes. The combination of cystoscopy and ureteroscopy allows for a more comprehensive evaluation of stone location, size, and the presence of multiple stones. Cystoscopy provides direct visualization of the bladder, while ureteroscopy accesses the upper urinary tract. Bladder endoscopes can assist ureteroscopes in reaching difficult stone locations, such as the lower pole calyces, thereby improving the efficiency of lithotripsy and reducing the likelihood of residual stones postoperatively [25].
After comparing the surgery and postoperative recovery of the two patient groups, it was found that using a combination of ureteroscopy and cystoscopy led to significantly longer operation times than using ureteroscopy alone. However, the research group experienced significantly reduced intraoperative bleeding and hospital stays. The time to ambulation post-surgery was not significantly different between the two groups. Pan et al. [26] mentioned that combined treatment not only needs to consider the patient’s stone clearance rate but also the impact on the operation time. Higher stone fragmentation efficiency can reduce the operation time and significantly lower surgical risks. The increased number of surgical steps due to using two endoscopes may have contributed to the longer operation times. Still, the operation duration remained within two hours, which did not increase patient surgical risk. Despite the longer surgical time, the combined use of the two flexible endoscopes can minimize intraoperative bleeding due to their minimally invasive nature, which reduces tissue damage [27]. Additionally, more precise endoscopic maneuvering and improved visualization mitigate the risk of complications, thereby shortening hospital stays. Zhao et al. [28] noted that combined cystoscopy minimizes postoperative renal impairment. Yang et al. [29] combined cystoscope lithotripsy with the treatment of patients with ureteropelvic junction obstruction and calyceal stones. They mentioned that when the ureteropelvic angle is very small or the patient has severe hydronephrosis, the cystoscope can more easily reach the lower renal calyx, improve the efficiency of stone removal, avoid the risk of secondary surgery, and reduce the patient’s bleeding risk and medical economic burden.
In the involved 146 patients, 31 had residual stones postoperatively. To better understand the causes of residual stones, it is essential to identify suitable patients preoperatively to enhance treatment success. Univariate and multivariate logistic regression analyses showed that multiple stones were an independent risk factor for stone retention. In contrast, stone locations in non-lower calyces and the treatment modality of cystoscopy combined with ureteroscopy were independent protective factors against stone retention.
Multiple stones indicate the presence of more than one stone in different parts of the kidney, including regions that are challenging to visualize or access, increasing the risk of residual stones [30]. Stones in non-lower calyceal regions are more accessible to locate and manage endoscopically. In contrast, anatomical limitations make stones in the lower calyces more challenging to remove [31]. Stones in non-lower calyceal regions are more accessible through ureteroscopes and cystoscopes, reducing the likelihood of residual stones.
The combination of cystoscopy and ureteroscopy provides a comprehensive view, enabling better stone localization and improving stone removal rates. Cystoscopy directly visualizes the bladder and upper urinary tract, while ureteroscopy accesses individual kidney calyces. The combined use of both scopes provides a more thorough assessment. The study evaluated the predictive value of these three independent factors for postoperative stone retention, with the AUC of the ROC curves being 0.647, 0.642, and 0.606, respectively. This suggests that identifying the number of stones, their location, and the treatment modality can more effectively predict and reduce the risk of stone retention.
This study has several limitations. The data collection period was relatively short, and the overall sample size was small, potentially leading to biased results due to subjectivity and selection bias in case selection. Additionally, intraoperative bleeding was influenced by factors such as fluid infusion, leading to potential bias in the results. The stone clearance rate was evaluated 2-3 days postoperatively, but this assessment could be influenced by factors such as intestinal gas interference and stone accumulation. Current studies suggest assessing surgical outcomes and stone retention 1-3 months postoperatively, but this is challenging due to patient loss to follow-up or incomplete case data [32,33]. Additionally, the cost of flexible cystoscopes is relatively high [34]. Many primary hospitals in some developing countries do not have access to flexible cystoscopes, so lower-cost treatment plans should be developed in the future.
In conclusion, the application of cystoscopy combined with ureteroscopy in treating patients with complex renal stones improves the stone clearance rate and postoperative recovery, with a more significant effect compared to ureteroscopy alone.
Disclosure of conflict of interest
None.
References
- 1.Fontenelle LF, Sarti TD. Kidney stones: treatment and prevention. Am Fam Physician. 2019;99:490–496. [PubMed] [Google Scholar]
- 2.Zeng G, Mai Z, Xia S, Wang Z, Zhang K, Wang L, Long Y, Ma J, Li Y, Wan SP, Wu W, Liu Y, Cui Z, Zhao Z, Qin J, Zeng T, Liu Y, Duan X, Mai X, Yang Z, Kong Z, Zhang T, Cai C, Shao Y, Yue Z, Li S, Ding J, Tang S, Ye Z. Prevalence of kidney stones in China: an ultrasonography based cross-sectional study. BJU Int. 2017;120:109–116. doi: 10.1111/bju.13828. [DOI] [PubMed] [Google Scholar]
- 3.Lemberger U, Pjevac P, Hausmann B, Berry D, Moser D, Jahrreis V, Ozsoy M, Shariat SF, Veser J. The microbiome of kidney stones and urine of patients with nephrolithiasis. Urolithiasis. 2023;51:27. doi: 10.1007/s00240-022-01403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zayed S, Goldfarb DS, Joshi S. Popular diets and kidney stones. Adv Kidney Dis Health. 2023;30:529–536. doi: 10.1053/j.akdh.2023.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Song H, Xiao B, Hu W, Zhang G, Fu M, Li J. PCNL cmbined with 3D printing technology for the treatment of complex staghorn kidney stones. J Healthc Eng. 2022;2022:7554673. doi: 10.1155/2022/7554673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Large T, Assmus MA, Valadon C, Emmott A, Forbes CM, Agarwal D, Nottingham C, Scotland K, Rivera M, Chew B, Krambeck A. A multi-institutional review of single-access percutaneous nephrolithotomy for complex staghorn stones. Eur Urol Focus. 2021;7:1170–1175. doi: 10.1016/j.euf.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Cui D, Yan F, Yi J, He D, Zhang Y, Zhang Z, Chen Y, Jiao Y, Zhang B. Efficacy and safety of 3D printing-assisted percutaneous nephrolithotomy in complex renal calculi. Sci Rep. 2022;12:417. doi: 10.1038/s41598-021-03851-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern KL, Borgert BJ, Wolf JS Jr. Steerable ureteroscopic renal evacuation (SURE) for large renal stones: a multi-institutional center study. J Endourol. 2023;37:1179–1183. doi: 10.1089/end.2023.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YH, Jhou HJ, Chou MH, Wu ST, Cha TL, Yu DS, Sun GH, Chen PH, Meng E. Endoscopic combined intrarenal surgery versus percutaneous nephrolithotomy for complex renal stones: a systematic review and meta-analysis. J Pers Med. 2022;12:532. doi: 10.3390/jpm12040532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta R, Mithal P, Klein I, Patel M, Gutierrez-Aceves J. Outcomes and costs following mini-percutaneous nephrolithotomy or flexible ureteroscopic lithotripsy for 1-2-cm renal stones: data from a prospective, randomized clinical trial. J Urol. 2023;209:1151–1158. doi: 10.1097/JU.0000000000003397. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Pan Y, Xiao M, Yang J, Wei Y. The outcomes of pre-stenting on renal and ureteral stones: a meta-analysis. Urol Int. 2022;106:495–503. doi: 10.1159/000519473. [DOI] [PubMed] [Google Scholar]
- 12.Gou L, Wang Z, Zhou Y, Zheng X. Comparison of nephroscopy and cystoscopy used in the treatment of bladder stones: a systematic review and meta-analysis of randomized controlled trials. BMC Surg. 2021;21:448. doi: 10.1186/s12893-021-01461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su J, Zhu Q, Yuan L, Zhang Y, Zhang Q, Wei Y, Shen L. Combined laparoendoscopic single-site ureterolithotomy and flexible cystoscopy in the treatment of concurrent large upper ureteral and renal stones. Scand J Urol. 2017;51:314–318. doi: 10.1080/21681805.2017.1310129. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Long Q, Chen X, He D, He H. Assessment of the SonixGPS system for its application in real-time ultrasonography navigation-guided percutaneous nephrolithotomy for the treatment of complex kidney stones. Urolithiasis. 2017;45:221–227. doi: 10.1007/s00240-016-0897-2. [DOI] [PubMed] [Google Scholar]
- 15.Zanaty F, Elshazly M, Kandeel H, Salman B. A single center comparative study of two single use digital flexible ureteroscopy in the management of renal stones less than 2 cm. World J Urol. 2023;41:777–782. doi: 10.1007/s00345-023-04290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirani KK, Ather MH, Kazmi Z, Aziz W. Access and fluoroscopy time difference in patients undergoing prone percutaneous nephrolithotomy (PCNL) with ureteric catheter placement in supine versus lithotomy position. Cureus. 2022;14:e26220. doi: 10.7759/cureus.26220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuoheti KB, Wang XH, Wang T, Wang YZ, Liu TZ, Wu ZH. A novel double-sheath negative-pressure versus conventional minimally invasive percutaneous nephrolithotomy for large kidney stone. Sci Rep. 2023;13:22972. doi: 10.1038/s41598-023-50237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen ZH, Lee KH, Tseng WH, Su CC, Hsieh KL, Lim CY, Huang SK. Comparison of mini endoscopic combined intrarenal surgery and multitract minimally invasive percutaneous nephrolithotomy specifically for kidney staghorn stones: a single-centre experience. BMC Urol. 2022;22:93. doi: 10.1186/s12894-022-01030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein I, Gutierrez-Aceves J. Preoperative imaging in staghorn calculi, planning and decision making in management of staghorn calculi. Asian J Urol. 2020;7:87–93. doi: 10.1016/j.ajur.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong C, Leveillee RJ. Single upper-pole percutaneous access for treatment of > or = 5-cm complex branched staghorn calculi: is shockwave lithotripsy necessary? J Endourol. 2002;16:477–481. doi: 10.1089/089277902760367430. [DOI] [PubMed] [Google Scholar]
- 21.Ma Y, Jian Z, Xiang L, Zhou L, Jin X, Luo D, Li H, Wang KJ. Development of a novel predictive model for a successful stone removal after flexible ureteroscopic lithotripsy based on ipsilateral renal function: a single-centre, retrospective cohort study in China. BMJ Open. 2022;12:e059319. doi: 10.1136/bmjopen-2021-059319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geavlete B, Mares C, Multescu R, Georgescu D, Geavlete P. Hybrid flexible ureteroscopy strategy in the management of renal stones - a narrative review. J Med Life. 2022;15:919–926. doi: 10.25122/jml-2022-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takazawa R, Kitayama S, Tsujii T. Appropriate kidney stone size for ureteroscopic lithotripsy: when to switch to a percutaneous approach. World J Nephrol. 2015;4:111–117. doi: 10.5527/wjn.v4.i1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv G, Wang K, Zhang Z, Zhou C, Li Y, Zhang D. Comparison of flexible ureteroscopy and mini-percutaneous nephrolithotomy in the treatment for renal calculi larger than 2 cm: a matched-pair analysis. Urolithiasis. 2022;50:501–507. doi: 10.1007/s00240-022-01336-z. [DOI] [PubMed] [Google Scholar]
- 25.Schlager D, Hein S, Obaid MA, Wilhelm K, Miernik A, Schoenthaler M. Performance of single-use FlexorVue vs reusable boavision ureteroscope for visualization of calices and stone extraction in an artificial kidney model. J Endourol. 2017;31:1139–1144. doi: 10.1089/end.2017.0454. [DOI] [PubMed] [Google Scholar]
- 26.Pan SY, Huang CP, Chen WC, Chen YH, Chou EC. Percutaneous nephrolithotomy combined antegrade flexible ureteroscope for complete staghorn stones: a case report of a new concept of stone surgery. Medicina (Kaunas) 2022;59:35. doi: 10.3390/medicina59010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olivero A, Riccardi N, Ndrevataj D, Balzarini F, Cerasuolo M, Bottino P, Borghesi M, Dodi F, Terrone C. Flexible cystoscopy for ureteral stent removal without antimicrobial prophylaxis. A prospective observational study. Urologia. 2021;88:130–134. doi: 10.1177/0391560320980897. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Z, Zhou J, Jing J, Ma Q, Chen M, Yang Y. Clinical efficacy and short-term outcome of combining flexible cystoscopy with percutaneous nephrolithotomy for complex renal stone management. Am J Transl Res. 2024;16:1256–1265. doi: 10.62347/OFET2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C, Zhou J, Lu ZX, Hao Z, Wang J, Zhang L, Liang C. Simultaneous treatment of ureteropelvic junction obstruction complicated by renal calculi with robotic laparoscopic surgery and flexible cystoscope. World J Urol. 2019;37:2217–2223. doi: 10.1007/s00345-018-2608-9. [DOI] [PubMed] [Google Scholar]
- 30.Sahan A, Dincer E, Ozkaptan O, Cubuk A, Ertas K, Eryildirim B, Akca O. The impact of anterior calyceal stones on the outcomes of percutaneous nephrolithotomy for complex kidney stones: a comparative study. Minerva Urol Nephrol. 2021;73:815–822. doi: 10.23736/S2724-6051.20.04002-3. [DOI] [PubMed] [Google Scholar]
- 31.Michel F, Negre T, Baboudjian M, Al-Balushi K, Oliva J, Gondran-Tellier B, Sichez PC, Delaporte V, Gaillet S, Aikiki A, Faure A, Karsenty G, Lechevallier E, Boissier R. Micro-percutaneous nephrolithotomy (Microperc) for renal stones, outcomes and learning curve. Prog Urol. 2021;31:91–98. doi: 10.1016/j.purol.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Oguz U, Sahin T, Senocak C, Ozyuvalı E, Bozkurt OF, Resorlu B, Unsal A. Factors associated with postoperative pain after retrograde intrarenal surgery for kidney stones. Turk J Urol. 2017;43:303–308. doi: 10.5152/tud.2017.58997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu C, Song R, Lu P, Jiang M, Zeng G, Zhang W. A retrospective study comparing super-mini percutaneous nephrolithotomy and flexible ureteroscopy for the treatment of 20-30 mm renal stones in obese patients. PeerJ. 2020;8:e8532. doi: 10.7717/peerj.8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Zeng X, Luo D, Ma Y, Li H, Wang K. Rigid ureteroscopy, a neglected choice for stent removal: a randomized controlled trial to compare rigid ureteroscopy, flexible cystoscopy, and rigid cystoscopy. Chin Med J (Engl) 2022;135:2767–2769. doi: 10.1097/CM9.0000000000002242. [DOI] [PMC free article] [PubMed] [Google Scholar]