Abstract
Objective: To assess the role of prothrombin time (PT), activated partial thromboplastin time (APTT), and thrombin time (TT) in guiding early rehabilitation following tibial fracture surgery. Methods: A retrospective analysis was conducted on 168 patients treated for tibial fractures from May 2020 to May 2022. Patients were divided into good and poor rehabilitation groups based on treatment outcomes. Data on age, gender, BMI, Schatzker classification, operation time, blood loss, PT, APTT, and TT were collected for univariate analysis. ROC curve analysis determined optimal cut-off values, followed by multivariable logistic regression to identify independent risk factors. A nomogram prediction model was then constructed. Results: Independent risk factors for early recovery included age ≥45 years, Schatzker type III, operation time ≥99.5 minutes, APTT≥28.5 seconds, and TT≥13.5 seconds. The nomogram model demonstrated high prediction accuracy with a C-index of 0.980. Conclusion: Prolonged APTT, extended TT, longer operation time, and higher Schatzker classification were identified as independent risk factors influencing early recovery post-surgery. A logistic regression-based prediction model was developed, facilitating the design of personalized rehabilitation training programs to improve patient outcomes.
Keywords: Tibia fracture, early recovery, coagulation function, prediction model
Introduction
Tibial fractures are a common occurrence in traumatic orthopedics, particularly among male adolescents and women aged 20 to 30 years [1]. In recent years, the incidence of tibial fractures has been rising due to increased transportation convenience and mechanization [2]. Tibial fractures account for approximately 13.7% of all fractures, with adult fractures and long bone fractures comprising 2% and 1-2 out of every 1,250,005 cases, respectively [3]. In the United States, there are approximately 490,000 cases of tibial fractures annually, with around 25% being open fractures. On average, inpatients with tibial fractures stay in the hospital for about 5 days, with treatment costs amounting to roughly $25,000 per case. Consequently, the overall medical expenses surpass $1.7 billion, placing a significant burden on both the public healthcare system and households [4].
Early rehabilitation training is crucial for patients with tibial fractures, offering significant value in promoting the recovery of physical function and improving quality of life. It can facilitate wound healing, reduce postoperative complications, accelerate recovery, enhance self-care abilities, and contribute to shorter hospital stays and reduced medical costs [5]. Additionally, early rehabilitation provides essential psychological support, aiding patients in quicker reintegration into society and daily life. However, challenges such as the lack of clear rehabilitation training guidelines and assessment indicators currently limit the promotion and implementation of early rehabilitation [6].
Coagulation indicators may be related to early rehabilitation in patients, primarily due to the impact of exercise and rehabilitation on coagulation and fibrinolysis markers [7]. Coagulation markers may reach higher baseline levels after exercise. For patients with heart failure, coagulation disorders can affect recovery and prognosis [8,9]. Early coagulation testing and dynamic monitoring of coagulation indicators may hold value in early identification of coagulation disorders, guiding treatment, and improving prognosis, which could be applicable in early rehabilitation [10].
However, there is a lack of specific research directly exploring the relationship between coagulation indicators and early rehabilitation. Additionally, the absence of standards and observational indicators for early rehabilitation may hinder understanding and application of this relationship. Therefore, this study aims to analyze the effects of pre-treatment prothrombin time (PT), activated partial thromboplastin time (APTT), and thrombin time (TT) in guiding early rehabilitation, providing a reference for clinical postoperative rehabilitation.
Methods and materials
Ethical statement
This research was approved by the Medical Ethics Committee of the Fourth Hospital of Wuhan (Ethical approval number: 20200438).
Sample collection
A retrospective analysis was performed on 168 patients with tibial fracture treated in the Department of Trauma Orthopedics of the Fourth Hospital of Wuhan from May 2020 to May 2022.
Inclusion and exclusion criteria
Inclusion criteria: patients treated at the Fourth Hospital of Wuhan who were not transferred from other hospitals; patients who received treatment; patients diagnosed with tibial fractures through imaging; patients with detailed clinical data required for the study; and patients aged over 18 years.
Exclusion criteria: patients with old fractures; patients with nerve injuries; patients with malignant tumors; pregnant women; patients with other conditions, such as serious blood diseases, autoimmune diseases, or heart, lung, liver, and kidney function abnormalities; and patients with conditions such as arteriovenous thrombosis, inflammation, or arthritis, requiring long-term medication for control.
Evaluation criteria for rehabilitation efficacy [11]
Markedly effective: The patient’s fracture site is not tender to touch and does not elicit pain upon percussion. X-ray results show complete healing of the fracture site with no displacement or deformity.
Effective: The patient’s tenderness and percussion pain at the fracture site are significantly reduced. X-ray results show that the fracture is basically healed.
Ineffective: The patient’s tenderness and percussion pain at the fracture site do not decrease. X-ray results show that the fracture is not healed.
Sample screening
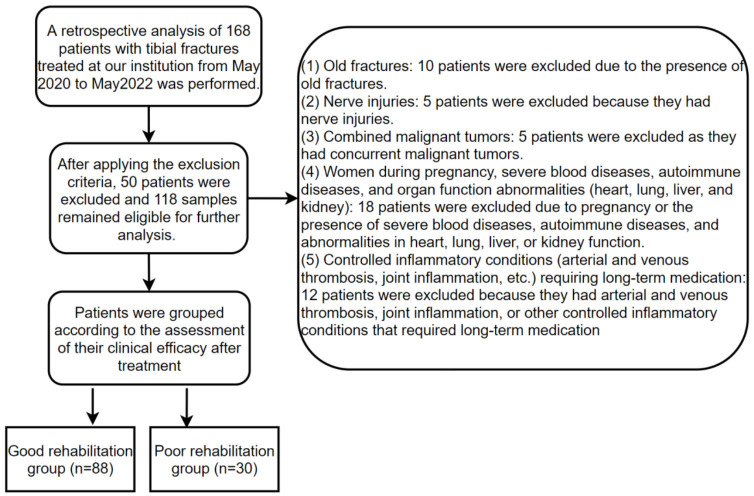
A total of 168 samples were collected for this study, meeting the inclusion criteria. After applying the exclusion criteria, 118 samples remained eligible for further analysis. Based on clinical efficacy assessment results post-treatment, patients were grouped as follows: those with markedly effective and effective outcomes were classified into the good rehabilitation group (n = 88), while those with ineffective outcomes were classified into the poor rehabilitation group (n = 30). The specific screening process is displayed in Figure 1.
Figure 1.
Flowchart of sample screening.
Clinical data
Clinical data were collected from electronic medical records and outpatient records, including age, gender, body mass index (BMI), Schatzker classification, operation time, hospitalization time, intraoperative blood loss, PT, APTT, and TT.
Outcome measures
Logistic regression analysis was performed to identify risk factors influencing early rehabilitation outcomes. Receiver operating characteristic (ROC) curve analysis was used to assess the predictive value of risk factors for poor rehabilitation outcomes. These risk factors were then visualized using a nomogram.
Statistical analysis
SPSS 26.0 was used for statistical analysis. Measurement data were expressed as mean ± standard deviation, and t-tests were conducted for comparisons between groups. Count data were expressed as cases (%), and comparisons between groups were performed using chi-square tests or Fisher’s exact tests. ROC curves were generated to evaluate the predictive value of operation time, PT, APTT, and TT on rehabilitation outcomes, and multivariate logistic regression analysis was conducted to identify independent risk factors affecting rehabilitation outcomes. A nomogram was generated using R software (version 4.3.2) and the “rms” package. Data visualization was performed using GraphPad Prism 8.00. Statistical significance was determined with a threshold of P<0.05.
Results
Univariate analysis of factors influencing early rehabilitation outcomes
Patients were grouped according to their rehabilitation outcomes, with those achieving markedly effective and effective outcomes classified into the good rehabilitation group (n = 88), and the others into the poor rehabilitation group (n = 30). The clinical data of the two groups were compared, revealing that the good rehabilitation group had a significantly higher number of patients aged ≥45 years and with higher Schatzker classifications compared to the poor rehabilitation group (P<0.01) (Table 1). Additionally, the poor rehabilitation group had longer operation times and higher PT, APTT, and TT values compared to the good rehabilitation group (all P<0.001) (Table 1).
Table 1.
Univariate analysis between the two groups
| Factors | Good rehabilitationgroup (n = 88) | Poor rehabilitation group (n = 30) | χ2/t | P |
|---|---|---|---|---|
| Age | 8.198 | 0.004 | ||
| ≥45 years old | 35 | 21 | ||
| <45 years old | 53 | 9 | ||
| Gender | 0.899 | 0.343 | ||
| Male | 50 | 20 | ||
| Female | 38 | 10 | ||
| Body mass index | 0.467 | 0.527 | ||
| ≥25 kg/m2 | 26 | 11 | ||
| <25 kg/m2 | 62 | 19 | ||
| Schatzker classification | 13.512 | 0.001 | ||
| I | 48 | 8 | ||
| II | 30 | 10 | ||
| III | 10 | 12 | ||
| Operation time (min) | 95.17±15.16 | 111.50±11.90 | 5.356 | <0.001 |
| Hospitalization time (d) | 8.96±2.80 | 9.06±3.02 | 0.161 | 0.871 |
| Intraoperative blood loss (mL) | 127.86±17.48 | 131.90±17.96 | 0.286 | 1.071 |
| PT (s) | 12.93±1.34 | 11.92±1.12 | 4.057 | <0.001 |
| APTT (s) | 27.70±3.17 | 24.88±3.03 | 4.355 | <0.001 |
| TT (s) | 13.70±0.88 | 12.23±1.65 | 4.673 | <0.001 |
| Comorbid hypertension | 2.031 | 0.154 | ||
| Yes | 11 | 7 | ||
| No | 77 | 23 | ||
| Comorbid diabetes mellitus | 0.567 | 0.451 | ||
| Yes | 10 | 5 | ||
| No | 78 | 25 | ||
| History of smoking | 2.317 | 0.128 | ||
| Yes | 15 | 9 | ||
| No | 73 | 21 | ||
| History of drinking | 3.420 | 0.064 | ||
| Yes | 13 | 9 | ||
| No | 75 | 21 | ||
| Place of residence | 1.856 | 0.173 | ||
| Rural areas | 67 | 19 | ||
| Urban areas | 21 | 11 |
Notes: PT, Prothrombin Time; APTT, Activated Partial Thromboplastin Time; TT, Thrombin Time.
Predication value of operation time, PT, APTT, and TT in rehabilitation outcomes
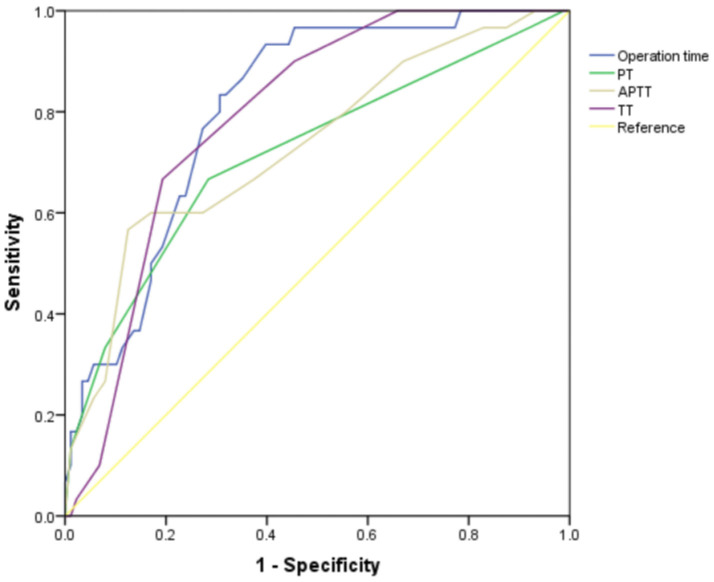
ROC curve analysis indicated that the area under the curve (AUC) for all four indicators was greater than 0.7, demonstrating their clinical predictive value (Table 2; Figure 2).
Table 2.
ROC curve parameters
| Predictor variables | Area under the curve (AUC) | Confidence interval (CI) | Sensitivity | Specificity | Youden index | Cut-off |
|---|---|---|---|---|---|---|
| Operation time | 0.807 | 0.725-0.888 | 60.23% | 93.33% | 53.56% | 99.5 |
| PT | 0.717 | 0.606-0.828 | 71.59% | 66.67% | 38.25% | 12.5 |
| APTT | 0.733 | 0.625-0.841 | 87.50% | 56.67% | 44.17% | 28.5 |
| TT | 0.791 | 0.711-0.871 | 80.68% | 66.67% | 47.35% | 13.5 |
Notes: PT, Prothrombin Time; APTT, Activated Partial Thromboplastin Time; TT, Thrombin Time.
Figure 2.
ROC curve of PT, APTT, TT and operation time before treatment for predicting rehabilitation outcomes. Notes: PT, Prothrombin Time; APTT, Activated Partial Thromboplastin Time; TT, Thrombin Time.
Risk factor assignment and multivariate logistics regression analysis
For multivariate logistic regression, variables with significant differences were assigned values, including age, Schatzker classification, operation time, PT, APTT, and TT (Table 3). The values for operation time, PT, APTT, and TT were assigned based on the cut-off values in Table 2. The cut-off values for operation time, PT, APTT, and TT were 99.5 minutes, 12.5 seconds, 28.5 seconds, and 13.5 seconds, respectively. Multivariate logistic regression analysis identified age, Schatzker classification, operation time, APTT, and TT as independent risk factors affecting rehabilitation outcomes (all P<0.05) (Table 4).
Table 3.
Assignment table
| Factors | Assignment |
|---|---|
| Age | ≥45 = 1, <45 = 0 |
| Schatzker classification | I = 0, II = 1, III = 2 |
| Operation time | ≥99.5 min = 1, <99.5 min = 0 |
| PT | ≥12.5 s = 1, <12.5 s = 0 |
| APTT | ≥28.5 s = 1, <28.5 s = 0 |
| TT | ≥13.5 s = 1, <13.5 s = 0 |
| Rehabilitation outcomes | Good = 0, poor = 1 |
Notes: PT, Prothrombin Time; APTT, Activated Partial Thromboplastin Time; TT, Thrombin Time.
Table 4.
Multivariate analysis
| Factors | β | Standard error | Wald | Sig. | Log OR value | Log 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower limit | Upper limit | ||||||
| Age | 3.444 | 1.213 | 8.055 | 0.005 | 1.496 | 0.463 | 2.529 |
| Schatzker classification | 2.442 | 0.716 | 11.639 | 0.001 | 1.061 | 0.451 | 1.670 |
| Operation time | 4.617 | 1.428 | 10.454 | 0.001 | 2.005 | 0.790 | 3.221 |
| PT | 1.773 | 0.956 | 3.438 | 0.064 | 0.770 | -0.044 | 1.584 |
| APTT | 3.809 | 1.14 | 11.17 | 0.001 | 1.654 | 0.684 | 2.625 |
| TT | 3.066 | 1.032 | 8.834 | 0.003 | 1.331 | 0.453 | 2.210 |
Notes: PT, Prothrombin Time; APTT, Activated Partial Thromboplastin Time; TT, Thrombin Time.
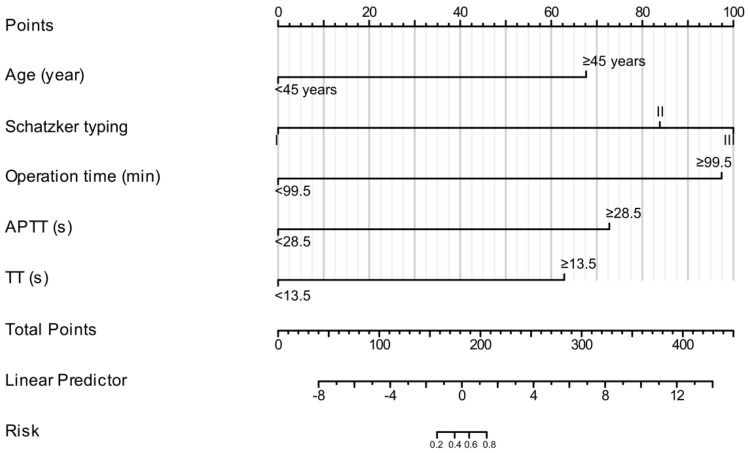
Nomogram construction
Based on the variables identified by multivariate logistic regression analysis (age, Schatzker classification, operation time, APTT, and TT), a risk model nomogram was constructed to predict the efficacy of early rehabilitation training for patients after tibial fracture surgery. The C-index of the nomogram was 0.980 (0.957-1.002), indicating high accuracy (Figure 3). Scores were assigned as follows: 67.5 for age, 100 for Schatzker classification, 97.5 for operation time, 72.5 for APTT, and 62.5 for TT. A higher score corresponds to a higher risk of poor rehabilitation outcomes. For example, if a patient is aged ≥45 years, with Schatzker Type I classification, operation time of 101 minutes, APTT of 29 seconds, and TT of 12 seconds, the calculated total score would be 165, with an estimated probability of 37.5% for experiencing poor rehabilitation outcomes.
Figure 3.
Nomogram for predicting the outcomes of early rehabilitation training in patients after tibial fracture surgery. Notes: APTT, Activated Partial Thromboplastin Time; TT, Thrombin Time.
Discussion
The primary value of early rehabilitation training after tibial fracture surgery lies in its ability to expedite the recovery process, improve patients’ quality of life, alleviate postopative complications, and reduce medical costs [12,13]. Early rehabilitation training is advantageous in restoring joint motion, enhancing muscle strength, reducing the risk of muscle atrophy and joint stiffness, alleviating postoperative pain and swelling, promoting wound healing, preventing complications such as deep vein thrombosis, and improving patients’ psychological well-being and self-confidence [14-17].
However, the current evaluation of early rehabilitation training lacks comprehensive scores or indicators, which hinders accurate assessment and continuous optimization of its effects [18,19]. This limitation affects the standardization and individualization of rehabilitation training programs [20]. Moreover, existing assessment indicators may not fully capture the significance of rehabilitation training in enhancing overall quality of life and reducing long-term disability. In this context, early coagulation testing and dynamic monitoring of coagulation indicators could be valuable in early rehabilitation training [10]. Coagulation disorders can impact recovery and prognosis, especially in patients with heart failure [8,9]. In our study, univariate analysis revealed that the poor rehabilitation group had a significantly higher number of patients aged ≥45 years and those with higher Schatzker grades compared to the good rehabilitation group. Additionally, this group had longer surgery times and elevated levels of PT, APTT, and TT. These findings suggest that age, fracture severity, surgery duration, and coagulation function are key factors influencing rehabilitation outcomes. As patients age, their metabolic rate and self-healing abilities tend to decline, impacting their recovery process [21]. Furthermore, increased fracture severity often indicates more complex fracture patterns and greater soft tissue damage [22,23]. Longer surgery times are typically associated with more intricate and extensive procedures, while abnormal coagulation function can elevate the risk of postoperative bleeding or thrombosis [24]. The interplay of these factors is crucial as they collectively influence the overall recovery process.
Multivariate logistic regression analysis in this study identified APTT, prolonged TT, longer operation time, and higher Schatzker classification as significant risk factors impacting rehabilitation outcomes. Coagulation function plays a critical role in both the surgical and rehabilitation processes, with APTT and TT serving as important indicators of coagulation system function. Prolonged APTT and TT times may reflect abnormal coagulation function, increasing the risk of postoperative bleeding and thrombus formation, potetially delaying rehabilitation. These coagulation abnormalities may require additional monitoring and intervention to prevent postoperative complications and ensure a smooth rehabilitation process [25].
The duration of surgery is often related to the complexity and difficulty of the procedure. Longer operations may indicate more complex surgeries, resulting in increased tissue trauma and blood loss [26]. Patients with tibial shaft fractures who underwent open reduction and internal fixation were reportedly more prone to surgical site complications, such as incision dehiscence and infections, compared to those who underwent intramedullary nail insertion [27]. Prolonged operation time may also increase the risk of postoperative complications, such as infection, bleeding, or blood clots, adversely affecting early recovery and extending the rehabilitation process.
Schatzker classification is a critical indicator for evaluating the severity of tibial plateau fractures. Higher classifications indicate more severe fractures, potentially requiring more complex surgical management. A recent study identified Schatzker type V and VI fractures, along with prolonged intraoperative ischemia, as risk factors for postoperative infection following tibial plateau fractures [28]. These factors may delay recovery and increase the risk of complications, necessitating more sophisticated surgical techniques and closer postoperative monitoring and rehabilitation to ensure a smooth recovery.
By considering these four factors, we can better understand patients’ rehabilitation needs and potential risks following tibial fracture surgery, enabling the development of personalized rehabilitation programs and more accurate evaluation of their effectiveness. Through in-depth analysis of these risk factors, we can improve the design and optimization of rehabilitation programs and enhance patient outcomes. ROC curve analysis showed that the AUC for surgery duration, PT, APTT, and TT were all greater than 0.7, indicating their predictive value for recovery outcomes and their association with factors such as surgical complexity, coagulation function abnormalities, and postoperative bleeding risks.
A nomogram is a practical statistical tool widely used in medical and health research. It visually represents the impact of multiple variables on a specific outcome, making the results easily understandable, even for non-experts [29-31]. In our study, we successfully constructed a risk model nomogram based on multivariate logistic regression analysis to predict the efficacy of early rehabilitation training for patients after tibial fracture surgery. The model exhibited high accuracy (C-index: 0.980), providing a valuable tool for clinical practice in developing individualized rehabilitation plans and monitoring strategies.
However, this study has limitations, including its retrospective design, small sample size, and the absence of standardized evaluation criteria for rehabilitation effectiveness. Future research should focus on increasing the sample size, employing prospective study designs, and developing standardized evaluation criteria. Additionally, calibration curves and external validation should be considered to interpret and generalize the findings. Although the risk model nomogram shows high accuracy, it requires validation in a broader patient population. The single-center and single-ethnic sample of this study may limit its generalizability. Future research should include multi-center and multi-ethnic studies to explore different rehabilitation training methods and interventions, optimizing programs, and improving rehabilitation outcomes and quality of life.
In summary, APTT, prolonged TT, extended operation time, and higher Schatzker classification were identified as independent risk factors affecting early recovery for patients after tibial fracture surgery. A precise prediction model based on logistic regression was established, offering a practical tool for developing individualized rehabilitation training programs.
Disclosure of conflict of interest
None.
Abbreviations
- PT
Prothrombin Time
- APTT
Activated Partial Thromboplastin Time
- TT
Thrombin Time
- ROC
Receiver Operating Characteristic
References
- 1.Kazley J, Jahangir A. Tibia diaphyseal fracture. StatPearls. Treasure Island (FL) ineligible companies; 2023. Disclosure: Alex Jahangir declares no relevant financial relationships with ineligible companies. [PubMed] [Google Scholar]
- 2.Larsen P, Elsoe R, Hansen SH, Graven-Nielsen T, Laessoe U, Rasmussen S. Incidence and epidemiology of tibial shaft fractures. Injury. 2015;46:746–750. doi: 10.1016/j.injury.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Adams BG, Houston MN, Cameron KL. The epidemiology of meniscus injury. Sports Med Arthrosc Rev. 2021;29:e24–e33. doi: 10.1097/JSA.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 4.Metcalf KB, Du JY, Lapite IO, Wetzel RJ, Sontich JK, Dachenhaus ER, Janes JL, Ochenjele G. Comparison of infrapatellar and suprapatellar approaches for intramedullary nail fixation of tibia fractures. J Orthop Trauma. 2021;35:e45–e50. doi: 10.1097/BOT.0000000000001897. [DOI] [PubMed] [Google Scholar]
- 5.Lin RC, Chiang SL, Heitkemper MM, Weng SM, Lin CF, Yang FC, Lin CH. Effectiveness of early rehabilitation combined with virtual reality training on muscle strength, mood state, and functional status in patients with acute stroke: a randomized controlled trial. Worldviews Evid Based Nurs. 2020;17:158–167. doi: 10.1111/wvn.12429. [DOI] [PubMed] [Google Scholar]
- 6.Xuefang L, Guihua W, Fengru M. The effect of early cognitive training and rehabilitation for patients with cognitive dysfunction in stroke. Int J Methods Psychiatr Res. 2021;30:e1882. doi: 10.1002/mpr.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsantes AG, Trikoupis IG, Papadopoulos DV, Tsante KA, Mavrogenis AF, Koulouvaris P, Savvidou OD, Kontogeorgakos VA, Piovani D, Kriebardis AG, Bonovas S, Papagelopoulos PJ, Tsantes AE. Higher coagulation activity in hip fracture patients: a case-control study using rotational thromboelastometry. Int J Lab Hematol. 2021;43:477–484. doi: 10.1111/ijlh.13409. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Wang Y, Zhou Z, Pan C, Jiang L, Zhou Z, Meng Y, Charugundla S, Li T, Allayee H, Seldin MM, Lusis AJ. Liver-heart cross-talk mediated by coagulation factor XI protects against heart failure. Science. 2022;377:1399–1406. doi: 10.1126/science.abn0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Y, Wei Z, Li J, Zhu L. Effects of metoprolol succinate combined with entresto on cardiac function indexes and coagulation function in patients with congestive heart failure. Comput Math Methods Med. 2022;2022:9765884. doi: 10.1155/2022/9765884. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Ma Y, Zhou Y, Wu F, Ji W, Zhang J, Wang X. The bidirectional interactions between inflammation and coagulation in fracture hematoma. Tissue Eng Part B Rev. 2019;25:46–54. doi: 10.1089/ten.TEB.2018.0157. [DOI] [PubMed] [Google Scholar]
- 11.Morris R, Pallister I, Trickett R. Measuring outcomes following tibial fracture. Injury. 2019;50:521–533. doi: 10.1016/j.injury.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Dyer SM, Perracini MR, Smith T, Fairhall NJ, Cameron ID, Sherrington C, Crotty M. Rehabilitation following hip fracture. In: Falaschi P, Marsh D, editors. Orthogeriatrics: The Management of Older Patients with Fragility Fractures. 2nd edition. CH: Cham: 2021. pp. 183–222. [Google Scholar]
- 13.Purcell K, Tiedemann A, Kristensen MT, Cunningham C, Hjermundrud V, Ariza-Vega P, Perracini M, Sherrington C. Mobilisation and physiotherapy intervention following hip fracture: snapshot survey across six countries from the Fragility Fracture Network Physiotherapy Group. Disabil Rehabil. 2022;44:6788–6795. doi: 10.1080/09638288.2021.1974107. [DOI] [PubMed] [Google Scholar]
- 14.Hulsbaek S, Bandholm T, Ban I, Foss NB, Jensen JB, Kehlet H, Kristensen MT. Feasibility and preliminary effect of anabolic steroids in addition to strength training and nutritional supplement in rehabilitation of patients with hip fracture: a randomized controlled pilot trial (HIP-SAP1 trial) BMC Geriatr. 2021;21:323. doi: 10.1186/s12877-021-02273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min K, Beom J, Kim BR, Lee SY, Lee GJ, Lee JH, Lee SY, Won SJ, Ahn S, Bang HJ, Cha Y, Chang MC, Choi JY, Do JG, Do KH, Han JY, Jang IY, Jin Y, Kim DH, Kim DH, Kim IJ, Kim MC, Kim W, Lee YJ, Lee IS, Lee IS, Lee J, Lee CH, Lim SH, Park D, Park JH, Park M, Park Y, Ryu JS, Song YJ, Yang S, Yang HS, Yoo JS, Yoo JI, Yoo SD, Choi KH, Lim JY. Clinical practice guideline for postoperative rehabilitation in older patients with hip fractures. Ann Rehabil Med. 2021;45:225–259. doi: 10.5535/arm.21110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unnanuntana A, Kuptniratsaikul V, Srinonprasert V, Charatcharoenwitthaya N, Kulachote N, Papinwitchakul L, Wattanachanya L, Chotanaphuti T. A multidisciplinary approach to post-operative fragility hip fracture care in Thailand - a narrative review. Injury. 2023;54:111039. doi: 10.1016/j.injury.2023.111039. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Roy I, Falvey J, Rudolph JL, Rivera-Hernandez M, Shaibi S, Sood P, Childers C, Karmarkar A. Effect of variation in early rehabilitation on hospital readmission after hip fracture. Phys Ther. 2023;103:pzac170. doi: 10.1093/ptj/pzac170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ficek K, Kedra N, Skowronek R, Kluczniok K, Strozik M, Gwiazdon P, Hajduk G. The fifth metatarsal bone fracture in athletes - modalities of treatment related to agility in soccer players. J Hum Kinet. 2021;79:101–110. doi: 10.2478/hukin-2020-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni M, Sun T, Zhang T, Jin J, Song Y. Quantitative initial safety range of early passive rehabilitation after ankle fracture surgery. Injury. 2022;53:2281–2286. doi: 10.1016/j.injury.2022.03.067. [DOI] [PubMed] [Google Scholar]
- 20.Goodloe JB, Cregar WM, Caughman A, Bailey EP, Barfield WR, Gross CE. Surgical management of proximal fifth metatarsal fractures in elite athletes: a systematic review. Orthop J Sports Med. 2021;9:23259671211037647. doi: 10.1177/23259671211037647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jura M, Kozak LP. Obesity and related consequences to ageing. Age (Dordr) 2016;38:23. doi: 10.1007/s11357-016-9884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabbagh H, Nikolova T, Kakoschke SC, Wichelhaus A, Kakoschke TK. Functional orthodontic treatment of mandibular condyle fractures in children and adolescent patients: an MRI follow-up. Life (Basel) 2022;12:1596. doi: 10.3390/life12101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polizzi A, Ronsivalle V, Lo Giudice A, Isola G, Bianchi A, Santonocito S, Leonardi R, Mummolo S. Orthodontic approaches in the management of mandibular fractures: a scoping review. Children (Basel) 2023;10:605. doi: 10.3390/children10030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zalunardo B, Panzavolta C, Bigolin P, Visonà A. Multidisciplinary care for the prevention and treatment of venous thromboembolism in patients with cancer-associated thrombosis (CAT): impact of educational interventions on CAT-related events and on patients’ and clinicians’ awareness. Life (Basel) 2022;12:1594. doi: 10.3390/life12101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong X, Liu X, Liu Y, Jiang L, Zhang H, Liu B. Clinical efficacy of conventional heparin anticoagulation combined with apixaban in the treatment of patients with cerebral venous thrombosis and its effect on serum D-Dimer and FIB expression. Comput Math Methods Med. 2021;2021:4979210. doi: 10.1155/2021/4979210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu H, Xiao H, Lu G, Fang S. Effect of transdermal fentanyl patch combined with enhanced recovery after surgery on the curative effect and analgesic effect of liver cancer. Biomed Res Int. 2022;2022:9722458. doi: 10.1155/2022/9722458. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Upfill-Brown A, Hwang R, Clarkson S, Brodke D, Devana S, Mayer E, Kelley B, Arshi A, Lee C. Rates and timing of short-term complications following operative treatment of tibial shaft fractures. OTA Int. 2021;4:e158. doi: 10.1097/OI9.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coelho A, Pares-Alfonso I, Companys R, Sanchez-Soler JF, Torres-Claramunt R, Alier A, Monllau JC. Risk factors for infection of tibial plateau fractures. Rev Esp Cir Ortop Traumatol. 2024;68:44–49. doi: 10.1016/j.recot.2023.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Huang D, Zheng S, Huang F, Chen J, Zhang Y, Chen Y, Li B. Prognostic nomograms integrating preoperative serum lipid derivative and systemic inflammatory marker of patients with non-metastatic colorectal cancer undergoing curative resection. Front Oncol. 2023;13:1100820. doi: 10.3389/fonc.2023.1100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng D, Cyr DP, Burtenshaw SM, Callegaro D, Gronchi A, Shultz D, Brar S, Chung P, Gladdy RA, Catton C, Swallow CJ. Effect of preoperative treatment on the performance of predictive nomograms in primary retroperitoneal sarcoma. Ann Surg Oncol. 2022;29:2304–2314. doi: 10.1245/s10434-021-11156-x. [DOI] [PubMed] [Google Scholar]
- 31.Dong DH, Zhang XF, Lopez-Aguiar AG, Poultsides G, Rocha F, Weber S, Fields R, Idrees K, Cho C, Maithel SK, Pawlik TM. Recurrence of non-functional pancreatic neuroendocrine tumors after curative resection: a tumor burden-based prediction model. World J Surg. 2021;45:2134–2141. doi: 10.1007/s00268-021-06020-8. [DOI] [PubMed] [Google Scholar]